Abstract
As seen over the past 20 years, calprotectin has evolved as a novel, non-invasive biomarker of gastrointestinal (GI) inflammation. We present this review of calprotectin in pediatrics. This article will focus on studies using calprotectin concentrations from different body fluids to monitor inflammation in different disease states and conditions. The ultimate goal of our group is to lay down a foundation as we consider using calprotectin prospectively as a marker of intestinal inflammation that could lead to further testing and possibly a marker of preparedness for feeding. We surveyed all published studies in English of calprotectin in neonates, infants, children, and adolescents through February 2014. We will discuss calprotectin's basic properties and analysis such as characteristics, identification, presence in body fluids, and maturational development. In addition, calprotectin's use in inflammatory diseases exploring both GI and non-GI conditions will be evaluated and compared with other serum markers presently available. Finally, a summary of our findings and discussion of future work that could be undertaken in order to render calprotectin as a more useful monitoring tool to the medical research community will complete the review.
INDEX TERMS: biomarker, inflammation, leukocyte L1 antigen complex, pediatric
INTRODUCTION
It is well-known that inflammation, either locally in the gastrointestinal (GI) tract or systemically, is a key factor that adversely affects nutrition and metabolic outcomes in patients. The quest for biomarkers depicting this complex process in the human body has been the interest of a wide variety of groups. Moreover, inflammation has often been identified as one of the root-causes of several chronic disease affecting patients nowadays. Having a biomarker that could reliably be detected in serum/plasma and/or urine could alleviate some of the difficulties that invasive procedures demand on follow-up for these patients. This would reduce part of the financial burden on our healthcare system today. Emerging studies are demonstrating the utility of calprotectin, increasing its clinical application in GI as well as other inflammatory diseases.
Currently in practice, there are other laboratory markers used to assess systemic inflammation such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). Other less-commonly used protein markers that will be mentioned in this review are: lactoferrin, M2 isoform of pyruvate kinase (M2-PK), and intestinal-fatty acid binding protein (I-FABP). Lactoferrin is a 80-kDa iron-binding glycoprotein thought to be released by neutrophils, along with other host defense factors, and possess antimicrobial and anti-inflammatory properties.1 M2-PK is an important enzyme, present in all cells, whose one of its functions is to catalyze transphosphorylation as part of the last step of glycolysis.2 Its role in innate immunity was observed as neutrophils with deficient PK activity led to ineffective intracellular killing and therefore susceptibility to infections.3 I-FABP is 1 of 2 cytosolic fatty-acid binding proteins present in enterocytes, thought to be involved in fat transport across the digestive mucosa.4 Recent evidence has shown that rises in serum levels of I-FABP as well as FABPs from other tissue sources could be reflective of tissue injury, depending on site of origin.5 We speculate that calprotectin could provide greater sensitivity or specificity to distinguish inflammation, and be useful for practitioners as suggested by some studies herein.
BASIC PROPERTIES AND ANALYSIS
Identification and Presence in Body Fluids
Calprotectin is a 36-kDa protein, with 2 heavy and 1 light non-covalently linked chains that binds calcium and zinc (see Figure 1).6,7 First isolated in the 1970s, calprotectin is found in the cytosolic fluid of neutrophils, monocytes, and macrophages.8 Calprotectin has been referred to by several names: leukocyte derived (L1) protein,9 MIF (Migration Inhibitory Factor) related protein 8 and 14 (MRP-8/14),10 and cystic fibrosis antigen (CFA).11 Wilkinson et al12 determined the similarities between these aforementioned 3 entities and proposed the name calgranulin A and B due to its calcium-binding properties and its main source, granulocytes.12 The structure was further categorized and found to belong to the S100 family of proteins,13 S100A8/S100A9 specifically. The S100 family of proteins contains several EF-hand (α-helix-loop-α-helix) calcium-binding proteins.14 It has been shown that altered expression of these proteins plays key roles in neurodegenerative and inflammatory disorders.15 These alterations can occur intracellularly as well as extracellularly, particularly for S100A8/S100A9 and a related protein, recently discovered, S100A12. The current name, calprotectin, was suggested following reports of its antimicrobial activity against Enterobacteriaceae in blood cultures and Cryptococcus spp. in cerebrospinal fluid (CSF) isolates.16
Figure 1.
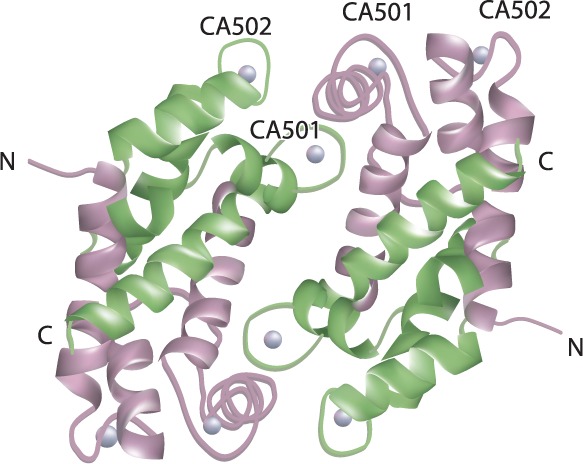
The heterotetramer structure of calprotectin.2 S100A8 chains (upper left); S100A9 chains (upper right); CA501 and CA502 sites where Ca2+ ions (spheres) bind. C and N denote protein termini (with permission from Elsevier)
Calprotectin has been detected in multiple types of body fluids, though most of the literature reporting content in fecal samples. Initially, as seen in the earlier studies, values were reported in μg/mL. With the optimization of fecal analytical kits, most of the literature currently reports on μg/gram of feces. This presumably was instituted in order to off-set the dilutional effects that diarrhea could have in a sample that was being quantified based on volume. Clinicians are advised to take into account the units of values reported, particularly in feces, when surveying the literature. Reference ranges have also been determined in serum/plasma. Data from 533 blood donors showed values of 0.09 to 0.53 mg/L and 0.12 to 0.66 mg/L for females and males, respectively.17 CSF calprotectin concentrations were 0.3 to 0.35 mg/L in human immunodeficiency virus (HIV)-infected patients with opportunistic infections compared to reference levels around 0.037 mg/L.18 Saliva samples from 12 healthy adults yielded mean levels of 3.2 mg/L from parotid saliva, 22.0 mg/L from stimulated whole saliva, and 40.9 mg/L from mucosal transudate.19 These findings highlighted the importance of sampling procedure and site of collection, and its likely role in host defense. Calprotectin concentrations in the urine of 7 patients with pyelonephritis was 1 mg/L compared to 0.024 mg/L in 63 healthy controls.20 Meconium from 131 neonates had a mean ± SD calprotectin concentration of 145 ± 78.5 μg/g.21 In patients with rheumatoid arthritis the median synovial fluid level was 18 mg/L compared to 0.9 mg/L in patients with osteoarthritis,22 suggesting it as a marker of local inflammation. Calprotectin has been isolated from almost every fluid possible in the human body as shown here and discussed in later sections. As the reader will encounter later on, investigators have mostly used fecal calprotectin (FC) concentrations to analyze and venture into other disease states, though clinical applications of calprotectin concentrations from other fluids have surfaced again. Research groups should still be cautious when assessing inflammatory status from raised calprotectin concentrations and determine if it depicts a generalized state or it only describes the local environment from which the sample was obtained.
Maturational Development
As more interest developed in calprotectin, researchers questioned if there were any age-dependent variations associated with its expression. Table 1 summarizes the studies that assessed FC concentrations in different pediatric age groups.23–26 Differences have been attributed to developmental factors among subjects such as gestational and/or postnatal age or feeding patterns with either breastmilk or formula. Zopelli et al27 associated FC values with postnatal and gestational age (GA) in 3 groups of premature infants. All groups experienced a decrease in FC levels in the first week of life; however, afterwards there was a statistically significant increase in those born between 26 and 32 weeks GA, compared to those born at less than 26 weeks GA who continued to decline. Likewise, a significant decrease was observed in FC concentrations of 52 preterm infants during the first week of life, which was followed by a significant increase over the next 7 weeks of life.28 Similar studies in comparable preterm infant cohorts did not support those results.29–31 Others thought differences, particularly in neonates and infants, may be explained by what was being fed to the gut. Savino et al32 analyzed FC from 39 healthy term infants receiving breast milk exclusively from their mothers. Median FC (range) was 555.00 (122.50–2000.00) μg/g in breast-fed compared to 206.60 (31.20–797.60) μg/g, (p < 0.001) of 35 formula-fed infants. This was contradictory to data from another group of 69 healthy term infants whose median FC was 167 μg/g.33 There were no significant associations whether the infant was fed breast-milk, standard or prebiotic formula.33 It begs the question “Can calprotectin be transmitted from mother to infant through breast milk?” To date no reports have been published of calprotectin being isolated from breast milk and/or colostrum. We hypothesize that there are many factors (Figure 2), yet to be fully elucidated, involved in the expression of calprotectin in the gut of early infants. If such factors create an effect, we should focus on investigating and determining new reference ranges for neonates/infants, premature and term, as well as older pediatric patients. Established references could help clinicians interpret calprotectin concentrations and be able to classify them as truly inflammatory, or just simply physiologic and part of ongoing development, much like bilirubin.
Table 1.
Pediatric Studies Analyzing Fecal Calprotectin by Age Group
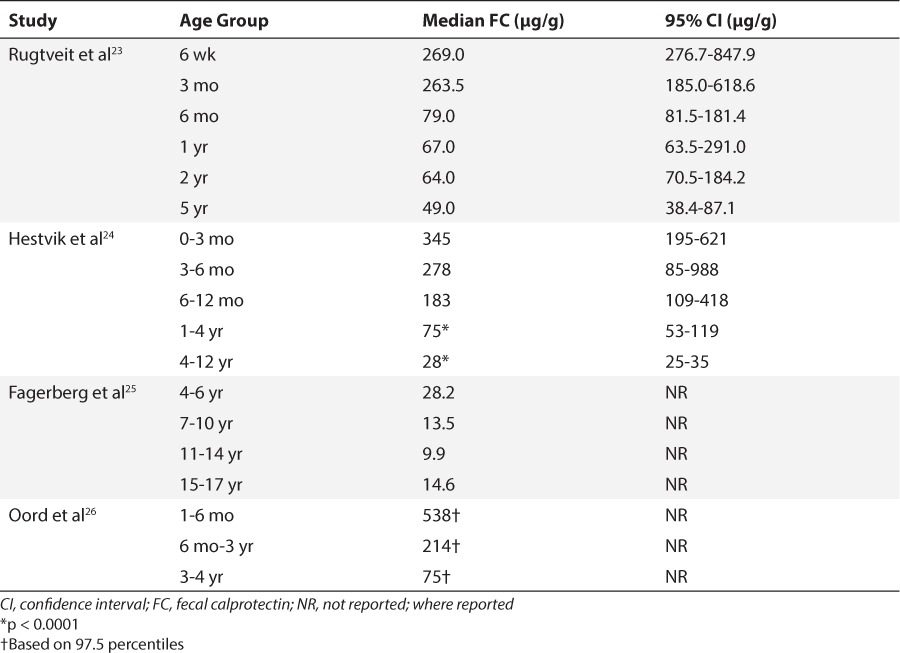
Figure 2.
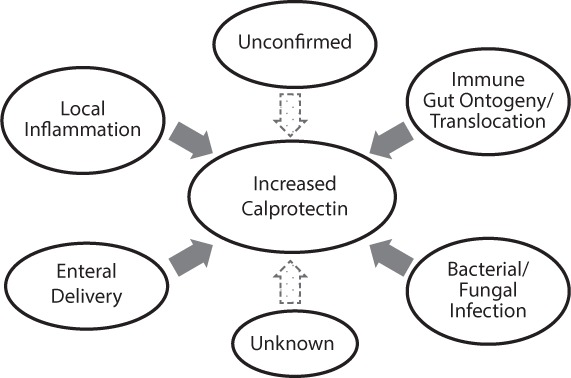
Factors possibly contributing to increased fecal expression of calprotectin in early infancy.
USE IN INFLAMMATORY DISEASES
A Marker of Inflammatory Bowel Disease
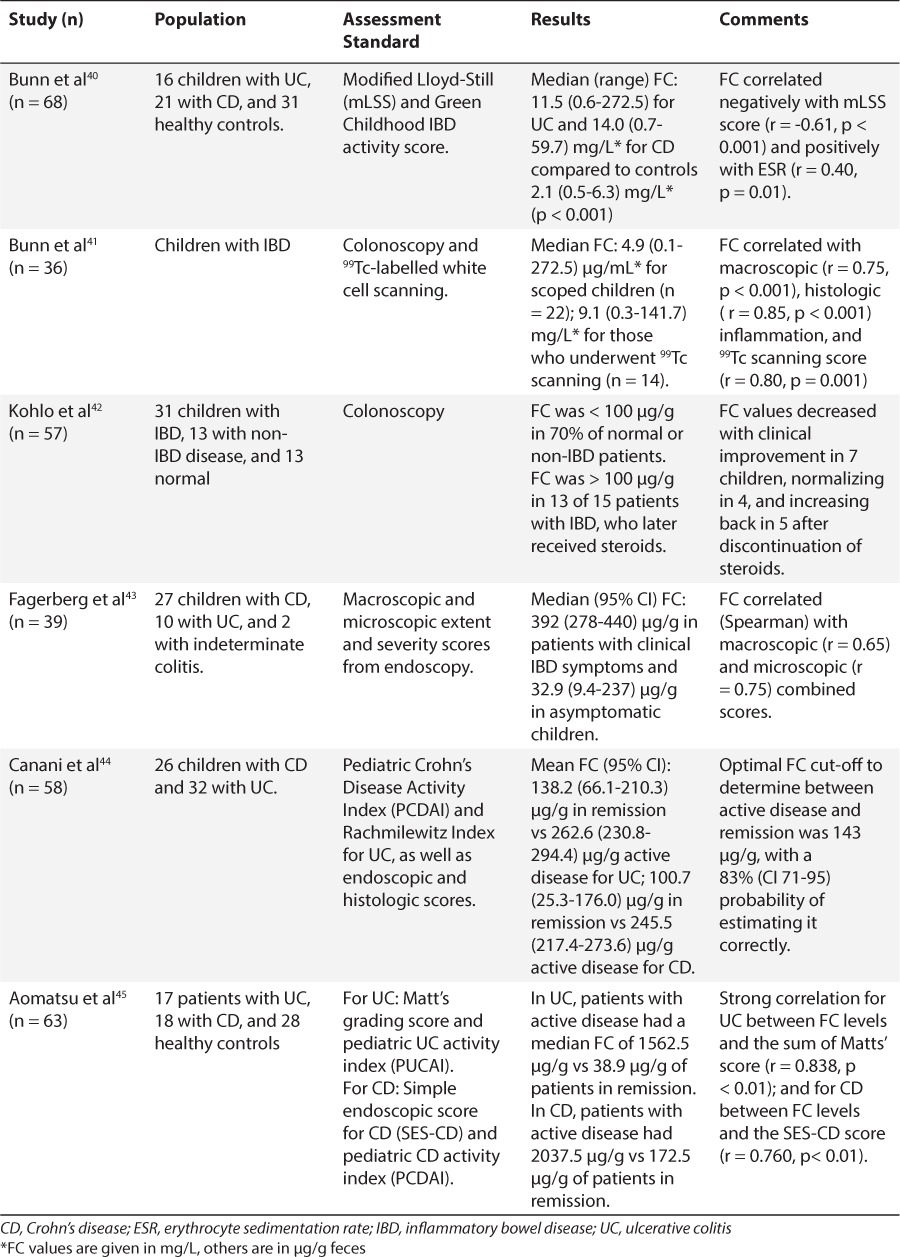
In the late 1990s to early 2000s, a few reports appeared in the literature, proposing the use of FC as a non-invasive marker of intestinal inflammation. Research focused initially in inflammatory bowel disease (IBD) and how calprotectin could be used to monitor disease activity. Up to that point inflammatory gut status could only be determined by histologic findings as a result of endoscopic procedures. Roseth et al34 studied 62 patients with ulcerative colitis (UC) undergoing colonoscopy. They reported median fecal concentrations of 68 mg/L, compared to 11.5 mg/L in those with low disease activity, and 6 mg/L in controls (p < 0.001).34 Similarly, Limburg et al35 found FC concentrations to be significantly associated with colorectal inflammation. As analytical assays were optimized, the application of this protein was tested in populations such as children, in whom invasive procedures are increasingly challenging. Table 2 shows select studies utilizing FC analysis in pediatric populations with suspected or soon after diagnosis of IBD.36–39 Table 3 lists pediatric studies in which FC was analyzed in patients with a long-standing diagnosis of IBD.40–45 Most of these research groups adopted the manufacturer's recommended cut-offs for normal FC, between 50 and 100 μg/g. These studies revealed substantial differences in FC concentrations between those with versus those without inflammation at the time of assessment. Even more, some of the studies hinted at establishing differences for the 2 main IBD types: UC and Crohn's disease (CD). Children with UC may exhibit higher fecal concentrations of calprotectin than children with CD depending on disease severity.46 Although there is conflicting evidence regarding which shows higher values given the different areas of involvement and invasiveness of the 2. A few investigators took a step forward to evaluate FC as a predictive marker of IBD relapse in children. Walkiewicz et al47 examined 32 children with IBD, 11 of those with CD. A FC value above 400 μg/g in asymptomatic CD patients, predicted relapse within 9 months of collection in 89% of patients (95% confidence interval [CI] 51.8–99.7, p = 0.03) compared to no clinical relapse within 9 months when FC values were below 400 μg/g.47 Similarly, van Rheenen48 reported that teenagers with a FC above 500 μg/g had a 53% (10/19) risk of progressing to symptomatic relapse within 3 months, whereas a value below 500 μg/g only had a 12% (5/43) risk of symptomatic relapse. A retrospective analysis of 73 children with IBD found that a FC concentration of 275 μg/g had sensitivity and negative predictive value (NPV) of 97% and specificity and a positive predictive value (PPV) of 97%, with a likelihood ratio of 6.4 for predicting IBD relapse.49 Here, we observe that investigators propose different cut-off values, as stated in several studies. The reason more than likely is that the stated value gave them the best sensitivity/specificity combination based on receiver-operator characteristic (ROC) curves. This can obviously create some confusion among clinicians as to which cut-off value they should use. In the meantime, clinicians would be advised to analyze intrapatient variability and have patients serve as their own controls to determine whether they are experiencing relapse or remission of their disease.
Table 2.
Fecal Calprotectin Pediatric Studies on Suspected or at Diagnosis of Inflammatory Bowel Disease
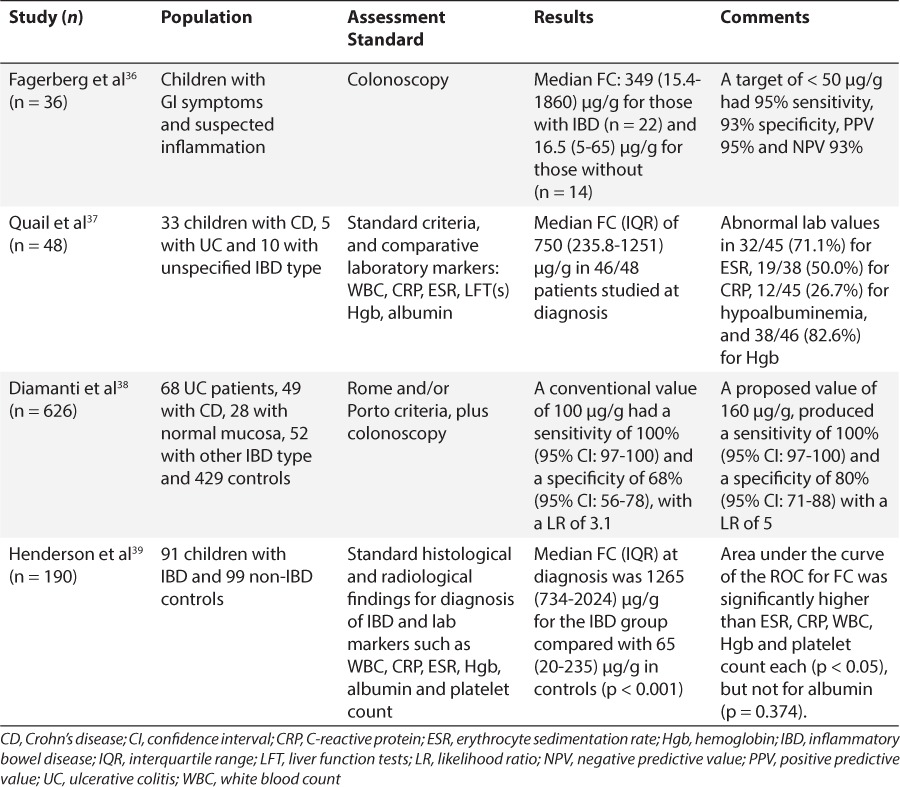
Table 3.
Fecal Calprotectin Pediatric Studies on Proven Inflammatory Bowel Disease
Additional studies have been published using FC in conjunction with other fecal protein markers, including other S100A proteins. Joishy et al50 reported a median FC of 251 μg/g for the active IBD group compared to 35.4 μg/g for a GI-control group and 14.1 μg/g for a non-GI control group (p < 0.001). Similar significant differences were established between the groups when fecal lactoferrin was used.50 M2-pyruvate kinase (M2-PK) is readily expressed in rapidly-dividing cells,51 which could be a helpful monitoring tool during active IBD disease due to increased GI mucosal-cell turnover. Low M2-PK expression could potentially mean flare-up resolution. Czub et al52 reported abnormal M2-PK levels in 49 of 75 UC patients and in 27 of 32 CD patients. Though in later studies, they were not able to demonstrate superiority in differentiating disease severity and remission compared with FC.53 Turner et al54 studied both of them used together in 101 children with UC. Their baseline fecal M2-PK and FC were significantly lower in responders to steroid treatment compared to those who did not.54 No differences were observed with fecal lactoferrin and fecal S100A12.54 Investigators have also evaluated S100A12, which elicits transient neutrophil infiltration and delayed monocyte recruitment. This response occurs as a result of gene up-regulation by known inflammatory cytokine, tumor necrosis factor-alpha (TNF-α), involved in the pathogenesis of IBD.55 Fecal S100A12 (median 55.2 μg/g) and FC of (median 1265 μg/g) in 31 children with IBD were higher compared to fecal S100A12 (median 1.1 μg/g) and FC (median 30.5 μg/g) in 30 children without IBD (p < 0.0001).56
In Other GI Diseases
Calprotectin became an acceptable marker of GI inflammation resulting from IBD. Investigators speculated about its use in detection of other acute and/or chronic inflammatory diseases. Limburg et al35 examined 110 subjects with chronic diarrhea referred for colonoscopy and found a median FC of 378 μg/g for subjects with colorectal inflammation, which were later diagnosed with collagenous or eosinophilic colitis among others; as opposed to a median of 31 μg/g for those who did not (p = 0.0001).35 Another study demonstrated that a FC cut-off of 50 μg/g had 64% sensitivity and 80% specificity with 70% positive and 74% NPVs in 70 adults; and 70% sensitivity, 93% specificity with 96% positive and 56% NPVs in 50 children (<18 years) with chronic diarrhea.57 Calprotectin was also used to distinguish between children with constitutive enterocyte disorders, such as epithelial dysplasia (ED) and microvillus atrophy (MVA). These children had fecal concentrations consistent with reports in similar age cohorts.23,24 Their values were either < 50 μg/g or undetectable, versus other immune-inflammatory disorders where the observed median (range) FC was 1145 (375–3095) μg/g at the onset of severe diarrhea, p < 0.01.58 In celiac disease, untreated adults were shown to have comparable FC (45.02 ± 24.18 μg/g), while healthy controls had values of 36.51 ± 21.67 μg/g.59 The application of calprotectin in celiac disease might be better suited for children and those receiving a specialized diet. There was a significant statistical difference in FC concentrations between newly diagnosed, untreated children and those receiving a gluten-free diet (GFD) for 1 year, while there was none between those receiving a GFD and healthy children.60,61 Another disease in which FC was used to monitor the efficacy of a change in diet is cow's milk protein (CMP) allergy. Beser et al62 evaluated 24 with Ig-E mediated and 8 with non-Ig-E mediated CMP allergic children. FC values were 392 ± 209 μg/g and 886 ± 278 μg/g before CMP elimination diet; and 218 ± 90 μg/g and 359 ± 288 μg/g after CMP elimination diet, respectively (p = 0.001 and p = 0.025). Chen et al63 evaluated FC's diagnostic value in predicting bacterial from viral infectious diarrhea in 153 children. The median (range) FC level was higher in patients with Salmonella infection, 765 (252–1246) μg/g, or Campylobacter infection, 689 (307–1046) μg/g; compared to patients with rotavirus infection 89 (11–426) μg/g, norovirus infection 93 (25–405) μg/g, or adeno-virus infection 95 (65–224) μg/g, p < 0.05. Even though there is some overlap with the ranges, it appears that FC expression would be higher as a result of bacterial compared to viral infections.
Particular interest was taken in the infant population as Campeotto et al29 showed calprotectin could be used as an acute marker of intestinal distress. Such distress was defined as GI bleeding, diarrhea with liquid stools, and/or abdominal distention. They reported decreased FC levels a week before onset and following a GI episode.29 Yang et al31 detected differences in mean FC ± SD levels between infants classified as “not sick” and as “sick.” They defined “sick” as those being evaluated for sepsis, requiring antibiotics or vasopressors, withholding enteral feeds, or requiring increased ventilator support. “Sick” infants showed higher FC concentrations 380.4 ± 246.3 μg/g, versus “not sick”, 122.8 ± 98.9 μg/g, (p < 0.001).31 These features could be useful for pediatric clinicians in determining temporal relations with disease causes at initial patient assessment. Calprotectin could also contribute to further understanding of disease severity or progression in other diseases that present with an inflammatory picture, such as necrotizing enterocolitis (NEC). Seven preterm infants with NEC showed higher mean ± SD FC at diagnosis 288.4 ± 49.1 mg/L compared to healthy matched controls, 98 ± 60.6 mg/L (p = 0.0006).64 Thirty-one neonates with mild enteropathy displayed median (range) FCs of 393 (52–996) μg/g compared to those with severe enteropathy, 832 (168–4775) μg/g, including patients with NEC Stage IIb and III.65 These results are consistent with those from recent studies that observed higher FC concentrations in preterm infants with NEC compared to controls66 and were linked with disease severity.67 I-FABP may be another marker that correlates well with extent of disease and mucosal damage in NEC.68,69 Recently, a combination of urinary I-FABP and FC provided a sensitivity of 94%, a specificity of 79%, a positive likelihood ratio of 4.48, and a negative likelihood ratio of 0.08, for diagnosing NEC in a cohort of neonates.70 In that case, those investigators looked to improve I-FABP's ability to diagnose NEC by adding FC assessment, as I-FABP alone had not been definitive in previous analyses of their cohort.71 FC was also analyzed to establish a possible relationship between gut inflammation and linear growth in 144 urban and rural Chinese infants. The median FC level was significantly higher in rural versus urban infants, 420.9 fig/g versus 140.1 fig/g (p < 0.0001), respectively, though there was wide variability observed in the ranges.72 Those authors pointed out a significant inverse relationship: for an increase of 100 μg/g in FC, there was an associated decrease of 0.06 in length-for-age Z-score. This could hint to an explanation of those yet to be found relationships, proposed in Figure 2. Relationships between raised calprotectin concentrations expressed initially in the gut, and inflammation, either locally or systemically, could affect outcomes such as growth and/or recovery from disease. FC has not been a useful marker with other infant GI illnesses including infantile colic, transient lactose intolerance, constipation, functional gastrointestinal disorders (FGID), or with small intestine bacterial overgrowth (SIBO).73–76
Applications in Other Diseases
The use of calprotectin as a possible screening or monitoring tool has paved its way into other disease states. Early on, it was suggested as a marker of inflammation in patients with cystic fibrosis (CF) who had median concentrations of 0.727 mg/L compared to controls with median 1.832 mg/L, p < 0.001, though exhibiting considerable overlap.77 More recently, calprotectin has been analyzed to monitor decreasing inflammation resulting from therapy after an acute exacerbation.78 In preeclampsia, Braekke et al79 examined 62 pregnant women reporting median maternal plasma concentrations were higher in the preeclampsia group compared with the control group (1.08 mg/L versus 0.55 mg/L, p < 0.001); others reported similar trends.80 Forty-one children with different types of juvenile idiopathic arthritis (JIA), unrelated connective tissue disorders (CTDs), or otherwise healthy were evaluated to see if FC could be used to assess subclinical gut inflammation.81 Median levels were highest in the children with arthritis, and lowest in the CTD controls.81 In a cohort of 74 adults and children undergoing intestinal transplant, more variance was seen in FC during rejection than in non-rejection.82 Serum calprotectin has been used in children with acute Kawasaki's disease as a monitoring tool to assess response to intravenous immunoglobulin (IVIG) therapy, and possibly lead to identification of those at risk of developing coronary injury.83,84 The ability to monitor inflammation is key, whether before or after a major intervention. Plasma calprotectin revealed a hazard ratio of 1.26, relative risk of mortality with increasing levels, in patients that had suffered an ST segment elevation myocardial infarction (MI) and had undergone successful percutaneous coronary intervention followed up to a year.85 Furthermore, one research group assessed serum and urine measurements of calprotectin in an obese population and its possible associations with insulin resistance and Type-II diabetes. Mean ± SD plasma calprotectin was significantly increased (p < 0.0001) in obese (131.4 ± 63.7 μg/L) compared with non-obese subjects (102.8 ± 71.7 μg/L).82 Hestvik et al86 determined reference values for children infected with HIV and undertaking highly active antiretroviral therapy for the first time. Median FC was 208 μg/g in infants 0 to 1 year, 171 μg/g among toddlers 1 to 4 years, and 62 μg/g for children 4 to 12 years, while also noticing that children with advanced disease and a low CD4 cell percentage had significantly higher median (range) FC concentrations, 203 (143–277) μg/g than those with a high CD4 cell percentage 99 (62–154) μg/g (p < 0.05). Muller et al87 reported that low maximal responses in serum calprotectin during zidovudine therapy were associated with short survival in 51 HIV patients. They also noticed inverse relationships between serum calprotectin and CD4 counts above 50 × 106/L, showing similar trends to the fecal data. In a state of generalized inflammation caused by disease progression, perhaps calprotectin can be found in multiple body fluids.
Comparison to Other Serum/Plasma Markers
CRP and ESR are examples of other markers that alert clinicians of ongoing inflammatory processes in the body. Gray et al88 analyzed serum and sputum samples during CF exacerbation and noticed that serum calprotectin predicted median time to exacerbation significantly better than CRP. In patients with rheumatic disease, calprotectin concentrations, but not CRP nor ESR, were significantly lower in those with no swollen joints compared to those with 1 or more swollen joints (2.614 mg/L versus 6.287 mg/L, p < 0.001).89 Similarly, calprotectin was the first to normalize, compared to CRP and ESR, in patients with reactive arthritis.90 In patients with JIA, it was shown to be a better diagnostic marker91 and predictor of response to methotrexate therapy.92 Terrin et al93 evaluated serum calprotectin as a diagnostic marker for sepsis in neonates. Mean ± SD serum concentrations were significantly higher (p < 0.001) in 62 newborns with confirmed sepsis (3.1 ± 1.0 mg/L) than in either 29 non-infected subjects (1.1 ± 0.3 mg/L) or 110 healthy controls (0.91 ± 0.58 mg/L); while showing greater sensitivity, 89%, and specificity, 96%, than common laboratory markers, such as white blood cell count (WBC) and CRP. Other investigations have been reported in the literature evaluating calprotectin concentrations in patients with appendicitis,94,95 congestive heart failure,96 and others.97–101 Results did not show significant clinical impact other than increased levels compared to control groups. We speculate that part of the reason why some of these studies have not shown greater merit or have not been followed up with additional investigations is that there has not been the same degree of development, in terms of performance of analytical kits, for serum/plasma and/or urine samples compared to fecal specimens.
SUMMARY AND FUTURE RESEARCH
In conclusion, calprotectin has been validated as a non-invasive marker of local GI inflammation in patients with IBD. Other protein markers discussed in this review could still be in their developmental phases or only available in large, research-driven facilities. Studies show potential for calprotectin to be used as a tool in other disease states that present with an inflammatory component. The majority of the medical community has relied on fecal samples to monitor this protein, though isolation from other body fluids appears feasible. Since there are perceived age-dependent variations in the expression of calprotectin, methods should be re-evaluated, as far as dilution technique and sample preparation, when handling pediatric as opposed to adult samples. Considering the manufacturer-reported stability of calprotectin in feces, it may serve better to utilize such sampling method for more stable patients or for those with routine follow-up. The utility of calprotectin in the clinical arena could be enhanced with development of assays that can reliably quantify it in serum/plasma or urine. This could prove extremely beneficial to clinicians when assessing more acutely-ill, hospitalized patients. Subsequent studies will be required to validate analytical methods extracting calprotectin from sources other than stool in order to show this protein can be an effective monitoring tool both in the in-patient and out-patient setting.
Abbreviations
- CD
Crohn's disease
- CF
cystic fibrosis
- CFA
cystic fibrosis antigen
- CI
confidence interval
- CMP
cow's milk protein
- CRP
C-reactive protein
- CSF
cerebrospinal fluid
- CTD
connective tissue disorder
- ED
epithelial dysplasia
- ESR
erythrocyte sedimentation rate
- FC
fecal calprotectin
- FGID
functional gastrointestinal disorders
- GA
gestational age
- GFD
gluten-free diet
- GI
gastrointestinal
- Hgb
hemoglobin
- HIV
human immunodeficiency virus
- IBD
inflammatory bowel disease
- I-FABP
intestinal-fatty acid binding protein
- IQR
interquartile range
- IVIG
intravenous immunoglobulin
- JIA
juvenile idiopathic arthritis
- L1
leukocyte-derived protein
- LFT
liver function tests
- LR
likelihood ratio
- M2-PK
M2-pyruvate kinase
- MI
myocardial infarction
- MRP 8/14
migration inhibitory factor related protein
- MVA
microvillus atrophy
- NEC
necrotizing enterocolitis
- NPV
negative predictive value
- PPV
positive predictive value
- ROC
receiver-operator characteristic
- SIBO
small intestine bacterial overgrowth
- TNF-α
tumor necrosis factor alpha
- UC
ulcerative colitis
- WBC
white blood count
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Vogel HJ. Lactoferrin, a bird's eye view. Biochem Cell Biol. 2012;90(3):233–244. doi: 10.1139/o2012-016. [DOI] [PubMed] [Google Scholar]
- 2.Gupta V, Bamezai RN. Human pyruvate kinase M2: a multifunctional protein. Protein Sci. 2010;19(11):2031–2044. doi: 10.1002/pro.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burge PS, Johnson WS, Hayward AR. Neutrophil pyruvate kinase deficiency with recurrent staphylococcal infections: first reported case. Br Med J. 1976;1(6012):742–745. doi: 10.1136/bmj.1.6012.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montoudis A, Delvin E, Menard D et al. Intestinal-fatty acid binding protein and lipid transport in human intestinal epithelial cells. Biochem Biophys Res Commun. 2006;339(1):248–254. doi: 10.1016/j.bbrc.2005.10.202. [DOI] [PubMed] [Google Scholar]
- 5.Pelsers MM, Hermens WT, Glatz JF. Fatty acid-binding proteins as plasma markers of tissue injury. Clin Chim Acta. 2005;352(1–2):15–35. doi: 10.1016/j.cccn.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Johne B, Fagerhol MK, Lyberg T et al. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol Pathol. 1997;50(3):113–123. doi: 10.1136/mp.50.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korndorfer IP, Brueckner F, Skerra A. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting alpha-helices can determine specific association of two EF-hand proteins. J Mol Biol. 2007;370(5):887–898. doi: 10.1016/j.jmb.2007.04.065. [DOI] [PubMed] [Google Scholar]
- 8.Fagerhol MK. Nomenclature for proteins: is calprotectin a proper name for the elusive myelomonocytic protein? Clin Mol Pathol. 1996;49(2):M74–M79. doi: 10.1136/mp.49.2.m74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dale I, Fagerhol MK, Frigard M. Quantitation of a highly immunogenic leukocyte antigen (L1) by radioimmunoassay: methodological evaluation. J Immunol Methods. 1983;65(1–2):245–255. doi: 10.1016/0022-1759(83)90321-6. [DOI] [PubMed] [Google Scholar]
- 10.Odink K, Cerletti N, Bruggen J et al. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987;330(6143):80–82. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- 11.Wilson GB, Jahn TL, Fonseca JR. Demonstration of serum protein differences in cystic fibrosis by isoelectric focusing in thin-layer polyacrylamide gels. Clin Chim Acta. 1973;49(1):79–91. doi: 10.1016/0009-8981(73)90346-x. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson MM, Busuttil A, Hayward C et al. Expression pattern of two related cystic fibrosis-associated calcium-binding proteins in normal and abnormal tissues. J Cell Sci. 1988;91(pt 2):221–230. doi: 10.1242/jcs.91.2.221. [DOI] [PubMed] [Google Scholar]
- 13.Freemont P, Hogg N, Edgeworth J. Sequence identity. Nature. 1989;339(6225):516. doi: 10.1038/339516b0. [DOI] [PubMed] [Google Scholar]
- 14.Meijer B, Gearry RB, Day AS. The role of S100A12 as a systemic marker of inflammation. Int J Inflam. 2012;2012:907078. doi: 10.1155/2012/907078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cmoch A, Groves P, Palczewska M et al. S100A proteins in propagation of a calcium signal in norm and pathology. Postepy Biochem. 2012;58(4):429–436. [PubMed] [Google Scholar]
- 16.Steinbakk M, Naess-Andresen CF, Lingaas E et al. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990;336(8718):763–765. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- 17.Dale I. Plasma levels of the calcium-binding L1 leukocyte protein: standardization of blood collection and evaluation of reference intervals in healthy controls. Scand J Clin Lab Invest. 1990;50(8):837–841. doi: 10.3109/00365519009104950. [DOI] [PubMed] [Google Scholar]
- 18.Dunlop O, Bruun JN, Myrvang B et al. Calprotectin in cerebrospinal fluid of the HIV infected: a diagnostic marker of opportunistic central nervous system infection? Scand J Infect Dis. 1991;23(6):687–689. doi: 10.3109/00365549109024294. [DOI] [PubMed] [Google Scholar]
- 19.Cuida M, Brun JG, Tynning T et al. Calprotectin levels in oral fluids: the importance of collection site. Eur J Oral Sci. 1995;103(1):8–10. doi: 10.1111/j.1600-0722.1995.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 20.Holt J, Fagerhol MK, Dale I. Quantitation of a leukocyte protein (L1) in urine. Acta Paediatr Scand. 1983;72(4):615–616. doi: 10.1111/j.1651-2227.1983.tb09781.x. [DOI] [PubMed] [Google Scholar]
- 21.Laforgia N, Baldassarre ME, Pontrelli G et al. Calprotectin levels in meconium. Acta Paediatr. 2003;92(4):463–466. doi: 10.1111/j.1651-2227.2003.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 22.Berntzen HB, Olmez U, Fagerhol MK et al. The leukocyte protein L1 in plasma and synovial fluid from patients with rheumatoid arthritis and osteoarthritis. Scand J Rheumatol. 1991;20(2):74–82. doi: 10.3109/03009749109165280. [DOI] [PubMed] [Google Scholar]
- 23.Rugtveit J, Fagerhol MK. Age-dependent variations in fecal calprotectin concentrations in children. J Pediatr Gastroenterol Nutr. 2002;34(3):323–324. doi: 10.1097/00005176-200203000-00022. author reply 324–325. [DOI] [PubMed] [Google Scholar]
- 24.Fagerberg UL, Loof L, Merzoug RD et al. Fecal calprotectin levels in healthy children studied with an improved assay. J Pediatr Gastroenterol Nutr. 2003;37(4):468–472. doi: 10.1097/00005176-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Hestvik E, Tumwine JK, Tylleskar T et al. Faecal calprotectin concentrations in apparently healthy children aged 0–12 years in urban Kampala, Uganda: a community-based survey. BMC Pediatr. 2011;11:9. doi: 10.1186/1471-2431-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oord T, Hornung N. Fecal calprotectin in healthy children. Scand J Clin Lab Invest. 2014;74(3):254–258. doi: 10.3109/00365513.2013.879732. [DOI] [PubMed] [Google Scholar]
- 27.Zoppelli L, Guttel C, Bittrich HJ et al. Fecal calprotectin concentrations in premature infants have a lower limit and show postnatal and gestational age dependence. Neonatology. 2012;102(1):68–74. doi: 10.1159/000337841. [DOI] [PubMed] [Google Scholar]
- 28.Josefsson S, Bunn SK, Domellof M. Fecal calprotectin in very low birth weight infants. J Pediatr Gastroenterol Nutr. 2007;44(4):407–413. doi: 10.1097/MPG.0b013e3180320643. [DOI] [PubMed] [Google Scholar]
- 29.Campeotto F, Kalach N, Lapillonne A et al. Time course of faecal calprotectin in preterm newborns during the first month of life. Acta Paediatr. 2007;96(10):1531–1533. doi: 10.1111/j.1651-2227.2007.00457.x. [DOI] [PubMed] [Google Scholar]
- 30.Rouge C, Butel MJ, Piloquet H et al. Fecal calprotectin excretion in preterm infants during the neonatal period. PLoS One. 2010;5(6):e11083. doi: 10.1371/journal.pone.0011083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Q, Smith PB, Goldberg RN et al. Dynamic change of fecal calprotectin in very low birth weight infants during the first month of life. Neonatology. 2008;94(4):267–271. doi: 10.1159/000151645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savino F, Castagno E, Viola S. Fecal calprotectin in infants with presumptive allergic colitis. J Pediatr. 2010;157(1):174. doi: 10.1016/j.jpeds.2010.03.010. author reply 174–175. [DOI] [PubMed] [Google Scholar]
- 33.Campeotto F, Butel MJ, Kalach N et al. High faecal calprotectin concentrations in newborn infants. Arch Dis Child Fetal Neonatal Ed. 2004;89(4):F353–F355. doi: 10.1136/adc.2002.022368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roseth AG, Aadland E, Jahnsen J et al. Assessment of disease activity in ulcerative colitis by faecal calprotectin, a novel granulocyte marker protein. Digestion. 1997;58(2):176–180. doi: 10.1159/000201441. [DOI] [PubMed] [Google Scholar]
- 35.Limburg PJ, Ahlquist DA, Sandborn WJ et al. Fecal calprotectin levels predict colorectal inflammation among patients with chronic diarrhea referred for colonoscopy. Am J Gastroenterol. 2000;95(10):2831–2837. doi: 10.1111/j.1572-0241.2000.03194.x. [DOI] [PubMed] [Google Scholar]
- 36.Fagerberg UL, Loof L, Myrdal U et al. Colorectal inflammation is well predicted by fecal calprotectin in children with gastrointestinal symptoms. J Pediatr Gastroenterol Nutr. 2005;40(4):450–455. doi: 10.1097/01.mpg.0000154657.08994.94. [DOI] [PubMed] [Google Scholar]
- 37.Quail MA, Russell RK, Van Limbergen JE et al. Fecal calprotectin complements routine laboratory investigations in diagnosing childhood inflammatory bowel disease. Inflamm Bowel Dis. 2009;15(5):756–759. doi: 10.1002/ibd.20820. [DOI] [PubMed] [Google Scholar]
- 38.Diamanti A, Panetta F, Basso MS et al. Diagnostic work-up of inflammatory bowel disease in children: the role of calprotectin assay. Inflamm Bowel Dis. 2010;16(11):1926–1930. doi: 10.1002/ibd.21257. [DOI] [PubMed] [Google Scholar]
- 39.Henderson P, Casey A, Lawrence SJ et al. The diagnostic accuracy of fecal calprotectin during the investigation of suspected pediatric inflammatory bowel disease. Am J Gastroenterol. 2012;107(6):941–949. doi: 10.1038/ajg.2012.33. [DOI] [PubMed] [Google Scholar]
- 40.Bunn SK, Bisset WM, Main MJ et al. Fecal calprotectin as a measure of disease activity in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;32(2):171–177. doi: 10.1097/00005176-200102000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Bunn SK, Bisset WM, Main MJ et al. Fecal calprotectin: validation as a noninvasive measure of bowel inflammation in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;33(1):14–22. doi: 10.1097/00005176-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Kolho KL, Raivio T, Lindahl H et al. Fecal calprotectin remains high during glucocorticoid therapy in children with inflammatory bowel disease. Scand J Gastroenterol. 2006;41(6):720–725. doi: 10.1080/00365520500419623. [DOI] [PubMed] [Google Scholar]
- 43.Fagerberg UL, Loof L, Lindholm J et al. Fecal calprotectin: a quantitative marker of colonic inflammation in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;45(4):414–420. doi: 10.1097/MPG.0b013e31810e75a9. [DOI] [PubMed] [Google Scholar]
- 44.Canani RB, Terrin G, Rapacciuolo L et al. Faecal calprotectin as reliable non-invasive marker to assess the severity of mucosal inflammation in children with inflammatory bowel disease. Dig Liver Dis. 2008;40(7):547–553. doi: 10.1016/j.dld.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 45.Aomatsu T, Yoden A, Matsumoto K et al. Fecal calprotectin is a useful marker for disease activity in pediatric patients with inflammatory bowel disease. Dig Dis Sci. 2011;56(8):2372–2377. doi: 10.1007/s10620-011-1633-y. [DOI] [PubMed] [Google Scholar]
- 46.Komraus M, Wos H, Wiecek S et al. Usefulness of faecal calprotectin measurement in children with various types of inflammatory bowel disease. Mediators Inflamm. 2012;2012:608249. doi: 10.1155/2012/608249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walkiewicz D, Werlin SL, Fish D et al. Fecal calprotectin is useful in predicting disease relapse in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2008;14(5):669–673. doi: 10.1002/ibd.20376. [DOI] [PubMed] [Google Scholar]
- 48.van Rheenen PF. Role of fecal calprotectin testing to predict relapse in teenagers with inflammatory bowel disease who report full disease control. Inflamm Bowel Dis. 2012;18(11):2018–2025. doi: 10.1002/ibd.22896. [DOI] [PubMed] [Google Scholar]
- 49.Diamanti A, Colistro F, Basso MS et al. Clinical role of calprotectin assay in determining histological relapses in children affected by inflammatory bowel diseases. Inflamm Bowel Dis. 2008;14(9):1229–1235. doi: 10.1002/ibd.20472. [DOI] [PubMed] [Google Scholar]
- 50.Joishy M, Davies I, Ahmed M et al. Fecal calprotectin and lactoferrin as noninvasive markers of pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2009;48(1):48–54. doi: 10.1097/MPG.0b013e31816533d3. [DOI] [PubMed] [Google Scholar]
- 51.Mazurek S, Grimm H, Oehmke M et al. Tumor M2-PK and glutaminolytic enzymes in the metabolic shift of tumor cells. Anticancer Res. 2000;20(6D):5151–5154. [PubMed] [Google Scholar]
- 52.Czub E, Herzig KH, Szafiarska-Popawska A et al. Fecal pyruvate kinase: a potential new marker for intestinal inflammation in children with inflammatory bowel disease. Scand J Gastroenterol. 2007;42(10):1147–1150. doi: 10.1080/00365520701320513. [DOI] [PubMed] [Google Scholar]
- 53.Czub E, Nowak JK, Szafiarska-Poplawska A et al. Comparison of fecal pyruvate kinase isoform M2 and calprotectin in assessment of pediatric inflammatory bowel disease severity and activity. Acta Biochim Pol. 2014;61(1):99–102. [PubMed] [Google Scholar]
- 54.Turner D, Leach ST, Mack D et al. Faecal calprotectin, lactoferrin, M2-pyruvate kinase and S100A12 in severe ulcerative colitis: a prospective multicentre comparison of predicting outcomes and monitoring response. Gut. 2010;59(9):1207–1212. doi: 10.1136/gut.2010.211755. [DOI] [PubMed] [Google Scholar]
- 55.Yang Z, Tao T, Raftery MJ et al. Proinflammatory properties of the human S100 protein S100A12. J Leukoc Biol. 2001;69(6):986–994. [PubMed] [Google Scholar]
- 56.Sidler MA, Leach ST, Day AS. Fecal S100A12 and fecal calprotectin as noninvasive markers for inflammatory bowel disease in children. Inflamm Bowel Dis. 2008;14(3):359–366. doi: 10.1002/ibd.20336. [DOI] [PubMed] [Google Scholar]
- 57.Carroccio A, Iacono G, Cottone M et al. Diagnostic accuracy of fecal calprotectin assay in distinguishing organic causes of chronic diarrhea from irritable bowel syndrome: a prospective study in adults and children. Clin Chem. 2003;49(6 pt 1):861–867. doi: 10.1373/49.6.861. [DOI] [PubMed] [Google Scholar]
- 58.Kapel N, Roman C, Caldari D et al. Fecal tumor necrosis factor-alpha and calprotectin as differential diagnostic markers for severe diarrhea of small infants. J Pediatr Gastroenterol Nutr. 2005;41(4):396–400. doi: 10.1097/01.mpg.0000178437.87546.06. [DOI] [PubMed] [Google Scholar]
- 59.Montalto M, Santoro L, Curigliano V et al. Faecal calprotectin concentrations in untreated coeliac patients. Scand J Gastroenterol. 2007;42(8):957–961. doi: 10.1080/00365520601173632. [DOI] [PubMed] [Google Scholar]
- 60.Ertekin V, Selimoglu MA, Turgut A et al. Fecal calprotectin concentration in celiac disease. J Clin Gastroenterol. 2010;44(8):544–546. doi: 10.1097/MCG.0b013e3181cadbc0. [DOI] [PubMed] [Google Scholar]
- 61.Balamtekin N, Baysoy G, Uslu N et al. Fecal calprotectin concentration is increased in children with celiac disease: relation with histopathological findings. Turk J Gastroenterol. 2012;23(5):503–508. doi: 10.4318/tjg.2012.0366. [DOI] [PubMed] [Google Scholar]
- 62.Beser OF, Sancak S, Erkan T et al. Can fecal calprotectin level be used as a markers of inflammation in the diagnosis and follow-up of cow's milk protein allergy? Allergy Asthma Immunol Res. 2014;6(1):33–38. doi: 10.4168/aair.2014.6.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen CC, Huang JL, Chang CJ et al. Fecal calprotectin as a correlative marker in clinical severity of infectious diarrhea and usefulness in evaluating bacterial or viral pathogens in children. J Pediatr Gastroenterol Nutr. 2012;55(5):541–547. doi: 10.1097/MPG.0b013e318262a718. [DOI] [PubMed] [Google Scholar]
- 64.Carroll D, Corfield A, Spicer R et al. Faecal calprotectin concentrations and diagnosis of necrotising enterocolitis. Lancet. 2003;361(9354):310–311. doi: 10.1016/S0140-6736(03)12333-1. [DOI] [PubMed] [Google Scholar]
- 65.Campeotto F, Baldassarre M, Butel MJ et al. Fecal calprotectin: cutoff values for identifying intestinal distress in preterm infants. J Pediatr Gastroenterol Nutr. 2009;48(4):507–510. doi: 10.1097/MPG.0b013e318186c4a6. [DOI] [PubMed] [Google Scholar]
- 66.Aydemir O, Aydemir C, Sarikabadayi YU et al. Fecal calprotectin levels are increased in infants with necrotizing enterocolitis. J Matern Fetal Neonatal Med. 2012;25(11):2237–2241. doi: 10.3109/14767058.2012.684172. [DOI] [PubMed] [Google Scholar]
- 67.Aydemir G, Cekmez F, Tanju IA et al. Increased fecal calprotectin in preterm infants with necrotizing enterocolitis. Clin Lab. 2012;58(7–8):841–844. [PubMed] [Google Scholar]
- 68.Evennett NJ, Hall NJ, Pierro A et al. Urinary intestinal fatty acid-binding protein concentration predicts extent of disease in necrotizing enterocolitis. J Pediatr Surg. 2010;45(4):735–740. doi: 10.1016/j.jpedsurg.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 69.Aydemir C, Dilli D, Oguz SS et al. Serum intestinal fatty acid binding protein level for early diagnosis and prediction of severity of necrotizing enterocolitis. Early Hum Dev. 2011;87(10):659–661. doi: 10.1016/j.earlhumdev.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 70.Reisinger KW, Van der Zee DC, Brouwers HA et al. Noninvasive measurement of fecal calprotectin and serum amyloid A combined with intestinal fatty acid-binding protein in necrotizing enterocolitis. J Pediatr Surg. 2012;47(9):1640–1645. doi: 10.1016/j.jpedsurg.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 71.Thuijls G, Derikx JP, van Wijck K et al. Non-invasive markers for early diagnosis and determination of the severity of necrotizing enterocolitis. Ann Surg. 2010;251(6):1174–1180. doi: 10.1097/SLA.0b013e3181d778c4. [DOI] [PubMed] [Google Scholar]
- 72.Liu JR, Sheng XY, Hu YQ et al. Fecal calprotectin levels are higher in rural than in urban Chinese infants and negatively associated with growth. BMC Pediatr. 2012;12:129. doi: 10.1186/1471-2431-12-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olafsdottir E, Aksnes L, Fluge G et al. Faecal calprotectin levels in infants with infantile colic, healthy infants, children with inflammatory bowel disease, children with recurrent abdominal pain and healthy children. Acta Paediatr. 2002;91(1):45–50. doi: 10.1080/080352502753457932. [DOI] [PubMed] [Google Scholar]
- 74.Mahjoub FE, Zahedi N, Ashjai B et al. Role of fecal calprotectin in differentiating between Hirschsprung's disease and functional constipation. Korean J Gastroenterol. 2013;62(5):288–291. doi: 10.4166/kjg.2013.62.5.288. [DOI] [PubMed] [Google Scholar]
- 75.Flagstad G, Helgeland H, Markestad T. Faecal calprotectin concentrations in children with functional gastrointestinal disorders diagnosed according to the Pediatric Rome III criteria. Acta Paediatr. 2010;99(5):734–737. doi: 10.1111/j.1651-2227.2010.01698.x. [DOI] [PubMed] [Google Scholar]
- 76.Fundaro C, Fantacci C, Ansuini V et al. Fecal calprotectin concentration in children affected by SIBO. Eur Rev Med Pharmacol Sci. 2011;15(11):1328–1335. [PubMed] [Google Scholar]
- 77.Golden BE, Clohessy PA, Russell G et al. Calprotectin as a marker of inflammation in cystic fibrosis. Arch Dis Child. 1996;74(2):136–139. doi: 10.1136/adc.74.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horsley AR, Davies JC, Gray RD et al. Changes in physiological, functional and structural markers of cystic fibrosis lung disease with treatment of a pulmonary exacerbation. Thorax. 2013;68(6):532–539. doi: 10.1136/thoraxjnl-2012-202538. [DOI] [PubMed] [Google Scholar]
- 79.Braekke K, Holthe MR, Harsem NK et al. Calprotectin, a marker of inflammation, is elevated in the maternal but not in the fetal circulation in preeclampsia. Am J Obstet Gynecol. 2005;193(1):227–233. doi: 10.1016/j.ajog.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 80.Holthe MR, Staff AC, Berge LN et al. Calprotectin plasma level is elevated in preeclampsia. Acta Obstet Gynecol Scand. 2005;84(2):151–154. doi: 10.1111/j.0001-6349.2005.00554.x. [DOI] [PubMed] [Google Scholar]
- 81.Stoll ML, Punaro M, Patel AS. Fecal calprotectin in children with the enthesitis-related arthritis subtype of juvenile idiopathic arthritis. J Rheumatol. 2011;38(10):2274–2275. doi: 10.3899/jrheum.110508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ortega FJ, Sabater M, Moreno-Navarrete JM et al. Serum and urinary concentrations of calprotectin as markers of insulin resistance and type 2 diabetes. Eur J Endocrinol. 2012;167(4):569–578. doi: 10.1530/EJE-12-0374. [DOI] [PubMed] [Google Scholar]
- 83.Abe J, Jibiki T, Noma S et al. Gene expression profiling of the effect of high-dose intravenous Ig in patients with Kawasaki disease. J Immunol. 2005;174(9):5837–5845. doi: 10.4049/jimmunol.174.9.5837. [DOI] [PubMed] [Google Scholar]
- 84.Hirono K, Foell D, Xing Y et al. Expression of myeloid-related protein-8 and -14 in patients with acute Kawasaki disease. J Am Coll Cardiol. 2006;48(6):1257–1264. doi: 10.1016/j.jacc.2006.02.077. [DOI] [PubMed] [Google Scholar]
- 85.Jensen LJ, Pedersen S, Bjerre M et al. Plasma calprotectin predicts mortality in patients with ST segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Interv Cardiol. 2010;23(2):123–129. doi: 10.1111/j.1540-8183.2010.00532.x. [DOI] [PubMed] [Google Scholar]
- 86.Hestvik E, Olafsdottir E, Tylleskar T et al. Faecal calprotectin in HIV-infected, HAART-naive Ugandan children. J Pediatr Gastroenterol Nutr. 2012;54(6):785–790. doi: 10.1097/MPG.0b013e318241a683. [DOI] [PubMed] [Google Scholar]
- 87.Muller F, Froland SS, Aukrust P et al. Elevated serum calprotectin levels in HIV-infected patients: the calprotectin response during ZDV treatment is associated with clinical events. J Acquir Immune Defic Syndr. 1994;7(9):931–939. [PubMed] [Google Scholar]
- 88.Gray RD, Imrie M, Boyd AC et al. Sputum and serum calprotectin are useful biomarkers during CF exacerbation. J Cyst Fibros. 2010;9(3):193–198. doi: 10.1016/j.jcf.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 89.Brun JG, Jonsson R, Haga HJ. Measurement of plasma calprotectin as an indicator of arthritis and disease activity in patients with inflammatory rheumatic diseases. J Rheumatol. 1994;21(4):733–738. [PubMed] [Google Scholar]
- 90.Hammer HB, Kvien TK, Glennas A et al. A longitudinal study of calprotectin as an inflammatory marker in patients with reactive arthritis. Clin Exp Rheumatol. 1995;13(1):59–64. [PubMed] [Google Scholar]
- 91.Frosch M, Ahlmann M, Vogl T et al. The myeloid-related proteins 8 and 14 complex, a novel ligand of toll-like receptor 4, and interleukin-1beta form a positive feedback mechanism in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2009;60(3):883–891. doi: 10.1002/art.24349. [DOI] [PubMed] [Google Scholar]
- 92.Moncrieffe H, Ursu S, Holzinger D et al. A subgroup of juvenile idiopathic arthritis patients who respond well to methotrexate are identified by the serum biomarker MRP8/14 protein. Rheumatology (Oxford) 2013;52(8):1467–1476. doi: 10.1093/rheumatology/ket152. [DOI] [PubMed] [Google Scholar]
- 93.Terrin G, Passariello A, Manguso F et al. Serum calprotectin: an antimicrobial peptide as a new marker for the diagnosis of sepsis in very low birth weight newborns. Clin Dev Immunol. 2011:1–6. doi: 10.1155/2011/291085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Horner D, Long AM. Towards evidence-based emergency medicine: best BETs from the Manchester Royal Infirmary. BET 3: Super calprotectin will not expedite your discharge. Emerg Med J. 2013;30(8):691–693. doi: 10.1136/emermed-2013-202918.3. [DOI] [PubMed] [Google Scholar]
- 95.Kharbanda AB, Rai AJ, Cosme Y et al. Novel serum and urine markers for pediatric appendicitis. Acad Emerg Med. 2012;19(1):56–62. doi: 10.1111/j.1553-2712.2011.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jensen LJ, Kistorp C, Bjerre M et al. Plasma calprotectin levels reflect disease severity in patients with chronic heart failure. Eur J Prev Cardiol. 2012;19(5):999–1004. doi: 10.1177/1741826711421078. [DOI] [PubMed] [Google Scholar]
- 97.Carroccio A, Rocco P, Rabitti PG et al. Plasma calprotectin levels in patients suffering from acute pancreatitis. Dig Dis Sci. 2006;51(10):1749–1753. doi: 10.1007/s10620-006-9078-4. [DOI] [PubMed] [Google Scholar]
- 98.Cobanoglu N, Dalkan C, Galip N et al. Is calprotectin a marker of tobacco smoke related inflammation? A pilot study in children. Inhal Toxicol. 2012;24(8):486–491. doi: 10.3109/08958378.2012.693137. [DOI] [PubMed] [Google Scholar]
- 99.Cobanoglu N, Galip N, Dalkan C et al. Leptin, ghrelin and calprotectin: inflammatory markers in childhood asthma? Multidiscip Respir Med. 2013;8(1):62. doi: 10.1186/2049-6958-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Malickova K, Brodska H, Lachmanova J et al. Plasma calprotectin in chronically dialyzed end-stage renal disease patients. Inflamm Res. 2010;59(4):299–305. doi: 10.1007/s00011-009-0103-x. [DOI] [PubMed] [Google Scholar]
- 101.Morandi F, Cangemi G, Barco S et al. Plasma levels of soluble HLA-E and HLA-F at diagnosis may predict overall survival of neuroblastoma patients. Biomed Res Int. 2013;2013:956878. doi: 10.1155/2013/956878. [DOI] [PMC free article] [PubMed] [Google Scholar]


