Abstract
Background:
Patients with subclinical hypothyroidism (SH) may have higher incidence of coronary heart disease and autonomic dysfunction.
Design of the Study:
Prospective case control study.
Aim and Objectives:
To evaluate beat-to-beat QT variability and vascular stiffness in patients with SH compared to normal controls.
Materials and Methods:
We compared linear and nonlinear measures of cardiac repolarization liability using beat-to-beat QT intervals derived from the surface electrocardiogram during supine posture and vascular indices including pulse wave velocity and ankle-brachial index (ABI) during supine posture between female patients with SH and age- and sex-matched normal controls. Spectral analysis was done at very low frequency (LF) (0.003–0.04 Hz), Low frequency (LF) (0.04–0.15 Hz), and high frequency (HF) (0.15–0.4 Hz). The HF represents vagal regulation (parasympathetic) and LF represents both parasympathetic and sympathetic regulation.
Results:
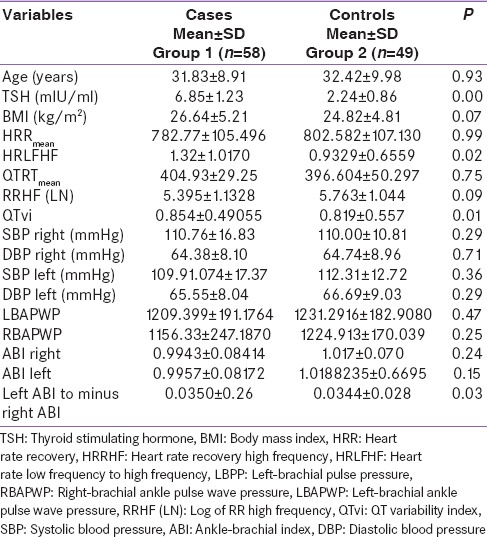
We recruited 58 women with a mean age of 31.83 ± 8.9 years and 49 controls with mean age of 32.4 ± 9.9 years (P = NS). QT variability index (QTvi) was higher in cases compared to controls (P = 0.01). The ratio of LF/HF of R-R interval which is an index of sympathovagal tone was significantly more in cases compared to controls (P = 0.02). The difference in the left minus the right ABI was significant between cases and controls (P = 0.03).
Conclusions:
The cases had lower parasympathetic activity as compared to controls, and there was a predominance of sympathetic activity in cases. QTvi may be an important noninvasive tool in this group of patients to study the risk of cardiovascular mortality.
Keywords: Ankle-brachial index, autonomic dysfunction, subclinical hypothyroidism
INTRODUCTION
Subclinical hypothyroidism (SH) is defined by elevated serum levels of thyroid stimulating hormone (TSH) with normal levels of free thyroid hormones, is common in adults, especially among women. Among individuals with this condition, almost half of them may progress to overt thyroid failure, the annual percent risk increasing with serum TSH level.[1] There is evidence that elevated TSH levels in patients with SH do not reflect pituitary compensation to maintain euthyroidism, but a mild tissue hypothyroidism sensu strictu. When lasting more than 6–12 months, SH may be associated with an atherogenic lipid profile, a hypercoagulable state, a subtle cardiac defect with mainly diastolic dysfunction, and impaired vascular profile.[2] The pattern of cardiovascular abnormalities is similar in subclinical and overt hypothyroidism, suggesting that a lesser degree of thyroid hormone deficiency may also affect the cardiovascular system. Hypothyroid patients, even those with SH, have impaired endothelial function, normal/depressed systolic function, left ventricular (LV) diastolic dysfunction at rest, and systolic and diastolic dysfunction on effort, which may result in poor physical exercise capacity.[3] Mild elevations of TSH are associated with changes in lipid profile significant enough to raise the cardiovascular risk ratio, and these changes are corrected, once the patients have been rendered euthyroid.[4] However, mild SH has not been shown to be associated with an increase in cardiovascular risk, as assessed by carotid intima-media thickness or formation of carotid artery plaque; however, there are studies in literature to support the association and improvement in these parameters after supplementation with levothyroxine.[5,6,7] Autonomic function abnormalities have been documented in patients with primary hypothyroidism.[8]
In premenopausal women, SH has a negative effect on the lipoprotein profile and may translate into a sizable cardiovascular risk if left untreated.[9]
MATERIALS AND METHODS
Methods
We recruited 58 women (in the age range of 18–50 years) diagnosed with SH, who were drug naïve after informed consent. Age- and sex-matched controls were also recruited. Patients who were pregnant or lactating, had diabetes mellitus, hypertension, postmenopausal, women on oral contraceptives or hormone replacement therapy, smokers and alcoholics, patients with body mass index (BMI) >30 kg/m2, patients on steroid therapy, high-dose beta blockers, amiodarone, lithium, patients with major depression, and severe anxiety disorder were excluded from the study. Patients with any serious medical illness including pulmonary, cardiac, hepatic, renal, and neurological illnesses were also excluded from the study. Both cases and controls were nondiabetic.
Diagnosis of subclinical hypothyroidism
Two readings of serum TSH (repeated at least 6 weeks apart) between 4.5 and 10 mU/L with normal freeT4 levels.[1]
Tests for cardiac autonomic function and vascular profiling were conducted in the morning time after the patients had a light breakfast and they were advised not to drink any caffeinated beverages at least for 3 h before the test. All patients were assessed in the same laboratory after they were acclimatized to the laboratory conditions for at least 10–15 min. The tests were performed while the patient lied down quietly on the bed and they were explained that the tests would be completely noninvasive.
The tests were carried out as follows the outcome measures for this study are discussed below in detail related to data acquisition and analysis.
Data acquisition
Electrocardiogram
Electrocardiogram (ECG) signal was obtained in eight leads at a sampling rate of 1000 Hz (with an accuracy of about 1 ms) using Delmar Digicorders (California, USA). These data were stored on the hard drive and then backed up on to compact discs.
Vascular indices
Using the Vascular Profiler-1000 (Colin Medical Instruments, Japan), we could obtain blood pressure (BP) in carotid and all four extremities, ankle-brachial index (ABI) of BP, ankle-brachial pulse wave velocity (PWV). The Colin VP-1000 (Model BP203RPE II, Form PWV/ABI, Japan) was approved by the US Food and Drug Administration. The device works on a waveform analysis and vascular evaluation technology that measures arterial compliance in central (large) arteries as well as the peripheral arteries that aid in the early detection of vascular disease. This concept revolves around the “Rotterdam Study”[10] that establishes the correlation between arterial stiffness and atherosclerosis and the clinical evidence of central (large) artery stiffness as an established independent marker of cardiovascular morbidity and mortality.
Heart period variability
Heart rate variability (HRV) can be expressed in both time and frequency domain measures. An important development in this area is the application of spectral analysis to heart period time series. The main advantage of spectral analysis of signals is the ability to study their frequency-specific oscillations. Thus, not only the amount of variability but also the oscillation frequency (number of heart rate [HR] fluctuations per second) can be obtained. Respiratory sinus arrhythmia is mediated by the parasympathetic system and gives a spectral peak between 0.15 and 0.5 Hz (high frequency [HF]). The baroreflex-related HR fluctuations are found as a spectral peak around 0.1 Hz (low frequency [LF]); this spectral peak is dually mediated by parasympathetic and sympathetic systems.[11] Spectral power below 0.04 Hz (very LF [VLF] and ultra LF) appears to be mediated by thermoregulatory and renin-angiotensin systems. A very useful derivative measure is the ratio of LF to HF powers, as this reflects the relative sympathovagal balance, though it is controversial. The upright position accentuates the baroreflex-mediated LF power while supine posture and controlled breathing enhance the respiratory sinus arrhythmia in the HF band.[12,13]
QT interval algorithm
This was done using a graphical interface of digitized ECG where the time of the “R” wave was obtained using a peak detection algorithm.[14,15,16] Then, the operator provided the program with the beginning and the end of the QT wave template. This algorithm finds the QT interval for each beat using the time-stretch model. If the operator chooses a longer QT template, all the QT intervals will be biased accordingly. This algorithm should only be used to study QT variability and not the mean QT. The R-R interval and QT interval data were sampled at 4 Hz to obtain instantaneous HR and QT intervals. Using a 4 Hz sampling rate allowed an accurate estimation of the power spectrum up to 1 Hz. These data were edited using software, which eliminated premature beats using a linear spline approach. The data were then detrended using the best-fit line before the computation of spectral analyses.
Four oscillometric BP cuffs were applied to the four limbs. Lead I ECG electrodes were applied, and a phonocardiogram (PCG) sensor was placed on the V2 position on the chest. The test was then initiated to inflate and deflate all the four cuffs together with lead I ECG and PCG monitoring. The carotid sensor was placed on the right carotid artery using a collar-shaped sensor. Two sets of records were taken, each of 30-s duration. The machine had built-in software, which automatically calculated all the vascular indices. The sampling rate was 1024 Hz for the signals. The following is a description of different measures that were obtained from the Vascular Profiler:
Systolic BP (SBP), diastolic BP (DBP), mean arterial pressure (MAP), and pulse pressure (PP: SBP-DBP) are the averages of 60 s of data. MAP was calculated as DBP + 1/3rd of PP (SBP-DBP). PWV is the speed at which the pulse is transmitted from the heart to the end artery when blood is expelled during contraction. It was mainly used to evaluate the hardness of the artery wall.
PWV + L (distance)/pulse transit time.
BAPWV indicates brachial to ankle PWV. The distance was measured by VP-1000 and is automatically calculated by patient's height based on statistical studies. An increase in this measure indicates arterial stiffening. Arterial stiffness percentage was calculated according to the normal values of the population and also taking gender into account. This is expressed in percentage.
For ABI data, the higher brachial SBP data were used. ABI is Ankle SBP/brachial SBP. This is mainly used to evaluate atherosclerosis. A low value of <0.9 indicates peripheral occlusive vascular disease in the lower extremities and may correlate with other regions as well. A high value over 1.2 or 1.3 may indicate a single or multiple vessel stenosis.
Measurement principle for the blood pressure
The machine detected the pulsation of the artery, caused by the contraction of the heart, as the pressure oscillation in the cuff. The relationship between the changes of cuff pressure and its oscillation was stored in memory and used to determine BP. When the cuff pressure oscillation increased rapidly, it was taken as SBP and when the oscillation decreased rapidly it was taken as DBP. Cuff pressure, when the oscillation reached a peak, was taken as MAP. This was not affected by external noise or electrical signal units.
ECG was continuously acquired in eight-lead-configuration in a noise-free environment. We used 256 s of artifact-free data sampled at 1024 Hz for the analyses of HRV and QT variability index (QTvi).
Analysis of the data
These have been described in great detail in the previous studies done by the coauthor.[17,18,19] We obtained R-R intervals, SBP, and DBP, using a peak and a trough detection program and then using Berger's technique. We constructed all the time series at 4 Hz using linear interpolation. The series were detrended using a linear detrending technique before performing spectral and cross-spectral analyses. The power spectrum was obtained as the magnitude squared of the Fourier transform using a rectangular window. The powers were integrated in the VLF (0.0033–0.04 Hz), LF (0.04–0.15 Hz), and HF (0.15–0.5 Hz) ranges. Relative powers were calculated as the percentages of total power.
QT intervals were obtained by the technique described by Berger et al.[20]
Statistical analysis
The effect sizes in our previous studies on QT variability, and arterial stiffness index for inter-group differences was at least 0.8–1. Thus comparing forty subjects in each group gave us a power of at least >0.6, as we used an alpha level of 0.05 for significance. This gave us about a 70% or more chance of being able to reject the null hypothesis for the degree of changes in the outcome measures between the two groups, especially in QT variability and arterial stiffness index.
The level of significance that we proposed to use was a probability value of ≤ 0.05 and all the tests were two-tailed.
RESULTS
These patients were taken from Endocrinology outpatient Department of M.S. Ramaiah Hospitals and Bangalore Diabetes Hospital. The autonomic function tests were carried out by a single physiologist. In patients with SH whether the abnormality exists in the sympathetic or parasympathetic system is not very clear.
This study was done to see the abnormalities in cardiovascular functions in the sympathetic and parasympathetic system in 58 female patients with SH, and they were compared with age- and sex- and BMI-matched 49 euthyroid controls.
The HRLFHF was higher in cases compared to controls (P = 0.02) which suggests an increase in the sympathetic activity. The difference in the left and right ABI was more in cases than controls. Log of RRHF showed a higher trend toward controls than cases (P = 0.09). QTvi was higher in cases than controls while RRHF was higher in controls than cases.
The HR recovery HF, QTRTmean, SBP and DBP, and PWV were comparable between cases and controls. Correlation between TSH and LF/HF ratio in cases was not found to be significant (P = 0.75, r = 0.04) [Table 1].
Table 1.
Parameters in cases and controls
DISCUSSION
The patients with SH in this study were found to have cardiac autonomic dysfunction and predisposition to early atherosclerosis.
The present study was a case–control study which took only females who were age, BMI, and gender-matched with controls.
Patients with SH have been shown to have a reduction in the parasympathetic activity. The HRHFLF was higher in cases compared to controls (P = 0.02). This suggests an increase in the sympathetic activity in our subjects with SH and similar results were reciprocated in the study done by Galetta et al., who also showed an increase in the sympathetic activity.[21] The patients who were included in the study done by Galleta had median TSH >3.6 mIU/ml and range was 3.8–12 mIU/ml and it included both men and women though the number of females was higher as compared to males, and the age was higher than compared to what has been taken in our study.[21] The mean age of females in our study was 31.8 ± 8.9 years. The previous study done by Mahajan et al.[8] also suggested that autonomic dysfunction may be seen in both subclinical and frank hypothyroidisms.[8] Inukai et al. showed that hypothyroidism is associated with increased sympathetic input to the heart.[22] They showed the power spectral analysis may be more sensitive than these tests. Low HF value suggests a reduction in the vagal tone, and though it was not significant, it was found to be lower in cases compared to controls. Kahaly also showed a hypofunctional parasympathetic system in patients with SH by analyzing time and frequency domain of HRV.[23]
The QTvi, which is an index of QT variability normalized for mean QT over HRV, normalized for mean HR, was higher in cases than controls (P = 0.01) which again shows a predominance of sympathetic activity in cases compared to controls. The QT interval represents the duration from the onset of depolarization to the completion of repolarization of the cardiac cycle. The autonomic modulation of HR can be altered in SH, and this may lead to ventricular recovery time inhomogeneity. QTvi was a better predictor of sudden death in cardiac patients compared to other measures such as ejection fraction, HRV, and T wave alterans in a study done by Atiga et al.[24] Although generally QT variability follows HRV, there may not be a complete coherence always between the two-time series.[25] A previous study showed that coherence between HR and QT interval fluctuations in the range of 0–0.5 Hz, and especially in the HF range (0.15–0.5 Hz), was significantly lower in adults than in children.[26]
There are studies in literature to show that intravenous isoproterenol and a change from supine to standing posture produces a significant increase in the QTvi, which again indirectly links it to an increase in the sympathetic activity.[14] And sympathomimetic agents such as Palmolein and Yohimbine have been shown to increase QTvi which again confirms that it is linked to increase in sympathetic activity.[27,28] Thus, the demonstration of increased QTvi in patients with SH points toward increased sympathetic activity and thus may indirectly be a pointer toward an increased predisposition to cardiovascular morbidity.
There are conflicting results available with regards to the sympathetic overactivity in patients with hypothyroidism Cacciatori et al. showed an increased sympathetic influence in hypothyroidism, but a study done by Xing et al. showed a higher level of vagal tone in these patients.[29,30] These conflicting results may be partially explained by the different selection of patients such as age, gender, type, severity, and duration of hypothyroidism and number of patients in the various studies and also to the diverse techniques used to monitor the sympathovagal imbalance. Log of RRHF showed a higher trend toward controls than cases. Galetta et al. showed that patients with SH showed higher QT dispersion and lower HRV measures than healthy controls, and QT dispersion was positively found to be positively related to TSH value and the measures became normal after treatment.[21]
Kahaly reported a defect in the autonomic function, i.e., the parasympathetic system by the analysis of heart and frequency domain of HRV in patients with hypothyroidism.[23] The study took women who were older compared to the women included in our study, and group of similar data was replicated by studying HR response to exercise and recovery. The HRV was lower in cases than controls. Lower HRV is associated with increased risk of arrhythmia, but it was not found to be significant in our study (P = not significant). Galetta et al. showed that there is an increase in the sympathetic tone with decrease in the HRV in patients with hypothyroidism suggesting a decrease in the vagal tone.[21] Similar results have been shown in our study where the HRV was found to be reduced in cases compared to controls. Sahin et al. also suggested that in patients with TSH >10 there was a decrease in the sympathetic tone and increase in the parasympathetic activity.[31] Inukai et al. showed a defect in parasympathetic activity in patients with severe hypothyroidism.[32] Celik et al. showed that HRV and HR turbulence were decreased in the patient group, and treatment with levothyroxine was not found to effectively restore the cardiac autonomic functions.[33] Falcone et al. showed that time domain parameters of HRV were definitely lower both in patients with SH than in the control group, with statistically significant differences in the standard deviation of all NN intervals (SDNN), mean of the sum of the square of the differences between adjacent NN intervals (root mean square of successive difference), triangular interpolation of NN interval, and mean response ratio for both subgroups. HF is a marker of vagal efferent signal that is modulated by ventilation.[34] HRV parameters were lower in cases as compared to controls in both time and frequency domains. HRV measures fluctuations in the autonomic inputs to the heart. Thus, it measures both autonomic withdrawal and saturating level of the sympathetic input which can lead to diminished HRV.
The mechanism for autonomic dysfunction in hypothyroidism has been contemplated to be due to high level of adrenaline along with a receptor or postreceptor sensitization, a decrease chronotropic response to beta-adrenergic stimulation, increase in thyrotropin-releasing hormone which has a direct influence on the sympathetic outflow.[35,36,37,38,39,40,41,42] The thyroid hormones can also have direct effect on the heart which includes increased protein deposition in the extracellular space leading to accumulation of water in the myocardial wall which can further cause fibrosis and increased regional inhomogeneity of ventricular repolarization.[43]
Impaired cardiac autonomic activity is closely related to lethal arrhythmias.[40] Sahin et al. showed a statistically significant difference in only time domain parameters of SDNN and SD of all 5-min mean normal NN intervals in patients with TSH >10 and controls and also a positive correlation was demonstrated between TSH value and root mean square of successive R-R interval.[31] The data on the effect of SH on sympathetic and parasympathetic activity are therefore contradictory. Sahin et al. showed that in patients with TSH >10 there was a decrease in the sympathetic tone and increase in parasympathetic activity while our study results show increase in sympathetic activity and reduction in parasympathetic activity which is similar to the results shown by Galetta et al.[31,44]
HRV measures the fluctuations in the autonomic inputs rather than mean of autonomic inputs; thus, both autonomic withdrawal and high level of sympathetic activity leads to diminished HRV.
Hamano and Inoue showed that vascular wall stiffness is increased in patients with SH and overt hypothyroidism, and appropriate treatment of the same may reverse the cardiovascular abnormalities.[45] Kosar F et al. also showed that SH is associated with impaired right ventricular diastolic function in addition to impaired LV diastolic function. In our study, the PWV on the right and left side was not found to be different among cases and controls.[2] Thus, autonomic dysfunction in SH would also justify the treatment of these patients as abnormality may lie in the sympathetic or parasympathetic system. There was no difference in SBP and DBP in the two groups. The BP was not recorded to be high in any of the subclinical hypothyroid group.
The difference in the left and right ABI was more in cases than controls (P = 0.03). This could point towards an increased cardiovascular risk as shown in the study done by Nead et al. who studied participants undergoing nonemergent coronary angiography and were subsequently followed for all-cause and cardiovascular mortality where it was shown that in patients who had no peripheral vascular disease, there was an 11% increased risk of all-cause mortality per 1 mmHg increased difference between the left and right brachial systolic pressures.[46] The results in our study show a difference in the right and the left ABI, and this could prove to be a marker of cardiovascular morbidity in future follow-up studies.
The limitations of our study include lack of follow-up data with repeat autonomic function tests after starting levothyroxine to see for improvement or any change in any of the parameters. The further studies are needed to follow-up these patients for improvement in the parameters after treatment. Another limitation of our study is that we included only females in our study, and further research is needed in males also to see if these findings can be extrapolated to males also.
CONCLUSIONS
Our study shows conclusively that patients with SH have significant sympathovagal dysfunction although few studies have shown predominant vagal dysfunction, but our study emphasizes that sympathetic dysfunction is also significantly present in this group of patients. The QTvi may be an important noninvasive tool in this group of patients to study the risk of cardiovascular mortality. The higher difference in the ABI between the two sides in cases confirms the previous findings that SH may lead to increased cardiovascular mortality if left untreated. These findings may explain higher cardiovascular morbidity and mortality in patients with SH and has further strengthened the evidence in favor of treatment with replacement therapy. It may lead onto follow-up studies to show how the vascular changes may progress over time.
Financial support and sponsorship
The study has been funded by grant from Endocrine Society of India
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Mr Gopinath for Technical Assistance.
REFERENCES
- 1.Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988–1028. doi: 10.4158/EP12280.GL. [Erratum in: Endocr Pract 2013;19:175] [DOI] [PubMed] [Google Scholar]
- 2.Kosar F, Sahin I, Turan N, Topal E, Aksoy Y, Taskapan C. Evaluation of right and left ventricular function using pulsed-wave tissue Doppler echocardiography in patients with subclinical hypothyroidism. J Endocrinol Invest. 2005;28:704–10. doi: 10.1007/BF03347553. [DOI] [PubMed] [Google Scholar]
- 3.Berezin AE, Kremzer AA, Martovitskaya YV, Samura TA, Berezina TA. Pattern of circulating endothelial-derived microparticles among chronic heart failure patients with dysmetabolic comorbidities: The impact of subclinical hypothyroidism. Diabetes Metab Syndr. 2016;10:29–36. doi: 10.1016/j.dsx.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Zha K, Zuo C, Wang A, Zhang B, Zhang Y, Wang B, et al. LDL in patients with subclinical hypothyroidism shows increased lipid peroxidation. Lipids Health Dis. 2015;14:95. doi: 10.1186/s12944-015-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delitala AP, Filigheddu F, Orrù M, AlGhatrif M, Steri M, Pilia MG, et al. No evidence of association between subclinical thyroid disorders and common carotid intima medial thickness or atherosclerotic plaque. Nutr Metab Cardiovasc Dis. 2015;25:1104–10. doi: 10.1016/j.numecd.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isik-Balci Y, Agladioglu S, Agladioglu K, Kilic-Toprak E, Kilic-Erkek O, Ozhan B, et al. Impaired hemorheological parameters and increased carotid intima-media thickness in children with subclinical hypothyroidism. Horm Res Paediatr. 2016;85:250–6. doi: 10.1159/000444363. [DOI] [PubMed] [Google Scholar]
- 7.del Busto-Mesa A, Cabrera-Rego JO, Carrero-Fernández L, Hernández-Roca CV, González-Valdés JL, de la Rosa-Pazos JE. Changes in arterial stiffness, carotid intima-media thickness, and epicardial fat after L-thyroxine replacement therapy in hypothyroidism. Endocrinol Nutr. 2015;62:270–6. doi: 10.1016/j.endonu.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Mahajan AS, Lal R, Dhanwal DK, Jain AK, Chowdhury V. Evaluation of autonomic functions in subclinical hypothyroid and hypothyroid patients. Indian J Endocrinol Metab. 2013;17:460–4. doi: 10.4103/2230-8210.111642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikhail GS, Alshammari SM, Alenezi MY, Mansour M, Khalil NA. Increased atherogenic low-density lipoprotein cholesterol in untreated subclinical hypothyroidism. Endocr Pract. 2008;14:570–5. doi: 10.4158/EP.14.5.570. [DOI] [PubMed] [Google Scholar]
- 10.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: The Rotterdam study. Circulation. 2006;113:657–63. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 11.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–92. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 12.Yeragani VK. Heart rate and blood pressure variability: Implications for psychiatric research. Neuropsychobiology. 1995;32:182–91. doi: 10.1159/000119234. [DOI] [PubMed] [Google Scholar]
- 13.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–2. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 14.Yeragani VK, Pohl R, Jampala VC, Balon R, Kay J, Igel G. Effect of posture and isoproterenol on beat-to-beat heart rate and QT variability. Neuropsychobiology. 2000;41:113–23. doi: 10.1159/000026642. [DOI] [PubMed] [Google Scholar]
- 15.Haigney MC, Zareba W, Gentlesk PJ, Goldstein RE, Illovsky M, McNitt S, et al. QT interval variability and spontaneous ventricular tachycardia or fibrillation in the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J Am Coll Cardiol. 2004;44:1481–7. doi: 10.1016/j.jacc.2004.06.063. [DOI] [PubMed] [Google Scholar]
- 16.Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485–90. doi: 10.1161/01.hyp.26.3.485. [DOI] [PubMed] [Google Scholar]
- 17.Rachow T, Berger S, Boettger MK, Schulz S, Guinjoan S, Yeragani VK, et al. Nonlinear relationship between electrodermal activity and heart rate variability in patients with acute schizophrenia. Psychophysiology. 2011;48:1323–32. doi: 10.1111/j.1469-8986.2011.01210.x. [DOI] [PubMed] [Google Scholar]
- 18.Boettger MK, Schulz S, Berger S, Tancer M, Yeragani VK, Voss A, et al. Influence of age on linear and nonlinear measures of autonomic cardiovascular modulation. Ann Noninvasive Electrocardiol. 2010;15:165–74. doi: 10.1111/j.1542-474X.2010.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koschke M, Boettger MK, Macholdt C, Schulz S, Yeragani VK, Voss A, et al. Increased QT variability in patients with anorexia nervosa – An indicator for increased cardiac mortality? Int J Eat Disord. 2010;43:743–50. doi: 10.1002/eat.20765. [DOI] [PubMed] [Google Scholar]
- 20.Berger RD, Kasper EK, Baughman KL, Marban E, Calkins H, Tomaselli GF. Beat-to-beat QT interval variability: Novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation. 1997;96:1557–65. doi: 10.1161/01.cir.96.5.1557. [DOI] [PubMed] [Google Scholar]
- 21.Galetta F, Franzoni F, Fallahi P, Rossi M, Carpi A, Rubello D, et al. Heart rate variability and QT dispersion in patients with subclinical hypothyroidism. Biomed Pharmacother. 2006;60:425–30. doi: 10.1016/j.biopha.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Inukai T, Takanashi K, Kobayashi H, Fujiwara Y, Tayama K, Aso Y, et al. Power spectral analysis of variations in heart rate in patients with hyperthyroidism or hypothyroidism. Horm Metab Res. 1998;30:531–5. doi: 10.1055/s-2007-978927. [DOI] [PubMed] [Google Scholar]
- 23.Kahaly GJ. Cardiovascular and atherogenic aspects of subclinical hypothyroidism. Thyroid. 2000;10:665–79. doi: 10.1089/10507250050137743. [DOI] [PubMed] [Google Scholar]
- 24.Atiga WL, Calkins H, Lawrence JH, Tomaselli GF, Smith JM, Berger RD. Beat-to-beat repolarization lability identifies patients at risk for sudden cardiac death. J Cardiovasc Electrophysiol. 1998;9:899–908. doi: 10.1111/j.1540-8167.1998.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 25.Kumar R, Ramachandraiah CT, Chokka P, Yeragani VK. Mean and variability of QT-interval: Relevance to psychiatric illness and psychotropic medication. Indian J Psychiatry. 2009;51:6–8. doi: 10.4103/0019-5545.44898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeragani VK, Berger R, Pohl R, Balon R. Effect of age on diurnal changes of 24-hour QT interval variability. Pediatr Cardiol. 2005;26:39–44. doi: 10.1007/s00246-004-0676-7. [DOI] [PubMed] [Google Scholar]
- 27.Pohl R, Balon R, Jayaraman A, Doll RG, Yeragani V. Effect of fluoxetine, pemoline and placebo on heart period and QT variability in normal humans. J Psychosom Res. 2003;55:247–51. doi: 10.1016/s0022-3999(02)00478-6. [DOI] [PubMed] [Google Scholar]
- 28.Yeragani VK, Tancer M, Uhde T. Heart rate and QT interval variability: Abnormal alpha-2 adrenergic function in patients with panic disorder. Psychiatry Res. 2003;121:185–96. doi: 10.1016/s0165-1781(03)00235-x. [DOI] [PubMed] [Google Scholar]
- 29.Cacciatori V, Gemma ML, Bellavere F, Castello R, De Gregori ME, Zoppini G, et al. Power spectral analysis of heart rate in hypothyroidism. Eur J Endocrinol. 2000;143:327–33. doi: 10.1530/eje.0.1430327. [DOI] [PubMed] [Google Scholar]
- 30.Xing H, Shen Y, Chen H, Wang Y, Shen W. Heart rate variability and its response to thyroxine replacement therapy in patients with hypothyroidism. Chin Med J (Engl) 2001;114:906–8. [PubMed] [Google Scholar]
- 31.Sahin I, Turan N, Kosar F, Taskapan C, Gunen H. Evaluation of autonomic activity in patients with subclinical hypothyroidism. J Endocrinol Invest. 2005;28:209–13. doi: 10.1007/BF03345374. [DOI] [PubMed] [Google Scholar]
- 32.Inukai T, Kobayashi I, Kobayashi T, Ishii A, Yamaguchi T, Yamaguchi Y, et al. Parasympathetic nervous system activity in hypothyroidism determined by R-R interval variations on electrocardiogram. J Intern Med. 1990;228:431–4. doi: 10.1111/j.1365-2796.1990.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 33.Celik A, Aytan P, Dursun H, Koc F, Ozbek K, Sagcan M, et al. Heart rate variability and heart rate turbulence in hypothyroidism before and after treatment. Ann Noninvasive Electrocardiol. 2011;16:344–50. doi: 10.1111/j.1542-474X.2011.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falcone C, Matrone B, Bozzini S, Guasti L, Falcone R, Benzi A, et al. Time-domain heart rate variability in coronary artery disease patients affected by thyroid dysfunction. Int Heart J. 2014;55:33–8. doi: 10.1536/ihj.13-198. [DOI] [PubMed] [Google Scholar]
- 35.Mittag J, Lyons DJ, Sällström J, Vujovic M, Dudazy-Gralla S, Warner A, et al. Thyroid hormone is required for hypothalamic neurons regulating cardiovascular functions. J Clin Invest. 2013;123:509–16. doi: 10.1172/JCI65252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva JE, Bianco SD. Thyroid-adrenergic interactions: Physiological and clinical implications. Thyroid. 2008;18:157–65. doi: 10.1089/thy.2007.0252. [DOI] [PubMed] [Google Scholar]
- 37.Dratman MB, Goldman M, Crutchfield FL, Gordon JT. Nervous system role of iodocompounds in blood pressure regulation. Life Sci. 1982;30:611–8. doi: 10.1016/0024-3205(82)90277-6. [DOI] [PubMed] [Google Scholar]
- 38.Hugues FC, Munera Y, Julien D, Marche J. Exploration of beta adrenergic functions in man. Isoprenaline test. Coeur Med Interne. 1974;13:527–33. [PubMed] [Google Scholar]
- 39.Heemstra KA, Burggraaf J, van der Klaauw AA, Romijn JA, Smit JW, Corssmit EP. Short-term overt hypothyroidism induces sympathovagal imbalance in thyroidectomized differentiated thyroid carcinoma patients. Clin Endocrinol (Oxf) 2010;72:417–21. doi: 10.1111/j.1365-2265.2009.03655.x. [DOI] [PubMed] [Google Scholar]
- 40.Manhem P, Bramnert M, Hallengren B, Lecerof H, Werner R. Increased arterial and venous plasma noradrenaline levels in patients with primary hypothyroidism during hypothyroid as compared to euthyroid state. J Endocrinol Invest. 1992;15:763–5. doi: 10.1007/BF03347648. [DOI] [PubMed] [Google Scholar]
- 41.Bilezikian JP, Loeb JN. The influence of hyperthyroidism and hypothyroidism on alpha- and beta-adrenergic receptor systems and adrenergic responsiveness. Endocr Rev. 1983;4:378–88. doi: 10.1210/edrv-4-4-378. [DOI] [PubMed] [Google Scholar]
- 42.Polikar R, Kennedy B, Maisel A, Ziegler M, Smith J, Dittrich H, et al. Decreased adrenergic sensitivity in patients with hypothyroidism. J Am Coll Cardiol. 1990;15:94–8. doi: 10.1016/0735-1097(90)90182-o. [DOI] [PubMed] [Google Scholar]
- 43.Polikar R, Burger AG, Scherrer U, Nicod P. The thyroid and the heart. Circulation. 1993;87:1435–41. doi: 10.1161/01.cir.87.5.1435. [DOI] [PubMed] [Google Scholar]
- 44.Galetta F, Franzoni F, Fallahi P, Tocchini L, Braccini L, Santoro G, et al. Changes in heart rate variability and QT dispersion in patients with overt hypothyroidism. Eur J Endocrinol. 2008;158:85–90. doi: 10.1530/EJE-07-0357. [DOI] [PubMed] [Google Scholar]
- 45.Hamano K, Inoue M. Increased risk for atherosclerosis estimated by pulse wave velocity in hypothyroidism and its reversal with appropriate thyroxine treatment. Endocr J. 2005;52:95–101. doi: 10.1507/endocrj.52.95. [DOI] [PubMed] [Google Scholar]
- 46.Nead KT, Cooke JP, Olin JW, Leeper NJ. Alternative ankle-brachial index method identifies additional at-risk individuals. J Am Coll Cardiol. 2013;62:553–9. doi: 10.1016/j.jacc.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]


