Abstract
BACKGROUND: Advances in cardiac operations over the last few decades, including corrective operations in early life, have dramatically increased the survival of children with congenital heart disease. However, postoperative care has been associated with neurologic complications, with seizures being the most common manifestation. The primary objective of this study is to describe the outcomes in pediatric patients who received an antiepileptic drug (AED) post–cardiac surgery.
METHOD: A retrospective cohort study was performed in all patients less than 18 years of age who received an AED in the cardiovascular intensive care unit at Texas Children's Hospital from June 2002 until June 2012. Cardiac surgical patients initiated on phenobarbital, phenytoin, and levetiracetam were queried. Patients were excluded if the AED was not initiated on the admission for surgery. Patients who received 1 AED were compared to patients who received 2 AED, and differences in outcomes examined between the 3 AEDs used were evaluated.
RESULTS: A total of 37 patients met the study criteria. Patients were initiated on an AED a median of 4 days following surgery and became seizure free a median of 1 day after initiation, with 65% remaining seizure free after the first dose. Half of all patients required 2 AEDs for seizure control, with a higher proportion of adolescents requiring 2 AEDs (p = 0.04). No differences were found when comparing the collected outcomes between phenobarbital, fosphenytoin, or levetiracetam.
CONCLUSION: No adverse events were reported with the AEDs reviewed. Further work is necessary to evaluate long-term neurodevelopmental outcomes in this population and whether outcomes are a result of the AED or of other clinical sequelae.
INDEX TERMS: antiepileptic drug, cardiac surgery, fosphenytoin, levetiracetam, pediatric, phenobarbital, phenytoin
BACKGROUND
Advances in cardiac operations over the last few decades, including corrective operations in early life, have dramatically increased the survival of children with congenital heart disease. However, postoperative care has been associated with neurologic complications, including seizures, which are the most common manifestation of neurologic dysfunction post–cardiac surgery.1 The reported incidence of seizures following cardiac surgery ranges from 2% to 25%.2–4 Seizure onset usually occurs in the second 24 hours following surgery and can last for hours to days and can progress to status epilepticus.5 Patients are therefore often placed on an antiepileptic drug (AED), but no guidelines exist with regard to medication or duration. Most commonly used agents include first-generation AEDs, phenobarbital and phenytoin/fosphenytoin. In recent years, newer AEDs have become available, such as levetiracetam, and some clinicians have shifted their practice to utilizing this agent over the older medications based on its less worrisome cardiac side effect profile.
Unfortunately, there are few studies examining the effects of AEDs in children, and well-designed, long-term, prospective studies are greatly needed. Additionally, the short-term efficacy and safety of these AEDs is unknown in the postoperative pediatric cardiac surgical population.
Reviewing the outcomes in post–cardiac surgery patients following use of newer AEDs may be beneficial in developing an understanding of the consequences of seizures post–cardiac surgery. The objective of this study is to describe the efficacy of AEDs, as evidenced by seizure control, and safety, as evidenced by adverse events, in post–cardiac surgery pediatric patients who received an AED.
METHODS
A retrospective cohort study was designed, institutional review board approval was obtained, and patient charts were queried from June 2002 through June 2012 to identify patients 18 years of age or less in the cardiovascular intensive care unit who received either phenobarbital, fosphenytoin or phenytoin, or levetiracetam following their cardiac surgery, who were not previously taking an AED. Patients were excluded if AED use began prior to surgery or if surgery was a separate admission from the AED initiation. All data were collected from the inpatient medical record by the primary investigator. Data collection included baseline demographics, such as the age of the patient at surgery, sex, surgery type, cardiac bypass time, and requirement for extracorporeal membrane oxygenation (ECMO). Medication use information, such as initial AED used, duration of therapy, need for a second agent, and continuation of AED therapy upon discharge, was collected. Lastly, outcomes were reviewed, including days until “seizure-freedom,” hospital length of stay, occurrence of stroke, mortality, and meeting of developmental milestones. These data were then compared between patients who received 1 AED to those who required 2 AEDs; we also evaluated whether there were any differences among the 3 AEDs.
Data were analyzed using Student's t-test and analysis of variance for normally distributed data; Wilcoxon Rank Sum and Kruskal-Wallis tests for non-normally distributed data; and chi-square analysis for categorical data.
RESULTS
Eight thousand cardiac surgeries were performed over the 10-year study period; 101 patients were on AEDs and 64 were excluded, leaving a sample size of 37 patients who met criteria. Patient demographics are listed in Table 1. The majority of patients were male and less than 1 year of age. Seizures were diagnosed clinically in 21 patients (57%), with confirmed seizures on electroencephalography for 16 (43%) patients. The mean cardiac bypass time was 149 minutes for the 32 patients who required bypass during surgery. A variety of cardiac surgeries were performed and are listed in Table 2. In-hospital mortality occurred in 6 (16%) patients, while an additional 3 patients died after discharge, for an overall mortality rate of 24% (Table 3).
Table 1.
Patient Demographics

Table 2.
Cardiac Surgical Procedures
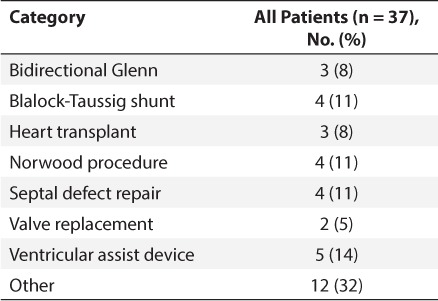
Table 3.
Outcomes *

Initial therapy consisted of 14 patients treated with phenobarbital, 14 with fosphenytoin or phenytoin, and 9 with levetiracetam (Table 4). Patients were initiated on an AED a median of 4 days following surgery and became seizure free a median of 1 day after initiation, with 65% of patients remaining seizure free after the first dose. Approximately half (49%) of all patients eventually required a second agent for seizure control prior to discharge. Median duration of AED administration was 9 days (range, 1–163 days). Fifty-seven percent of patients were discharged home on an AED; the most common was levetiracetam, followed by oxcarbazepine or phenobarbital, and one patient was sent home on phenytoin. Doses used were appropriate doses for each drug by age and weight of the patient. According to chart review, there were no adverse events reported, including bradycardia, hypotension, cardiac arrhythmias, or behavioral changes.
Table 4.
Antiepileptic Drug Use *

Twenty-one patients had documentation of developmental milestones after discharge; 13 (62%) of those did not meet milestones. The most common reported delay in development was language, as more than half (54%) of the patients not meeting developmental milestones were noted to be in speech therapy. Seven of the 13 patients not meeting developmental milestones had a history of stroke, but there was no statistical association between stroke and failure to meet developmental milestones (p > 0.05) (Table 3). Seven of the 13 patients (54%) not meeting developmental milestones received phenobarbital, while 4 patients (31%) received fosphenytoin, and 2 (15%) received levetiracetam.
All of the collected data were then compared between patients who received 1 AED to those who required 2 AEDs, and the only statistically significant difference found was that adolescents often required 2 AEDs (p = 0.04). There were no statistically significant differences in any of the data when comparing the outcomes among the 3 AEDs studied.
DISCUSSION
It has been shown that infants with perioperative seizures have worse neurodevelopmental outcomes, including delayed motor skills, language, and development.2 The evidence is fairly strong with regard to phenobarbital and its association with cognitive deficits in pediatric patients,6 some data showing phenytoin has an effect on mental speed, while levetiracetam does not seem to have a negative impact on cognition.7
This is the first study to report the outcomes of post–cardiac surgical pediatric patients who received AED therapy as a result of postoperative seizures. Overall, the use of these agents at our institution is very low, considering that approximately 900 cardiac surgical procedures are performed each year. An evaluation of safety and efficacy endpoints is warranted for newer AEDs used for postoperative seizures. Even though the use of these agents is infrequent, the outcomes seen in patients who received an AED are less than ideal. Close to two-thirds of patients were discharged home on an AED. Continuation of AED therapy at discharge was at the discretion of the physician, and appropriateness cannot be determined as a result of the retrospective nature of the study. Hospital mortality rate was 16%, which is much higher than the 3.1% national discharge mortality rate for the most difficult and invasive pediatric cardiac surgical procedures.8
While the use of these agents did not result in any adverse events, half of all patients required 2 AEDs for seizure control, and no differences were found in any of the outcomes collected when comparing among patients who received phenobarbital, fosphenytoin, or levetiracetam. It is speculated that those patients who required 2 agents had less seizure control, as dosing was appropriate for these patients, but without consistent concentrations drawn on patients, no conclusions can be drawn. Levetiracetam has been used more often in the present day, but, according to our data, may not be more effective than other first-line agents. Without a head-to-head study of these agents in this setting, no recommendations can be given regarding which AED should be considered first-line. In addition, it appears that adolescents were more likely to require 2 medications for adequate control of seizures. It is unclear why adolescents were more likely to require 2 agents, but perhaps adding a second agent earlier on if seizures are not controlled may be warranted.
Recently, a large collaborative study9 from 22 institutions has been published analyzing the neurodevelopmental outcomes after cardiac surgery in infancy alone. Of their 1770 subjects, the psychomotor development index and mental development index scores were lower than normative means (p < 0.001). Risk factors included lower birth weight, white race, less maternal education, male sex, and presence of a genetic/extracardiac anomaly. The authors9 conclude that as more high-risk surgeries are being performed with greater rates of survival, additional societal resources will be required for this population.
Similar to the above reported data, the patients with follow-up in our data set had significant deficits in neurodevelopmental outcomes. Thirteen patients (62%) did not meet their developmental milestones upon follow-up after discharge. The majority of these patients were male (n = 9, 69%) and of infant age, with a median of 4.1 months of age (range, 3 days to 7 years), all born at term. No adolescents had reported delays in developmental milestones, but this may be due to their age, and other follow-up may be needed in this patient population. Since it appears that developmental delay is common in these patients, assessment of development and appropriate treatment should strongly be considered during follow-up visits in these patients.
The limitations associated with this study are those common to retrospective reviews. As this is the first investigation into this area, it is primarily hypothesis generating and should be used to drive future investigations into AED therapy in postoperative pediatric cardiac surgical patients. The sample size associated with this review was small, and future research should likely be multicenter and include a control group. Utilization of newer medications, as they are developed, should be evaluated in a systematic manner to determine best practices. This study could not draw definitive conclusions associating the various outcomes caused by the AED, but rather was a descriptive study reporting outcomes observed in those patients who received an AED. Further studies evaluating the risks AEDs pose in the postoperative period may help guide practitioners in choosing which AED to use in the case of postoperative seizures in pediatric patients.
CONCLUSION
Antiepileptic drug use in pediatric patients who have undergone cardiac surgery was effective in terminating seizure activity in most patients. Those who do not respond quickly to therapy may require a second agent. No adverse events were reported with the AEDs used in this cohort. Further work is necessary to evaluate long-term neurodevelopmental outcomes in this population.
Abbreviations
- AED
antiepileptic drug
- ECMO
extracorporeal membrane oxygenation
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Saliba RM, Annegers JF, Waller DK et al. Incidence of neonatal seizures in Harris County, Texas, 1992–1994. Am J Epidemiol. 1999;150(7):763–769. doi: 10.1093/oxfordjournals.aje.a010079. [DOI] [PubMed] [Google Scholar]
- 2.Newburger J, Jonas R, Wernovsky G et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med. 1993;329(15):1057–1064. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- 3.Menache CC, du Plessis AJ, Wessel DL et al. Current incidence of acute neurologic complications after open-heart operations in children. Ann Thorac Surg. 2002;73(6):1752–1758. doi: 10.1016/s0003-4975(02)03534-8. [DOI] [PubMed] [Google Scholar]
- 4.Gaynor JW, Jarvik GP, Bernbaum J et al. The relationship of postoperative electrographic seizures to neurodevelopmental outcome at 1 year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. 2006;131(1):181–189. doi: 10.1016/j.jtcvs.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wernovsky G, Hanley FL. Neurologic disorders. In: Chang AC, Hanley Fl, Wernovsky G, Wessel DL, editors. Pediatric Cardiac Intensive Care. Baltimore, MD: Williams and Wilkins; 1998. [Google Scholar]
- 6.Loring DW, Meador KJ. Cognitive side effects of antiepileptic drugs in children. Neurology. 2004;62(6):872–877. doi: 10.1212/01.wnl.0000115653.82763.07. [DOI] [PubMed] [Google Scholar]
- 7.Ijff DM, Aldenkamp AP. Cognitive side-effects of antiepileptic drugs in children. Handbook Clin Neurol. 2013;111:707–718. doi: 10.1016/B978-0-444-52891-9.00073-7. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins KJ, Gaurveau K, Newburger JW et al. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123(1):110–118. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 9.Gaynor JW, Stopp C, Wypij D et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics. 2015;135(5):816–825. doi: 10.1542/peds.2014-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]


