Abstract
Background:
A controversy exists regarding the association between subclinical hypothyroidism (SH) and dyslipidemia. Moreover, studies on lipid ratios and atherogenic index of plasma (AIP) in SH are rare, particularly in the Indian scenario.
Aim:
This study aimed to investigate abnormalities in conventional lipid profile, lipid ratios, and AIP in SH and attempted to correlate thyroid stimulating hormone (TSH) and AIP in SH.
Materials and Methods:
In this retrospective analysis of patient records of SH subjects and euthyroid subjects, age, free triiodothyronine, free thyroxine, TSH, total cholesterol, triglycerides, high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol, lipid ratios, and AIP were compared between the two groups. The correlation of TSH and AIP in SH was studied. Spearman's correlation, Mann–Whitney U-test and logistic regression analysis were performed.
Results:
Triglyceride, triglyceride/HDL-C, and AIP were significantly higher in SH as compared to euthyroid group, but there was no correlation between TSH and AIP in SH. AIP emerged as the significant single factor associated with SH in multiple logistic regressions.
Conclusion:
The positive association of dyslipidemia and SH indicates a need for regular screening of these patients to enable early diagnosis and treatment of dyslipidemia. Even in patients who have a normal conventional lipid profile, lipid ratios, and AIP have to be calculated for better assessment of atherogenic risk.
Keywords: Atherogenic index of plasma, dyslipidemia, lipid profile, lipid ratios, subclinical hypothyroidism
INTRODUCTION
Subclinical hypothyroidism (SH), described as a condition characterized by elevated serum level of thyroid stimulating hormone (TSH) with normal levels of free thyroxine (fT4) and free triiodothyronine (fT3), is a common disorder having a prevalence of about 7.5–8.5% in females and 2.8–4.4% in males, worldwide.[1,2]
Several studies have established overt hypothyroidism to be associated with abnormalities of lipid metabolism such as elevated levels of total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C), and triglycerides (TG), thereby predisposing to cardiovascular diseases.[3,4,5] However, the association of SH with abnormalities in lipid parameters have been studied with conflicting results.[4,6] Some studies have also established a link between SH and metabolic syndrome while others have failed to do so.[7,8] It has also been indicated that small changes in thyroid hormone levels within the reference range may influence the severity of atherosclerosis.[9]
Currently, serum lipid ratios such as TC/high-density lipoprotein-cholesterol (TC/HDL-C), TG/HDL-C, and LDL-C/HDL-C have been shown to be better predictors of cardiovascular risk compared to conventional lipid profile.[10] Small dense LDL (sdLDL), a subtype of LDL has been described as the main determinant of the atherogenicity of LDL-C.[10] Atherogenic index of plasma (AIP) has been defined as the logarithmically transformed ratio of TG to HDL-C.[11] AIP, which is a good surrogate marker of sdLDL, has been indicated as a superior predictor of cardiovascular risk than the previously used lipid parameters.[10,12] However, research on the complex interrelationship between SH and lipid ratios and AIP has been extremely rare.
Thus, though the effect of overt hypothyroidism on abnormalities in lipid parameters has already been established, the relationship between SH and lipid metabolism needs further study. Furthermore, there is insufficient information regarding the effect of SH on lipid ratios and AIP. This is particularly true in the Indian scenario where conflicting results have been obtained, especially in the female population where SH is more prevalent. Therefore, this study was conducted to understand the association of SH with abnormalities in the serum lipid profile and AIP in apparently healthy adult females. The study also aims to find out whether any correlation exists between AIP and serum TSH levels among SH cases.
MATERIALS AND METHODS
The study was a retrospective, time-bound, hospital-based, case–control study carried out by detailed analysis of patient records in the Department of Biochemistry at a medical college in Southern India. The study was approved by the institutional research committee and ethics committee, which follow the guidelines set by the Helsinki declaration (Reference number - RC/14/123).
Data collection was done for 2 months from May 1, 2015, to June 30, 2015. Study subjects were taken from the records of apparently healthy adult females coming for a master health check to our hospital from June 1, 2014, to May 30, 2015. Adult females with high TSH (>4.2 μIU/mL) but normal fT3 (2.0–4.4 pg/mL) and fT4 (0.93–1.7 ng/dL) levels were included in the SH group. Adult females having normal TSH (0.27–4.2 μIU/mL), fT3 (2.0–4.4 pg/mL), and fT4 (0.93-1.7 ng/dL) levels were included in the euthyroid (EU) group. Individuals with a history of overt hypothyroidism or levothyroxine replacement therapy, history of radioiodine treatment or thyroid surgery, history of alcohol abuse and smoking, history of drugs such as estrogens, diabetic individuals, subjects with renal, hepatic or other systemic diseases, and known history of dyslipidemia, were excluded from the study. Totally, 504 female subjects came for master health check during the period that had been selected. Of these, 300 patient records could be studied during the 2 months when the study was carried out. Based on the fT3, fT4, and TSH values, the subjects were classified into SH and EU groups according to the inclusion criteria. Based on age-matching between the SH and EU groups, seventy consecutive patients in each group, according to their date of presentation were taken for statistical analysis.
Detailed epidemiological information along with a detailed history was taken from the hospital records of the patients who came for a master health check to our hospital. Specific emphasis was given to age, history of smoking and alcohol intake, history of overt hypothyroidism or levothyroxine replacement therapy, history of thyroid surgery, history of hypertension, diabetes or dyslipidemia, history of liver, hepatic or any other systemic disease and history of medications such as oral contraceptive pills. Next, the reports of the subjects were collected with special emphasis on serum fasting glucose, liver function tests, renal function tests, complete blood count with peripheral smear, and routine examination of urine. These results helped to exclude patients if any abnormality was noted. For the study, the following results were noted: serum TSH, fT3, fT4, TC, TG, HDL-C, and LDL-C. For each subject included in SH group and EU group, the lipid ratios were calculated as TC/HDL-C, TG/HDL-C, and LDL-C/HDL-C. AIP was calculated by the formula: log([TG]/[HDL-C]).[11]
The routine serum parameters in all the study subjects had been measured using automated routine chemistry analyzer (Roche Cobas Integra 400 plus, Roche Diagnostics Limited, Switzerland) with commercial kits (Roche Cobas C packs, Roche Diagnostics Limited, Switzerland) according to manufacturer's protocol. Thyroid profile of all the study subjects had been measured using automated electrochemiluminescence immunoassay analyzer (Roche Cobas e411, Roche Diagnostics Limited, Switzerland) with commercial kits (Cobas cassettes containing working solutions) according to manufacturer's protocol.
Statistical study
The data were analyzed using SPSS statistical software (v 16; IBM Corporation, Armonk, NY, USA). To describe continuous variables, mean and standard deviation was used. Since the data were not normally distributed, the difference in lipid parameters, lipid ratios, and AIP between SH group and EU group, were compared using Mann–Whitney U-test and a P < 0.05 was considered statistically significant. In the SH group, AIP and TSH were correlated using Spearman's correlation. Logistic regression was performed to ascertain the effects of age, TC, TG, HDL, LDL, TG/HDL, TC/HDL, LDL/HDL, and AIP on SH. Variables which are significant at <0.1 in simple regression were considered for multiple regression analysis and because of multicollinearity, only one of the following variables were included in the model at a time: TG, TG/HDL, and AIP. The final model was found by the Enter procedure.
RESULTS
The descriptive statistics of all the study subjects are shown in Table 1. There was no statistically significant difference (P > 0.05) among the mean ages of SH and EU groups. However, there was a statistically significant (P < 0.05) difference between the means of fT3, fT4, and TSH of the SH and EU groups.
Table 1.
Comparison of baseline characteristics of the study subjects
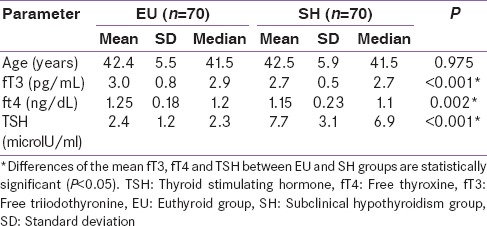
The data in Table 2 represent a comparison of lipid profile parameters of EU group and SH group. It includes mean and standard deviations of TC, TG, HDL-C, and LDL-C of EU and SH groups. The differences between the means of TC, TG, and LDL-C were found to be statistically significant while that for HDL-C was found to be insignificant (P = 0.712). The data presented in Table 3 shows the comparison of lipid ratios such as TC/HDL-C, TG/HDL-C, and LDL-C/HDL-C between SH and EU groups. The difference in the mean TG/HDL-C between the two groups was found to be significant (P < 0.005).
Table 2.
Comparison of lipid profile parameters between the study groups
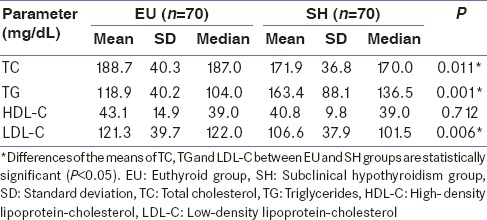
Table 3.
Comparison of lipid ratios between the study groups
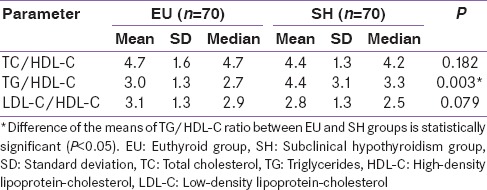
Figure 1 represents the comparison between the mean AIP of SH group and EU group. The mean AIP of EU group was 0.078, and that of SH group was 0.211. When the means were compared using Mann–Whitney U-test, the difference was found to be statistically significant (P = 0.002). Figure 2 shows the correlation between AIP and TSH in SH groups. The Spearman's correlation coefficient r is 0.174 (P = 0.149) indicating that there is no significant correlation between AIP and TSH in the SH group.
Figure 1.
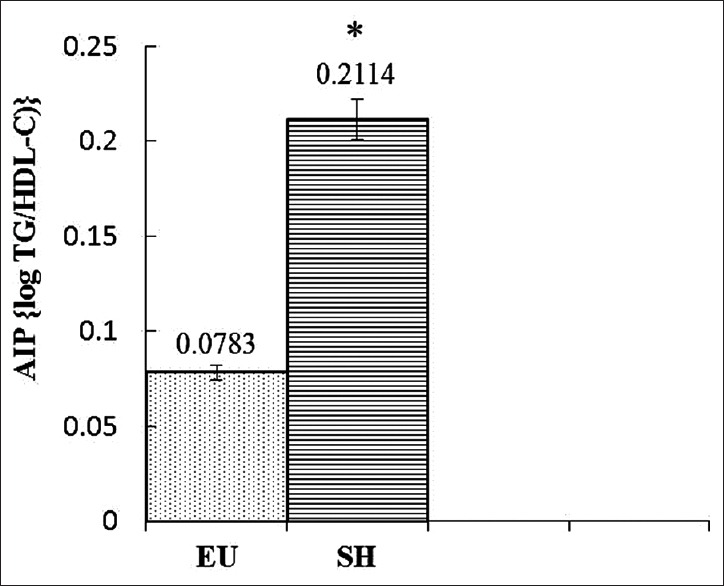
Comparison of atherogenic index of plasma between the study groups. EU: Euthyroid group, SH: Subclinical hypothyroidism group, AIP: Atherogenic index of plasma. *Difference of the means of AIP between EU and SH groups is statistically significant (P = 0.002, <0.05)
Figure 2.
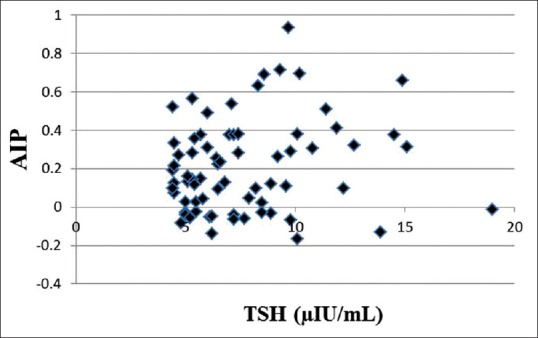
Correlation between atherogenic index of plasma and thyroid stimulating hormone in subclinical hypothyroidism group. AIP: Atherogenic index of plasma, TSH: Thyroid stimulating hormone. No significant correlation, Spearman's correlation coefficient r is 0.174 (P = 0.149)
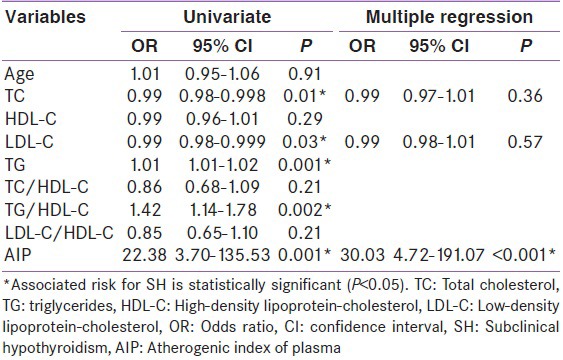
Table 4 represents the results of logistic regression analysis. AIP has a significantly higher risk (OR = 22.38) than TG (OR = 1.01) or TG/HDL (OR = 1.42). The variable AIP emerged as a significant single factor in multiple regression analysis.
Table 4.
Variables associated with subclinical hypothyroidism
DISCUSSION
The link between dyslipidemia and SH has been explored in a large number of studies of varying methodologies in different parts of the world.[2,9,13,14,15] However, studies on the abnormalities in lipid ratios and AIP which are better markers of cardiovascular risk than conventional lipid profile are rare. With this background, we conducted a hospital-based case–control study, where we retrospectively studied patient records to investigate the abnormalities in conventional lipid profile, lipid ratios, and AIP in SH. The study also aimed to find out if there was any correlation between TSH and AIP levels in SH.
In our study, the descriptive variable-age did not differ significantly between SH and EU. This confirms the age matching in our study. The mean age of SH patients in our study was 42.5 similar to other studies done in Tamil Nadu (India), Greece, and Turkey but higher than studies done in Kashmir (India), Italy, and Turkey.[4,9,16,17,18] The differences can be explained by the different study populations used in the different studies which differed on ethnicity, gender preponderance, race, etc., However, TSH levels were significantly higher in SH as compared to EU similar to other studies.[9] In our study, we also reported that the mean fT3 and fT4 was significantly lower in SH as compared to EU though in both groups the values were within normal reference ranges. This is in contrast to the other study done in Tamil Nadu where no significant difference was noted.[9] The difference could be explained by our larger sample size whereby the study in Tamil Nadu had a sample size of 30 whereas our study had a sample size of 70.[9]
Regarding lipid profile parameters, our study showed a significantly lower TC and LDL-C level and a significantly higher TG level in SH as compared to EU. Several other studies conducted in India and abroad have also reported similarly higher TG levels, but TC and LDL-C were also reported to be higher in SH as opposed to our study.[2,5] Since our study was based on retrospective analysis of patient records, it can be suggested that a prospective study could lead to a better agreement. On the other hand, a contrasting result was obtained in National Health and Nutrition Examination Survey III, a large study of 215 subjects with SH and 8013 euthyroid individuals, where no significant difference in lipid parameters between SH and euthyroid individuals was reported, when adjusted for age, race, sex, and the use of lipid-lowering agents.[19] In overt hypothyroidism, the molecular basis of this dyslipidemic pattern has been elucidated. In overt hypothyroidism, due to the reduced activity of LDL-C receptors, the catabolism of LDL-C, and intermediate density lipoprotein-cholesterol are affected, consequently increasing serum TC and LDL-C.[20,21] Moreover, in overt hypothyroidism, the activity of the enzyme lipoprotein lipase is lowered, thereby inhibiting the removal of TG-rich lipoproteins leading to a high serum TG level.[22] In our study, the mean HDL-C was lower in SH than in EU, but the difference was not statistically significant. This result is in contrast to the study done in Tamil Nadu where the HDL-C was significantly lower in SH compared to EU.[9] In our study, the lower than desirable HDL-C levels in both SH and EU can be explained by the lack of exercise and sedentary lifestyle among the study participants. In the Indian context, this poses a serious health risk and needs to be investigated further by large population-based studies as well as experimental studies to elucidate the molecular etiopathogenetic links. Several studies have also shown that levothyroxine therapy has led to significant improvement in dyslipidemia in SH patients, but we have not studied this aspect.[23] In contrast, in overt hypothyroidism, elevation of serum HDL-C levels may be seen due to increased concentration of HDL2 particles.[24]
Considering our results about the lipid ratios, our study showed a significantly higher TG/HDL-C in SH as compared to EU, but no significant difference was seen in TC/HDL-C and LDL-C/HDL-C levels. In a study, it was proved that 6-month treatment with levothyroxine led to normalization of TSH, and this improved the adverse lipid profile of elderly women with SH. This effect was reflected in the lipid ratios and thereby concentrations of TC/HDL-C, TC/LDL-C, Lp(a) and apoB were significantly reduced but apoAI and HDL-C were also reduced but not significantly.[25] However, the difference in the means of lipid ratios between SH and EU have not been studied. Thus, though controversies exist regarding the association of SH with cardiovascular risk, to the best of our knowledge, no other study has investigated the status of lipid ratios in SH. Thus, to fill this knowledge lacuna, we decided to include lipid ratios in our study.
In our study, AIP was significantly higher among SH subjects than EU subjects. The mean value of AIP in SH subjects was found to be 0.211 which falls in the at risk category (AIP >0.11) for cardiovascular diseases. AIP has already been established as strongly correlating with serum sdLDL level, which is the main atherogenic fraction of LDL.[10] On the other hand, SH has been shown to be associated with highly atherogenic pattern B of LDL-C subfractions.[26] It has been reported that as a marker of lipoprotein particle size, AIP adds predictive value beyond that of the individual lipids, and/or TC/HDL-C ratio.[27] This is corroborated in our multiple regression analysis, where AIP emerged as the single most significant factor associated with SH among the study parameters. This could also indicate that SH is associated with high AIP and thereby a highly atherogenic lipid profile. Nevertheless, despite extensive literature search, no study was found to explore the association between AIP and SH. In other metabolic disease states such as metabolic syndrome, hyperuricemia, and diabetes mellitus, AIP was found to be a good predictor of cardiovascular risk.[10,28] In overt hypothyroidism also AIP levels have not been studied, but conflicting results were seen in studies investigating the impact of overt hypothyroidism on LDL-C subfractions, particularly sdLDL.[5] Though a recent study conducted on newly diagnosed hypothyroid patients showed an association between hypothyroidism and elevated sdLDL, other studies could not conclusively prove such an association.[29,30,31] All these results reiterate the need for studies on the association of AIP and abnormal lipid ratios with SH. In our study, we have attempted to bridge this gap and from our results, it could be speculated that though serum LDL-C was lower in SH subjects, their pattern is significantly atherogenic with a high level of sdLDL.
We failed to find any correlation between AIP and TSH levels in SH subjects thereby indicating that though SH patients were at a greater risk of cardiovascular diseases, the risk did not proportionately increase with an increase in TSH levels. Several studies have reported that dyslipidemic changes were not apparent when serum TSH levels were below 10 mIU/L but above this level, dyslipidemia was more overt. An Indian study reported that there was no association between lipid abnormalities and SH when the value of serum TSH was <10 mIU/L.[32] On the other hand, patients with TSH concentrations >10 mU/l had significantly greater levels of TC and LDL-C as well as lower HDL-C compared with controls.[32] Based on these findings, it would not be wrong to speculate that since a majority of our patients had a TSH level <10 mIU/L, AIP could not be correlated with TSH levels in SH patients.
However, our study has certain limitations. Ours is a retrospective, hospital-based, time-bound study where hospital records of study subjects were analyzed. Large, well-planned, community-based, prospective studies need to be conducted to gain a better understanding of dyslipidemia in SH, particularly the study of AIP.
Thus, the positive association of dyslipidemia and SH indicates a need for regular screening of SH patients for dyslipidemia to enable early diagnosis and treatment of the condition. Even in patients who have a normal conventional lipid profile, lipid ratios, and AIP have to be calculated for better assessment of cardiovascular risk in SH patients. The high prevalence of low HDL levels reiterates the need for counseling of patients regarding healthy dietary habits and regular exercise. High AIP among the SH population too underlines the need for large-scale, community-based programs for health awareness and lifestyle modification. However, this is a cross-sectional study and so, whether SH leads to high AIP could not be determined. Moreover, the molecular basis for this association can only be elucidated by experimental studies with animal models.
CONCLUSION
This study helped to bridge the knowledge gap as well as paucity of Indian data available regarding the complex interplay between thyroid hormones and atherogenic lipid parameters, particularly in SH. The study also emphasizes the importance of AIP in the detection of atherogenic risk in SH patients, even in the presence of minor dyslipidemia. Abnormalities found in conventional lipid profile as well as in lipid ratios and AIP imply SH to be a risk factor for dyslipidemia and atherosclerosis. An atherogenic lipid profile is a prequel to cardiovascular and cerebrovascular diseases, and hence, its association with SH in general and calculation of AIP, in particular, could provide avenues for early diagnosis and rigorous management of dyslipidemia in hypothyroidism.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to acknowledge the management and Dean Research Office of Pondicherry Institute of Medical Sciences for awarding the PIMS fellowship to SRJ under the guidance of LR.
REFERENCES
- 1.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–34. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 2.Lioudaki E, Mavroeidi NG, Mikhailidis DP, Ganotakis ES. Subclinical hypothyroidism and vascular risk: An update. Hormones (Athens) 2013;12:495–506. doi: 10.14310/horm.2002.1437. [DOI] [PubMed] [Google Scholar]
- 3.Pucci E, Chiovato L, Pinchera A. Thyroid and lipid metabolism. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S109–12. doi: 10.1038/sj.ijo.0801292. [DOI] [PubMed] [Google Scholar]
- 4.Efstathiadou Z, Bitsis S, Milionis HJ, Kukuvitis A, Bairaktari ET, Elisaf MS, et al. Lipid profile in subclinical hypothyroidism: Is L-thyroxine substitution beneficial? Eur J Endocrinol. 2001;145:705–10. doi: 10.1530/eje.0.1450705. [DOI] [PubMed] [Google Scholar]
- 5.Rizos CV, Elisaf MS, Liberopoulos EN. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc Med J. 2011;5:76–84. doi: 10.2174/1874192401105010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahaly GJ. Cardiovascular and atherogenic aspects of subclinical hypothyroidism. Thyroid. 2000;10:665–79. doi: 10.1089/10507250050137743. [DOI] [PubMed] [Google Scholar]
- 7.Lee YK, Kim JE, Oh HJ, Park KS, Kim SK, Park SW, et al. Serum TSH level in healthy Koreans and the association of TSH with serum lipid concentration and metabolic syndrome. Korean J Intern Med. 2011;26:432–9. doi: 10.3904/kjim.2011.26.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang JY, Wang CY, Pei D, Lai CC, Chen YL, Wu CZ, et al. Association between thyroid function and metabolic syndrome in elderly subjects. J Am Geriatr Soc. 2010;58:1613–4. doi: 10.1111/j.1532-5415.2010.02998.x. [DOI] [PubMed] [Google Scholar]
- 9.Karthick N, Dillara K, Poornima KN, Subhasini AS. Dyslipidaemic changes in women with subclinical hypothyroidism. J Clin Diagn Res. 2013;7:2122–5. doi: 10.7860/JCDR/2013/5777.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baliarsingh S, Sharma N, Mukherjee R. Serum uric acid: Marker for atherosclerosis as it is positively associated with “atherogenic index of plasma”. Arch Physiol Biochem. 2013;119:27–31. doi: 10.3109/13813455.2012.732580. [DOI] [PubMed] [Google Scholar]
- 11.Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)) Clin Biochem. 2001;34:583–8. doi: 10.1016/s0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 12.Dobiásová M. AIP – Atherogenic index of plasma as a significant predictor of cardiovascular risk: From research to practice. Vnitr Lek. 2006;52:64–71. [PubMed] [Google Scholar]
- 13.Fatourechi V. Subclinical hypothyroidism: An update for primary care physicians. Mayo Clin Proc. 2009;84:65–71. doi: 10.4065/84.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laway BA, War FA, Shah S, Misgar RA, Kumar Kotwal S. Alteration of lipid parameters in patients with subclinical hypothyroidism. Int J Endocrinol Metab. 2014;12:e17496. doi: 10.5812/ijem.17496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu XL, He S, Zhang SF, Wang J, Sun XF, Gong CM, et al. Alteration of lipid profile in subclinical hypothyroidism: A meta-analysis. Med Sci Monit. 2014;20:1432–41. doi: 10.12659/MSM.891163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caraccio N, Ferrannini E, Monzani F. Lipoprotein profile in subclinical hypothyroidism: Response to levothyroxine replacement, a randomized placebo-controlled study. J Clin Endocrinol Metab. 2002;87:1533–8. doi: 10.1210/jcem.87.4.8378. [DOI] [PubMed] [Google Scholar]
- 17.Erem C. Blood coagulation, fibrinolytic activity and lipid profile in subclinical thyroid disease: Subclinical hyperthyroidism increases plasma factor X activity. Clin Endocrinol (Oxf) 2006;64:323–9. doi: 10.1111/j.1365-2265.2006.02464.x. [DOI] [PubMed] [Google Scholar]
- 18.Cikim AS, Oflaz H, Ozbey N, Cikim K, Umman S, Meric M, et al. Evaluation of endothelial function in subclinical hypothyroidism and subclinical hyperthyroidism. Thyroid. 2004;14:605–9. doi: 10.1089/1050725041692891. [DOI] [PubMed] [Google Scholar]
- 19.Hueston WJ, Pearson WS. Subclinical hypothyroidism and the risk of hypercholesterolemia. Ann Fam Med. 2004;2:351–5. doi: 10.1370/afm.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson GR, Soutar AK, Spengel FA, Jadhav A, Gavigan SJ, Myant NB. Defects of receptor-mediated low density lipoprotein catabolism in homozygous familial hypercholesterolemia and hypothyroidism in vivo. Proc Natl Acad Sci U S A. 1981;78:2591–5. doi: 10.1073/pnas.78.4.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abrams JJ, Grundy SM. Cholesterol metabolism in hypothyroidism and hyperthyroidism in man. J Lipid Res. 1981;22:323–38. [PubMed] [Google Scholar]
- 22.Nikkilä EA, Kekki M. Plasma triglyceride metabolism in thyroid disease. J Clin Invest. 1972;51:2103–14. doi: 10.1172/JCI107017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: Effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: A quantitative review of the literature. J Clin Endocrinol Metab. 2000;85:2993–3001. doi: 10.1210/jcem.85.9.6841. [DOI] [PubMed] [Google Scholar]
- 24.Pearce EN, Wilson PW, Yang Q, Vasan RS, Braverman LE. Thyroid function and lipid subparticle sizes in patients with short-term hypothyroidism and a population-based cohort. J Clin Endocrinol Metab. 2008;93:888–94. doi: 10.1210/jc.2007-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganotakis ES, Mandalaki K, Tampakaki M, Malliaraki N, Mandalakis E, Vrentzos G, et al. Subclinical hypothyroidism and lipid abnormalities in older women attending a vascular disease prevention clinic: Effect of thyroid replacement therapy. Angiology. 2003;54:569–76. doi: 10.1177/000331970305400506. [DOI] [PubMed] [Google Scholar]
- 26.Hernández-Mijares A, Jover A, Bellod L, Bañuls C, Solá E, Veses S, et al. Relation between lipoprotein subfractions and TSH levels in the cardiovascular risk among women with subclinical hypothyroidism. Clin Endocrinol (Oxf) 2013;78:777–82. doi: 10.1111/cen.12064. [DOI] [PubMed] [Google Scholar]
- 27.Dobiásová M. Atherogenic index of plasma [log(triglycerides/HDL-cholesterol)]: Theoretical and practical implications. Clin Chem. 2004;50:1113–5. doi: 10.1373/clinchem.2004.033175. [DOI] [PubMed] [Google Scholar]
- 28.Sharma N, Baliarsingh S. High fasting serum glucose in non-diabetic subjects ≥45 years is an indicator of future cardiovascular events as it is positively associated with atherogenic index of plasma. Arch Physiol Biochem. 2012;118:43–6. doi: 10.3109/13813455.2012.677455. [DOI] [PubMed] [Google Scholar]
- 29.Abbas JM, Chakraborty J, Akanji AO, Doi SA. Hypothyroidism results in small dense LDL independent of IRS traits and hypertriglyceridemia. Endocr J. 2008;55:381–9. doi: 10.1507/endocrj.k07e-065. [DOI] [PubMed] [Google Scholar]
- 30.Kim CS, Kang JG, Lee SJ, Ihm SH, Yoo HJ, Nam JS, et al. Relationship of low-density lipoprotein (LDL) particle size to thyroid function status in Koreans. Clin Endocrinol (Oxf) 2009;71:130–6. doi: 10.1111/j.1365-2265.2008.03436.x. [DOI] [PubMed] [Google Scholar]
- 31.Roscini AR, Lupattelli G, Siepi D, Pagliaricci S, Pirro M, Mannarino E. Low-density lipoprotein size in primary hypothyroidism. Effects of hormone replacement therapy. Ann Nutr Metab. 1999;43:374–9. doi: 10.1159/000012806. [DOI] [PubMed] [Google Scholar]
- 32.Marwaha RK, Tandon N, Garg MK, Kanwar R, Sastry A, Narang A, et al. Dyslipidemia in subclinical hypothyroidism in an Indian population. Clin Biochem. 2011;44:1214–7. doi: 10.1016/j.clinbiochem.2011.07.003. [DOI] [PubMed] [Google Scholar]


