Abstract
Background
Hyperuricemia is implicated in cardiovascular and cerebrovascular diseases. This study evaluated associations between uric acid (UA), cardiovascular health and neurocognitive function in adolescent and adult survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only.
Methods
126 adolescent (mean[SD] age 14.6[5.0]; 7.8[1.7] years post-diagnosis) and 226 adult survivors (age 25.4[4.2] years; 18.1[4.4] years post-diagnosis) completed comprehensive neurocognitive testing. Concurrent UA measurements were conducted for both groups. For adult survivors, cardiovascular risk factors were assessed; and UA measurements during adolescence (12.3[4.0] years before neurocognitive testing) were also collected. UA levels were categorized into quartiles for age- and gender-based ranking, and associations with neurocognitive outcomes were examined.
Results
Survivors demonstrated worse attention, processing speed and executive functions than population norms (p's<0.05). Adolescent survivors with elevated UA had poorer attention (p=0.04), visual-processing speed (p=0.03) and cognitive flexibility (p=0.02). UA was not associated with neurocognitive outcomes in adult survivors. Adult survivors developed dyslipidemia (46%), hypertension (32%) and abdominal obesity (26%), and high UA during adolescence was associated with these cardiovascular risk factors as adults (all p's<0.01). Fine-motor processing speed was slower in adult survivors with dyslipidemia (p=0.04) and abdominal obesity (p=0.04). Poorer attention was marginally associated with hypertension (p=0.06).
Conclusions
Elevated UA is associated with neurocognitive performance in adolescent survivors. In adult survivors, relative elevation of UA during adolescence was predictive of cardiovascular health, which was associated with poorer neurocognitive outcomes.
Impact
Future studies should evaluate the mediating role of chronic cardiovascular health conditions between elevated UA and subsequent neurocognitive impairment in survivors.
Keywords: Cardiovascular, childhood acute lymphoblastic leukemia, neurocognitive, survivorship, uric acid
Introduction
Contemporary chemotherapy protocols have gradually replaced whole brain irradiation with intrathecal chemotherapy for prophylactic central nervous system treatment in childhood acute lymphoblastic leukemia (ALL).[1, 2] Despite excellent survival rates, survivors treated with only chemotherapy experience chronic health conditions, such as cardiovascular, pulmonary, neurological and endocrine complications, that can persist into long-term survival.[3–6] Specific to neurocognitive outcomes, we have recently demonstrated that at an average of 7.7 years post-diagnosis, ALL survivors treated with only chemotherapy experienced higher rates of impairment than expected on measures of processing speed, attention and executive functions.[7]
There is increasing interest in evaluating biomarkers relevant to the pathophysiology of chemotherapy-based neurotoxicity in long-term survivors.[8, 9] Higher levels of peripheral inflammation and oxidative stress, reflected through serum tumor necrosis factor-alpha (TNF-α) and oxidated phosphatidylcholine in the cerebrospinal fluid, have been associated with cognitive impairment in cancer patients.[9, 10] These biomarkers have been linked to vascular injury, which may be a key component of the pathophysiology of brain dysfunction.[11, 12]
Of interest to this study is the unique contribution of uric acid (UA) on neurocognitive outcomes in cancer survivors. UA can have a direct impact on vascular function by disrupting the renin-angiotensin system and inducing inflammation and oxidative stress.[13–15] The enzymatic degradation of xanthine, the precursor for the production of UA, generates superoxide anions, which are pro-oxidants that can induce extensive vascular injuries. Our recent work has shown that higher levels of post-methotrexate homocysteine during active treatment was related to poorer neurocognitive performance in long-term survivors of ALL.[7] Homocysteine is a physiological by-product of folate metabolism and its systemic elevation is associated with vascular injuries.[16, 17] Elevations in UA have also been associated with vascular disease, such as stroke, hypertension and dyslipidemia.[18–21]. Treatment exposures may lead to neurocognitive impairment through direct or indirect impacts on vascular function. Existing studies observed a relationship between UA and neurocognitive problems, even at subclinical levels.[22–25] Higher UA levels are correlated with brain white matter atrophy and poorer cognitive function.[22] It is proposed that higher circulating levels of UA at a young age may induce early microvascular injuries that lead to cognitive dysfunction as survivors age.
We examined the concurrent and longitudinal associations between UA and neurocognitive impairment, as well as the effects of UA on subsequent cardiovascular function and resultant neurocognitive outcomes (see Supplementary Fig. S1 for a summary of the theoretical framework). Specifically, we examined associations between concurrent UA levels and neurocognitive performance in adolescent survivors of ALL (5 to 10 years post-diagnosis), and associations between UA levels during adolescence and future neurocognitive performance and cardiovascular risk factors in adult survivors (more than 10 years post-diagnosis). We also explored associations between treatment factors and UA levels in survivors of ALL. This study is designed to test hypotheses that both adolescent and adult survivors with UA levels in the top quartile would demonstrate poorer neurocognitive outcomes than survivors with UA in the bottom quartiles; risk factors for cardiovascular conditions would be more prevalent in adult survivors with adolescent UA levels in the top quartile compared to those in the bottom quartiles; and adult survivors with cardiovascular factors would display poorer neurocognitive outcomes than those without.
Materials and Methods
Patients
This study included two groups of long-term survivors of childhood ALL: adolescent survivors who were 5–10 years post-diagnosis (N=126) and adult survivors who were > 10 years post diagnosis (N=226). All survivors were treated as children with chemotherapy without cranial irradiation.
Adolescent survivors were recruited as part of an established study to evaluate psychopathology and neurocognitive impairment in their early years of survivorship.[7] To be eligible, survivors had to have completed treatment on an institution-based chemotherapy protocol for childhood ALL (St. Jude Total Therapy XV)[2], be at least 5 years from diagnosis and at least 8 years of age. All survivors had routine measurement of serum UA during their visit to the long-term follow-up clinic.
Adult survivors were recruited as part of a large institutional study, the St. Jude Lifetime Cohort (SJLIFE) study, which evaluates medical and psychosocial late effects in adult survivors of childhood cancer.[26] To be eligible, survivors had to have been treated at St Jude Children's Research Hospital for ALL and be at least 10 years from diagnosis and at least 18 years of age. Neurocognitive outcomes of a subset of ALL survivors within the SJLIFE study had previously been reported.[6] However, this study also includes performance for survivors not previously reported. To be eligible for this current study, adult survivors must have serum UA measured within 5 to 10 years post-diagnosis, as well as during their current follow-up visit. Eligible adult survivors were previously treated with various protocols for ALL,[1, 2, 27–29] with a vast majority of them receiving the Total Therapy chemotherapy regimens (Supplementary Table S1).
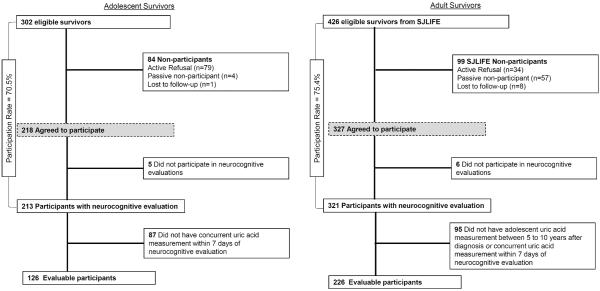
Survivors were excluded from the study if they received cranial irradiation or hematopoietic cell transplantation, developed relapse or secondary cancer, were not proficient in English, or had an unrelated neurological disorder associated with cognitive impairment. The participation rates for the adolescent and adult survivors are 70.5% and 75.4%, respectively (Figure 1).
Figure 1. Consort diagram.
A total of 302 adolescent survivors were eligible and 218 (70.5%) participated in the study. For adult survivors, there were 426 eligible survivors and 327 (75.4%) participated. Finally, complete data from 126 adolescent and 226 adult survivors were evaluated.
The study was approved by the institutional review board and written informed consent was obtained from all participants and/or their parents.
Neurocognitive assessment
The primary outcomes of this study were measures of neurocognitive function. The domains of interest included attention[30, 31], processing speed[32, 33] and executive function[31] (cognitive flexibility, verbal fluency and working memory). These specific measures were selected a priori based on our previous finding that impairments in these measures of executive function, processing speed and attention measures were found in long-term survivors of ALL who were treated with chemotherapy only.[6, 7] All neurocognitive tests were completed with licensed/certified examiners under the general supervision of a board-certified clinical neuropsychologist.
Treatment exposures
Cumulative chemotherapy doses were abstracted from medical records and are presented in Table 1. Given a previous association we observed between homocysteine and executive dysfunction within adolescent survivors[7], an exploratory analysis was conducted to evaluate the association between homocysteine and UA. For the adolescent survivors, plasma homocysteine concentrations were measured at baseline before intravenous (IV) high-dose (HD) methotrexate (MTX) infusion and at 6, 23 and 42 hours post-infusion for a total of four courses, and values were quantified as area-under-curve (AUC).[34, 35] Plasma homocysteine was not sampled during prior therapies on which the adult survivors were treated.
Table 1.
Demographics and treatment characteristics
| Adolescent survivors# (N=126) | Adult survivors+ (N=226) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Demographics | % | Mean (SD) | % | Mean (SD) | P | |
| Gender | Male | 44.4 | 48.2 | 0.49 | ||
| Race | White | 76.2 | 88.5 | 0.002 | ||
| Black | 12.7 | 9.3 | ||||
| Others | 11.1 | 2.2 | ||||
| Ethnicity | Hispanics | 7.9 | 4.9 | 0.24 | ||
| Current age | Years | 14.6 (5.0) | 25.4 (4.2) | NA | ||
|
| ||||||
| Clinical/Treatment characteristics | ||||||
|
| ||||||
| Age at diagnosis | Years | 6.8 (4.8) | 7.4 (4.4) | 0.067 | ||
| Time since diagnosis | Years | 7.8 (1.7) | 18.1 (4.4) | NA | ||
| Age at adolescent uric acid | Years | 13.1 (4.2) | NA | |||
| Estimated glomerular filtration rate* | ml/min | 94.6 (17.4) | 107.4 (20.1) | <0.0001 | ||
| Treatment risk stratum | Low | 54.0 | NA | NA | ||
| Standard/High | 46.0 | NA | NA | |||
| Oral corticosteroid | ||||||
| Dexamethasone | 100 | 38.9 | <0.0001 | |||
| Prednisone | 100 | 99.1 | 0.28 | |||
| IV High-dose cytarabine (g/m2) | 42.0 | 8.4 (3.5) | 7.5 | 15.3 (10.9) | 0.008 | |
| IV cyclophosphamide (g/m2) | 100 | 2.7 (1.9) | 56.2 | 8.3 (2.3) | <0.0001 | |
| IV daunorubicin (mg/m2) | 100 | 49.1 (10.3) | 88.0 | 81.7 (32.1) | <0.0001 | |
| IV doxorubicin (mg/m2) | 99.2 | 114.6 (58.0) | 6.6 | 155.0 (61.1) | 0.034 | |
| Methotrexate | IV standard-dose (g/m2) | 98.4 | 3.8 (1.2) | 72.6 | 2.4 (1.3) | <0.0001 |
| IV high-dose (g/m2) | 100 | 16.0 (7.6) | 98.7 | 15.9 (7.6) | 0.17 | |
| IT (ml) | 100 | 171.6 (58.6) | 100 | 170.0 (61.6) | 0.68 | |
| IV vincristine (mg/m2) | 99.2 | 59.1 (12.6) | 100 | 43.5 (22.9) | <0.0001 | |
| Triple IT chemotherapy^ | Number of counts | 99.2 | 15.0 (4.2) | NA | NA | NA |
Abbreviation: IQR: interquartile range; IM: intramuscular; IT: intrathecal; IV: intravenous; NA: not applicable; SD: standard deviation
Adolescent survivors (5 to 10 years post-diagnosis) are a subset of the study cited in reference [7]
Adult survivors are part of an ongoing, established study cited in references [6, 26]
Glomerular filtration rate is estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula for survivors above 18 years of age, and the Kidney Disease Improving Global Outcomes (KDIGO) for survivors who are 18 years of age and below
Triple IT chemotherapy: combination of methotrexate, hydrocortisone and cytarabine
Chronic cardiovascular conditions
For the adult survivors, cardiovascular risk factors were graded using the Common Terminology Criteria for Adverse Events version 4.03 (criteria outlined in Supplementary Table S2). Risk factors of interest were hypertension, dyslipidemia, abdominal obesity, high blood glucose, as well as overall metabolic syndrome, and were selected because associations between these factors and cognitive impairment in long-term survivors of childhood cancers had previously been studied (K.R. Krull; unpublished observations).
Statistical analysis
Neurocognitive performance was transformed into age-adjusted Z-scores (mean=0, standard deviation=1) using national normative data. One-sample t-tests or Wilcoxon signed-rank tests were used to compare performances between survivors and population norms. We did not expect clinical hyperuricemia to be prevalent in this population of relatively young survivors. Based on the assumption that the majority of the survivors were normouricemic, “high UA” was defined as survivors with UA levels in the top quartile. Survivors were categorized into 6 strata based on gender and age at UA measurement (<10 years of age; 10 to 18 years; >18 years). Within each stratum, UA levels were rank- ordered and grouped into quartiles. Neurocognitive scores were compared between survivors falling in the top v. three bottom quartiles using generalized linear models (GLM), after adjusting for age at diagnosis and years from diagnosis. Within adult survivors, Chi-square test was used to compare the proportion of survivors within the highest quartile of UA level for each cardiovascular risk factor. Independent t-tests were used to examine differences in neurocognitive test Z-scores between survivors with or without each cardiovascular risk factor and metabolic syndrome. Corrections for multiple testing were not made since UA as the predictor, as well as the specific neurocognitive measures and cardiovascular risk factors, were predetermined. Exploratory analysis was conducted to identify treatment predictors that are associated with UA using GLM, adjusting for current age and gender. As this analysis is exploratory in nature, false discovery rate (FDR) was applied.
Results
Demographics and treatment characteristics of the adolescent and adult survivors are presented in Table 1. Adolescent survivors had a mean age of 14.6 years at the time of neurocognitive assessment, while the adult survivors had a mean age of 25.4 years of age at assessment. Compared to adult survivors, adolescent survivors received higher doses of IV MTX and vincristine, but lower doses of anthracyclines and cyclophosphamide (p<0.0001). For adults, the mean time between adolescent and adult UA measurements was 12.3 (4.0) years. Both adolescent and adult survivors have normal renal function, as reflected by the estimated glomerular filtration rate. The UA values for each reference group are presented in Supplementary Table S3; in general, male survivors had higher UA levels than females across both age groups. For the adult survivors, UA measured during adolescence was associated with levels collected during adulthood; half of the adult survivors (53%) whose adolescent UA fell within the top quartile remained in the top quartile during adulthood while 83% of the survivors who fell within the bottom quartiles remained in those quartiles.
Within adolescent survivors, higher cumulative dose of daunorubicin was marginally associated with higher UA levels (p=0.06, corrected for FDR) (Supplementary Table S4). After adjusting for gender and age, higher UA level in survivors was associated with higher homocysteine levels following IV HDMTX (p=0.06, corrected for FDR). No association was identified between UA levels and treatment factors in adult survivors (Supplementary Table S5).
Adolescent survivors' scored below normative reference for measures of attention (p's=0.004 to 0.05), visual and fine-motor processing speed (p's<0.0001) and executive function (cognitive flexibility, fluency and working memory; p's=0.01, <0.0001 and 0.006, respectively; Table 2). After adjusting for age at diagnosis and years from diagnosis, adolescent survivors in the top quartile of UA level performed worse than those in the bottom three quartiles, for attention (signal detection; −0.52 vs. −0.05; p=0.04), visual processing speed (mean = −0.32 vs. 0.12; p=0.03) and cognitive flexibility (−1.01 vs. −0.37; p=0.02) (Table 2). Rates of impairment in attention, visual processing speed and cognitive flexibility were 22%, 19% and 46%, respectively, in survivors in the top UA quartile (Supplementary Table S6).
Table 2.
Neurocognitive Outcomes in Adolescent Survivors
| Domain | Z-scores Mean (SD) | Pop P* | Top Quartile (N=37) | Bottom 3 Quartiles (N=89) | Group P^ |
|---|---|---|---|---|---|
| Attention | Adj Mean#(95% CI) | Adj Mean#(95% CI) | |||
| Focused | −0.21 (1.06) | 0.03 | −0.44 (−0.78, −0.10) | −0.12 (−0.34, −0.10) | 0.12 |
| Sustained | −0.24 (1.58) | 0.01 | −0.21 (−0.72, 0.29) | −0.24 (−0.57, 0.08) | 0.93 |
| Impulsivity | −0.18 (1.25) | 0.05 | −0.51 (−0.91, −0.10) | −0.04 (−0.30, 0.22) | 0.06 |
| Variability | −0.22 (1.28) | 0.06 | −0.41 (−0.83, −0.00) | −0.14 (−0.41, 0.12) | 0.28 |
| Signal detection | −0.19 (1.23) | 0.004 | −0.52 (−0.90, −0.14) | −0.05 (−0.30, 0.19) | 0.04 |
| Processing Speed | |||||
| Fine motor | −1.35 (1.40) | <0.0001 | −1.21 (−1.69, −0.73) | −1.37 (−1.69, −1.06) | 0.58 |
| Visual | −0.01 (1.03) | 0.95 | −0.32 (−0.65, 0.02) | 0.12 (−0.09, 0.34) | 0.03 |
| Visual motor | −0.36 (0.98) | <0.0001 | −0.42 (−0.74, 0.10) | −0.33 (−0.54, 0.13) | 0.64 |
| Executive Function | |||||
| Cognitive flexibility | −0.56 (1.16) | 0.01 | −1.01 (−1.38, −0.64) | −0.37 (−0.60, −0.13) | 0.02 |
| Cognitive fluency | −0.41 (0.90) | <.0001 | −0.70 (−0.99, −0.41) | −0.29 (−0.48, −0.11) | 0.14 |
| Working memory | −0.27 (1.00) | 0.006 | −0.44 (−0.77, −0.12) | −0.20 (−0.42, 0.01) | 0.23 |
Abbreviation: CI, confidence interval; CPT, Conners Continuous Performance Test
Adolescent survivors are a subset of the study cited in reference [7]
Comparison of Z-scores was conducted with population mean of 0 and standard deviation of 1
Adjusted for age at diagnosis and years from diagnosis
Comparison of Z-scores was conducted between subjects in the top and bottom 3 quartiles
Neurocognitive performances of adult survivors (Table 3) were lower than population norms on measures of focused attention (p=0.007), impulsivity (p=0.002), motor and visual processing speed (p<0.0001 and 0.02, respectively) and executive functions (cognitive flexibility, fluency and working memory; p's<0.001 and 0.003). There were no differences in neurocognitive scores between those with high v. low UA levels during adolescence. Higher UA collected concurrently during adulthood was associated with poorer attention (p=0.05). The rates of impairment within adult survivors in the top and bottom 3 quartiles are presented in Supplementary Table S6.
Table 3.
Neurocognitive Outcomes in Adult Survivors
| Uric acid levels measured at adolescence | Uric acid levels measured at adulthood | |||||||
|---|---|---|---|---|---|---|---|---|
| Domain | Z-scores Mean (SD) | Pop P* | Top Quartile (N=60) | Bottom 3 Quartiles (N=166) | P ^ | Top Quartile (N=61) | Bottom 3 Quartiles (N=165) | P ^ |
| Attention | Adj Mean# (95% CI) | Adj Mean# (95% CI) | Adj Mean# (95% CI) | Adj Mean# (95% CI) | ||||
| Focused | −0.22 (1.22) | 0.007 | −0.27 (−0.58, 0.05) | −0.20 (−0.39, −0.02) | 0.74 | −0.22 (−0.53, 0.09) | −0.22 (−0.41, −0.03) | 0.97 |
| Sustained | −0.09 (1.26) | 0.30 | −0.17 (−0.50, 0.15) | −0.05 (−0.25, 0.14) | 0.53 | −0.29 (−0.61, 0.03) | −0.01 (−0.20, 0.18) | 0.13 |
| Impulsivity | −0.23 (1.14) | 0.002 | −0.47 (−0.76, −0.18) | −0.15 (−0.32, 0.03) | 0.06 | −0.48 (−0.76, −0.19) | −0.14 (−0.32, 0.03) | 0.05 |
| Variability | −0.08 (1.04) | 0.28 | −0.13 (−0.40, 0.14) | −0.06 (−0.22, 0.10) | 0.64 | −0.09 (−0.35, 0.18) | −0.07 (−0.23, 0.09) | 0.93 |
| Signal detection | −0.12 (1.04) | 0.088 | −0.21 (−0.47, 0.06) | −0.09 (−0.25, 0.07) | 0.45 | −0.27 (−0.54, −0.01) | −0.06 (−0.22, 0.10) | 0.17 |
| Processing Speed | ||||||||
| Fine motor | −1.03 (1.36) | <0.0001 | −1.27 (−1.61, −0.92) | −0.94 (−1.15, −0.74) | 0.11 | −1.15 (−1.49, −0.81) | −0.99 (−1.19, −0.78) | 0.42 |
| Visual | 0.15 (0.97) | 0.02 | 0.21 (−0.04, 0.46) | 0.13 (−0.02, 0.28) | 0.57 | 0.05 (−0.20, 0.29) | 0.19 (0.04, 0.34) | 0.33 |
| Visual motor | −0.10 (1.00) | 0.13 | −0.14 (−0.40, 0.11) | −0.09 (−0.24, 0.07) | 0.72 | −0.26 (−0.51, −0.01) | −0.04 (−0.20, 0.11) | 0.15 |
| Executive Function | ||||||||
| Cognitive flexibility | −0.64 (1.68) | <0.001 | −0.64 (−1.07, −0.21) | −0.64 (−0.90, −0.38) | 0.99 | −0.56 (−0.99, −0.14) | −0.67 (−0.93, −0.41) | 0.66 |
| Cognitive fluency | −0.21 (1.03) | 0.003 | −0.38 (−0.65, −0.12) | −0.15 (−0.31, 0.01) | 0.14 | −0.28 (−0.54, −0.01) | −0.18 (−0.34, −0.03) | 0.55 |
| Working memory | −0.24 (0.87) | <0.001 | −0.09 (−0.31, 0.13) | −0.30 (−0.43, −0.16) | 0.12 | −0.23 (−0.45, −0.01) | −0.25 (−0.38, −0.11) | 0.90 |
Adult survivors are part of an ongoing, established study cited in references [6, 26]
Abbreviation: CI, confidence interval; CPT, Conners Continuous Performance Test
Comparison of Z-scores was conducted with population mean of 0 and standard deviation of 1
Adjusted for age at diagnosis and years from diagnosis.
Comparison of Z-scores was conducted between subjects in the top and bottom 3 quartiles
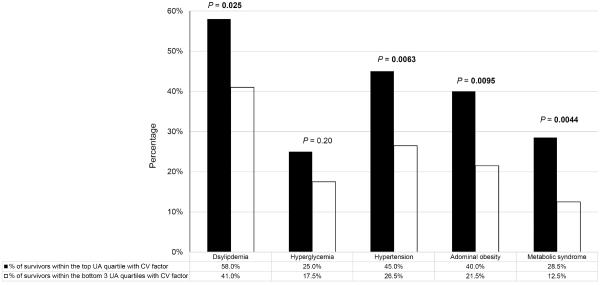
Risk factors for cardiovascular conditions were relatively common in adult survivors; dyslipidemia (46%), hypertension (32%) and abdominal obesity (26%) were the most prevalent. Collectively, 17% of adult survivors fulfilled the criteria for metabolic syndrome. With the exception of hyperglycemia, the proportion of survivors with cardiovascular risk factors were significantly higher in survivors with high UA level collected during adolescence (all p's<0.01) (Figure 2).
Figure 2. Association between Uric Acid and Cardiovascular Risk Factors.
The proportion of adult survivors with cardiovascular risk factors was significantly higher in survivors with high uric acid level collected during adolescence.
CV: Cardiovascular; UA: Uric acid
Processing speed and sustained attention were associated with cardiovascular risk factors (Supplementary Table S7). Mildly slower fine motor processing speed was found in survivors with dyslipidemia (p=0.04) and abdominal obesity (p=0.04). Visual and visual-motor processing speeds tended to be poorer in survivors with dyslipidemia (p=0.05) and abdominal obesity (p=0.06), as did poorer attention performance in survivors with hypertension (p=0.06). Cognitive performance was not related to hyperglycemia or metabolic syndrome.
Discussion
The results of this study demonstrate an association between UA and neurocognitive function in long-term survivors of childhood ALL. Adolescent survivors whose UA levels were at the higher end of the average range during survivorship had poorer neurocognitive performances compared to those with UA in the low to moderate range. Neurocognitive performance during adulthood was not globally associated with UA levels during adulthood or UA levels collected during prior adolescence. However, in these adults, UA during adolescence was predictive of cardiovascular health during adulthood, and cardiovascular health during adulthood was associated with neurocognitive performance.
Higher serum UA levels in young ALL survivors, between 5 to 10 years post-diagnosis were associated with neurocognitive impairment in measures of attention, processing speed and cognitive flexibility. Although the statistical significance of these associations is modest, it is worthwhile to highlight that the group differences of half to two-third of a standard deviation is considered a substantial effect size. Our finding is also consistent with other studies associating elevated UA with poorer neurocognitive function in non-cancer populations at a much older age. One study reported in a community sample of 96 older adults (male: 73.7±7.6 years; female: 72.4±7.8 years), those with high to normal serum UA concentrations were 2.7 to 5.9 times more likely to score in the bottom quartile on measures of processing speed, verbal memory, and working memory.[23] Likewise in relatively healthy elderly adults, it has been reported that higher UA levels were associated with poorer global cognitive performance on the mini-mental state examination.[25] Another study found hyperuricemia to be a marker of global vascular brain disease and related to brain white matter atrophy.[22] Approximately 23% of survivors of childhood ALL developed mild to moderate leukoencephalopathy (Grade 2 and below) during chemotherapy treatment.[36] UA levels at long-term follow-up was not related to on-therapy leukoencephalopathy (data not presented). However, we found associations between UA and neurocognitive function specifically in the domains of processing speed and attention, which are more closely tied to white matter integrity.[37, 38] Future studies should investigate the impact of high UA on microstructural changes in the brain and functional outcomes in survivors.
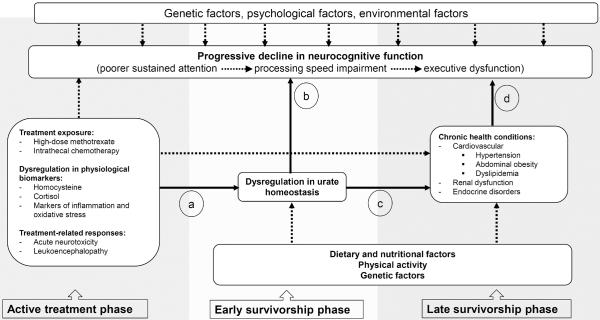
UA levels during adolescence were not predictive of neurocognitive performance in adult survivors. However, higher UA levels during adolescence were associated with cardiovascular risk factors in adult survivors an average of 10 years later, and these cardiovascular risk factors were related to poor processing speed and attention that are known to be affected by hypertension and microvascular infarction.[39–41] Existing literature suggests that hyperuricemia co-exists with other clusters of metabolic and hemodynamic abnormalities, such as abdominal obesity, glucose intolerance, insulin resistance, dyslipidemia and hypertension, in patients with metabolic syndromes.[18, 42] A review of this subject matter also highlights that hyperuricemia carries an increased relative risk for hypertension developing within 5 to 10 years, independent of other risk factors.[20] This is a particularly important finding within the population of young cancer survivors who experience excess risk compared to siblings for chronic health conditions. A previous Childhood Cancer Survivor Study investigation estimated that by 35 years of age, 47% of survivors of childhood ALL, as compared to 32% in their healthy siblings, reported more than one chronic health disorder, of which hypertension was one of the most frequently reported.[43] Individuals with these collective cardiovascular conditions are also at an increased risk for neurocognitive decline.[39, 44, 45] As cancer survivors are more susceptible to experiencing late cardiac, metabolic, and endocrine complications due to the anti-cancer treatments, the effect of UA on neurocognitive functioning may be mediated through these cardiovascular and metabolic risk factors. Figure 3 provides our hypothesized model of the interplay among treatment exposures, UA dysregulation and chronic health conditions, leading to survivors' progressive decline in their neurocognitive function. During the early phase of survivorship, the link between mildly elevated UA levels and various cognitive measures may reflect the presence of subclinical processes such as inflammation and oxidative stress. These processes may lead to chronic health conditions, reflected through UA measured in adolescence predicting the development of risk factors for cardiovascular diseases. The lack of association between UA and neurocognitive function in the adult survivors may be attributed to added variance from factors related to the cardiovascular system, as well as the presence of other intrinsic and extrinsic factors, such as genetic and dietary factors, that contribute to UA dysregulation and neurocognitive impairment. Studies have demonstrated that genetic predispositions related to oxidative stress are associated with poorer neurocognitive outcomes and higher risk of hyperuricemia.[46–48] It can be inferred from our current findings that in survivors of childhood ALL, who are already at risk for chronic morbidities due to primary treatment exposures, cardiovascular health may mediate the relationship between elevated UA levels and neurocognitive impairment. Longitudinal studies are needed to verify this multifactorial process.
Figure 3.
Uric Acid Dysregulation, Chronic Health Conditions and Neurocognitive Function in Cancer Survivors: A Theoretical model
Solid arrows: Relationships among uric acid dysregulation, chronic health conditions and neurocognitive function that this study aimed to address
Dashed arrows: Other factors that might contribute to dysregulation in uric acid levels and neurocognitive impairment in cancer survivors
a. Treatment exposures may affect urate homeostasis. Results from this current study suggests that higher on-therapy homocysteine exposure and cumulative doses of methotrexate were associated with elevated uric acid levels in survivors. Chemotherapy exposure and acute physiological response to the treatments may potentially initiate the process of urate hemostasis disruption in the early years of cancer survivorship.
b. Uric acid is associated with brain function and neurocognitive performance. Uric acid can also have a direct impact on brain vascular function by inducing inflammation and oxidative stress.[13–15] One large study has found that higher uric acid levels were also associated with white matter atrophy, and poorer cognitive function was found in individuals with hyperuricemia.[22]
c. Hyperuricemic is commonly observed in patients with hypertension, metabolic, endocrinal and renal disorders. Studies have shown that metabolic syndrome can occur in patients with elevated (>10mg/dL) uric acid, even though they have a normal body mass index.[55] Waist circumference and the level of triglycerides were found to be strongly correlated with serum uric acid level.[56] In an animal model, inhibiting xanthine oxidase results in lower uric acid and leads to a partial reduction of metabolic-like syndrome and hypertension.[57] Elevated uric acid level is an independent predictor of the development of both microalbuminuria and renal dysfunction in subjects with normal renal function and lowering levels of uric acid may slow progression of renal disease in patients with hyperuricemia.[58, 59]
d. Hypertension, metabolic and renal disorders are associated with neurocognitive impairment. Patients with vascular complications, such as hypertension and microvascular diseases, are at increased risk for cognitive impairment and dementia.[24, 39, 40, 44] Among metabolic syndrome components, hypertriglyceridemia and low high-density lipoprotein cholesterol levels were associated with global cognitive impairment while diabetes was associated with declines in executive function.[60] Lower estimated glomerular filtration rate at baseline was associated with a more rapid rate of cognitive decline, particularly in memory function.[61] Patients in end-stage renal failure with cognitive impairment have cortical defects consistent with infarcts.[62]
The molecular and biochemical mechanisms linking UA to neurocognitive function remain unclear. While some studies suggest neuroprotective properties of UA, acting through suppression of oxyradical accumulation, stabilization of calcium homeostasis, and preservation of mitochondrial function,[24, 49, 50] others report that excess UA is neurotoxic, through inducing systemic inflammation and endothelial dysfunction.[13, 14] Our study found that higher UA was associated with poorer neurocognitive and cardiovascular outcomes, which are both known to be associated with vascular injury.[15, 18, 20, 41, 44] Hence our results are suggestive that higher UA may induce cellular proliferation, inflammation and oxidative stress, and may not function as an antioxidant in this context. Interestingly, adolescent survivors with UA in the top quartile were treated with modestly higher doses of daunorubicin than those in the bottom quartiles. Both human and animal studies have suggested that anthracycline could increase the level of circulating cytokines, such as TNF-α, which could penetrate the blood-brain-barrier, leading to poorer neurocognitive outcomes.[10, 51, 52] This observation may suggest that elevated UA level may be a result of local inflammation and oxidative processes that are induced by primary treatment exposures. Serum UA is also related to increased cell apoptosis and necrosis, and implicated in the development of oxidative and nitrosative stress and subsequent inflammatory diseases.[13–15] This may have an indirect impact on the brain vasculature and subsequently, neurocognitive dysfunction.
Our research team previously demonstrated that homocysteine levels following HDMTX administration were predictive of poorer neurocognitive function.[7] The current findings suggests that higher plasma concentration of homocysteine following HDMTX during active chemotherapy was positively associated with UA levels in young survivors during the earlier years of long-term survivorship. Homocysteine is a physiological byproduct of folate metabolism and its systemic elevation in ALL patients is an acute response to the inhibition of dihydrofolate reductase by MTX. The prolonged elevation of serum homocysteine, even in subclinical levels, has been implicated in cardiovascular and cerebrovascular complications in the general population.[16, 17, 53] Recent literature also demonstrates that folic acid therapy lowers UA levels in patients with mild to moderate hypertension.[54] These preliminary findings suggest that the acute response to the anti-cancer treatment may initiate chronic vascular dysfunction by the disruption of UA homeostasis. However, this speculation needs to be further explored, as our current study did not examine the association between concurrent UA and homocysteine levels in the survivors, or associations with markers of oxidative stress and inflammation.
These findings should be interpreted in the context of potential limitations. Measuring serum UA level at a single time point may not be predictive of chronic UA levels. However, UA level is reported to be relatively stable without large biological fluctuations, and the prognostic value of UA across years prior to the development of chronic health conditions has been verified in existing literature.[20] Drawing parallel experiences from the clinical diagnosis of gout, this single UA measurement may still be valid in exploring its association with neurocognitive function. Our finding of association between UA level during adolescent and adult follow-up for our adult sample further supports the notion of UA stability. Lastly, non-English speaking patients were excluded due to the language dependent nature of the neurocognitive tests adopted in this study. As the overarching aim of this study was to look at the pathophysiology behind neurocognitive impairment in cancer survivors, we believe that the associations observed from our study may still be generalizable to the non-English speaking populations even though studies have shown that treatment outcomes may differ across ethnic groups. This study also did not examine other factors such as physical activity, diet, and genetic vulnerability, all of which might be related to both UA metabolism and neurocognitive function. Future studies should include prospective UA assessment with these health behaviors and genetic factors.
Limitations notwithstanding, this study demonstrates an association between concurrent UA levels and neurocognitive function in adolescent survivors of childhood ALL treated on a contemporary chemotherapy-only protocol. UA acid during adolescence was not related to neurocognitive performance in the adult survivors but it was predictive of cardiovascular risk factors that were associated with those survivors processing speed and attention impairments. We believe that UA may potentially be an important marker of brain injury and neurocognitive decline in patients during their early years of cancer survivorship. Future studies should prospectively evaluate the patterns of change in UA from adolescence to adulthood, and the mediating role of chronic health conditions between UA and neurocognitive function.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Dr. Cara Kimberg, Ms. Cindy Jones, Ms. Debbie Stewart and Ms. Adrienne Studaway for administering the neurocognitive tests; Ms. Joycelynn Butler for extracting and cleaning the data; Ms. Chenghong Li for providing support for statistical analysis.
Funding: Support was provided by the National Institute of Mental Health (MH085849 to K.R. Krull), the National Cancer Institute (U01CA195547-01 to M.M. Hudson and L.L. Robison), and by the American Lebanese Syrian Associated Charities (ALSAC). Sponsors had no involvement in the design or conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript
Footnotes
Conflict of interest: All authors declare no competing interests.
Reference
- 1.Pui CH. Toward optimal central nervous system-directed treatment in childhood acute lymphoblastic leukemia. J Clin Oncol. 2003;21:179–81. doi: 10.1200/JCO.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–41. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diller L, Chow EJ, Gurney JG, Hudson MM, Kadin-Lottick NS, Kawashima TI, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: A review of published findings. J Clin Oncol. 2009;27:2339–55. doi: 10.1200/JCO.2008.21.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang TT, Hudson MM, Stokes DC, Krasin MJ, Spunt SL, Ness KK. Pulmonary outcomes in survivors of childhood cancer: A systematic review. Chest. 2011;140:881–901. doi: 10.1378/chest.10-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation. 2013;128:1927–55. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 6.Krull KR, Brinkman TM, Li C, Armstrong GT, Ness KK, Kumar Srivastava D, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: A report from the St Jude Lifetime Cohort Study. J Clin Oncol. 2013;31:4407–15. doi: 10.1200/JCO.2012.48.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krull KR, Cheung YT, Fellah S, Liu W, Ogg RJ, Srivastava D, et al. Chemotherapy Pharmacodynamics, Neuroimaging and Neurocognitive Outcomes in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. J Clin Oncol. 2016 doi: 10.1200/JCO.2015.65.4574. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saykin AJ, de Ruiter MB, McDonald BC, Deprez S, Silverman DHS. Neuroimaging biomarkers and cognitive function in non-CNS cancer and its treatment: Current status and recommendations for future research. Brain Imaging Behav. 2013;7:363–73. doi: 10.1007/s11682-013-9283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caron JE, Krull KR, Hockenberry M, Jain N, Kaemingk K, Moore IM. Oxidative stress and executive function in children receiving chemotherapy for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2009;53:551–56. doi: 10.1002/pbc.22128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers JS. The possible role of cytokines in chemotherapy-induced cognitive deficits. Adv Exp Med Biol. 2010;678:119–23. doi: 10.1007/978-1-4419-6306-2_15. [DOI] [PubMed] [Google Scholar]
- 11.Zhang C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol. 2008;103:398–406. doi: 10.1007/s00395-008-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–52. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–42. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 14.Hong Q, Qi K, Feng Z, Huang Z, Cui S, Wang L, et al. Hyperuricemia induces endothelial dysfunction via mitochondrial Na +/Ca 2+ exchanger-mediated mitochondrial calcium overload. Cell Calcium. 2012;51:402–10. doi: 10.1016/j.ceca.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y, Lai X, Chen G, Xu Y, Huang B, Wu Y, et al. Association among serum uric acid, cardiovascular risk, and arterial stiffness: A cross-sectional study in She ethnic minority group of Fujian Province in China. J Endocrinol Invest. 2012;35:290–97. doi: 10.3275/7765. [DOI] [PubMed] [Google Scholar]
- 16.Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6. doi: 10.1186/1475-2891-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catena C, Colussi G, Nait F, Capobianco F, Sechi LA. Elevated homocysteine levels are associated with the metabolic syndrome and cardiovascular events in hypertensive patients. Am J Hypertens. 2015;28:943–50. doi: 10.1093/ajh/hpu248. [DOI] [PubMed] [Google Scholar]
- 18.Mankovsky B, Kurashvili R, Sadikot S. Is serum uric acid a risk factor for atherosclerotic cardiovascular disease?: A review of the clinical evidence. Part 1. Diabetes Metab Synd. 2010;4:176–84. [Google Scholar]
- 19.Ryu WS, Kim CK, Kim BJ, Lee SH. Serum Uric Acid Levels and Cerebral Microbleeds in Patients with Acute Ischemic Stroke. PLoS One. 2013;8:e55210. doi: 10.1371/journal.pone.0055210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feig DI, Kang DH, Johnson RJ. Medical progress: Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–21. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vannorsdall TD, Jinnah HA, Gordon B, Kraut M, Schretlen DJ. Cerebral ischemia mediates the effect of serum uric acid on cognitive function. Stroke. 2008;39:3418–20. doi: 10.1161/STROKEAHA.108.521591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verhaaren BFJ, Vernooij MW, Dehghan A, Vrooman HA, De Boer R, Hofman A, et al. The relation of uric acid to brain atrophy and cognition: The Rotterdam Scan Study. Neuroepidemiology. 2013;41:29–34. doi: 10.1159/000346606. [DOI] [PubMed] [Google Scholar]
- 23.Schretlen DJ, Inscore AB, Jinnah HA, Rao V, Gordon B, Pearlson GD. Serum uric acid and cognitive function in community-dwelling older adults. Neuropsychology. 2007;21:136–40. doi: 10.1037/0894-4105.21.1.136. [DOI] [PubMed] [Google Scholar]
- 24.Euser SM, Hofman A, Westendorp RGJ, Breteler MMB. Serum uric acid and cognitive function and dementia. Brain. 2009;132:377–82. doi: 10.1093/brain/awn316. [DOI] [PubMed] [Google Scholar]
- 25.Cicero AFG, Desideri G, Grossi G, Urso R, Rosticci M, D'Addato S, et al. Serum uric acid and impaired cognitive function in a cohort of healthy young elderly: data from the Brisighella Study. Intern Emerg Med. 2015;10:25–31. doi: 10.1007/s11739-014-1098-z. [DOI] [PubMed] [Google Scholar]
- 26.Hudson MM, Ness KK, Nolan VG, Armstrong GT, Green DM, Morris EB, et al. Prospective medical assessment of adults surviving childhood cancer: Study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2011;56:825–36. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hijiya N, Gajjar A, Zhang Z, Sandlund JT, Ribeiro RC, Rubnitz JE, et al. Low-dose oral etoposide-based induction regimen for children with acute lymphoblastic leukemia in first bone marrow relapse. Leukemia. 2004;18:1581–86. doi: 10.1038/sj.leu.2403467. [DOI] [PubMed] [Google Scholar]
- 28.Pui CH, Boyett JM, Rivera GK, Hancock ML, Sandlund JT, Ribeiro RC, et al. Long-term results of Total Therapy Studies 11, 12 and 13A for childhood acute lymphoblastic leukemia at St Jude Children's Research Hospital. Leukemia. 2000;14:2286–94. doi: 10.1038/sj.leu.2401938. [DOI] [PubMed] [Google Scholar]
- 29.Pui CH, Relling MV, Sandlund JT, Downing JR, Campana D, Evans WE. Rationale and design of Total Therapy Study XV for newly diagnosed childhood acute lymphoblastic leukemia. Ann Hematol. 2004;83:S124–26. doi: 10.1007/s00277-004-0850-2. [DOI] [PubMed] [Google Scholar]
- 30.Conners CK. Conners' Continuous Performance Performance Test II. Multi-Health Systems; North Tonawanda, NY: 2001. [Google Scholar]
- 31.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- 32.Wechsler D. Wechsler Adult Intelligence Scale. Fourth Edition Pearson; San Antonio: 2008. [Google Scholar]
- 33.Wechsler D. Wechsler Intelligence Scale for Children. Fourth Edition Harcourt Assessment, Inc; San Antonio, TX: 2003. [Google Scholar]
- 34.Kishi S, Griener J, Cheng C, Das S, Cook EH, Pei D, et al. Homocysteine, pharmacogenetics, and neurotoxicity in children with leukemia. J Clin Oncol. 2003;21:3084–91. doi: 10.1200/JCO.2003.07.056. [DOI] [PubMed] [Google Scholar]
- 35.Rühs H, Becker A, Drescher A, Panetta JC, Pui CH, Relling MV, et al. Population PK/PD Model of Homocysteine Concentrations after High-Dose Methotrexate Treatment in Patients with Acute Lymphoblastic Leukemia. PLoS One. 2012;7:e46015. doi: 10.1371/journal.pone.0046015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhojwani D, Sabin ND, Pei D, Yang JJ, Khan RB, Panetta JC, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32:949–59. doi: 10.1200/JCO.2013.53.0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santiago C, Herrmann N, Swardfager W, Saleem M, Oh PI, Black SE, et al. White matter microstructural integrity is associated with executive function and processing speed in older adults with coronary artery disease. Am J Geriatr Psychiatry. 2015;23:754–63. doi: 10.1016/j.jagp.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Penke L, Mañiega SM, Bastin ME, Hernández MCV, Murray C, Royle NA, et al. Brain-wide white matter tract integrity is associated with information processing speed and general intelligence. Mol Psychiatry. 2012;17:1026–30. doi: 10.1038/mp.2012.127. [DOI] [PubMed] [Google Scholar]
- 39.Köhler S, Baars MAE, Spauwen P, Schievink S, Verhey FRJ, Van Boxtel MJP. Temporal evolution of cognitive changes in incident hypertension: Prospective cohort study across the adult age span. Hypertension. 2014;63:245–51. doi: 10.1161/HYPERTENSIONAHA.113.02096. [DOI] [PubMed] [Google Scholar]
- 40.Chen KHM, Henderson VW, Stolwyk RJ, Dennerstein L, Szoeke C. Prehypertension in midlife is associated with worse cognition a decade later in middle-aged and older women. Age Ageing. 2015;44:439–45. doi: 10.1093/ageing/afv026. [DOI] [PubMed] [Google Scholar]
- 41.Jacobs HI, Leritz EC, Williams VJ, Van Boxtel MP, Elst WVD, Jolles J, et al. Association between white matter microstructure, executive functions, and processing speed in older adults: The impact of vascular health. Hum Brain Mapp. 2013;34:77–95. doi: 10.1002/hbm.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42:474–80. doi: 10.1161/01.HYP.0000091371.53502.D3. [DOI] [PubMed] [Google Scholar]
- 43.Essig S, Li Q, Chen Y, Hitzler J, Leisenring W, Greenberg M, et al. Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2014;15:841–51. doi: 10.1016/S1470-2045(14)70265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seidel UK, Gronewold J, Volsek M, Todica O, Kribben A, Bruck H, et al. The prevalence, severity, and association with HbA1c and fibrinogen of cognitive impairment in chronic kidney disease. Kidney Int. 2014;85:693–702. doi: 10.1038/ki.2013.366. [DOI] [PubMed] [Google Scholar]
- 46.Cole PD, Finkelstein Y, Stevenson KE, Blonquist TM, Vijayanathan V, Silverman LB, et al. Polymorphisms in genes related to oxidative stress are associated with inferior cognitive function after therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2015;33:2205–11. doi: 10.1200/JCO.2014.59.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDonagh EM, Thorn CF, Callaghan JT, Altman RB, Klein TE. PharmGKB summary: Uric acid-lowering drugs pathway, pharmacodynamics. Pharmacogenet Genomics. 2014;24:464–76. doi: 10.1097/FPC.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krull KR, Bhojwani D, Conklin HM, Pei D, Cheng C, Reddick WE, et al. Genetic mediators of neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2013;31:2182–88. doi: 10.1200/JCO.2012.46.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Méndez-Hernández E, Salas-Pacheco J, Ruano-Calderón L, Téllez-Valencia A, Cisneros-Martínez J, Barraza-Salas M, et al. Lower uric acid linked with cognitive dysfunction in the elderly. CNS Neurol Disord Drug Targets. 2015;14:564–66. doi: 10.2174/1871527314666150430161659. [DOI] [PubMed] [Google Scholar]
- 50.Wu Y, Zhang D, Pang Z, Jiang W, Wang S, Tan Q. Association of serum uric acid level with muscle strength and cognitive function among Chinese aged 50–74 years. Geriatr Gerontol Int. 2013;13:672–77. doi: 10.1111/j.1447-0594.2012.00962.x. [DOI] [PubMed] [Google Scholar]
- 51.Ganz PA, Bower JE, Kwan L, Castellon SA, Silverman DHS, Geist C, et al. Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun. 2013;30:S99–S108. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aluise CD, Sultana R, Tangpong J, Vore M, St. Clair D, Moscow JA, et al. Chemo Brain (Chemo Fog) as a potential side effect of doxorubicin administration: Role of cytokine-induced, oxidative/nitrosative stress in cognitive dysfunction. Adv Exp Med Biol. 2010;678:147–56. doi: 10.1007/978-1-4419-6306-2_19. [DOI] [PubMed] [Google Scholar]
- 53.Santilli F, Davì G, Patrono C. Homocysteine, methylenetetrahydrofolate reductase, folate status and atherothrombosis: A mechanistic and clinical perspective. Vascul Pharmacol. 2016;78:1–9. doi: 10.1016/j.vph.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Li H, Qin X, Xie D, Tang G, Zhang Y, Li J, et al. Effects of combined enalapril and folic acid therapy on the serum uric acid levels in hypertensive patients: a multicenter, randomized, double-blind, parallel-controlled clinical trial. Intern Med. 2015;54:17–24. doi: 10.2169/internalmedicine.54.2931. [DOI] [PubMed] [Google Scholar]
- 55.Choi HK, Ford ES. Prevalence of the Metabolic Syndrome in Individuals with Hyperuricemia. Am J Med. 2007;120:442–47. doi: 10.1016/j.amjmed.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 56.You L, Liu A, Wuyun G, Wu H, Wang P. Prevalence of hyperuricemia and the relationship between serum uric acid and metabolic syndrome in the Asian Mongolian area. J Atheroscler Thromb. 2014;21:355–65. doi: 10.5551/jat.20529. [DOI] [PubMed] [Google Scholar]
- 57.Sánchez-Lozada LG, Tapia E, Bautista-García P, Soto V, Ávila-Casado C, Vega-Campos IP, et al. Effects of febuxostat on metabolic and renal alterations in rats with fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2008;294:F710–F18. doi: 10.1152/ajprenal.00454.2007. [DOI] [PubMed] [Google Scholar]
- 58.Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44:642–50. [PubMed] [Google Scholar]
- 59.Lee JE, Kim YG, Choi YH, Huh W, Kim DJ, Oh HY. Serum uric acid is associated with microalbuminuria in prehypertension. Hypertension. 2006;47:962–67. doi: 10.1161/01.HYP.0000210550.97398.c2. [DOI] [PubMed] [Google Scholar]
- 60.Raffaitin C, Féart C, Le Goff M, Amieva H, Helmer C, Akbaraly TN, et al. Metabolic syndrome and cognitive decline in French elders: The Three-City Study. Neurology. 2011;76:518–25. doi: 10.1212/WNL.0b013e31820b7656. [DOI] [PubMed] [Google Scholar]
- 61.Feng L, Yap KB, Yeoh LY, Ng TP. Kidney function and cognitive and functional decline in elderly adults: Findings from the Singapore Longitudinal Aging Study. J Am Geriatr Soc. 2012;60:1208–14. doi: 10.1111/j.1532-5415.2012.04043.x. [DOI] [PubMed] [Google Scholar]
- 62.Lass P, Buscombe JR, Harber M, Davenport A, Hilson AJW. Cognitive impairment in patients with renal failure is associated with multiple-infarct dementia. Clin Nucl Med. 1999;24:561–65. doi: 10.1097/00003072-199908000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.