Abstract
Objective:
To systematically review the updated information about the gut microbiota-brain axis.
Data Sources:
All articles about gut microbiota-brain axis published up to July 18, 2016, were identified through a literature search on PubMed, ScienceDirect, and Web of Science, with the keywords of “gut microbiota”, “gut-brain axis”, and “neuroscience”.
Study Selection:
All relevant articles on gut microbiota and gut-brain axis were included and carefully reviewed, with no limitation of study design.
Results:
It is well-recognized that gut microbiota affects the brain's physiological, behavioral, and cognitive functions although its precise mechanism has not yet been fully understood. Gut microbiota-brain axis may include gut microbiota and their metabolic products, enteric nervous system, sympathetic and parasympathetic branches within the autonomic nervous system, neural-immune system, neuroendocrine system, and central nervous system. Moreover, there may be five communication routes between gut microbiota and brain, including the gut-brain's neural network, neuroendocrine-hypothalamic-pituitary-adrenal axis, gut immune system, some neurotransmitters and neural regulators synthesized by gut bacteria, and barrier paths including intestinal mucosal barrier and blood-brain barrier. The microbiome is used to define the composition and functional characteristics of gut microbiota, and metagenomics is an appropriate technique to characterize gut microbiota.
Conclusions:
Gut microbiota-brain axis refers to a bidirectional information network between the gut microbiota and the brain, which may provide a new way to protect the brain in the near future.
Keywords: Gut Microbiota, Gut Microbiota-brain Axis, Metagenomics, Microbiome
Introduction
The latest research showed that changes in gut microbiota could affect the brain's physiological, behavioral, and cognitive functions.[1,2,3,4,5] In 2013, the United States launched a special research project on gut microbiota-brain axis. Since then, this field, especially the interaction between gut microbiota and the brain, has gradually become the focus of neuroscience.[2,3,4] Although the exact mechanism of gut microbiota-brain axis has not yet been fully understood and clarified, the evidence from animals and human studies has showed that gut microbiota can play an important role in brain behavior and cognitive development by producing hormones, immune factors, and metabolites, which also indicated that altering the gut microbiota may improve or even cure brain diseases.[6,7,8,9,10,11,12,13,14]
The gut microbiota and the brain interact with each other, and the gut microbiota can be regarded as an independent variable in gut microbiota-brain axis, its effect on the brain regarded as a dependent variable. Therefore, this article focused on the influence of gut microbiota on the brain and described the latest progress in gut microbiota, cognitive process of influence of gut microbiota on the brain in neuroscience domain, and the possible measurement and detection of gut microbiota.
Gut Microbiota
Gut microbiota is a complex community that helps to maintain dynamic metabolic ecological balance.[15] There are an estimated 100 trillion bacteria in an adult's body, 80% of which exist in the gut, about ten times as many as cells in human body. The gut microbiome host more than 100 bacterial species that encode 150 times as many genes as the human genome.[16,17] It is well-recognized that human microbiome is composed of more than 5000 strains of microbes and greater than 1000 kinds of microflora.[16,17] The bacteria, mainly anaerobic bacteria, dominate this environment, and others including virus, protozoa, archaea, and fungi also involve in this environment.[18,19] The microbiome is mainly defined by two bacterial phylotypes, Bacteroidetes and Firmicutes, and the amounts of Proteobacteria, Actinomyces, Fusobacterium, and Verrucomicrobia are relatively small.[20]
Gut microbiota is changing with human development and influenced by various stress factors. Babies receive the initial microbiome from their mothers.[20,21] After 1-year-old, the infants form a complex gut microbiome like adults.[22,23] The compositions of gut microbiota are not fixed, and change with increasing age. The dynamic changes are markedly different among different individuals, but the common effect is a macrobalance.[24] The changes of beneficial bacteria can significantly affect the health of individuals, while some factors such as infection, drug, illness, and diet may change the microbiome.[25,26,27]
Some evidence showed that stress in the first few years of life could lead to the change of microbiota, and this change is a risk factor for stress-related disorders in adulthood.[28,29] Maternal separation could lead to the reducing number of Lactobacillus in the feces of baby rats three days later, which had a long-term effect on the gut microbiome.[28] Prenatal stress changed the compositions of microbiota by reducing the total number of Bifidobacterium and Lactobacillus in the rhesus monkey.[30] Moreover, prenatal antibiotic treatment increased offspring's susceptibility to experimental colitis.[31] These results indicated that stress could change the gut microbiome.
Gut microbiota has multiple functions. First, gut microbiota constitutes the intestinal barrier, promotes the continuous existence of gut microbiota, stimulates intestinal epithelial cell regeneration, and produces mucus and nourishes mucosa by producing short-chain fatty acids (SCFAs).[32] Gut microbiota is involved in the maturation of immune system by stimulating innate immune system in the early stage of life, which leads to the maturity of intestinal-related lymphoid tissue, inspires the acquired immunity by stimulating local and systemic immune responses,[33] intestinal synthesis and metabolism of certain nutrients, hormones and vitamins, and plays an important role in drug and poison removal. Under physiological conditions, gut microbiota continues to stimulate the immune system, leading to a state of “low degree of physiological inflammation”, which is a rapid and effective mechanism for defending against pathogens.[34] In addition, bacterial colony also plays a role of protective competition in gut, producing nutrition for the survival of pathogen and cytokines that can inhibit the growth of the microorganism.[35]
Influence of Gut Microbiota on Cognitive Process of Brain
The effect of gut microbiota on the brain was seldom recognized by people at first, except the pathogenic microorganism in the gut can pass through the blood-brain barrier and affect the brain, for instance, rabies virus could elicit aggression, agitation, and a fear of water when it enters the brain.[3] However, a public health emergency aroused people's attention to the possible relationship between the gut microbiota and the brain. In the year 2000, the flood occurred in the town of Walkerton, Canada, making the drinking water polluted by Escherichia coli and Campylobacter jejuni. Among the 4561 infected participants, 2451 of them completed a reassessment 8 years later, and 1166 of them were diagnosed with irritable bowel syndrome (IBS). Among these IBS patients, anxiety and depression were found to be independent risk factors for continuous IBS.[13] But at that time, the interaction between gut microbiota and the brain had not been taken seriously by neuroscientists.
In the year 2011, a study by Diaz Heijtz et al.[5] showed that compared with conventional mice who were growing in specific-pathogen-free (SPF) environment, germ-free (GF) mice under the experimental conditions had less anxiety-like behaviors and increased 5-HT synthesis in the thalamus. When moving the adult GF mice to SPF environment, its reduced anxiety-like behavior did not increase, but its offspring's anxiety behavior returned to the normal state, which indicated that there was a critical time window for the influence of gut microbiota on behavior development. At that time, although there were an increasing number of related studies, most of them were conducted by gastrointestinal scientists alone, few were performed by gastrointestinal scientists together with psychologists. The focuses of the studies were peripheral and behavioral variations rather than variation in the brain. However, the study of Diaz Heijtz et al. sparked an interest of scientists in this field, and they hoped to directly study the underlying mechanism about the role of gut microbiota in the brain.
In the year 2013, the National Institute of Mental Health (NIMH) launched a special project on exploring the mechanism involved in gut microbiota-brain communication, with a view to develop new medications or noninvasive treatments for mental diseases. Since then, studies on the influence of gut microbiota on the brain have been increasing, and gut microbiota-brain axis has become one of the focuses of neuroscience. The core of the axis was the interaction between the gut microbiota and the brain.[2,3,4] Gut microbiota has an important influence on the brain through the neural network, neuroendocrine system, and immune system.[2,3,4,6,7]
During 2014 and 2015, NIMH offered a special fund of 1 million US dollars to study the gut microbiota-brain axis. In the year 2015, the United States Navy Institute planned to provide a special fund of 14.5 million US dollars in the next 6–7 years to research the role of the gut in cognitive and stress disorders. The European Union has launched a 5-year MyNewGut project (10.1 million US dollars) for research on brain development and related disorders.[3]
How Does Gut Microbiota Affect the Brain
Currently, the exact mechanism of communication between the gut microbiota and the brain has not yet been fully understood and clarified. Generally speaking, gut microbiota exerts effects on the brain not only through the nervous system (gut-brain's neuroanatomical pathway) but also through the endocrine system, immune system, and metabolic system. A bidirectional communication between the gut and the brain is referred to as the gut-brain axis.[36,37] Interaction of gut microbiota and gut-brain axis is referred to as the gut microbiota-gut-brain axis (hereinafter referred to as the gut microbiota-brain axis).[38,39] In the gut microbiota-brain axis, because gut microbiota can be used as an independent variable and changed intentionally, more emphases are placed on the role of microbes in gut microbiota-brain axis.[40]
Neuroanatomical pathways
The gut can interact with the brain through two neuroanatomical pathways. The one is mutual information exchange directly between gut and brain by the autonomic nervous system (ANS) and vagus nerve (VN) in the spinal cord; another one is a bidirectional communication between gut and brain through the bi-communication between enteric nervous system (ENS) in the gut and ANS and VN within the spinal cord. The neural anatomical pathways for controlling gut functions form a hierarchic four-level integrative organization:[41,42] the first level is the ENS, including myenteric ganglia, submucous ganglion, and gut glial cells;[43,44] the second level is prevertebral ganglia regulating peripheral visceral reflex responses;[45] The third level is the ANS in the spinal cord (from T5-L2 sympathetic nerve and S2-S4 parasympathetic nervous system) and brain stem nucleus tractus solitarius and dorsal motor nucleus of VN, which receive and give the origin of afferent and efferent fiber of VN, respectively. The most important effect of the dorsal motor nucleus of VN is prominent in the upper gastrointestinal tract, and the cholinergic neurons on myenteron of upper gastrointestinal tract regulate vagal excitability effect;[46] and the fourth level is the higher brain centers. Information from cortex and subcortical centers including basal ganglia and funnels down to peculiar brainstem nuclei. Brainstem nuclei control many gut functions. The afferent fiber of VN stops at the brain stem nucleus tractus solitarius, which then gives fiber upward and arrives at thalamus, lobus limbicus, and insular cortex through parabrachial nucleus. Spinal afferent fiber goes upward within spinothalamic tract and spinal tract to the thalamus (spinothalamic tract) and gracile nucleus and cuneate nucleus of medulla oblongata (spinal tract), respectively, then project fiber to thalamus through lemniscus medialis. Fiber is gave from thalamus and projected to the primary sensorimotor areas and insular cortex. Damages and abnormalities at the above-mentioned levels can influence the regulation of intestinal function, including local intestinal reflexes, and external neural control.[42]
Direct neural communication between gut microbiota and the brain is mainly realized through VN, i.e., bacteria stimulates afferent neurons of ENS,[47] and the vagal signal from the gut can stimulate the anti-inflammatory response, preventing against pyosepticemia caused by microorganisms. Further research showed that many effects of gut microbiota or potential probiotics on brain functions were independent on vagal activation,[39,48] and bacteria settled in the gut played a critically important role in individual's postnatal development and the maturation of the immune system, the endocrine system, and the nervous system.[47]
Neuroendocrine-hypothalamic-pituitary-adrenal axis
Gut microbiota is helpful to the maturation of neuroendocrine. Lacking gut microbiota and low/lack of expression of toll-like receptors (TLRs) contribute to producing a neuroendocrine response to the pathogen in the gut.[49,50] For example, the response of TLR4-knockout mouse to lipopolysaccharide (LPS) produced by Gram-negative bacteria was reduced.[51] Griseofulvin (GF) mouse is one of the most appropriate models to study the hypothalamic-pituitary-adrenal (HPA) axis regulated by the microorganism. Compared with SPF mice, mild restraint stress led to significantly elevated corticosterone and adrenocorticotropic hormone in GF mice. GF mouse's stress response could be partially reversed by fecal microbial transplant, and completely reversed over time by single Bifidobacterium infantis.[52] The study clearly showed the feces containing gut microbiota were vital for the postnatal development of appropriate stress reaction, and the timing that microbiota appeared in early life was a very narrow window, which was extremely important for normal development of HPA axis.
Gut microbiota can affect neural circuits and behavior related with the stress response. Compared with SPF mice, GF male mice had the reduced brain-derived neurotrophic factor (BDNF) and 2A subtype of N-methyl-D-aspartic acid receptor (NMDA receptor) expressions in cortex and hippocampus.[52] Neufeld et al.[53] have found that mRNA of BDNF in the hippocampal area was improved in female mice, which conflicted with the results reported by the earlier studies. Clarke et al.[54] have also found that mRNA of hippocampus BDNF in male mice was reduced and the 5-HT functional system was significantly changed, but these changes could not be found in female mice. These findings suggested that the regulation of gut microbiota-brain axis may be dependent on gender. Many studies have shown the changes of hippocampal NMDA and 5-HT1A receptor of GF animals.[52] These receptors affect the release and expression of the corticotropin-releasing hormone of the hypothalamus and then change the function of HPA.
Stress and HPA axis can affect the composition of the gut microbiome. Early stress and maternal separation could lead to a long-term change of HPA, and also had a long effect on the microbiome.[55,56] When compared with rats nonseparated from the mother, the diversity of 16S ribosomal RNA in adult rats, who received mother separation for 3 h/day from day 2 to day 12 after birth, revealed that stress significantly changed microbiome in feces.[28] Microbiome composition in mouse exposed to a long-term restraint stress was significantly different from that of a nonstressed mouse.[57] Recently, using the above method and the repeated social interaction, stress can reduce the quantity of Bacteroides at cecum and increase the number of Clostridium.[58] Stress also increased interleukin-6 and monocyte chemotactic protein 1 (MCP-1) levels in blood, and MCP-1 was significantly related with the changes of three kinds of stress-induced bacteria of Enterococcus faecalis, Pseudobutyrivibrio, and aerogenic bacteria Dorea strain.
Gut immune system
Development of gut immune system depends on gut microbiota.[59,60] GF mice almost had no immune activity, but they could generate immune function when giving certain microbiota.[61] For example, the segmented filamentous bacterium in the gut can restore the full functions of gut B and T lymphocytes.[62,63,64] Bacteria communicate with the host through a variety of ways, and the receptors-TLRs of host cell play a key role in the communication between bacteria and host. There are ten kinds of TLRs in the human innate immune system, which have been identified as pattern recognition receptors.[65] These receptors are a part of the innate immune system, which is the first step to produce cytokine response and is also widely distributed on neurons.[66] Hence, neurons also respond to bacterial and viral components. Intestinal epithelial cells can transport microbial composition or metabolites into the inner environment, and the nervous system also interacts with these bacterial and viral components.[67] The balance of gut microbiota may change the regulation of inflammatory response, and this mechanism may also get involved in the regulation of emotion and behavior.[41,61,68,69,70]
Neurotransmitters and neural regulators synthesized by intestinal bacteria
Gut bacteria can synthesize gamma amino acid, butyric acid, 5-HT, dopamine, and SCFAs,[48,71] and these substances can exchange between cells of microorganism,[48] especially intestinal cells in the gut can produce many 5-HT that have an effect on the brain. Bacterial enzymes can also produce neurotoxin products such as D-lactic acid and ammonia.[3,72] Hence, a lot of necessary neurotransmitters in the body are generated by the gut microbiota, exerting influence on the human body including the brain, among which many of neurotransmitters in the human gut microbiota are also critical molecules.[73]
Intestinal mucosal barrier and blood-brain barrier (barrier system)
Evidence from rodent studies showed that stress changed intestinal mucosal barrier function, made LPS and other cytokines entering blood circulation, and stimulated TLR4 and other TLRs producing inflammatory cytokine.[74] Peripheral produced inflammatory factors could increase the permeability of blood-brain barrier, thus make it possible for peripheral produced inflammatory factors to directly influence the brain.[75]
Therefore, vast evidence of animals and human studies showed that gut microbiota plays a critical role in the brain development and function.[76,77]
Use of Microbiome and Metagenomics to Analyze Composition and Characteristics in Gut Microbiota Respectively
Evidence of the animal studies showed that gut microbiota composition and metabolic products could be obtained through feces analysis.[73]
Microbiome define the composition and functional characteristics of gut microbiota
To effectively understand the role of symbiotic microorganisms of mammals, in particular bacteria, on health and disease, terms and indicators must be used to describe complex ecological gut microbiota. Some bacteria phyla represent its characteristics in gut, and symbiotic bacteria represent the possible diversity, there are about 1000 different bacteria in the gut.[78,79,80] Two main bacteria phyla are Bacteroidetes and Firmicutes, which account for at least 70–75% of the microbiome.[79,80,81] There are also Proteobacteria, Actinomyces, Fusobacterium, and Verrucomicrobia, which are relatively small in number.[81] How to describe a variety of gut microbiota? Scientists use microbiome to give an overview description, and microbiome refers to the living bacteria in the gut and its genetic materials, including the archaea, protozoa, fungi, and virus.[41,82]
Dynamic characteristics and diversity of microbiome are beyond people's imagination. Currently, scientists have only begun to understand the distribution and diversity of bacteria phyla which are helpful for health and disease. Metagenomics approach has already revealed that certain bacterial colonies could be as phenotype which shared with a human.[81] Beyond hierarchical features of bacteria phyla, detailed analysis showed that there was a big bacteria variation between the individuals who were relating or nonrelating.[82,83] The microbiome is a dynamic entity, influenced by factors such as gene, diet, metabolites, age, geography, use of antibiotics, and stress.[26,84,85,86,87,88,89,90,91] Hence, the characteristics of gut microbiota are a good representative of individual's environment, which is helpful for understanding individual disease risk, disease progression, and treatment effect. These tools are now being used in the human and animals studies.
Metagenomics is used to be an appropriate technique to characterize gut microbiota
The recent developments in molecular biology and metagenomics allow researchers to better understand the structure and function of gut microbiota. Metagenomics is an emerging subject that uses the method of nonmicrobial culture to study microbial colony in the environment,[92] the main research objects are bacteria, archaea, fungi, viruses, and other microbes in bacterial colony, and its main purpose is to reveal the deeper genetic and evolutionary laws through analysis of aspects of microbial diversity, population structure and its dynamic change, relationship between members, and the relationship with environment within microbial colony. Metagenomics allows us to discover new genes and proteins, or even new method that is more accurate than traditional microorganisms or molecular biology, and complete comprehensive nonculturable microbial genome in a shorter time.[92]
The differences between metagenomics sequencing and 16S/18S rDNA sequencing are as follows:[93] 16S/18S rDNA gene sequencing is mainly bacterial 16S rRNA or fungal 18S rRNA gene sequencing, and the cores of study are species taxonomy, species abundance, and system evolution within samples. Metagenomics sequencing takes microbiota genome in the environmental samples as research object, directly extracts DNA of all microbiota from environmental samples, constructs metagenomic library, uses high-throughput sequencing technology to analyze population genetic composition, function and participated metabolic pathway of all microbiota contained in environmental samples, interprets the diversity and abundance of the microbial population, seeks for the relationship between microbiota and the environment and relationship between microbiota and the host, and explores and studies new genes with specific functions.
The metagenomic study can be used for evolution analysis, gene discovery, environmental and ecological research, and disease and individual medicine,[78,94,95] especially in individual medical field. Vast evidence showed that population and diversity of human gut microbiota have obvious correlation with the occurrence of human diseases, such as obesity, cardiovascular disease, and tumor, but the impact on health and disease of the human brain is underway.
Conclusion
In summary, gut microbiota-brain axis is a “bottom-up” term as opposed to a “top-down” term of “brain-gut-microbiota axis”, no matter what is called, its meaning refers to a bidirectional communication network between gut and brain. Its composition includes gut microbiota and their metabolic products, ENS, sympathetic and parasympathetic branches, neural-immune system, neuroendocrine system, and central nervous system. Moreover, there might have possible five routes of communicating between gut microbiota and brain, including the gut-brain's neural network, neuroendocrine-HPA axis, gut immune system, some neurotransmitters and neural regulators synthesized by gut bacteria, and barriers including intestinal mucosal barrier and blood-brain barrier [Figure 1]. In this communicating network, the brain affects gut movement, sensory and secretion function, and viscera signal from the gut also affects brain function. For example, incoming and outgoing branches of VN play an important role in gut message transmission. Vagal activation has anti-inflammatory effect. Positive effects of many gut microbiota and probiotics on brain function are dependent on the vagal activity.
Figure 1.
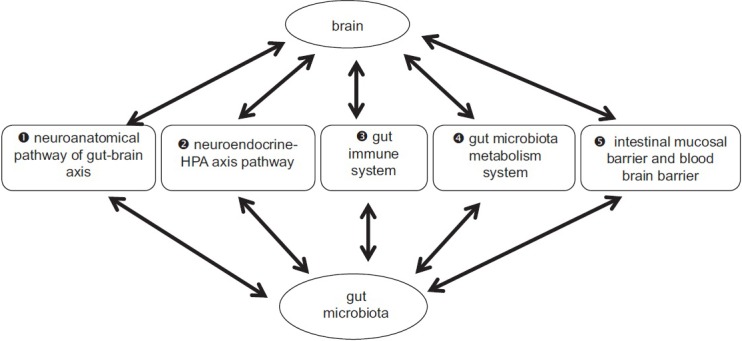
Gut microbiota-brain axis. Five possible communication routes (❶–❺) between gut microbiota and brain: intestinal mucosal barrier and blood-brain barrier (❺) is the important base for neuroendocrine-HPA axis pathway (❷), gut immune system (❸), and gut microbiota metabolism system (❹). Substances produced by neuroendocrine-HPA axis pathway (❷), gut immune system (❸), and gut microbiota metabolism system (❹), only into the system circulation and brain through the intestinal mucosal barrier and blood-brain barrier system can play effect of gut microbiota on the brain. HPA: Hypothalamic-pituitary-adrenal.
It is believed that the gut microbiota-brain axis will provide more information and possible route for people to know the brain, understand the brain, and protect the brain.
Financial support and sponsorship
This study was supported by grants from Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. XMLX201401), the National Natural Science Foundation of China (No. 81301138), National High-Tech R&D Program of China (863 Program, No. 2015AA020514), National Hundred, Thousand, and Ten Thousand Talents Project of Beijing (No. 2010-005).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
References
- 1.Jenkins TA, Nguyen JC, Polglaze KE, Bertrand PP. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016;8 doi: 10.3390/nu8010056. pii: E56. doi: 10.3390/nu8010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt C. Mental health: Thinking from the gut. Nature. 2015;518:S12–5. doi: 10.1038/518S13a. doi: 10.1038/518S13a. [DOI] [PubMed] [Google Scholar]
- 3.Smith PA. The tantalizing links between gut microbes and the brain. Nature. 2015;526:312–4. doi: 10.1038/526312a. doi: 10.1038/526312a. [DOI] [PubMed] [Google Scholar]
- 4.Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: Paradigm shift in neuroscience. J Neurosci. 2014;34:15490–6. doi: 10.1523/JNEUROSCI.3299-14.2014. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047–52. doi: 10.1073/pnas.1010529108. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogbonnaya ES, Clarke G, Shanahan F, Dinan TG, Cryan JF, O’Leary OF. Adult hippocampal neurogenesis is regulated by the microbiome. Biol Psychiatry. 2015;78:e7–9. doi: 10.1016/j.biopsych.2014.12.023. doi: 10.1016/j.biopsych.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 7.Jašarevic E, Howerton CL, Howard CD, Bale TL. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology. 2015;156:3265–76. doi: 10.1210/en.2015-1177. doi: 10.1210/en.2015-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–76. doi: 10.1016/j.cell.2015.02.047. Erratum in: Cell 2015;163:258. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. Erratum in: Sci Transl Med 2014;6:266er7. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. doi: 10.1053/j.gastro.2011.04.052. 609e1-3. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 11.Arentsen T, Raith H, Qian Y, Forssberg H, Diaz Heijtz R. Host microbiota modulates development of social preference in mice. Microb Ecol Health Dis. 2015;26:29719. doi: 10.3402/mehd.v26.29719. doi: 10.3402/mehd.v26.29719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108(Suppl1):4615–22. doi: 10.1073/pnas.1000082107. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall JK, Thabane M, Garg AX, Clark WF, Moayyedi P, Collins SM Walkerton Health Study Investigators. Eight year prognosis of postinfectious irritable bowel syndrome following waterborne bacterial dysentery. Gut. 2010;59:605–11. doi: 10.1136/gut.2009.202234. doi: 10.1136/gut.2009.202234. [DOI] [PubMed] [Google Scholar]
- 14.De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 2013;8:e76993. doi: 10.1371/journal.pone.0076993. doi: 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alander M, Satokari R, Korpela R, Saxelin M, Vilpponen-Salmela T, Mattila-Sandholm T, et al. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosu s GG, after oral consumption. Appl Environ Microbiol. 1999;65:351–4. doi: 10.1128/aem.65.1.351-354.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Vos WM, de Vos EA. Role of the intestinal microbiome in health and disease: From correlation to causation. Nutr Rev. 2012;70(Suppl 1):S45–56. doi: 10.1111/j.1753-4887.2012.00505.x. doi: 10.1111/j.1753-4887.2012.00505.x. [DOI] [PubMed] [Google Scholar]
- 17.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–30. doi: 10.1038/nature11550. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dave M, Higgins PD, Middha S, Rioux KP. The human gut microbiome: Current knowledge, challenges, and future directions. Transl Res. 2012;160:246–57. doi: 10.1016/j.trsl.2012.05.003. doi: 10.1016/j.trsl.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 19.O’Toole PW. Changes in the intestinal microbiota from adulthood through to old age. Clin Microbiol Infect. 2012;18(Suppl4):44–6. doi: 10.1111/j.1469-0691.2012.03867.x. doi: 10.1111/j.1469-0691.2012.03867.x. [DOI] [PubMed] [Google Scholar]
- 20.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Relman DA. The human microbiome: Ecosystem resilience and health. Nutr Rev. 2012;70(Suppl 1):S2–9. doi: 10.1111/j.1753-4887.2012.00489.x. doi: 10.1111/j.1753-4887.2012.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vallès Y, Gosalbes MJ, de Vries LE, Abellán JJ, Francino MP. Metagenomics and development of the gut microbiota in infants. Clin Microbiol Infect. 2012;18(Suppl4):21–6. doi: 10.1111/j.1469-0691.2012.03876.x. doi: 10.1111/j.1469-0691.2012.03876.x. [DOI] [PubMed] [Google Scholar]
- 23.Vaishampayan PA, Kuehl JV, Froula JL, Morgan JL, Ochman H, Francino MP. Comparative metagenomics and population dynamics of the gut microbiota in mother and infant. Genome Biol Evol. 2010;2:53–66. doi: 10.1093/gbe/evp057. doi: 10.1093/gbe/evp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–84. doi: 10.1038/nature11319. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 25.Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol Motil. 2011;23:187–92. doi: 10.1111/j.1365-2982.2010.01664.x. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 26.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davey KJ, O’Mahony SM, Schellekens H, O’Sullivan O, Bienenstock J, Cotter PD, et al. Gender-dependent consequences of chronic olanzapine in the rat: Effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology (Berl) 2012;221:155–69. doi: 10.1007/s00213-011-2555-2. doi: 10.1007/s00213-011-2555-2. [DOI] [PubMed] [Google Scholar]
- 28.O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, et al. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–7. doi: 10.1016/j.biopsych.2008.06.026. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Sudo N. Stress and gut microbiota: Does postnatal microbial colonization program the hypothalamic-pituitary-adrenal system for stress response? Int Congr Ser. 2006;1287:350–4. doi: 10.1016/j.ics.2005.12.019. [Google Scholar]
- 30.Bailey MT, Coe CL. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobiol. 1999;35:146–55. [PubMed] [Google Scholar]
- 31.Munyaka PM, Khafipour A, Wang H, Eissa N, Khafipour E, Ghia JE. Prenatal antibiotic treatment increases offspring's susceptibility to experimental colitis: A role of the gut microbiota. Gastroenterology. 2015;148:S708. doi: 10.1371/journal.pone.0142536. doi.org/10.1016/S0016-5085(15)32404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burger-van Paassen N, Vincent A, Puiman PJ, van der Sluis M, Bouma J, Boehm G, et al. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. Biochem J. 2009;420:211–9. doi: 10.1042/BJ20082222. doi: 10.1042/BJ20082222. [DOI] [PubMed] [Google Scholar]
- 33.Nell S, Suerbaum S, Josenhans C. The impact of the microbiota on the pathogenesis of IBD: Lessons from mouse infection models. Nat Rev Microbiol. 2010;8:564–77. doi: 10.1038/nrmicro2403. doi: 10.1038/nrmicro2403. [DOI] [PubMed] [Google Scholar]
- 34.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 36.Montiel-Castro AJ, González-Cervantes RM, Bravo-Ruiseco G, Pacheco-López G. The microbiota-gut-brain axis: Neurobehavioral correlates, health and sociality. Front Integr Neurosci. 2013;7:70. doi: 10.3389/fnint.2013.00070. doi: 10.3389/fnint.2013.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cryan JF, Dinan TG. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–12. doi: 10.1038/nrn3346. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 38.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–14. doi: 10.1038/nrgastro.2009.35. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauer KC, Huus KE, Finlay BB. Microbes and the mind: Emerging hallmarks of the gut microbiota-brain axis. Cell Microbiol. 2016;18:632–44. doi: 10.1111/cmi.12585. doi: 10.1111/cmi.12585. [DOI] [PubMed] [Google Scholar]
- 40.Al Omran Y, Aziz Q. The brain-gut axis in health and disease. Adv Exp Med Biol. 2014;817:135–53. doi: 10.1007/978-1-4939-0897-4_6. doi: 10.1007/978-1-4939-0897-4_6. [DOI] [PubMed] [Google Scholar]
- 41.Foster JA, McVey Neufeld KA. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–12. doi: 10.1016/j.tins.2013.01.005. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson's disease. World J Gastroenterol. 2015;21:10609–20. doi: 10.3748/wjg.v21.i37.10609. doi: 10.3748/wjg.v21.i37.10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schemann M, Neunlist M. The human enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl1):55–9. doi: 10.1111/j.1743-3150.2004.00476.x. doi: 10.1111/j.1743-3150.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 44.Anlauf M, Schäfer MK, Eiden L, Weihe E. Chemical coding of the human gastrointestinal nervous system: Cholinergic, VIPergic, and catecholaminergic phenotypes. J Comp Neurol. 2003;459:90–111. doi: 10.1002/cne.10599. doi: 10.1002/cne.10599. [DOI] [PubMed] [Google Scholar]
- 45.Szurszewski JH. Physiology of mammalian prevertebral ganglia. Annu Rev Physiol. 1981;43:53–68. doi: 10.1146/annurev.ph.43.030181.000413. doi: 10.1146/annurev.ph.43.030181.000413. [DOI] [PubMed] [Google Scholar]
- 46.Chang HY, Mashimo H, Goyal RK. Musings on the wanderer: What's new in our understanding of vago-vagal reflex? IV. Current concepts of vagal efferent projections to the gut. Am J Physiol Gastrointest Liver Physiol. 2003;284:G357–66. doi: 10.1152/ajpgi.00478.2002. doi: 10.1152/ajpgi.00478.2002. [DOI] [PubMed] [Google Scholar]
- 47.Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol Med. 2014;20:509–18. doi: 10.1016/j.molmed.2014.05.002. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol. 2014;817:115–33. doi: 10.1007/978-1-4939-0897-4_5. doi: 10.1007/978-1-4939-0897-4_5. [DOI] [PubMed] [Google Scholar]
- 49.Dissanayake D, Hall H, Berg-Brown N, Elford AR, Hamilton SR, Murakami K, et al. Nuclear factor-kB1 controls the functional maturation of dendritic cells and prevents the activation of autoreactive T cells. Nat Med. 2011;17:1663–7. doi: 10.1038/nm.2556. doi: 10.1038/nm.2556. [DOI] [PubMed] [Google Scholar]
- 50.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–93. doi: 10.1038/sj.embor.7400731. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gosselin D, Rivest S. MyD88 signaling in brain endothelial cells is essential for the neuronal activity and glucocorticoid release during systemic inflammation. Mol Psychiatry. 2008;13:480–97. doi: 10.1038/sj.mp.4002122. doi: 10.1038/sj.mp.4002122. [DOI] [PubMed] [Google Scholar]
- 52.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558(Pt 1):263–75. doi: 10.1113/jphysiol.2004.063388. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23:255–64. e119. doi: 10.1111/j.1365-2982.2010.01620.x. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 54.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–73. doi: 10.1038/mp.2012.77. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 55.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43:164–74. doi: 10.1016/j.jpsychires.2008.03.009. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 56.Barouei J, Moussavi M, Hodgson DM. Effect of maternal probiotic intervention on HPA axis, immunity and gut microbiota in a rat model of irritable bowel syndrome. PLoS One. 2012;7:e46051. doi: 10.1371/journal.pone.0046051. doi: 10.1371/journal.pone.0046051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bangsgaard Bendtsen KM, Krych L, Sørensen DB, Pang W, Nielsen DS, Josefsen K, et al. Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PLoS One. 2012;7:e46231. doi: 10.1371/journal.pone.0046231. doi: 10.1371/journal.pone.0046231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50. doi: 10.1038/nature12721. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 60.Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125:926–38. doi: 10.1172/JCI76304. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain Behav Immun. 2010;24:9–16. doi: 10.1016/j.bbi.2009.05.058. doi: 10.1016/j.bbi.2009.05.058. [DOI] [PubMed] [Google Scholar]
- 62.Umesaki Y, Okada Y, Matsumoto S, Imaoka A, Setoyama H. Segmented filamentous bacteria are indigenous intestinal bacteria that activate intraepithelial lymphocytes and induce MHC class II molecules and fucosyl asialo GM1 glycolipids on the small intestinal epithelial cells in the ex-germ-free mouse. Microbiol Immunol. 1995;39:555–62. doi: 10.1111/j.1348-0421.1995.tb02242.x. doi: 10.1111/j.1348-0421.1995.tb02242.x. [DOI] [PubMed] [Google Scholar]
- 63.Umesaki Y, Setoyama H, Matsumoto S, Imaoka A, Itoh K. Differential roles of segmented filamentous bacteria and clostridia in development of the intestinal immune system. Infect Immun. 1999;67:3504–11. doi: 10.1128/iai.67.7.3504-3511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Talham GL, Jiang HQ, Bos NA, Cebra JJ. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect Immun. 1999;67:1992–2000. doi: 10.1128/iai.67.4.1992-2000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. doi: 10.1016/j.cell.2010.01.022. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 66.McKernan DP, Dennison U, Gaszner G, Cryan JF, Dinan TG. Enhanced peripheral toll-like receptor responses in psychosis: Further evidence of a pro-inflammatory phenotype. Transl Psychiatry. 2011;1:e36. doi: 10.1038/tp.2011.37. doi: 10.1038/tp.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Brien SM, Scott LV, Dinan TG. Cytokines: Abnormalities in major depression and implications for pharmacological treatment. Hum Psychopharmacol. 2004;19:397–403. doi: 10.1002/hup.609. doi: 10.1002/hup.609. [DOI] [PubMed] [Google Scholar]
- 68.Levkovich T, Poutahidis T, Smillie C, Varian BJ, Ibrahim YM, Lakritz JR, et al. Probiotic bacteria induce a ‘glow of health’. PLoS One. 2013;8:e53867. doi: 10.1371/journal.pone.0053867. doi: 10.1371/journal.pone.0053867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–73. doi: 10.1126/science.1223490. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kopp MV, Goldstein M, Dietschek A, Sofke J, Heinzmann A, Urbanek R. Lactobacillus GG has in vitro effects on enhanced interleukin-10 and interferon-gamma release of mononuclear cells but no in vivo effects in supplemented mothers and their neonates. Clin Exp Allergy. 2008;38:602–10. doi: 10.1111/j.1365-2222.2007.02911.x. doi: 10.1111/j.1365-2222.2007.02911.x. [DOI] [PubMed] [Google Scholar]
- 71.Lyte M. Microbial endocrinology: Host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes. 2014;5:381–9. doi: 10.4161/gmic.28682. doi: 10.4161/gmic.28682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–53. doi: 10.1126/science.1188510. Erratum in: Science 2011;334:594. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dinan TG, Stanton C, Cryan JF. Psychobiotics: A novel class of psychotropic. Biol Psychiatry. 2013;74:720–6. doi: 10.1016/j.biopsych.2013.05.001. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 74.Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37:1885–95. doi: 10.1016/j.psyneuen.2012.03.024. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 75.McCusker RH, Kelley KW. Immune-neural connections: How the immune system's response to infectious agents influences behavior. J Exp Biol. 2013;216(Pt 1):84–98. doi: 10.1242/jeb.073411. doi: 10.1242/jeb.073411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Galland L. The gut microbiome and the brain. J Med Food. 2014;17:1261–72. doi: 10.1089/jmf.2014.7000. doi: 10.1089/jmf.2014.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tillisch K, Labus JS. Neuroimaging the microbiome-gut-brain axis. Adv Exp Med Biol. 2014;817:405–16. doi: 10.1007/978-1-4939-0897-4_18. doi: 10.1007/978-1-4939-0897-4_18. [DOI] [PubMed] [Google Scholar]
- 78.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. Ahuman gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8. doi: 10.1126/science.1110591. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lay C, Sutren M, Rochet V, Saunier K, Doré J, Rigottier-Gois L. Design and validation of 16S rRNA probes to enumerate members of the Clostridium leptum subgroup in human faecal microbiota. Environ Microbiol. 2005;7:933–46. doi: 10.1111/j.1462-2920.2005.00763.x. doi: 10.1111/j.1462-2920.2005.00763.x. [DOI] [PubMed] [Google Scholar]
- 81.Diamant M, Blaak EE, de Vos WM. Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes Rev. 2011;12:272–81. doi: 10.1111/j.1467-789X.2010.00797.x. doi: 10.1111/j.1467-789X.2010.00797.x. [DOI] [PubMed] [Google Scholar]
- 82.Dash S, Clarke G, Berk M, Jacka FN. The gut microbiome and diet in psychiatry: Focus on depression. Curr Opin Psychiatry. 2015;28:1–6. doi: 10.1097/YCO.0000000000000117. doi: 10.1097/YCO.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 83.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–7. doi: 10.1126/science.1177486. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–33. doi: 10.1146/annurev.mi.31.100177.000543. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 85.Cho I, Yamanishi S, Cox L, Methé BA, Zavadil J, Li K, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–6. doi: 10.1038/nature11400. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Panagiotidis G, Bäckström T, Asker-Hagelberg C, Jandourek A, Weintraub A, Nord CE. Effect of ceftaroline on normal human intestinal microflora. Antimicrob Agents Chemother. 2010;54:1811–4. doi: 10.1128/AAC.01716-09. doi: 10.1128/AAC.01716-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schloss PD, Iverson KD, Petrosino JF, Schloss SJ. The dynamics of a family's gut microbiota reveal variations on a theme. Microbiome. 2014;2:25. doi: 10.1186/2049-2618-2-25. doi: 10.1186/2049-2618-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J, et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci U S A. 2010;107:18933–8. doi: 10.1073/pnas.1007028107. doi: 10.1073/pnas.1007028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karlsson CL, Onnerfält J, Xu J, Molin G, Ahrné S, Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity (Silver Spring) 2012;20:2257–61. doi: 10.1038/oby.2012.110. doi: 10.1038/oby.2012.110. [DOI] [PubMed] [Google Scholar]
- 90.Serino M, Fernández-Real JM, García-Fuentes E, Queipo-Ortuño M, Moreno-Navarrete JM, Sánchez A, et al. The gut microbiota profile is associated with insulin action in humans. Acta Diabetol. 2013;50:753–61. doi: 10.1007/s00592-012-0410-5. Erratum in: Acta Diabetol 2013;50:763. doi: 10.1007/s00592-012-0410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7. doi: 10.1038/nature11053. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Escobar-Zepeda A, Vera-Ponce de León A, Sanchez-Flores A. The road to metagenomics: From microbiology to DNA sequencing technologies and bioinformatics. Front Genet. 2015;6:348. doi: 10.3389/fgene.2015.00348. doi: 10.3389/fgene.2015.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lozupone CA, Stombaugh J, Gonzalez A, Ackermann G, Wendel D, Vázquez-Baeza Y, et al. Meta-analyses of studies of the human microbiota. Genome Res. 2013;23:1704–14. doi: 10.1101/gr.151803.112. doi: 10.1101/gr.151803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thomas T, Gilbert J, Meyer F. Metagenomics – A guide from sampling to data analysis. Microb Inform Exp. 2012;2:3. doi: 10.1186/2042-5783-2-3. doi: 10.1186/2042-5783-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang N, He QS. Commensal microbiome promotes resistance to local and systemic infections. Chin Med J. 2015;128:2250–5. doi: 10.4103/0366-6999.162502. doi: 10.4103/0366-6999.162502. [DOI] [PMC free article] [PubMed] [Google Scholar]


