Abstract
Context:
The available data concerning oxidant stress and antioxidant capacity in hypothyroidism are scanty and inconclusive. While some authors suggest that tissues may be protected from oxidant damage because of a hypometabolic state in hypothyroidism, others report increased oxidative stress in hypothyroidism. Selenium acts as a cofactor for the thyroid hormone (TH) deiodinases that activate and then deactivate various THs and their metabolites. Selenium may inhibit thyroid autoimmunity.
Aims:
The study was designed, first, to study the impact of oxidative stress in patients of primary hypothyroidism due to autoimmune thyroiditis, by estimation of serum malondialdehyde (MDA) as a biomarker of oxidative stress. Second, to study the change in MDA level pre- and post-L-thyroxine treatment. Finally, to look into the possible role of selenium supplementation on oxidative stress in autoimmune hypothyroidism.
Subjects and Methods:
Patients attending endocrine outpatient department (OPD) services of IPGMER and SSKM hospital were considered for the study. Sixty treatment-naive adult patients (age > 18 years) with hypothyroidism were included in the study. The patients were divided into two groups, each comprised thirty patients. One group was treated with L-thyroxine and placebo (Group A). The other group received L-thyroxine replacement along with selenium (100 mcg twice a day) as antioxidant supplementation (Group B). The patients were blinded about selenium and placebo. The study duration for both groups was 6 months. The starting dose of L-thyroxine was 1.6 mcg/kg body weight free thyroxine (FT4), and thyroid-stimulating hormone (TSH) was repeated after 12 weeks. L-thyroxine dose adjustments were done if needed. MDA was assessed at the beginning and at the end of the study, i.e., after 6 months of treatment. The control cohort was composed of thirty healthy adults. Only overt hypothyroidism (OH) cases were included in the study.
Statistical Analysis Used:
Normality of data was determined using Anderson–Darling test, Shapiro–Wilk test, and QQ plot. P values were calculated using ANOVA and post hoc Bonferroni tests for normally distributed data. Correlation analysis was carried out using Pearson correlation test. P < 0.05 considered to be statistically significant.
Results:
After treatment in Group A patients, FT4 showed a significant increment while TSH value decreased. MDA level reduced after treatment, (P < 0.001). After treatment in Group B patients, FT4 showed increment while TSH value decreased (P < 0.05). After treatment, there was a drop in estimated MDA level (P < 0.001). MDA level shows a significant drop in both groups after treatment. In Group B, there is more decline in the MDA percentage but did not reach statistical significance. By performing repeated measure MANOVA, no significant difference was found in the MDA levels between the two groups. MDA reduction when expressed as percentage showed reduction of 39.5% in patients of Group A. Similarly, Group B patients showed a percentage reduction of 45.4%.
Conclusions:
Oxidative stress compounds hypothyroidism. Hypothyroidism is a state of increased oxidative stress. In this study, biomarker, MDA level is high in treatment-naive primary hypothyroid patients. After treatment with L-thyroxine, the stress marker is reduced to a significant extent. MDA can be used as a useful biomarker to measure and monitor oxidative stress. The role of the addition of antioxidant in the form of selenium remained inconclusive.
Keywords: Antioxidant supplementation, overt hypothyroidism, oxidative stress, serum malondialdehyde
INTRODUCTION
Oxidative stress results are an imbalance between the antioxidant defense systems and the rate of production of reactive oxygen species (ROS). It not only leads to lipid peroxidation and oxidative DNA damage but also interferes with physiologic adaptation and intracellular signal transduction. The resulting change in the intracellular redox status leads to the activation of protein kinases, for example, tyrosine kinase, protein kinase C, and the mitogen-activated protein kinase cascade leading to altered cellular functions.[1]
The thyroid hormones (THs), namely, tetraiodothyronine (thyroxin, T4) and a much smaller proportion of triiodothyronine, exert actions at the cellular level by binding to a set of specialized receptors that couple to both genomic and nongenomic signaling pathways. They are subjected to transformations in the peripheral tissues, mainly in the form of deiodination. The general metabolic effect of THs is a relative acceleration of the basal metabolism that includes an increase of the rate of both catabolic and anabolic reactions. This results in increased energy expenditure, oxygen consumption, respiratory rate, and heat production. While ROS production depends largely on the mitochondria, THs do not directly determine the respiratory state of the mitochondria; they also affect the cell antioxidant status.[2] Hypothyroidism-associated ROS is the consequence of both increased production of free radicals and reduced capacity of the antioxidative defense. Excess thyroid-stimulating hormone (TSH) per se might alter oxidative stress processes.[3] Hypothyroidism-induced dysfunction of the mitochondrial respiratory chain can lead to the accelerated production of free radicals.[4] Lipid peroxidation is reported to be high in hyperlipidemia, which is a consistent biochemical feature in hypothyroidism. The presence of OS in hypothyroidism correlates with the lipid risk factors of atherosclerosis.[5] Metabolic disorder from autoimmune-based hypothyroidism can also increase oxidative stress.[6] Low-grade chronic inflammation causes endothelial dysfunction and impairs nitric oxide availability, leading to increased OS in Hashimoto's thyroiditis.[7] The available data concerning oxidant stress and antioxidant capacity in hypothyroidism are scanty and inconclusive. While some authors suggest that tissues may be protected from oxidant damage because of a hypometabolic state in hypothyroidism, others report increased oxidative stress in hypothyroidism. Selenium acts as a cofactor for the TH deiodinases that activate and then deactivate various THs and their metabolites. Selenium may inhibit thyroid autoimmunity.
SUBJECTS AND METHODS
Patients attending endocrine OPD services of IPGMER and SSKM hospital were considered. Sixty treatment-naive adult patients (age >18 years) with hypothyroidism were included in the study. The control cohort was composed of thirty (30) healthy adults. Only overt hypothyroidism (OH) cases were included in the study. The diagnosis of OH was based on the finding of low free thyroxine (FT4) level with elevated TSH level. Thyroid autoantibody in the form of anti-thyroid peroxidase antibody (anti-TPO Ab) was tested. Appropriate exclusion criteria were observed. The patients were divided into two groups, each comprised thirty patients. One group was treated with L-thyroxine and placebo (Group A). The other group received L-thyroxine replacement along with selenium (100 mcg twice a day) as antioxidant supplementation (Group B). They patients were blinded about selenium and placebo. The study duration for both groups was 6 months. The starting dose of L-thyroxine was 1.6 mcg/kg body weight. FT4 and TSH were repeated after 12 weeks. L-thyroxine dose adjustments were done if needed. Malondialdehyde (MDA) was assessed at the beginning and at the end of the study, i.e. after 6 months of treatment. Patients diagnosed with hypothyroidism but already on the treatment for the disease with L-thyroxine, patients taking lipid-lowering drugs or antioxidant supplements, smokers, patients with hypertension, alcoholics, pregnant women and patients suffering from diabetes mellitus, hepatic and renal impairment, CAD, and heart failure were excluded from the study. Blood samples were collected in the morning between 8:00 am and 9.00 am after an overnight fast. The samples were centrifuged for 15 min at 2500 rpm, and aliquots of serum were kept at − 20°C until assays for analysis. Thyroid profile was assessed by the estimation of serum FT4 and TSH by chemiluminescence method. Anti-TPO Ab was also tested. This was done by Immulite 1000 machine of Siemens Medical Solutions Diagnostics. This is a continuous random access instrument that performs automated chemiluminescent immunoassay. Its coefficient of variance is 4–5%. The normal reference ranges are - FT4: 0.8–1.8 ng/dl, TSH: 0.4–4 μIU/ml. The normal range of anti-TPO Ab was <35 IU/ml. Lipid peroxidation was assessed by MDA as thiobarbituric acid reactive substances after precipitating it with trichloroacetic acid. Two moles of thiobarbituric acid react with one mole of MDA to give a colored adduct, which is extracted by butanol, which further eliminates colored interfering substances present in the aqueous phase. The color was read at 532 nm by an ultraviolet spectrophotometer. Serum MDA was calculated from standard curve prepared using tetraethoxypropane as standard and expressed as μmol/L (27), (28), and (29). Serum MDA levels were measured at baseline and then, the same measurements were repeated after 6 months of drug therapy in both groups. They were also tested for blood sugar (fasting), serum creatinine, and lipid profile at baseline. Pure selenium preparation for oral use is available as 100 and 200 μg capsules. It is available as per order placed through the internet, and is not readily available and costly too. Providing that to thirty patients for a period of 6 months was beyond our financial limit. Common pharmaceutical companies do not have the requisite manufacturing license. The commonly available medicines having selenium as a mixed preparation but without the other common antioxidant molecules were used. The preparation used contained per capsule: Vitamin B6 (pyridoxine hydrochloride) I.P. - 3.0 mg, nicotinamide I.P. - 100 mg, Vitamin B12 (cyanocobalamin) I.P. - 15 mcg, chromium picolinate USP - 250 mcg, selenium (as selenious acid) USP - 100 mcg, zinc sulfate monohydrate USP - 61.8 mg, excipients q.s. The capsule was given in twice daily dosing. Similar placebo capsules were used in control. The patients were explained about the study and only those who gave informed written consent were included in the final study. Clearance from the Institutional Ethics committee was obtained before the study initiation. The finally included patients in the study were given an appointment to attend the OPD services after an overnight fast. Data on age, sex, exclusion criteria and other disorders were investigated at baseline. All patients underwent detailed clinical examination. Blood samples were then collected. There are not much of Indian studies describing the prevalence of oxidative stress in hypothyroidism, so the minimum number of study subjects recommended to minimize the statistical error, i.e. 25 for each group were taken and rounded off to total sixty patients. A total period of 2 years from January 2013 to December 2014 was spent to get the requisite number of study subjects and subsequent follow-up. The collected data were analyzed using SPSS for windows (Version 20.0 for Windows, SPSS, Inc., Chicago, IL, USA). The results of the study were analyzed by paired t-test (measurements before and after thyroxine treatment) and unpaired t-test (comparison between two groups). The values were expressed as mean ± standard deviation correlation analysis was carried out using Pearson's correlation test. P < 0.05 was considered statistically significant.
RESULTS
The study subjects were grouped into two groups, namely, Group A and Group B. Group A received L-thyroxine and placebo, whereas Group B received L-thyroxine and selenium. They were compared with a control group (n = 30). The baseline characteristics included patients’ age, sex, body mass index, blood pressure (BP), presence, or absence of goiter. They were comparable between control and the two study groups. In each study groups, 18 subjects out of 30, i.e., 60% were female. On neck examination, goiter was found in 18 patients (60%) of Group A and 15 patients of Group B (50%). Anti-TPO Ab was positive in 18 patients in each group (60%) [Tables 1 and 2]. There was no significant difference between Group A and B (P > 0.05) in baseline TSH, FT4, and MDA, but Groups A and B have significant higher TSH and MDA, lower FT4 than control (P < 0.05).
Table 1.
Baseline Characteristics
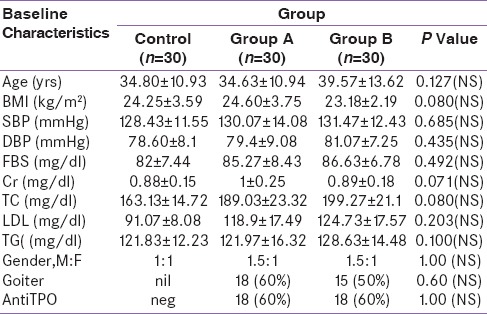
Table 2.
Baseline comparison of thyroid profile and (Malondialdehyde) MDA

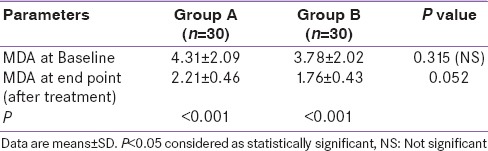
After treatment in Group A patients, FT4 showed increment while TSH value decreased [Table 3]. The magnitude of change reached statistical significance. MDA level reduced after treatment [Table 3]. The reduction reached statistical significance with P < 0.001. After treatment in Group B patients, FT4 showed increment while TSH value decreased. The magnitude of change reached statistical significance. After treatment, there was a drop in estimated MDA level. The reduction reached statistical significance with P < 0.001 [Table 4]. Table 5 shows the intragroup as well as intergroup comparison of MDA level. MDA level shows a significant drop in both groups after treatment. In Group B, there is more decline in the MDA percentage, but that did not reach statistical significance. By performing repeated-measure MANOVA, no significant difference was found in the MDA levels between the two groups. MDA reduction when expressed as percentage showed reduction of 39.5% in patients of Group A. Similarly, Group B patients showed a percentage reduction. Here, the reduction was even more –45.4%. Although the reduction is more, it did not reach statistical significance with P = 0.29. Baseline TSH and MDA level showed a positive correlation. A significant positive correlation is observed between MDA levels and the baseline TSH values.
Table 3.
Comparison of biochemical characteristics of Group A. (Paired t Test)
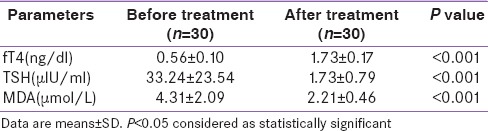
Table 4.
Comparison of biochemical characteristics of Group B (Paired t Test)
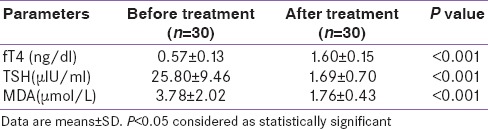
Table 5.
Comparison of MDA level between two groups (Group A vs. Group B) (unpaired t Test)
DISCUSSION
The biomarkers that can be used to assess oxidative stress have attracted much interest. Many such markers have been proposed. We used MDA, one such marker of lipid peroxidation, to measure oxidative stress for our study. Previous studies have found MDA to be useful is studying oxidative stress[8,9] and it does not vary across the age and gender of the study population. This study also came across similar results. Selenium is a micronutrient that plays an important role in antioxidant defense system of the body being part of different antioxidant enzymes, for example, glutathione peroxidase. It also has role in the proper functioning of THs as it is the trace element in the deiodinases group of enzymes. MDA level was found to be higher in the treatment-naive patients as compared to controls suggesting increased oxidative stress in hypothyroid subjects. Many previous studies have documented similar observations.[8,9] A study by Baskol et al. showed that the study group comprised 33 patients with primary hypothyroidism. The patients were compared with 26 normal healthy controls. Serum MDA was measured according to an enzymatic spectrophotometric method. MDA levels were found higher in patients with hypothyroidism before the treatment than the controls. MDA levels were also found to be decreased after the treatment in patients with hypothyroidism.[9,10,11,12] In our study too, MDA level reduced in post-treatment analysis in both groups, which was of statistical significance. It was found to be reduced more in the group of patients who received selenium, but that reduction did not reach statistical significance. A positive correlation between TSH and MDA level was found. Similar was the observation by an Indian study by Haribabu et al.[13] They postulated the role of excess TSH to directly produce oxidative stress. Hypothyroidism is associated with increased oxidative stress response. Treatment with L-thyroxine is effective in bringing reduction in the level of stress markers. Results after the addition of selenium remained inconclusive. Future studies with a larger sample size may come up with some more definite and positive conclusion so that addition of selenium as antioxidant may make physicians able to reduce the dose requirement of L-thyroxine. This study had its limitations of small sample size. More numbers of oxidative stress markers for logistic and economic constraints were not included in the study. The study with a larger sample size and possibly a few more markers could have drawn a much clearer conclusion.
CONCLUSION
Oxidative stress compounds hypothyroidism. Hypothyroidism is a state of increased oxidative stress. In this study, biomarker, MDA level is high in treatment-naive primary hypothyroid patients. After treatment with L-thyroxine, the stress marker is reduced to a significant extent. MDA can be used as a useful biomarker to measure and monitor oxidative stress. The role of the addition of antioxidant in the form of selenium remained inconclusive.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Yoshikawa T, Naito Y. What is oxidative stress? JMAJ. 2002;45:271–6. [Google Scholar]
- 2.Schwartz HL, Oppenheimer JH. Ontogenesis of 3,5,3’-triiodothyronine receptors in neonatal rat brain: Dissociation between receptor concentration and stimulation of oxygen consumption by 3,5,3’-triiodothyronine. Endocrinology. 1978;103:943–8. doi: 10.1210/endo-103-3-943. [DOI] [PubMed] [Google Scholar]
- 3.Nanda N, Bobby Z, Hamide A. Association of thyroid stimulating hormone and coronary lipid risk factors with lipid peroxidation in hypothyroidism. Clin Chem Lab Med. 2008;46:674–9. doi: 10.1515/CCLM.2008.139. [DOI] [PubMed] [Google Scholar]
- 4.Resch U, Helsel G, Tatzber F, Sinzinger H. Antioxidant status in thyroid dysfunction. Clin Chem Lab Med. 2002;40:1132–4. doi: 10.1515/CCLM.2002.198. [DOI] [PubMed] [Google Scholar]
- 5.Nanda N, Bobby Z, Hamide A. Oxidative stress and protein glycation in primary hypothyroidism. Male/female difference. Clin Exp Med. 2008;8:101–8. doi: 10.1007/s10238-008-0164-0. [DOI] [PubMed] [Google Scholar]
- 6.Komosinska-Vassev K, Olczyk K, Kucharz EJ, Marcisz C, Winsz-Szczotka K, Kotulska A. Free radical activity and antioxidant defense mechanisms in patients with hyperthyroidism due to Graves’ disease during therapy. Clin Chim Acta. 2000;300:107–17. doi: 10.1016/s0009-8981(00)00306-5. [DOI] [PubMed] [Google Scholar]
- 7.Taddei S, Caraccio N, Virdis A, Dardano A, Versari D, Ghiadoni L, et al. Low-grade systemic inflammation causes endothelial dysfunction in patients with Hashimoto's thyroiditis. J Clin Endocrinol Metab. 2006;91:5076–82. doi: 10.1210/jc.2006-1075. [DOI] [PubMed] [Google Scholar]
- 8.Lakshmi LJ, Mohapatra E, Zephy D, Kumari S. Serum lipids and oxidative stress in hypothyroidism. J Adv Res Biol Sci. 2013;5:63–6. [Google Scholar]
- 9.Baskol G, Atmaca H, Tanriverdi F, Baskol M, Kocer D, Bayram F. Oxidative stress and enzymatic antioxidant status in patients with hypothyroidism before and after treatment. Exp Clin Endocrinol Diabetes. 2007;115:522–6. doi: 10.1055/s-2007-981457. [DOI] [PubMed] [Google Scholar]
- 10.Erdamar H, Demirci H, Yaman H, Erbil MK, Yakar T, Sancak B, et al. The effect of hypothyroidism, hyperthyroidism, and their treatment on parameters of oxidative stress and antioxidant status. Clin Chem Lab Med. 2008;46:1004–10. doi: 10.1515/CCLM.2008.183. [DOI] [PubMed] [Google Scholar]
- 11.Drutel A, Archambeaud F, Caron P. Selenium and the thyroid gland: More good news for clinicians. Clin Endocrinol (Oxf) 2013;78:155–64. doi: 10.1111/cen.12066. [DOI] [PubMed] [Google Scholar]
- 12.Bhimte B, Agrawal BK, Sharma VK, Chauhan SS. Oxidative stress status in hypothyroid patients. Biomed Res. 2012;23:286–8. [Google Scholar]
- 13.Haribabu A, Reddy VS, Pallavi CH, Bitla AR, Sachan A, Pullaiah P, et al. Evaluation of protein oxidation and its association with lipid peroxidation and thyrotropin levels in overt and subclinical hypothyroidism. Endocrine. 2013;44:152–7. doi: 10.1007/s12020-012-9849-y. [DOI] [PubMed] [Google Scholar]


