Abstract
Objective
To explore the relationship of intracranial pressure with intensive care unit length of stay in a large cohort of severe traumatic brain injury patients and to identify factors that associate with prolonged ICU course.
Design
Single-center, database review of de-identified research data that had been collected prospectively as a part of Institutional Review Board–approved research studies.
Setting
Neurosurgical ICU, Ben Taub General Hospital.
Patients
438 critically ill, severe TBI patients.
Interventions
None
Measurements and Main Results
438 patients with severe TBI were identified, 149 (34%) had a motor GCS of 1–3 on admission and 284 (65%) had 4–5. Bilaterally unreactive pupils were encountered in 109 patients (25%). Intracranial pressure, during the ICU course, for the group was 19.8 ± 11.2 mm Hg. Favorable outcome was obtained in 148 patients (34%), unfavorable in 211 (48%) with a mortality of 28%. Intensive care unit LOS was 19.4 ± 13.9 days. Joint modeling of ICP and ICU LOS was undertaken, adjusted for admission prognostic indicators such as Age, Sex, Marshall CT classification, Pupil reactivity, Glasgow Coma Scale motor score and Injury Severity Score. A non-significant association was seen between higher ICP and longer ICU LOS (p = 0.4). Presence of a mass lesion on admission head CT was strongly correlated with a prolonged ICU LOS (p = 0.0007), the result was near significance for the presence of diffuse injury categories 3 and 4 (p = 0.053).
Conclusions
Intracranial pressure, as monitored and managed according to BTF guidelines, is not associated with ICU length of stay. Patients with severe TBI and a mass lesion on admission head CT were found to have prolonged ICU LOS independently of indicators of injury severity and intracranial pressure course.
Keywords: traumatic brain injury, intensive care unit, length of stay, intracranial pressure, computed tomography, diffuse injury, mass lesions
Traumatic brain injury (TBI) remains a major public health issue worldwide. The annual incidence is estimated at 500/100,000 in the U.S. and Europe, resulting in over 200 per 100,000 individuals being admitted to hospitals each year in Europe (1–3). About 1.5 million people die globally from TBI, the majority in developing countries and it is the leading cause of mortality between 1–44 years of age. In addition, TBI affords a large societal and economic toll. The estimated economic cost in 2010, including direct and indirect medical costs, is estimated to be approximately $76.5 billion. Fatal TBI and TBI requiring hospitalization account for approximately 90% of the total TBI medical costs (5, 6).
Length of stay (LOS) is an important measure of health care utilization and determinant of hospitalization costs. Although there are accepted prognostic models for severe TBI, little has been published on factors influencing intensive care unit (ICU) LOS in this population. Monitoring and control of intracranial pressure (ICP) is one of the defining components of ICU management in these patients; the association of ICP with ICU LOS has not been thoroughly explored. Proper analysis ought to consider confounding factors that accompany high ICP and the fact that patients suffering refractory intracranial hypertension may have shorter ICU LOS. The aim of this current study is to explore the relationship of ICP and ICU LOS after adjusting for prognostically important clinical and radiologic features on admission in a large cohort of severe TBI patients. The identification of factors associated with prolonged ICU stay and insight in the relationship of ICP and LOS are important in allowing clinicians to identify high-risk patients, assist with clinical decision-making, and facilitate the appropriate allocation of hospital resources. Furthermore, and in view of the contemporary debate over the significance of monitoring and management of ICP, the relation with ICU course is a highly relevant question to answer.
MATERIALS AND METHODS
The study design was a database review of de-identified research data that had been collected prospectively as a part of Institutional Review Board–approved research studies.
Patients and Management
A total of 438 patients who were admitted to Ben Taub General Hospital in the period 1995–2005? and received critical care for severe TBI, including the standardized monitoring and management of ICP and cerebral perfusion pressure (CPP), were studied. Inclusion criteria were the following: TBI, motor component of the Glasgow Coma Scale (GCS) score ≤ 5 (either after resuscitation or after subsequent deterioration), valid ICP data collected as a part of an ongoing research protocol, and demographic and injury characteristics and neurological outcome collected as a part of an ongoing research protocol. Exclusion criteria included GCS score of 3 with fixed, dilated pupils and loss to follow-up before 3 months after injury. A standard management protocol was employed that emphasized the prevention of secondary insults and the prompt evacuation of intracranial masses. General management goals were PaO2 > 100 mm Hg, PaCO2 of 35 to 40 mm Hg, systolic blood pressure > 120 mm Hg, central venous pressure of 5 to 10 mm Hg, and urine output > 0.5 to 1 mL/kg/h. Phenytoin was given for 7 days as prophylaxis for seizures. Invasive monitoring of ICP was usually via ventriculostomy. The goals of management were ICP < 20 mm Hg and CPP > 60 mm Hg [for some patients a higher CPP was targeted based on jugular bulb oxygen saturation (Sjvo2) and/or brain partial tissue oxygenation (Pbto2) readings]. Treatment of intracranial hypertension was based on Brain Trauma Foundation (BTF) guidelines for management of severe TBI and involved surgical removal of mass lesions, cerebrospinal fluid drainage via ventriculostomy, sedation, neuromuscular paralysis, mannitol, and mild to moderate hyperventilation. Barbiturate coma, moderate hypothermia, and decompressive craniectomy were treatment options used for refractory intracranial hypertension (7). Monitoring of ICP was continued until the ICP was < 20 mm Hg for 24 hours without treatment.
Data Collection
The demographic and clinical data collected included age, sex, race, mechanism of injury, GCS, pupil reactivity, and Injury Severity Score (ISS) upon admission. Intracranial pressure and other physiological parameters were recorded hourly within a few hours after ICU admission and for the duration of the monitoring. The Marshall CT category (8) was used to describe the admission CT scan findings, and the results were collapsed into the following 3 groups: mild diffuse injury (diffuse injury 1 and 2, D12), severe diffuse injury (diffuse injury 3 and 4, D34), and mass lesions (evacuated and non-evacuated, M). The GCS score on admission was also classified into the following 2 categories according to the motor GCS score: motor GCS score 4–5 and 1–3. Pupil reactivity was classified as pupils reactive, 1 unreactive pupil, or both pupils unreactive. Six month Glasgow Outcome Scale (GOS) was collected and dichotomized as favorable recovery (good recovery or moderate disability) and unfavorable recovery (severe disability, vegetative, or dead).
Statistical Analysis
We used joint modeling of longitudinal measurements and event time data in order to examine the relationship between ICU LOS and ICP, adjusting for important prognostic, admission factors (Age, Sex, Pupil reactivity, GCS, CT characteristics, PaCO2) (9). Intracranial pressure measurements are repeatedly measured overtime, i.e. time dependent; we can not simply take the mean of ICP and use it as the covariate since during the ICU stay patients with higher ICP may die earlier resulting in a shorter ICU LOS. So, we consider these patients as censored cases to adjust for the effect of higher ICP on ICU LOS. This is accomplished by using the joint modeling method in which ICU LOS is treated as a time to event process and simultaneously, ICP is longitudinally modeled. Specifically,
where (t) is the time to event (ICU discharge), i.e. ICU LOS; mi (t) is ICP measurements for subject i at time t; wi are time independent variables for subject i; xi are fixed effects covariates and zi are random effects covariates, they can be either time dependent or independent.
RESULTS
Patient Characteristics
Demographic and injury characteristics of all 438 patients, which are summarized in Table 1, were typical for a severe TBI population. Men predominated in the group, 379 (86.5%) compared with 59 women (13.5%). The mean age for the group was 33.2 ± 14.5 years, and the mean ISS was 27.7 ± 5.6. The mechanism of injury was motor vehicle collision in 217 (49.5%), assault in 98 (22.4%), fall/jump in 41 (9.4%), and other in 6 (1.4%). In 19 patients (4.3%), the mechanism was unknown. The motor component of the GCS score was 1–3 in 149 patients (34%) and 4–5 in 284 patients (64.8%). In 5 patients (1.1%), an admission GCS score could not be obtained. Pupils were reactive on admission in 181 patients (41.3%), 1 pupil was unreactive in 44 patients (10%), and both pupils were unreactive in 109 patients (24.9%). For 104 patients (23.7%), the pupils could not be assessed. The CT scan of the head on admission was classified as D12, D34, and M in 115 patients (26.2%), 82 patients (18.7%), and 240 patients (54.8%), respectively. Mean and standard deviation for ICU physiologic monitoring variables can be found in Table 2.
Table 1.
Demographic and Injury Severity Characteristics
| Variable | Mean ± sd or n(%) |
|---|---|
| Age | 33.2 ± 14.5 |
| Sex | |
| Male | 379 (86.5) |
| Female | 59 (13.5) |
| Race | |
| White | 150 (34.2) |
| Black | 113 (25.8) |
| Hispanic | 162 (37.0) |
| Asian | 12 (2.7) |
| Other | 1 (0.2) |
| Mechanism of injury | |
| MVA | 217 (49.5) |
| Fall/jump | 41 (9.4) |
| Assault | 98 (22.4) |
| Gunshot wound | 57 (13.0) |
| Others | 6 (1.4) |
| Unknown | 19 (4.3) |
| Moto GCS | |
| 1–3 | 149 (34.0) |
| 4–5 | 284 (64.8) |
| Untestable | 5 (0.01) |
| Pupils | |
| Both reactive | 181 (41.3) |
| 1 Unreactive | 44 (10.0) |
| Both unreactive | 109 (24.9) |
| Untestable | 104 (23.7) |
| Type of injury (Marshall CT category) | |
| Diffuse injury 1 or 2 | 115 (26.2) |
| Diffuse injury 3 or 4 | 82 (18.7) |
| Mass lesion | 240 (54.8) |
| Unknown | 1 (0.2) |
| ISS | 27.7 ± 5.6 |
Table 2.
Physiologic Monitoring Data
| Variable | Mean ± sd or % | No. of patients |
|---|---|---|
| ICP, mm Hg | 19.8 ± 11.2 | 438 |
| MAP, mm Hg | 92.0 ± 13.7 | 438 |
| CPP, mm Hg | 72.3 ± 17.6 | 438 |
| SjvO2, % | 71.2 ± 10.8 | 373 |
| SaO2, % | 98.8 ± 1.8 | 429 |
| PCO2, % | 32.4 ± 6.1 | 438 |
| PbtO2, % | 29.5 ± 21.0 | 35 |
The GOS score was assessed at 6 months. A total of 148 patients (33.8%) had a favorable outcome, whereas 211 patients (48.1%) had an unfavorable outcome. One hundred twenty-four patients died (28.3%). No outcome data were available in 48 patients (11%) (Table 3).
Table 3.
Long-term Neurological Outcomes and Intensive Care Unit Length of Stay
| Variable | Mean ± sd or n(%) | No. of patients |
|---|---|---|
| Glasgow Outcome Scale (Month 6) | 438 | |
| Good recovery | 71 (16.2) | |
| Moderate disability | 77 (17.6) | |
| Severe disability | 65 (14.8) | |
| Vegetative | 22 (5.0) | |
| Dead | 124 (28.3) | |
| Unknown | 79 (18.0) | |
| ICU LOS | 19.4 ± 13.9 | 379 |
Intracranial Pressure and ICU LOS
The mean ICU LOS was obtained from 379 patients and it was 19.4 ± 13.9 days. Joint modeling of longitudinal measurements of ICP and event time data (ICU discharge as an event) was employed. In the longitudinal model, where ICP is the outcome, the following variables were found to be statistically significant and to predict a higher ICP: Younger age, male gender, presence of CT D34 and lower GCS on admission. When this model is jointly analyzed with the event process analysis it can be seen that the only significant predictor of longer ICU LOS is CT M (presence of mass lesion, p = 0.0007). Of marginal significance is CT D34 (p = 0.0565) (Table 4). Figure 1 depicts the proportions of discharges versus deaths in the ICU per CT classification category. Intracranial pressure is not significantly associated with ICU LOS, nevertheless the association is in the direction of higher ICP relating to a longer LOS.
Table 4.
Joint Modeling for ICP and Intensive Care Unit LOS
| Event Process | Longitudinal Process | ||||||
|---|---|---|---|---|---|---|---|
| Value | Std.Err | p-value | Value | Std.Err | p-value | ||
| Age | −0.01 | 0.01 | 0.2639 | (Intercept) | 20.38 | 2.09 | < 0.0001 |
| Gender Male | −0.04 | 0.31 | 0.9053 | Age | −0.16 | 0.03 | < 0.0001 |
| One Reactive | −0.50 | 0.45 | 0.2736 | Gender Male | 3.45 | 1.51 | 0.0224 |
| Both Reactive | −0.00 | 0.24 | 0.9858 | One Reactive | 2.82 | 1.43 | 0.0485 |
| CTD34 | −0.64 | 0.33 | 0.0534 | Both Reactive | −0.78 | 0.84 | 0.3545 |
| CTM | −0.91 | 0.27 | 0.0007 | CTD34 | 4.42 | 1.23 | 0.0003 |
| ISS | −0.01 | 0.02 | 0.7341 | CTM | 4.03 | 1.03 | 0.0001 |
| ICP | −0.02 | 0.02 | 0.4681 | GCS.sum | −0.32 | 0.11 | 0.0052 |
| log(σ) | 2.05 | 0.02 | |||||
| D11 | 37.80 | 11.19 | |||||
Figure 1.
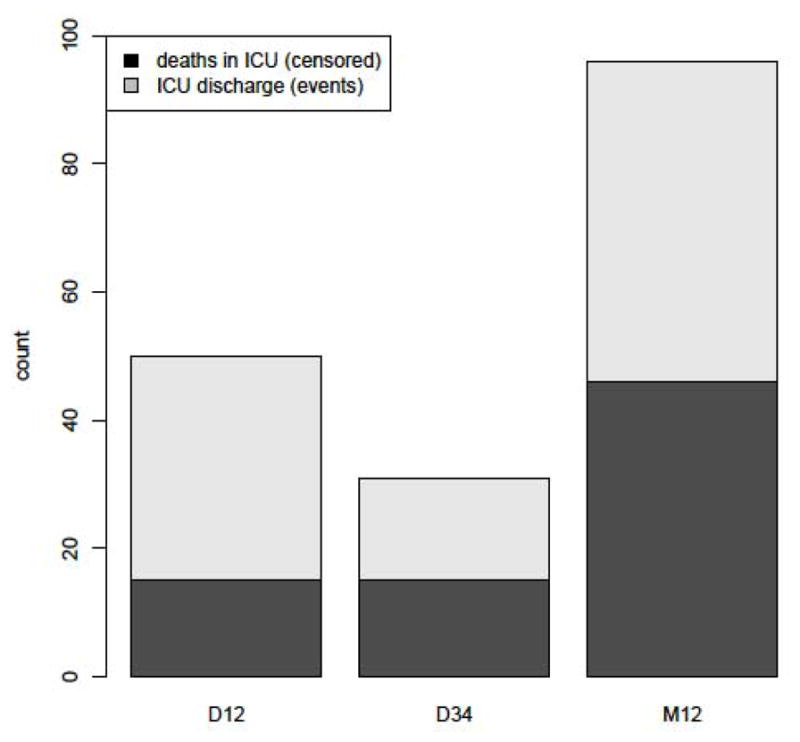
CT classification categories and proportion of deaths vs. discharges from the ICU
DISCUSSION
The morbidity, use of resources and cost of a prolonged ICU stay cannot be overemphasized. One of the main goals of ICU management in severe TBI is the monitoring and control of ICP. To our knowledge, this is the first study to examine the association of ICP, controlled for confounders, with ICU LOS using joint modeling of longitudinal data with event time data in a large cohort of severe TBI patients. Our main findings are that ICP is not associated with ICU LOS (despite a trend of higher ICP associating with prolonged LOS), and that CT classification indicating the presence of a mass lesion on admission is strongly correlated with a prolonged ICU stay.
Intracranial Pressure and ICU LOS
The IMPACT study group analyzed individual patient data from over 9000 patients with severe or moderate TBI merged from 11 studies, and confirmed age, GCS motor score, pupillary response, and CT characteristics as the most powerful independent prognostic variables (10). We used these factors in a joint model to identify predictors of ICU LOS and to investigate the relationship of ICP with ICU LOS. Prior studies have sought to examine the relationship between BTF-guided ICP management and outcomes, including LOS, comparing with groups of patients that were not monitored; conflicting results have been obtained. Talving et al. in a recent prospective observational single-center study found that patients who were monitored and managed according to the BTF experienced significantly lower in-hospital mortality in conjunction with significantly longer ICU and hospital LOS (11). Bulger et al. collected retrospective data from 34 trauma centers and compared the outcomes of “aggressive” centers (defined as those placing ICP monitors in >50% of patients meeting BTF criteria) versus others (12). Management at an aggressive center was associated with a significant reduction in the risk of mortality. Adjusted LOS for survivors at aggressive centers was shorter. Cremer et al. performed an observational study in two trauma centers in different regions of the Netherlands (13). Compared with supportive intensive care without ICP monitoring, the use of an ICP/CPP-targeted treatment protocol resulted in a much longer period of mechanical ventilation and a more extensive use of medical therapies to control intracranial hypertension without an effect on functional status or survival. Alkhoury and Kyriakides surveyed the National Trauma Data Bank to determine the effect of ICP monitoring on outcomes in pediatric patients with severe TBI (14). After adjustment for admission GCS score, age group, sex, Revised Trauma Score, and ISS, ICP monitoring was associated with longer hospital and ICU LOS. A tendency of extended ICU LOS in ICP-monitored patients was found in a meta-analysis of two randomized controlled trials and seven cohort studies by Su et al. (15). The only relevant data we have from an RCT is the data from the BEST-TRIP trial (16). This study randomized severe TBI patients in two different protocols for the management of intracranial hypertension; one group received ICP monitoring and treatment based on a threshold of 20 mm Hg and the other was treated based on clinical and radiologic findings without the monitoring of intracranial pressure. There was no difference in the primary clinical outcome. The median LOS in the ICU was similar in the two groups (12 days in the ICP group and 9 days in the imaging–clinical examination group; P = 0.25); The two groups differed though in the number of days of brain-specific treatments (e.g., administration of hyperosmolar fluids and the use of hyperventilation) in the ICU, favoring the ICP group (4.8 days without vs. 3.4 with ICP monitoring, P = 0.002).
We found that neither ICP nor ISS were associated with LOS. This can be explained taking into consideration that patients with very high or very low levels of intracranial pressure and injury severity will likely experience a shorter ICU stay. These patients are at increased risk for dying or being discharged early in the course of ICU admission. This may not be reflected unless the appropriate analysis of time dependent data (ICP) and event-time data (ICU discharge) is undertaken, i.e. jointly modeled.
In the current debate about the best use of ICP monitoring, some of the above referenced literature has suggested that BTF-based ICP monitoring and treatment may increase ICU LOS without a benefit in outcome. Based on our data and the data from BEST-TRIP we conclude that ICP monitoring and management as guided by BTF, do not prolong ICU stay and may in fact decrease the intensity of treatment during the ICU course.
Marshall CT score and ICU LOS
The Marshall CT classification is widely accepted for descriptive purposes and as major predictor of outcome in TBI. Various studies have confirmed the predictive value of the CT classification (17–19), and the international guidelines on prognosis include the CT classification as a major CT predictor based on Class I evidence (20). The relationship between CT characteristics on admission and ICU course has not been thoroughly investigated before. We found that patients admitted with severe TBI and a mass lesion on CT had a significantly prolonged ICU stay. A similar trend, but of marginal statistical significance, was seen in patients with severe diffuse injury (D34). Maas et al. analyzed individual patient data from the IMPACT database with the aim of describing and quantifying the relationship between CT characteristics and 6-month GOS (21). The CT classification was strongly related to outcome, with worst outcome for patients with diffuse injuries in CT class 3 (OR 2.50; CI 2.09–3.0) or CT class 4 (OR 3.03; CI 2.12–4.35). The prognosis in patients with mass lesions was better for patients with an epidural hematoma (OR 0.64; CI 0.56–0.72) and poorer for an acute subdural hematoma (OR 2.14; CI 1.87–2.45). One of the limitations of our report is that we grouped all mass lesions into one category and did not differentiate based on pathology nor status of evacuation. The Marshall CT classification does not permit any distinction on type of mass lesion. Furthermore, many have argued that evacuation status reflects a clinical decision and does not in itself constitute a CT parameter; in clinical practice this has led to confusion and it has been proposed not to include this differentiation (18, 22).
Limitations
This is a retrospective review of single-center data collected over a decade ago and the argument could be made that they are not representative of contemporary cohorts. Management of these patients spans over periods with changing BTF guidelines; it is likely that some patients were managed to a CPP target over 70 mm Hg, some over 60 mm Hg, and some had individualized goals based on other monitoring variables. Nevertheless, our demographic data are reflective of a typical severe TBI population and the outcome data are consistent with current TBI series. The ICP goal has consistently been less than 20 mm Hg and the average physiologic monitoring data for our cohort are reflective of the ICU course of a severe TBI group of patients.
Kahraman et al. recently quantified ICP insults as the product of “pressure times time dose” (PTD) (23). They used high resolution data from 30 severe TBI patients and found that total PTD for ICP > 20 mm Hg and CPP < 60 mm Hg had high predictive power for functional outcome, in-hospital mortality and were strongly correlated with ICU LOS. The predictive ability of automated data was superior to manual data. We used hourly ICP data that were manually recorded and ICP was treated as a time-dependent variable; ICP dose was not calculated. Despite lacking automated data and ICP dose, our findings are derived by employing a statistically robust method to model the association of ICP and ICU LOS.
CONCLUSIONS
Intracranial pressure, as monitored and managed according to BTF guidelines, is not associated with ICU length of stay. Patients with severe TBI and a mass lesion on admission head CT were found to have prolonged ICU LOS independently of indicators of injury severity and intracranial pressure course.
Footnotes
The authors report no conflicts of interest in relation to this manuscript. No funding has been received in relation to this manuscript. No reprints will be ordered.
References
- 1.Langlois JA, Rutland-Brown W, Thomas KE. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta: 2006. [Google Scholar]
- 2.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 3.Maas AI, Menon DK, Lingsma HF, Pineda JA, Sandel ME, Manley GT. Reorientation of clinical research in traumatic brain injury: report of an international workshop on comparative effectiveness research. J Neurotrauma. 2012 Jan 1;29(1):32–46. doi: 10.1089/neu.2010.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO/OMS. Global status report on road safety: Time for action. Geneva: World Health Organisation; 2009. http://whqlibdoc.who.int/publications/2009/9789241563840_eng.pdf. [Google Scholar]
- 5.Finkelstein E, Corso P, Miller T, et al. The Incidence and Economic Burden of Injuries in the United States. New York (NY): Oxford University Press; 2006. [Google Scholar]
- 6.Coronado, McGuire, Faul, Sugerman, Pearson . The Epidemiology and Prevention of TBI. 2012. (in press) [Google Scholar]
- 7.Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(suppl 1):S7–S95. doi: 10.1089/neu.2007.9995. [DOI] [PubMed] [Google Scholar]
- 8.Marshall LF, Marshall SB, Klauber MR, et al. A new classification of head injury based on computerized tomography. J Neurosurg. 1991;75(5 suppl):S14–S20. [Google Scholar]
- 9.Henderson R, Diggle P, Dobson A. Joint modelling of longitudinal measurements and event time data. Biostatistics. 2000 Dec;1(4):465–80. doi: 10.1093/biostatistics/1.4.465. [DOI] [PubMed] [Google Scholar]
- 10.Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24:329–37. doi: 10.1089/neu.2006.0035. [DOI] [PubMed] [Google Scholar]
- 11.Talving P, Karamanos E, Teixeira PG, et al. Intracranial pressure monitoring in severe head injury: compliance with Brain Trauma Foundation guidelines and effect on outcomes: a prospective study. J Neurosurg. 2013 Nov;119(5):1248–54. doi: 10.3171/2013.7.JNS122255. [DOI] [PubMed] [Google Scholar]
- 12.Bulger EM, Nathens AB, Rivara FP, et al. Brain Trauma Foundation. Management of severe head injury: institutional variations in care and effect on outcome. Crit Care Med. 2002 Aug;30(8):1870–6. doi: 10.1097/00003246-200208000-00033. [DOI] [PubMed] [Google Scholar]
- 13.Cremer OL, van Dijk GW, van Wensen E, et al. Effect of intracranial pressure monitoring and targeted intensive care on functional outcome after severe head injury. Crit Care Med. 2005;33:2207–2213. doi: 10.1097/01.ccm.0000181300.99078.b5. [DOI] [PubMed] [Google Scholar]
- 14.Alkhoury F, Kyriakides TC. Intracranial Pressure Monitoring in Children With Severe Traumatic Brain Injury: National Trauma Data Bank-Based Review of Outcomes. JAMA Surg. 2014 Apr 30; doi: 10.1001/jamasurg.2013.4329. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Su SH, Wang F, Hai J, et al. The effects of intracranial pressure monitoring in patients with traumatic brain injury. PLoS One. 2014 Feb 21;9(2):e87432. doi: 10.1371/journal.pone.0087432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chesnut RM, Temkin N, Carney N, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367(26):2471–2481. doi: 10.1056/NEJMoa1207363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lobato RD, Cordobes F, Rivas JJ, de la Fuente M, Montero A, Barcena A, Perez C, Cabrera A, Lamas E. Outcome from severe head injury related to the type of intracranial lesion: A computerized tomography study. J Neurosurg. 1983;59:762–774. doi: 10.3171/jns.1983.59.5.0762. [DOI] [PubMed] [Google Scholar]
- 18.Servadei F, Murray GD, Penny K. The value of the “worst” computed tomographic scan in clinical studies of moderate and severe head injury. Neurosurgery. 2000;46:70–77. doi: 10.1097/00006123-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Hukkelhoven CW, Steyerberg EW, Habbema JD, et al. Predicting outcome after traumatic brain injury: development and validation of a prognostic score based on admission characteristics. J Neurotrauma. 2005 Oct;22(10):1025–39. doi: 10.1089/neu.2005.22.1025. [DOI] [PubMed] [Google Scholar]
- 20.Chesnut RM, Ghajar J, Maas AR. Guidelines for the Management and prognosis of severe traumatic brain injury part II: Early indicators of prognosis in severe traumatic brain injury. J Neurotrauma. 2000;17:556–627. [Google Scholar]
- 21.Maas AI, Steyerberg EW, Butcher I, et al. Prognostic value of computerized tomography scan characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007 Feb;24(2):303–14. doi: 10.1089/neu.2006.0033. [DOI] [PubMed] [Google Scholar]
- 22.Maas AI, Hukkelhoven CW, Marshall LF, et al. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005 Dec;57(6):1173–82. doi: 10.1227/01.neu.0000186013.63046.6b. [DOI] [PubMed] [Google Scholar]
- 23.Kahraman S, Dutton RP, Hu P, et al. Automated measurement of “pressure times time dose” of intracranial hypertension best predicts outcome after severe traumatic brain injury. J Trauma. 2010 Jul;69(1):110–8. doi: 10.1097/TA.0b013e3181c99853. [DOI] [PubMed] [Google Scholar]


