Abstract
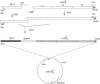
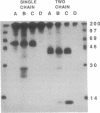
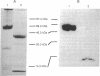
Heterodimeric class I major histocompatibility complex molecules, which consist of a 45-kDa heavy-chain and a 12-kDa beta 2-microglobulin (beta 2m) light chain, bind endogenously synthesized peptides for presentation to antigen-specific T cells. We have synthesized a gene encoding a single-chain, soluble class I molecule derived from mouse H-2Dd, in which the carboxyl terminus of beta 2m is linked via a peptide spacer to the amino terminus of the heavy chain. The chimeric protein is secreted efficiently from transfected L cells, is thermostable, and when loaded with an appropriate antigenic peptide, stimulates an H-2Dd-restricted antigen-specific T-cell hybridoma. Thus, functional binding of peptide does not require the complete dissociation of beta 2m, implying that a heavy chain/peptide complex is not an obligate intermediate in the assembly of the heavy-chain/beta 2m/peptide heterotrimer. Single-chain major histocompatibility complex molecules uniformly loaded with peptide have potential uses for structural studies, toxin or fluor conjugates, and vaccines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen H., Fraser J., Flyer D., Calvin S., Flavell R. Beta 2-microglobulin is not required for cell surface expression of the murine class I histocompatibility antigen H-2Db or of a truncated H-2Db. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7447–7451. doi: 10.1073/pnas.83.19.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird R. E., Hardman K. D., Jacobson J. W., Johnson S., Kaufman B. M., Lee S. M., Lee T., Pope S. H., Riordan G. S., Whitlow M. Single-chain antigen-binding proteins. Science. 1988 Oct 21;242(4877):423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- Boyd L. F., Kozlowski S., Margulies D. H. Solution binding of an antigenic peptide to a major histocompatibility complex class I molecule and the role of beta 2-microglobulin. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2242–2246. doi: 10.1073/pnas.89.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., Queen C., Junghans R. P., Waldmann T. A., FitzGerald D. J., Pastan I. A recombinant immunotoxin consisting of two antibody variable domains fused to Pseudomonas exotoxin. Nature. 1989 Jun 1;339(6223):394–397. doi: 10.1038/339394a0. [DOI] [PubMed] [Google Scholar]
- Elliott T., Cerundolo V., Elvin J., Townsend A. Peptide-induced conformational change of the class I heavy chain. Nature. 1991 May 30;351(6325):402–406. doi: 10.1038/351402a0. [DOI] [PubMed] [Google Scholar]
- Elliott T. How do peptides associate with MHC class I molecules? Immunol Today. 1991 Nov;12(11):386–388. doi: 10.1016/0167-5699(91)90134-f. [DOI] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Shykind B., Seidman J. G., Ozato K. Exon shuffling: mapping polymorphic determinants on hybrid mouse transplantation antigens. Nature. 1982 Dec 23;300(5894):755–757. doi: 10.1038/300755a0. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Deres K., Metzger J., Jung G., Rammensee H. G. Identification of naturally processed viral nonapeptides allows their quantification in infected cells and suggests an allele-specific T cell epitope forecast. J Exp Med. 1991 Aug 1;174(2):425–434. doi: 10.1084/jem.174.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Garboczi D. N., Hung D. T., Wiley D. C. HLA-A2-peptide complexes: refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3429–3433. doi: 10.1073/pnas.89.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godeau F., Casanova J. L., Luescher I. F., Fairchild K. D., Delarbre C., Saucier C., Gachelin G., Kourilsky P. Binding of low concentration of peptide to H-2Kd produced in insect cells requires mouse beta 2-microglobulin co-expression. Int Immunol. 1992 Feb;4(2):265–275. doi: 10.1093/intimm/4.2.265. [DOI] [PubMed] [Google Scholar]
- Gunning P., Leavitt J., Muscat G., Ng S. Y., Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T. H., Myers N. B., Lee D. R. Studies of two antigenic forms of Ld with disparate beta 2-microglobulin (beta 2m) associations suggest that beta 2m facilitate the folding of the alpha 1 and alpha 2 domains during de novo synthesis. J Immunol. 1988 May 15;140(10):3522–3527. [PubMed] [Google Scholar]
- Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989 Apr 15;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Huston J. S., Levinson D., Mudgett-Hunter M., Tai M. S., Novotný J., Margolies M. N., Ridge R. J., Bruccoleri R. E., Haber E., Crea R. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B. H., Smithies O. Inactivating the beta 2-microglobulin locus in mouse embryonic stem cells by homologous recombination. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8932–8935. doi: 10.1073/pnas.86.22.8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski S., Takeshita T., Boehncke W. H., Takahashi H., Boyd L. F., Germain R. N., Berzofsky J. A., Margulies D. H. Excess beta 2 microglobulin promoting functional peptide association with purified soluble class I MHC molecules. Nature. 1991 Jan 3;349(6304):74–77. doi: 10.1038/349074a0. [DOI] [PubMed] [Google Scholar]
- Krangel M. S., Orr H. T., Strominger J. L. Assembly and maturation of HLA-A and HLA-B antigens in vivo. Cell. 1979 Dec;18(4):979–991. doi: 10.1016/0092-8674(79)90210-1. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Stam N. J., Ohlén C., Neefjes J. J., Höglund P., Heemels M. T., Bastin J., Schumacher T. N., Townsend A., Kärre K. Empty MHC class I molecules come out in the cold. Nature. 1990 Aug 2;346(6283):476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- Margulies D. H., Evans G. A., Ozato K., Camerini-Otero R. D., Tanaka K., Appella E., Seidman J. G. Expression of H-2Dd and H-2Ld mouse major histocompatibility antigen genes in L cells after DNA-mediated gene transfer. J Immunol. 1983 Jan;130(1):463–470. [PubMed] [Google Scholar]
- Margulies D. H., Ramsey A. L., Boyd L. F., McCluskey J. Genetic engineering of an H-2Dd/Q10b chimeric histocompatibility antigen: purification of soluble protein from transformant cell supernatants. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5252–5256. doi: 10.1073/pnas.83.14.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey J., Boyd L., Foo M., Forman J., Margulies D. H., Bluestone J. A. Analysis of hybrid H-2D and L antigens with reciprocally mismatched aminoterminal domains: functional T cell recognition requires preservation of fine structural determinants. J Immunol. 1986 Dec 15;137(12):3881–3890. [PubMed] [Google Scholar]
- Mottez E., Jaulin C., Godeau F., Choppin J., Levy J. P., Kourilsky P. A single-chain murine class I major transplantation antigen. Eur J Immunol. 1991 Feb;21(2):467–471. doi: 10.1002/eji.1830210232. [DOI] [PubMed] [Google Scholar]
- Otten G. R., Bikoff E., Ribaudo R. K., Kozlowski S., Margulies D. H., Germain R. N. Peptide and beta 2-microglobulin regulation of cell surface MHC class I conformation and expression. J Immunol. 1992 Jun 15;148(12):3723–3732. [PubMed] [Google Scholar]
- Parker K. C., Silver M. L., Wiley D. C. An HLA-A2/beta 2-microglobulin/peptide complex assembled from subunits expressed separately in Escherichia coli. Mol Immunol. 1992 Mar;29(3):371–378. doi: 10.1016/0161-5890(92)90024-r. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Rothbard J. B., Koszinowski U. H. A pentapeptide as minimal antigenic determinant for MHC class I-restricted T lymphocytes. Nature. 1989 Feb 16;337(6208):651–653. doi: 10.1038/337651a0. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Gamble S., Rothstein L., Benacerraf B. Reassociation with beta 2-microglobulin is necessary for Db class I major histocompatibility complex binding of an exogenous influenza peptide. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):301–304. doi: 10.1073/pnas.88.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Gramm C., Benacerraf B. Low temperature and peptides favor the formation of class I heterodimers on RMA-S cells at the cell surface. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4200–4204. doi: 10.1073/pnas.88.10.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Rothstein L. E., Gamble S. R., Benacerraf B. Reassociation with beta 2-microglobulin is necessary for Kb class I major histocompatibility complex binding of exogenous peptides. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7517–7521. doi: 10.1073/pnas.87.19.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Deres K., Schild H., Norda M., Metzger J., Jung G., Rammensee H. G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990 Nov 15;348(6298):252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- Saper M. A., Bjorkman P. J., Wiley D. C. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6 A resolution. J Mol Biol. 1991 May 20;219(2):277–319. doi: 10.1016/0022-2836(91)90567-p. [DOI] [PubMed] [Google Scholar]
- Shirai M., Pendleton C. D., Berzofsky J. A. Broad recognition of cytotoxic T cell epitopes from the HIV-1 envelope protein with multiple class I histocompatibility molecules. J Immunol. 1992 Mar 15;148(6):1657–1667. [PubMed] [Google Scholar]
- Silver M. L., Parker K. C., Wiley D. C. Reconstitution by MHC-restricted peptides of HLA-A2 heavy chain with beta 2-microglobulin, in vitro. Nature. 1991 Apr 18;350(6319):619–622. doi: 10.1038/350619a0. [DOI] [PubMed] [Google Scholar]
- Tarleton R. L., Koller B. H., Latour A., Postan M. Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature. 1992 Mar 26;356(6367):338–340. doi: 10.1038/356338a0. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Bastin J., Gould K., Brownlee G. G. Cytotoxic T lymphocytes recognize influenza haemagglutinin that lacks a signal sequence. Nature. 1986 Dec 11;324(6097):575–577. doi: 10.1038/324575a0. [DOI] [PubMed] [Google Scholar]
- Townsend A., Elliott T., Cerundolo V., Foster L., Barber B., Tse A. Assembly of MHC class I molecules analyzed in vitro. Cell. 1990 Jul 27;62(2):285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Vitiello A., Potter T. A., Sherman L. A. The role of beta 2-microglobulin in peptide binding by class I molecules. Science. 1990 Dec 7;250(4986):1423–1426. doi: 10.1126/science.2124002. [DOI] [PubMed] [Google Scholar]
- Williams D. B., Barber B. H., Flavell R. A., Allen H. Role of beta 2-microglobulin in the intracellular transport and surface expression of murine class I histocompatibility molecules. J Immunol. 1989 Apr 15;142(8):2796–2806. [PubMed] [Google Scholar]
- Winter G., Milstein C. Man-made antibodies. Nature. 1991 Jan 24;349(6307):293–299. doi: 10.1038/349293a0. [DOI] [PubMed] [Google Scholar]