Abstract
Background:
Diabetes mellitus (DM) remains a major health problem worldwide. Several clinical trials have shown the superiority of the Traditional Chinese Medicine in delaying or reversing the development and progression of DM. This study aimed to evaluate the efficacy of Jinlida (JLD) granule, a Chinese herbal recipe, in the treatment of impaired glucose tolerance (IGT) and its effect on the prevention of DM.
Methods:
Sixty-five IGT patients were randomized to receive one bag of JLD granules three times daily (JLD group, n = 34) or no drug intervention (control group, n = 31) for 12 weeks. Oral glucose tolerance test, glycated hemoglobin A1c (HbA1c), body mass index, blood lipids levels, fasting insulin, and insulin resistance calculated using homeostatic model assessment (HOMA-IR) of all the patients were observed and compared before and after the treatment.
Results:
Sixty-one participants completed the trial (32 in JLD group and 29 in the control group). There were statistically significant decreases in HbA1c (P < 0.001), 2-h plasma glucose (P < 0.001), and HOMA-IR (P = 0.029) in JLD group compared with the control group after 12 weeks of treatment. After 12 weeks of treatment, two (6.9%) patients returned to normal blood glucose, and five (17.2%) patients turned into DM in control group, while in the JLD group, 14 (43.8%) returned to normal blood glucose and 2 (6.2%) turned into DM. There was a significant difference in the number of subjects who had normal glucose at the end of the study between two groups (P = 0.001).
Conclusions:
JLD granule effectively improved glucose control, increased the conversion of IGT to normal glucose, and improved the insulin resistance in patients with IGT. This Chinese herbal medicine may have a clinical value for IGT.
Keywords: Clinical Observation, Diabetes Mellitus, Impaired Glucose Tolerance, Jinlida Granule
Introduction
Type 2 diabetes is a growing public health challenge globally. In the year 2013, 382 million people had diabetes mellitus (DM), this number is expected to rise to 592 million by the year 2035.[1] An even larger segment of the world's population has impaired glucose tolerance (IGT).[2] IGT, which is a risk factor for type 2 diabetes,[3,4] is characterized by an increase in the postprandial glucose levels[5] and considered as an intermediate metabolic state between normal blood glucose and DM. The overall prevalence of DM is estimated to be 11.6% in the Chinese adult population according to the recent national survey.[6] Some studies have discovered that about 5–10% of IGT will develop into DM within one year.[7] IGT could be reversed to normal blood glucose by timely and effective intervention. Therefore, effective interventions for patients with IGT are necessary, which can prevent and delay the development of DM. Several studies have shown that lifestyle or pharmacological therapy could delay the development of DM in patients with IGT,[8,9,10] and clinical research has shown the superiority of the Traditional Chinese Medicine (TCM) in this field.[11,12,13] Jinlida (JLD) granule, a TCM, is an herbal formula developed under the theory that Pi (Spleen)-deficiency appears in the onset of DM. By nourishing Pi and regulating body fluid of DM subjects, JLD granule was used for diabetes management with positive data from the animal and human studies.[14,15,16,17,18] However, the efficacy of JLD granule in the treatment of IGT patients and its effect on the prevention of DM have never been observed. This study therefore was designed to evaluate the efficacy and safety of JLD granule for the IGT treatment and prevention of DM.
Methods
Study design
The research procedures were performed in abidance with standards of quality control in Chinese clinical trial and in accordance with the Declaration of Helsinki. The study was approved by the Medical Ethical Committee of The First Affiliated Hospital of Xiamen University (No. KYZ-2014-004), and the written informed consent was obtained from all the participants.
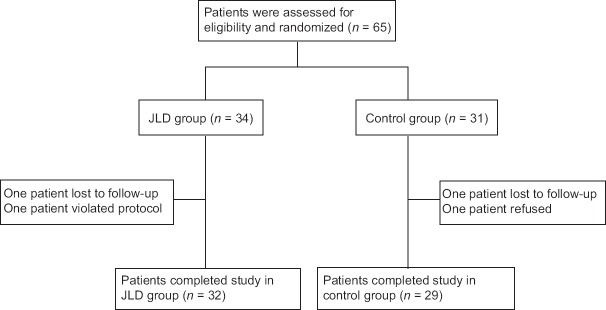
The overall design of the study consisted of a screening visit and a 12-week treatment period. After the initial screening, 65 eligible participants from Department of Endocrinology and Diabetes, The First Affiliated Hospital of Xiamen University, between April 2014 and October 2014 were randomly assigned to the JLD group (n = 34) and control group (n = 31) based on the random block procedure produced by the Random Allocation Software (version 1.0; Isfahan, Iran) [Figure 1]. Participants in JLD group orally took one bag of JLD granules (9 g) three times daily with 120–150 ml warm water after each meal and participants in the control group received no drug treatment. All the participants engaged in standard diet control and exercise therapy. Smoking and alcohol consumption were controlled. During treatment, the use of other TCM that shares similar functional components with JLD granule was prohibited.
Figure 1.
Flow diagram of the participants of this study. JLD: Jinlida.
Inclusion criteria were as follows: (1) patients with IGT, which was defined as fasting plasma glucose (FPG) concentration <7.0 mmol/L and 2-h plasma glucose (2-h PG) concentration ≥7.8 mmol/L and <11.1 mmol/L, according to the World Health Organization criteria,[19] and diagnosis will have been made after undertaking an oral glucose tolerance test (OGTT); (2) age between 20 years and 80 years; and (3) body mass index (BMI) of 18–30 kg/m2.
Exclusion criteria were as follows: (1) Type 1 DM and type 2 DM (T2DM), gestational DM, secondary diabetics, and other special types of DM; (2) ketoacidosis or hyperosmotic coma within 12 weeks; (3) previous history or treatment for type 2 diabetes; (4) serious hepatic and renal function; (5) complicated with severe cardiovascular diseases, severe gastrointestinal disease, or psychiatric diseases; (6) former use of antidiabetic or lipid-lowering drugs; (7) concomitant medication known to interfere with glucose metabolism, such as systemic corticosteroids glucocorticoid within 8 weeks of start date of this study; (8) previous use of growth hormones within 6 months of start date of this study; (9) pregnancy, lactation, or being prepared pregnant women; (10) participated in other clinical trial within the last 2 weeks; or (11) refusing to provide consent for the study.
Study medication
The JLD granules were provided by Shijiazhuang Yiling Pharmaceutical Company (Hebei, China). This herbal drug, prepared in small granules, is dark brown in color and has a bitter taste. The JLD granule contain over a dozen Chinese medicinal herbs, including ginseng (Renshen), puerarin (Gegen), pale white atractylodes rhizome (Cangbaizhu), Coptis chinensis (Huanglian), poria cocos (Fuling), radix polygonati officinalis (Yuzhu), and so on. The quality of these herbs and decoction preparation was in accordance with the requirement of Chinese Pharmacopoeia (2005 edition).
Study assessments
Participants meeting preliminary criteria were invited to attend a clinic visit by a physician to confirm eligibility and have signed an informed consent before any study assessments. All the participants received diabetic education and an exercise program formulated according to age and gender. The following clinical data were collected: demographic data (i.e., gender, age and family history of diabetes), medical history, body weight, height, waist circumference, hip circumference, vital signs, PG, plasma insulin, glycated hemoglobin A1c (HbA1c), total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), cholesterol triglycerides (TG), and liver and kidney function.
During this 12-week period, the study assessments were performed at 0, 4, 8, and 12 weeks. Participants received frequent reminders on healthy lifestyle principles. FPG was checked at each visit. At 0 and 12 weeks, OGTT, HbA1c, routine laboratory tests, serum lipids, and physical examination (body weight, BMI, waist circumference and hip circumference, etc.) were checked, plasma insulin levels were measured, and the homeostatic model assessment (HOMA) was performed to quantify insulin resistance (HOMA-IR).
Body weight will be measured in participants wearing light clothes without shoes using a digital scale to the nearest 0.1 kg. Height will be measured using a portable stadiometer to the nearest 0.1 cm. Waist circumference and hip circumference were measured at the first visit and final visit, as well as BMI. The waist circumference measurement was taken at the midpoint between the lower rib margin and the iliac crest in centimeters to the nearest 0.1 cm, and hip circumference was measured at the most prominent area of hip in centimeter to the nearest 0.1 cm.
PG and insulin levels were tested by OGTT. We collected venous blood samples at fasting, 30 min and 2 h after 75 g glucose ingestion. Blood samples collected by venipuncture for metabolic parameters (i.e., FPG, total cholesterol, LDL-cholesterol, HDL-cholesterol, and TGs) were obtained in the early morning after an 8 h fast and were analyzed by Hitachi Automatic Analyzer (Hitachi, Japan). The HbA1c level was measured using a Bio-Rad D10 Automated HbA1c Analyzer (Bio-Rad, Hercules, CA, USA). Patients having symptoms such as polyuria and polydipsia suggesting DM during the study visits would have a random PG measurement taken by a blood glucose monitor (Contour Plus Blood Glucose Monitoring System, Bayer, Germany). A glucose measurement ≥ 11.1 mmol/L was followed up using a venipuncture PG sample for the patient's safety.
HOMA was used to calculate insulin resistance. The calculation was based on FPG and insulin. In general, a high PG level indicates insulin resistance despite hyperinsulinemia. HOMA-IR was calculated as FPG (mmol/L) × fasting insulin (μU/ml)/22.5.[20,21]
Efficacy evaluation
The primary outcome was the changes in HbA1c, FPG, and 2-h PG levels. The secondary outcome included the conversion of IGT to normal blood glucose, the conversion of IGT to T2DM, and changes in insulin resistance (HOMA-IR).
Safety evaluation
The safety evaluations included routine blood tests, liver function, kidney function, and adverse events (AEs) during the therapeutic period. Subjects were interviewed monthly to record adherence and query about AEs. Any AEs were assessed for severity and possible relationship to study drugs and were followed until they either being resolved or no clinical significance determined by the investigators.
Statistical analysis
We calculated the sample size required to detect an effect size of 0.6 (i.e., mean change in the outcome variable over time differs by at least 0.6 standard deviations (SDs) between the two groups) and thirty patients were required in each group to obtain an alpha of 0.05 and a beta of 0.80. We sought to enroll 35 participants per group to allow for 10% withdrawal and noncompliance. These calculations were based on the changes found in an earlier study of JLD granule.[18]
The statistical analyses were performed using the SPSS Statistics (version 16.0; SPSS Inc., Chicago, IL, USA). Data are expressed as mean ± SD or median (Q1, Q3) for continuous variables and frequencies (percentages) for categorical variables. For between-group comparisons, Student's t-test was performed on variables with normal distributions, Chi-square test for categorical data, and Mann-Whitney's U-test for variables with nonnormal distributions. For in-group comparisons, Paired t-test or Wilcoxon test was performed. Analysis of covariance (ANCOVA) was used to model outcome at 12 weeks by treatment group corrected for baseline level of the outcome variable. All statistical tests were two-sided and assumed to be statistically significant at a level of P < 0.05.
Results
Subject characteristics
A total of 65 patients were enrolled in the study, and 61 (93.8%) patients completed this study. Of these 61 patients, 32 (52.5%) were in the JLD group and 29 (47.5%) in the control group. There were no statistically significant differences between groups in gender, age, BMI, and laboratory data (all P >0.05) [Table 1]. No participants were prescribed other diabetes medications during the study. As the test drug could be taken conveniently, the compliance of patients was generally pretty good.
Table 1.
Patient demographic and clinical characteristics of JLD and control groups at baseline
| Characteristics | JLD group (n = 32) | Control group (n = 29) | t | P |
|---|---|---|---|---|
| Age (years) | 47.1 ± 7.1 | 49.9 ± 7.2 | −1.527 | 0.132 |
| Male | 17 (53.1) | 14 (48.3) | 0.143 | 0.800 |
| Weight (kg) | 61.7 ± 9.0 | 62.7 ± 8.2 | −0.453 | 0.652 |
| BMI (kg/m2) | 22.9 ± 2.5 | 23.6 ± 2.7 | 0.945 | 0.280 |
| Waist (cm) | 83.4 ± 9.1 | 81.5 ± 5.4 | 0.960 | 0.341 |
| Waist/hip ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.957 | 0.343 |
| FPG (mmol/L) | 6.2 ± 0.5 | 6.3 ± 0.6 | −0.956 | 0.343 |
| 2-h PG (mmol/L) | 8.5 ± 2.1 | 9.4 ± 1.6 | −1.841 | 0.071 |
| HbA1c (%) | 6.5 ± 0.7 | 6.5 ± 0.5 | 0.007 | 0.994 |
| Fasting insulin (μU/ml) | 9.2 (7.0, 15.3) | 9.0 (8.1, 10.7) | −0.159 | 0.874 |
| HOMA-IR | 2.4 (2.0, 4.0) | 2.5 (2.1, 3.0) | −0.116 | 0.908 |
| Triglycerides (mg/L) | 15.3 ± 7.8 | 15.4 ± 7.9 | −0.026 | 0.979 |
| Total cholesterol (mg/L) | 56.1 ± 10.5 | 55.5 ± 9.1 | 0.244 | 0.808 |
| HDL-C (mg/L) | 13.5 ± 3.1 | 14.2 ± 2.7 | −0.905 | 0.369 |
| LDL-C (mg/L) | 33.8 ± 6.2 | 32.4 ± 8.3 | 0.740 | 0.462 |
Data are shown as mean ± SD, n (%), or median (Q1, Q3). The difference between groups was nonsignificant at the level P>0.05 at baseline tested by Student’s t-test or Chi-square test. BMI: Body mass index; FPG: Fasting plasma glucose; 2-h PG: 2-h plasma glucose; HbA1c: Glycated hemoglobin A1c; HOMA-IR: Insulin resistance index of homeostasis model assessment; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; JLD: Jinlida; SD: Standard deviation.
Comparisons of blood glucose and glycated hemoglobin A1c
After 12 weeks of treatment, 2-h PG was significantly decreased in JLD group (t = 2.180, P = 0.037) and not significantly changed in control group (t = −2.030, P = 0.052), compared with baseline. An ANCOVA found that 2-h PG showed a statistically significant difference between JLD group and control group after 12 weeks of treatment (F = 14.425, P < 0.001). HbA1c was very significantly decreased in JLD group (t = 4.107, P < 0.001) and not significantly changed in control group (t = −0.946, P = 0.352), compared with baseline. The ANCOVA showed a statistically significant difference in HbA1c between two groups after 12 weeks of treatment (F = 23.340, P < 0.001). FPG was significantly decreased in both groups compared with baseline (t = 4.828, P < 0.001 for JLD group; t = 3.720, P = 0.001 for control group), but the difference between two groups after 12 weeks of treatment was not statistically significant (F = 1.874, P = 0.176) [Table 2].
Table 2.
Clinical variables at baseline and after 12 weeks treatment in JLD and control groups
| Variables | JLD group (n = 32) | Control group (n = 29) | After 12 weeks treatment, JLD group versus control group | |||
|---|---|---|---|---|---|---|
| Baseline | After 12 weeks | Baseline | After 12 weeks | Statistical values | P | |
| FPG (mmol/L) | 6.2 ± 0.5 | 5.8 ± 0.6* | 6.3 ± 0.55 | 6.0 ± 0.6* | 1.874‡ | 0.176 |
| 2-h PG (mmol/L) | 8.5 ± 2.1 | 7.7 ± 2.0† | 9.4 ± 1.6 | 10.3 ± 2.5 | 14.425‡ | <0.001 |
| HbA1c (%) | 6.5 ± 0.7 | 6.0 ± 0.6* | 6.5 ± 0.5 | 6.5 ± 0.5 | 23.340‡ | <0.001 |
| Fasting insulin (μU/ml) | 9.2 (7.0, 15.3) | 9.5 (6.5, 11.9)† | 9.0 (8.1, 10.7) | 11.4 (8.3, 12.4)* | –1.813§ | 0.070 |
| HOMA-IR | 2.4 (2.0, 4.0) | 2.41 (1.7, 3.9)† | 2.5 (2.1, 3.0) | 3.0 (2.1, 3.3)* | –2.181§ | 0.029 |
| Weight (kg) | 61.7 ± 9.0 | 60.2 ± 9.6* | 62.7 ± 8.2 | 61.1 ± 7.6* | 0.005‡ | 0.944 |
| BMI (kg/m2) | 22.9 ± 2.5 | 22.3 ± 2.4* | 23.6 ± 2.7 | 23.0 ± 2.2* | 0.271‡ | 0.605 |
| Waist (cm) | 83.4 ± 9.1 | 82.6 ± 9.4† | 81.5 ± 5.4 | 80.9 ± 4.5 | 0.002‡ | 0.965 |
| Waist/hip ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.0 | 0.135‡ | 0.715 |
| Triglycerides (mg/L) | 15.3 ± 7.8 | 15.0 ± 7.1 | 15.4 ± 7.9 | 13.4 ± 5.4 | 3.289‡ | 0.075 |
| Total cholesterol (mg/L) | 56.1 ± 10.5 | 52.4 ± 10.1* | 55.5 ± 9.1 | 49.7 ± 5.6* | 2.362‡ | 0.130 |
| HDL-C (mg/L) | 13.5 ± 3.1 | 13.1 ± 2.7 | 14.2 ± 2.7 | 13.3 ± 2.4† | 0.268‡ | 0.606 |
| LDL-C (mg/L) | 33.8 ± 6.2 | 32.6 ± 6.1 | 32.4 ± 8.3 | 31.2 ± 5.9 | 0.267‡ | 0.607 |
Data are shown as mean ± SD, or median (Q1, Q3). *P<0.01, †P<0.05, compared with baseline in the same group using paired t-test or Wilcoxon test. ‡Analysis of covariance; §Mann-Whitney’s U-test. BMI: Body mass index; FPG: Fasting plasma glucose; 2-h PG: 2-h plasma glucose; HbA1c: Glycated hemoglobin A1c; HOMA-IR: Insulin resistance index of homeostasis model assessment; HDL: High-density lipoprotein cholesterol; LDL: Low-density lipoprotein cholesterol; JLD: Jinlida; SD: Standard deviation.
Comparison of insulin resistance index of homeostatic model assessment
After 12 weeks of treatment, HOMA-IR was significantly lower in JLD group (Z = −2.412, P = 0.015) and significantly higher in control group (Z = −3.104, P = 0.001), compared with baseline. There was a statistically significant difference in HOMA-IR between two groups after 12 weeks of treatment (Z = −2.181, P = 0.029) [Table 2].
Comparisons of weight, body mass index, waist/hip ratio, and total cholesterol
After 12 weeks of treatment, significant reduction in weight, BMI, and total cholesterol were observed in both groups compared with baseline (JLD group: t = 4.238, P < 0.001 for weight, t = 4.268, P < 0.001 for BMI, t = 3.207, P = 0.003 for total cholesterol; control group: t = 3.228, P = 0.003 for weight, t = 3.274, P = 0.003 for BMI, t = 3.915, P = 0.001 for total cholesterol); but no significant changes in waist/hip ratio were observed between baseline and after 12 weeks of treatment in both groups (all P >0.05). After 12 weeks of treatment, the differences in weight, BMI, waist/hip ratio, and total cholesterol between two groups were not significantly different (all P >0.05) [Table 2].
Comparison of the conversion of impaired glucose tolerance
After 12 weeks of treatment, 2 (6.9%) patients returned to normal blood glucose, 22 (75.9%) patients remained in IGT, and 5 (17.2%) patients developed DM in control group, while in the JLD group, 14 patients (43.8%) returned to normal blood glucose, 16 (50.0%) patients remained in IGT, and 2 (6.2%) patients developed DM. There was a significant difference in the number of subjects who had normal glucose at the end of the study between JLD group and control group (χ2 = 10.678, P = 0.001). The Chi-square test showed no significant difference in comparison of the incidence of DM between the two groups (χ2 = 1.809, P = 0.173).
Adverse reactions
Results of liver and kidney function in both groups examined before and after treatment were all in the normal range. Following the treatment for 12 weeks, AEs were noted in two patients. All reported AEs were gastrointestinal reactions and mild to moderate in severity. One patient in the JLD group experienced diarrhea and one subject in the control group had nausea. No patients were withdrawn from the study due to these AEs. No hypoglycemia or other serious AEs were reported during the study period.
Discussion
Several large-scale intervention trials have demonstrated that lifestyle changes (including dieting and exercising)[22,23,24] and pharmacological interventions[25,26,27,28,29,30] (acarbose, voglibose, metformin, thiazolidinediones, etc.) were effective in preventing T2DM. However, lifestyle intervention is impractical to some extent as it could hardly be carried out by patients to achieve satisfactory improvement. There are also many disadvantages of the Western medicine listed above, such as costly price and AEs. In this field, TCM has been used in the prevention of diabetes and showed certain superiority.[11,12,31,32,33,34]
Several clinical trials have revealed that JLD granule can control blood glucose levels and have systemic benefits in patients with diabetes.[14,15,16,17,18] In an early clinical observational study of 186 patients with T2DM ineffectively managed by metformin monotherapy, 12 weeks of JLD granule treatment as add-on medication decreased HbA1c by 0.92 ±1.09% and reduced FPG and 2-h PG levels.[18] Elevated HbA1c levels that integrated PG overtime was now validated as another indicator of IGT.[5] Our study showed that patients in JLD group had significantly reduced FPG, 2-h PG, and HbA1c after 12 weeks of treatment, and the levels of 2-h PG and HbA1c were very significantly different between two groups after 12 weeks of treatment. Our data indicated that JLD granule had a good hypoglycemic effect, in accord with the result of the previous clinical study on JLD granule.
Chinese herbal medicine is often used in the prevention of different diseases. To date, no clinical trials have been conducted to evaluate whether JLD granule is effective in treating IGT. IGT can be observed as an intermediate stage in diabetes.[19] In this study, the JLD granule effectively enhanced the conversion of IGR to normal blood glucose, and the incidence of DM showed a decreased tendency in JLD group compared with control group.
IGT is associated with the insulin resistance syndrome. Insulin resistance syndrome, which is also known as the metabolic syndrome, is characterized by the presence of insulin resistance, dyslipidemia, increased weight circumference (abdominal obesity), dysglycemia, hypertension, etc.[35] The clinical characters listed above are risk factors that increase the risk of type 2 diabetes, cardiovascular disease, and all-cause mortality.[36] Our data showed that insulin resistance, which directly involved in the pathogenesis of T2DM, was significantly decreased in JLD group after 12 weeks of treatment, compared with baseline. Both JLD and control groups showed lowered weight, BMI, and total cholesterol after 12 weeks of treatment, indicating that our subjects may benefit from standard diet control and exercise therapy. Noticing that mean waist circumference of JLD group was reduced after 12 weeks of treatment (P = 0.037) while no changes were observed in the control group, which implied that patients with abdominal obesity might benefit from JLD granule.
JLD granule is composed of seventeen Chinese medical herbs. Many components of JLD granule, such as ginsenosides, puerarin, and C. chinensis, have been demonstrated to have a hypoglycemic effect.[37,38,39] Besides, Wang et al.[40] supposed that the mechanism underlying the antihyperglycemic effect of JLD granule might be associated with its protection of pancreatic β-cells through AMP-activated protein kinase activation. In the current study, no obvious AEs occurred, which suggested that this herbal drug was safe to use. Although the results of our study need to be confirmed by larger and longer clinical trials in the future, JLD granule had promising potential as an effective means in slowing the progression of IGT to T2DM.
Our study had some limitations, such as relatively short study period and small sample size. Given the limitation of this study, the conversion of IGT to T2DM did not reach the significant level based on statistical analysis. In the future, further interventional studies with longer length of treatment and follow-up and larger sample size are needed to verify it.
In summary, the JLD granule effectively improved glucose control, increased the conversion of IGT to normal glucose, and improved the insulin resistance in individuals with IGT. For IGT individuals who are at high risk of developing T2DM, this Chinese herbal medicine may help to prevent diabetes.
Financial support and sponsorship
This study was supported by a grant from the Xiamen Systems Biology Research Program for Metabolic Disease (No. 3502Z20100001).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
References
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–49. doi: 10.1016/j.diabres.2013.11.002. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Bergman M. Pathophysiology of prediabetes and treatment implications for the prevention of type 2 diabetes mellitus. Endocrine. 2013;43:504–13. doi: 10.1007/s12020-012-9830-9. doi: 10.1007/s12020-012-9830-9. [DOI] [PubMed] [Google Scholar]
- 3.Gerstein HC, Santaguida P, Raina P, Morrison KM, Balion C, Hunt D, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: A systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007;78:305–12. doi: 10.1016/j.diabres.2007.05.004. doi: 10.1016/j.diabres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: A high-risk state for diabetes development. Lancet. 2012;379:2279–90. doi: 10.1016/S0140-6736(12)60283-9. doi: 10.1016/s0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buysschaert M, Bergman M. Definition of prediabetes. Med Clin North Am. 2011;95:289–97. doi: 10.1016/j.mcna.2010.11.002. vii. doi: 10.1016/j.mcna.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–59. doi: 10.1001/jama.2013.168118. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 7.Sicree R, Shaw J, Zimmet P. The global burden of diabetes. In: Gan D, editor. Diabetes Atlas. Brussels, Belgium: International Diabetes Federation; 2003. pp. 15–71. [Google Scholar]
- 8.Fruchter O. Prevention of type 2 diabetes mellitus by changes in lifestyle. N Engl J Med. 2001;345:696. doi: 10.1056/NEJM200108303450912. [PubMed] [Google Scholar]
- 9.Ratner R, Goldberg R, Haffner S, Marcovina S, Orchard T, Fowler S, et al. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care. 2005;28:888–94. doi: 10.2337/diacare.28.4.888. doi: 10.2337/diacare.28.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lian F, Li G, Chen X, Wang X, Piao C, Wang J, et al. Chinese herbal medicine Tianqi reduces progression from impaired glucose tolerance to diabetes: A double-blind, randomized, placebo-controlled, multicenter trial. J Clin Endocrinol Metab. 2014;99:648–55. doi: 10.1210/jc.2013-3276. doi: 10.1210/jc.2013-3276. [DOI] [PubMed] [Google Scholar]
- 12.Fang Z, Zhao J, Shi G, Shu Y, Ni Y, Wang H, et al. Shenzhu Tiaopi granule combined with lifestyle intervention therapy for impaired glucose tolerance: A randomized controlled trial. Complement Ther Med. 2014;22:842–50. doi: 10.1016/j.ctim.2014.08.004. doi: 10.1016/j.ctim.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Guess ND, Dornhorst A, Oliver N, Bell JD, Thomas EL, Frost GS. A randomized controlled trial: The effect of inulin on weight management and ectopic fat in subjects with prediabetes. Nutr Metab (Lond) 2015;12:36. doi: 10.1186/s12986-015-0033-2. doi: 10.1186/s12986-015-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pang J, Gao HL, Wang HT, Wei C, Wu YL. A study on the effect of Jinlida on blood sugar regulating hormone and islet cell function in diabetic rats (in Chinese) Chin J Basic Med Tradit Chin Med. 2014;20:605–7. [Google Scholar]
- 15.Zheng Y, Yan FQ, Wang HY, Yang L, Liu WQ, Wang W, et al. The impact of Jinlida granule on the islet function and TNF-α level in patients with type 2 diabetes, and evaluation on its efficacy and safety (in Chinese) Chin J Basic Med Tradit Chin Med. 2013;19:654–6. [Google Scholar]
- 16.Guo MH, Lu YL. Effects of Jinlida granule combined with metformin on treatment of patients with initial type 2 diabetes (in Chinese) J Clin Med Pract. 2013;17:35–7. [Google Scholar]
- 17.Li JB, Wang DK, Lu FE, Liu C. Evaluation on the efficacy and safety of Jinlida granule in the treatment of type 2 diabetes (in Chinese) Eval Anal Drug Use Hosp Chin. 2013;13:591–4. [Google Scholar]
- 18.Lian F, Tian J, Chen X, Li Z, Piao C, Guo J, et al. The efficacy and safety of Chinese herbal medicine jinlida as add-on medication in type 2 diabetes patients ineffectively managed by metformin monotherapy: A Double-Blind, Randomized, Placebo-Controlled, Multicenter Trial. PLoS One. 2015;10:e0130550. doi: 10.1371/journal.pone.0130550. doi: 10.1371/journal.pone.0130550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. doi: 10.2337/diacare.26.2007.S5. [DOI] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 22.Kitabchi AE, Temprosa M, Knowler WC, Kahn SE, Fowler SE, Haffner SM, et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: Effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404–14. doi: 10.2337/diabetes.54.8.2404. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: A 20-year follow-up study. Lancet. 2008;371:1783–9. doi: 10.1016/S0140-6736(08)60766-7. doi: 10.1016/s0140-6736(08)60766-7. [DOI] [PubMed] [Google Scholar]
- 24.Khemayanto H, Shi B. Role of Mediterranean diet in prevention and management of type 2 diabetes. Chin Med J. 2014;127:3651–6. doi: 10.3760/cma.j.issn.0366-6999.20141358. [PubMed] [Google Scholar]
- 25.Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: Systematic review and meta-analysis. BMJ. 2007;334:299. doi: 10.1136/bmj.39063.689375.55. doi: 10.1136/bmj.39063.689375.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M STOP-NIDDM Trail Research Group. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. Lancet. 2002;359:2072–7. doi: 10.1016/S0140-6736(02)08905-5. doi: 10.1016/s0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 27.Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Kaku K Voglibose Ph- Study Group. Voglibose for prevention of type 2 diabetes mellitus: A randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet. 2009;373:1607–14. doi: 10.1016/S0140-6736(09)60222-1. doi: 10.1016/s0140-6736(09)60222-1. [DOI] [PubMed] [Google Scholar]
- 28.Cai XL, Chen YL, Zhao JJ, Shan ZY, Qiu MC, Li CJ, et al. Efficacy and safety of avandamet or uptitrated metformin treatment in patients with type 2 diabetes inadequately controlled with metformin alone: A multicenter, randomized, controlled trial. Chin Med J. 2015;128:1279–87. doi: 10.4103/0366-6999.156735. doi: 10.4103/0366-6999.156735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knowler WC, Hamman RF, Edelstein SL, Barrett-Connor E, Ehrmann DA, Walker EA, et al. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes. 2005;54:1150–6. doi: 10.2337/diabetes.54.4.1150. doi: 10.2337/diabetes.54.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, et al. DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet. 2006;368:1096–105. doi: 10.1016/S0140-6736(06)69420-8. doi: 10.1016/s0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 31.Sun X, Guo L, Shang H, Ren M, Wang Y, Huo D, et al. The cost-effectiveness analysis of JinQi Jiangtang tablets for the treatment on prediabetes: A randomized, double-blind, placebo-controlled, multicenter design. Trials. 2015;16:496. doi: 10.1186/s13063-015-0990-9. doi: 10.1186/s13063-015-0990-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei Y, Hong YZ, Ye X. Effect of Tang No. 1 granule (1) in treating patients with impaired glucose tolerance. Chin J Integr Med. 2008;14:298–302. doi: 10.1007/s11655-008-0298-7. doi: 10.1007/s11655-008-0298-7. [DOI] [PubMed] [Google Scholar]
- 33.Gao Y, Zhou H, Zhao H, Feng X, Feng J, Li Y, et al. Clinical research of traditional Chinese medical intervention on impaired glucose tolerance. Am J Chin Med. 2013;41:21–32. doi: 10.1142/S0192415X1350002X. doi: 10.1142/s0192415x1350002x. [DOI] [PubMed] [Google Scholar]
- 34.Tong XL, Dong L, Chen L, Zhen Z. Treatment of diabetes using traditional Chinese medicine: Past, present and future. Am J Chin Med. 2012;40:877–86. doi: 10.1142/S0192415X12500656. doi: 10.1142/s0192415x12500656. [DOI] [PubMed] [Google Scholar]
- 35.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 36.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014. 2014 doi: 10.1155/2014/943162. 943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Attele AS, Zhou YP, Xie JT, Wu JA, Zhang L, Dey L, et al. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–8. doi: 10.2337/diabetes.51.6.1851. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Shangguan Z, Liu Y, Wang J, Li X, Yang S, et al. Puerarin protects pancreatic ß-cell survival via PI3K/Akt signaling pathway. J Mol Endocrinol. 2014;53:71–9. doi: 10.1530/JME-13-0302. doi: 10.1530/jme-13-0302. [DOI] [PubMed] [Google Scholar]
- 39.Yin J, Hu R, Chen M, Tang J, Li F, Yang Y, et al. Effects of berberine on glucose metabolism in vitro. Metabolism. 2002;51:1439–43. doi: 10.1053/meta.2002.34715. doi: 10.1053/meta.2002.34715. [DOI] [PubMed] [Google Scholar]
- 40.Wang D, Tian M, Qi Y, Chen G, Xu L, Zou X, et al. Jinlida granule inhibits palmitic acid induced-intracellular lipid accumulation and enhances autophagy in NIT-1 pancreatic ßcells through AMPK activation. J Ethnopharmacol. 2015;161:99–107. doi: 10.1016/j.jep.2014.12.005. doi: 10.1016/j.jep.2014.12.005. [DOI] [PubMed] [Google Scholar]