Abstract
Background:
Long-term efficacy of liraglutide, a glucagon-like peptide-1 analog, on body weight and glycemic control has not been studied in Indian Type 2 diabetes mellitus (T2DM) subjects.
Aim:
To evaluate the effect of liraglutide on glycemic control and body weight for 1 year in Indian T2DM patients.
Methods:
Liraglutide was prescribed to 96 obese patients with T2DM and followed up for 1 year. Clinical parameters were measured at baseline and 3, 6, 9, and 12 months. Dosage of liraglutide and other medications was adjusted according to clinical judgment.
Results:
1 year data were available for 74 patients. Mean age was 50.9 ± 9.6 years. Mean duration of diabetes was 11.6 ± 6.3 years. Glycosylated hemoglobin (HbA1c) significantly decreased from 8.9 ± 1.3% at baseline to 7.4 ± 1.2% at 1 year. Body weight significantly declined from 98.9 ± 16.0 kg at baseline to 93.8 ± 15.0 kg at 1 year. After an initial decline, subset of patients had an increase in mean HbA1c (n = 30/74) and mean body weight (n = 33/74) after 6 months of liraglutide initiation. Baseline HbA1c and baseline body weight were positively associated with a reduction of HbA1c and body weight at 1 year, respectively. No major side effects occurred.
Conclusion:
Liraglutide treatment resulted in a significant and sustained reduction in HbA1c and body weight over 1 year in Indian T2DM patients. Magnitude of reduction of HbA1c and body weight at 1 year was positively associated with baseline HbA1c and baseline weight, respectively.
Keywords: Diabetes, India, liraglutide, obesity
INTRODUCTION
Liraglutide is a once-daily injectable glucagon-like peptide-1 (GLP-1) analog approved for the treatment of Type 2 diabetes mellitus (T2DM). Liraglutide treatment led to significant reduction in glycosylated hemoglobin (HbA1c) and weight in patients with T2DM in various liraglutide effect and action in diabetes (LEAD) trials.[1] However, the duration of all LEAD trials was short (26 weeks, except LEAD 3, which was 52 weeks). Long-term data on efficacy and safety of liraglutide in real-world clinical practice is limited. Few countries have published their data recently, on efficacy and safety of liraglutide in T2DM, but the duration of these studies was mostly 6 months, which might not truly reflect long-term effects.[2,3,4,5] Our group has demonstrated the beneficial effects of liraglutide on body weight and glycemic control in the real life clinical practice setting until 3 months after liraglutide initiation in Indian obese T2DM subjects.[6] However, it is not known in Indian T2DM subjects whether the effect of liraglutide on body weight and glycemic control would be sustained for a longer period.
In this study, we present 1 year follow-up data on effectiveness and tolerability of liraglutide in Indian patients with T2DM in the real-world clinical setting. Aim of the study was to evaluate the effect of liraglutide on glycemic control and weight in obese patients with T2DM for 1 year after liraglutide introduction.
METHODS
This was an observational study conducted at the outpatient clinic of a tertiary care hospital. Methodology has been described in detail in our previously published study.[6] Liraglutide was prescribed to 96 patients with T2DM and obesity and followed up at 3 months intervals up to 1 year. Of 96 patients, 22 patients discontinued liraglutide before completion of 1 year for a variety of reasons; adverse events (n = 10), financial constraints (n = 2), lost to follow-up (n = 6), and lack of weight loss (n = 4). Therefore, 1 year data were available for 74 patients.
Clinical parameters including, body weight, body mass index (BMI), and metabolic parameters (fasting plasma glucose [FPG], postprandial plasma glucose [PPG] and HbA1c) were assessed at baseline and 3 months, 6 months, 9 months, and 1 year after liraglutide introduction. Adverse events noted by patients were recorded at each visit. Dosage adjustment of other antidiabetic medications and up-titration of liraglutide dose (0.6 mg/day, 1.2 mg/day or 1.8 mg/day) was done according to clinical judgment. Informed written consent was obtained from all the subjects. The study protocol was approved by institutional review board.
Statistical methods
Data are presented as mean ± standard deviation. Data analysis was done using SPSS software (SAS 9.1.3, SAS Institute Inc., Cary, North Carolina). Comparison between mean pretreatment and posttreatment value of various parameters was done using paired t-test. Comparison between two groups was done using Student's t-test. The significance level was set at P ≤ 0.05.
RESULTS
Baseline parameters of the subjects are given in Table 1. The mean age of patients was 50.9 ± 9.6 years and mean duration of diabetes was 11.6 ± 6.3 years. At baseline, mean HbA1c was 8.9 ± 1.3% and mean body weight was 98.9 ± 16.0 kg. Table 2 shows the clinical outcomes for liraglutide at 6 and 12 months follow-up. A significant decline in mean HbA1c, FPG, PPG, and body weight was seen at 6 months and 12 months of follow-up. Mean HbA1c levels at 6 and 12 months of follow-up were 7.3 ± 1.1% and 7.4 ± 1.2%, respectively [Figure 1]. The mean absolute decline in HbA1c levels at 6 and 12 months of follow-up were − 1.5 ± 1.2% and − 1.4 ± 1.6% (both P < 0.01) compared with baseline, respectively. Similarly, significant reduction in mean body weight was observed at 6 months and 12 months of follow-up [Figure 2]. Average body weight at 6 and 12 months of follow-up were 94.1 ± 14.9 kg and 93.8 ± 15.0 kg, respectively.
Table 1.
Baseline characteristics

Table 2.
Various parameters at 6 months and 12 months after liraglutide introduction (mean ± standard deviation)
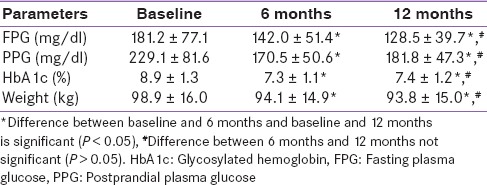
Figure 1.
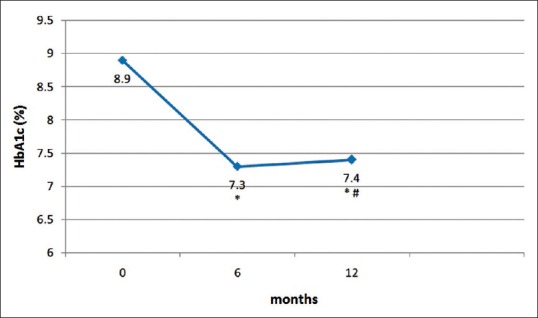
Change in mean glycosylated hemoglobin from baseline to 1 year after liraglutide introduction. *Difference from baseline is significant (P < 0.05), #Difference between 6 months and 12 months not significant (P > 0.05)
Figure 2.
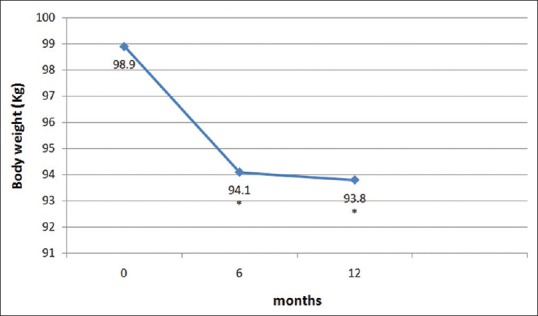
Change in mean body weight from baseline. *Difference from baseline is significant (P < 0.05)
We further categorized the patients into six groups based on the response to liraglutide treatment at 6 months and 12 months. Group 1 included patients who showed continued lowering of mean HbA1c from baseline up to 1 year (n = 32). Group 2 included patients in whom mean HbA1c declined initially from baseline up to 6 months but subsequently increased (n = 30). Group 3 included patients in whom mean HbA1c did not lower at all or continued rising from baseline (n = 12). Group 4 included patients who showed continued lowering of mean weight from baseline up to 1 year (n = 37). Group 5 included patients in whom mean weight declined initially from baseline up to 6 months but subsequently increased (n = 33). Group 6 included patients in whom mean weight did not lower at all or continued rising from baseline (n = 4).
Tables 3 and 4 show a comparison between Groups 1 and 2 and Groups 4 and 5, respectively. Groups 3 and 6 were not compared due to small numbers. Group 1 patients had significantly higher mean HbA1c at baseline as compared to Group 2 patients (9.4% vs. 8.7% P = 0.04). There was no significant difference in any of the parameters between Groups 4 and 5. Figure 3 shows the change in mean HbA1c from baseline in Group 1 and Group 2. Mean HbA1c was significantly higher at 12 months as compared to 6 months in Group 2 (6.8 ± 0.9% vs. 7.9 ± 1.2%, P < 0.001), however, it was significantly lower compared to baseline (8.7 ± 1.1% vs. 7.9 ± 1.2%, P = 0.001). Mean weight was significantly higher at 12 months as compared to 6 months in Group 5 (92.4 ± 14.1 kg vs. 94.5 ± 14.6 kg, P < 0.001), however, it was significantly lower compared to baseline (97.2 ± 14.7 kg vs. 94.5 ± 14.6 kg, P < 0.001). Reduction of HbA1c at 1 year was associated positively with baseline HbA1c (r = 0.336, P < 0.001) and body weight reduction at 1 year was associated positively with baseline weight (r = 0.664, P = 0.003).
Table 3.
Comparison of various parameters (mean±standard deviation) between Group 1 (patients with consistent lowering of glycosylated hemoglobin) and Group 2 (patients in whom glycosylated hemoglobin lowered initially but again increased)
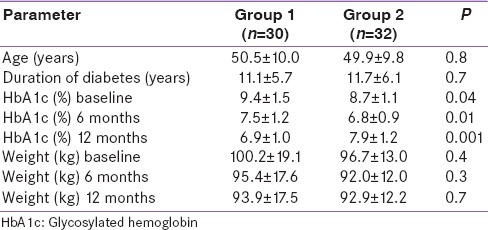
Table 4.
Comparison of various parameters (mean±standard deviation) between Group 4 (patients in whom weight declined consistently) and Group 5 (patients in whom weight declined initially but increased again)
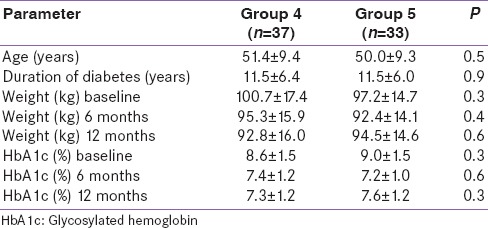
Figure 3.
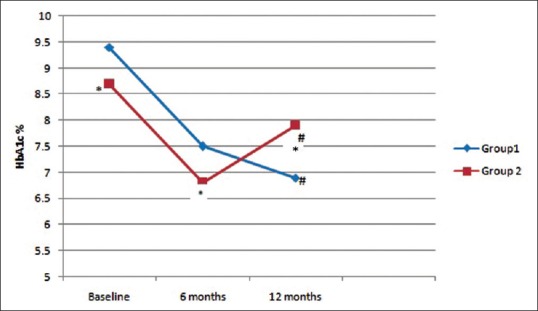
Comparison of mean glycosylated hemoglobin between Group 1 (patients with consistent lowering of glycosylated hemoglobin) and Group 2 (patients in whom glycosylated hemoglobin lowered initially but again increased). *Significant difference between the groups (P < 0.05), #Significant difference from baseline and 6 months (P < 0.05)
Adverse events were; diarrhea (n = 11), nausea (n = 14), vomiting (n = 2), pain abdomen (n = 1), dizziness (n = 2), and lethargy (n = 2). None of the subjects had major hypoglycemia. The maximal daily dose of liraglutide, i.e., 1.8 mg was reached in 18/74 patients, and in the rest, the dose was maintained at 1.2 mg daily.
DISCUSSION
Present study for the first time demonstrates the efficacy of liraglutide on weight reduction and fair glycemic control over 1 year in Indian T2DM subjects. We found that initiating liraglutide was associated with significant reductions in mean HbA1c and mean weight at 12 months of follow-up. Liraglutide treatment for 1 year resulted in 1.4% decline in mean HbA1c level and mean weight loss of 5.1 kg. However, in significant proportion of patients (30/74), mean HbA1c decreased initially from baseline until 6 months after initiating liraglutide (8.7 ± 1.1% at baseline vs. 6.8 ± 0.9% at 6 months, P < 0.001) but increased thereafter (6.8 ± 0.9% at 6 months vs. 7.9 ± 1.2% at 12 months, P < 0.001). Similarly, in subset of patients (n = 33/74), mean weight decreased initially from baseline until 6 months (97.2 ± 14.7 at baseline vs. 92.4 ± 14.1 kg at 6 months, P < 0.001) but increased thereafter (92.4 ± 14.1 kg at 6 months vs. 94.5 ± 14.6 kg at 12 months, P < 0.001).
Significant reductions in body weight and HbA1c were obtained with liraglutide in all LEAD trials.[7,8,9,10,11,12] The duration of all LEAD trials was 26 weeks, except LEAD 3, which was 52 weeks. Real world clinical effectiveness of liraglutide has been evaluated by others where liraglutide was shown to be effective in reducing HbA1c and weight in T2DM subjects.[2,3,4,5,13] However, follow-up of most of these studies was up to 6 months. Recently, our group has demonstrated the beneficial effects of liraglutide on body weight and glycemic control in the real life clinical practice setting until 3 months after liraglutide initiation in Indian obese T2DM subjects.[6] Short-term results (until 6 months) on HbA1c and weight of the current study are in accordance with earlier studies evaluating the efficacy of liraglutide in T2DM patients over 12–24 weeks follow-up. However, we found the fading effect of liraglutide on glycemic control and body weight after 6 months of initiation, in significant proportion of patients.
Studies describing the effect of liraglutide on glycemic control and body weight over long-term, in different populations have started appearing recently in literature.[14,15,16,17,18,19,20] The LEAD-3 sub-analysis showed the significant weight reduction by liraglutide treatment for 2 years.[21] Similarly long-term liraglutide treatment (over 2 years) in Japanese T2DM subjects, effectively maintained the reduction of body weight and the fair glycemic control.[16] Another observational study[14] concluded that adding liraglutide to insulin in daily clinical practice reduced HbA1c significantly within 6 months; however, there was nonsustainable effect during long-term treatment. In this study, median HbA1c re-increased (7.7% at baseline, 6.9% at 6 months, and 7.5% at 12 months) and median weight rose again (99.8 kg at baseline, 97.5 kg at 6 months, and 100.5 kg at 12 months), after 6 months of liraglutide initiation.
Our study similarly showed a rise in HbA1c (n = 30/74) and body weight (n = 33/74) after an initial decline, following 6 months of liraglutide initiation in a subset of patients. Nevertheless, their HbA1c and body weight stayed significantly lower at 12 months compared to baseline.
We compared the group of patients who continued to show decline in mean HbA1c from baseline up to 1 year with the group of patients in whom HbA1c increased after 6 months of initial decline. Patients who showed a continuous decline in mean HbA1c after liraglutide initiation had higher mean HbA1c at baseline, but there was no significant difference in any other parameter including weight or duration of diabetes, compared to the group of patients in whom HbA1c increased after 6 months of initial decline. Although mean HbA1c re-increased in the latter group, weight continued to decline up to 1 year. We found significant positive association between baseline HbA1c and reduction of HbA1c at 1 year and between baseline weight and body weight reduction at 1 year. Magnitude of reduction of HbA1c at 1 year was not associated with duration of diabetes or baseline weight. Similarly, magnitude of reduction of weight at 1 year was not associated with duration of diabetes or baseline HbA1c. These results may imply that liraglutide has an independent effect on body weight and glycemic control, may be by different mechanisms in GLP-1 actions. Furthermore, it suggests that liraglutide effectiveness is not influenced by duration of diabetes, and good glycemic control can be achieved with liraglutide even in patients with long-standing T2DM. The mean duration of diabetes in our study was 11.6 years. Our results are consistent with that of a recent study evaluating long-term impact of liraglutide use on body weight and glycemic control in Japanese T2DM subjects which showed that significant determinants for the reduction of body weight or HbA1c from baseline to 2 years were baseline BMI and insulin dose, or baseline HbA1c, respectively.[16] Other studies have also shown that baseline BMI and HbA1c may be important predictors for weight reduction and glycemic control, respectively, before liraglutide therapy.[20,22] Patients’ nonadherence regarding dietary restrictions could be one of the reasons accounting for the fading effect of liraglutide on body weight and HbA1c beyond 6 months in a subset of patients presented here. In our observation, patients do not tend to adhere to a diet plan, once the gastrointestinal side effects of liraglutide disappear after few months of liraglutide initiation.
Of 74 subjects, 32 (43.2%) experienced adverse drug reactions. Most common adverse events were nausea (18.9%) and diarrhea (14.8%). None of the patients experienced severe hypoglycemia.
Limitations of the study; this is an observational study, not a randomized clinical trial study.
CONCLUSION
Our study demonstrated the effectiveness of liraglutide at 6 and 12 months follow-up in real-world clinical practice in Indian T2DM subjects. A significant proportion of patients showed a fading effect of liraglutide on weight reduction and glycemic control after 6 months; however, their weight and HbA1c still remained significantly lower compared to baseline. Baseline HbA1c and baseline weight were significant predictors of glycemic control and weight reduction respectively at 1 year suggesting that liraglutide may be more effective in patients who are obese and have poor glycemic control on oral antidiabetic drugs with or without insulin.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wajcberg E, Amarah A. Liraglutide in the management of type 2 diabetes. Drug Des Devel Ther. 2010;4:279–90. doi: 10.2147/DDDT.S10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponzani P, Corsi A. Incretin-based therapies in clinical practice: From efficacy to effectiveness. Focus on liraglutide. Minerva Endocrinol. 2012;37:1–8. [PubMed] [Google Scholar]
- 3.Seino Y, Rasmussen MF, Nishida T, Kaku K. Efficacy and safety of the once-daily human GLP-1 analogue, liraglutide, vs glibenclamide monotherapy in Japanese patients with type 2 diabetes. Curr Med Res Opin. 2010;26:1013–22. doi: 10.1185/03007991003672551. [DOI] [PubMed] [Google Scholar]
- 4.Zenari L. Efficacy and tolerability of liraglutide in combination with other antidiabetic drugs in type 2 diabetes. Acta Biomed. 2011;82:251–3. [PubMed] [Google Scholar]
- 5.Mezquita-Raya P, Reyes-Garcia R, Moreno-Perez O, Escalada-San Martin J, Ángel Rubio Herrera M, Lopez de la Torre Casares M. Clinical effects of liraglutide in a real-world setting in Spain: EDiabetes-Monitor SEEN Diabetes Mellitus Working Group Study. Diabetes Ther. 2015;6:173–85. doi: 10.1007/s13300-015-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur P, Mishra SK, Mithal A, Saxena M, Makkar A, Sharma P. Clinical experience with Liraglutide in 196 patients with type 2 diabetes from a tertiary care center in India. Indian J Endocrinol Metab. 2014;18:77–82. doi: 10.4103/2230-8210.126572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): A randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–81. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- 8.Marre M, Shaw J, Brändle M, Bebakar WM, Kamaruddin NA, Strand J, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26:268–78. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: The LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): A randomised controlled trial. Diabetologia. 2009;52:2046–55. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD) Diabetes Care. 2009;32:1224–30. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: A 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 13.Yang W, Chen L, Ji Q, Liu X, Ma J, Tandon N, et al. Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: A 16-week, randomized, double-blind, active control trial(*) Diabetes Obes Metab. 2011;13:81–8. doi: 10.1111/j.1463-1326.2010.01323.x. [DOI] [PubMed] [Google Scholar]
- 14.Lipowsky C, Sze L, Krull I, Brändle M. Liraglutide as add-on therapy to insulin in type 2 diabetes mellitus: A retrospective, observational study from a daily clinical practice setting in Switzerland. Diabetes Ther. 2015;6:41–7. doi: 10.1007/s13300-014-0093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nauck M, Frid A, Hermansen K, Thomsen AB, During M, Shah N, et al. Long-term efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2 diabetes: 2-year results from the LEAD-2 study. Diabetes Obes Metab. 2013;15:204–12. doi: 10.1111/dom.12012. [DOI] [PubMed] [Google Scholar]
- 16.Inoue K, Maeda N, Fujishima Y, Fukuda S, Nagao H, Yamaoka M, et al. Long-term impact of liraglutide, a glucagon-like peptide-1 (GLP-1) analogue, on body weight and glycemic control in Japanese type 2 diabetes: An observational study. Diabetol Metab Syndr. 2014;6:95. doi: 10.1186/1758-5996-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponzani P. Long-term effectiveness and safety of liraglutide in clinical practice. Minerva Endocrinol. 2013;38:103–12. [PubMed] [Google Scholar]
- 18.Buysschaert M, D’Hooge D, Preumont V Roots Study Group. ROOTS: A multicenter study in Belgium to evaluate the effectiveness and safety of liraglutide (Victoza®) in type 2 diabetic patients. Diabetes Metab Syndr. 2015;9:139–42. doi: 10.1016/j.dsx.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 19.van Velsen EF, Lamers J, Blok V, van Leendert RJ, Kiewiet-Kemper RM. A prospective study of concomitant GLP-1 analogue and insulin use in type 2 diabetes in clinical practice. Neth J Med. 2014;72:523–7. [PubMed] [Google Scholar]
- 20.Lapolla A, Frison V, Bettio M, Dal Pos M, Rocchini P, Panebianco G, et al. Correlation between baseline characteristics and clinical outcomes in a large population of diabetes patients treated with liraglutide in a real-world setting in Italy. Clin Ther. 2015;37:574–84. doi: 10.1016/j.clinthera.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Garber A, Henry RR, Ratner R, Hale P, Chang CT, Bode B LEAD- (Mono) Study Group. Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:348–56. doi: 10.1111/j.1463-1326.2010.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fadini GP, Simioni N, Frison V, Dal Pos M, Bettio M, Rocchini P, et al. Independent glucose and weight-reducing effects of Liraglutide in a real-world population of type 2 diabetic outpatients. Acta Diabetol. 2013;50:943–9. doi: 10.1007/s00592-013-0489-3. [DOI] [PubMed] [Google Scholar]


