Abstract
OBJECTIVES: To determine if the incidence of nephrotoxicity is higher in pediatric patients treated with the combination of vancomycin and piperacillin-tazobactam, compared to patients treated with vancomycin alone. Secondary objectives were to determine if admission to an intensive care unit (ICU), higher serum vancomycin trough concentrations (>15 mg/L), or receipt of other nephrotoxic agents were related to the development of nephrotoxicity.
METHODS: This was a retrospective, single-center, cohort study of 79 patients treated with vancomycin and 106 patients treated with vancomycin and pipracillin/tazobacatam (TZP). Serum creatinine was trended to determine if patients had nephrotoxicity, which was defined as at least a 100% increase in serum creatinine or an increase of ≥0.5 mg/dL from the baseline value. Fisher's exact test was used to compare the incidence of nephrotoxicity in the vancomycin group to the combination group. Secondary objectives were evaluated by using relative risk (RR).
RESULTS: Nephrotoxicity developed in 3 of 79 patients (3.8%) in the vancomycin group and in 25 of 106 patients (23.6%) on combination therapy (p = 0.0001). In patients receiving only vancomycin, there was no statistically significant increase in nephrotoxicity for patients in the ICU (RR 1.85, 95% confidence interval [CI] 0.175–19.62, p = 0.61), those with higher vancomycin troughs (RR 2.32, CI 0.226–23.86, p = 0.48), or those receiving other nephrotoxic medications (RR 2.94, CI 0.2779–31.05, p = 0.37). In the combination group, having higher serum vancomycin trough concentrations increased the risk of nephrotoxicity (RR 5.22, CI 2.407–11.306, p < 0.0001).
CONCLUSIONS: Combination therapy with vancomycin and TZP is potentially more nephrotoxic than vancomycin alone. ICU admissions, high vancomycin troughs (>15 mg/L), and concomitant nephrotoxic medications cannot be excluded as risk factors for the observed increase in nephrotoxicity in patients receiving vancomycin and TZP.
INDEX TERMS: acute kidney injury, adverse drug effect, nephrotoxicity, pediatric, piperacillin-tazobactam, vancomycin
INTRODUCTION
Vancomycin is a glycopeptide antimicrobial agent that is widely used in the hospital setting for treatment of life-threatening Gram-positive infections and infections caused by methicillin-resistant Staphylococcus aureus.1 Clinically, vancomycin has been in use for more than 50 years and has long been associated with the development of adverse effects including infusion-related reactions, nephrotoxicity, and possible ototoxicity.2 The first reports of nephrotoxicity with vancomycin were attributed to impurities and poor manufacturing processes. In fact, vancomycin was once referred to as “Mississippi mud” because of the product's muddy brown appearance, which was caused by the impurities. Improvements in manufacturing and the availability of extensive pharmacokinetic information have contributed to a decrease in vancomycin-related nephrotoxicity. Recent data suggest that vancomycin, especially when used alone and at conventional doses, has little potential for nephrotoxicity or ototoxicity.3
The incidence of vancomycin-induced nephrotoxicity ranges from 5% to 35%,2 and is dependent on the population studied, the presence or absence of risk factors, and the definition of nephrotoxicity used by the investigators. Available literature suggests that intensive care unit (ICU) admission, concomitant administration of a nephrotoxin, and higher vancomycin serum trough concentrations (>15 mg/L) may increase the risk for vancomycin-associated nephrotoxicity; however, most data come from adult studies.4 McKamy et al1 reviewed the incidence and risk factors associated with vancomycin-related nephrotoxicity in 167 children and found the incidence of nephrotoxicity to be 14%. Additionally, patients with vancomycin trough concentrations ≥ 15 mg/L were more likely to have nephrotoxicity than those with troughs < 15 mg/L (28% vs. 7.3%). The authors also indicated that administration of furosemide in the ICU was associated with higher incidences of nephrotoxicity.1 Although limited, pediatric literature suggests that many of the risk factors for vancomycin-associated nephrotoxicity in adults may also apply to the pediatric population. A consensus report that reviewed the vancomycin literature in adults noted that nephrotoxicity due to vancomycin monotherapy occurs in 5% to 7% of patients, but that the incidence increased to 35% in those who receive vancomycin plus other nephrotoxic medications.3
Since June 2012 both case reports and studies have noted the development of nephrotoxicity attributed to the use of vancomycin in conjunction with piperacillin-tazobactam (TZP) (Zosyn, AuroMedics, Dayton, NJ).2,5–7 In hospitalized patients, vancomycin and TZP are often used as empiric therapy for serious infections or infections caused by drug-resistant organisms. TZP is not widely considered to produce nephrotoxicity; however, the manufacturer's prescribing information for TZP notes that increased blood urea nitrogen (BUN) and serum creatinine are adverse events that occur in 1.8% of the population.5 There are also case reports that potentially implicate TZP as a cause of nephrotoxicity in pediatric oncology patients; however, no specific doses of TZP were mentioned.8
Nephrotoxicity appears to increase when vancomycin and TZP are used simultaneously. An article6 in Pharmacy Practice News from June 2012 states that as many as 50% of patients treated with the combination of vancomycin and TZP develop acute kidney injury, with acute kidney injury defined as a 100% increase or greater in serum creatinine or an increase of at least 0.5 mg/dL from the baseline serum creatinine. Burgess and Drew2 observed an 8.1% rate of occurrence of nephrotoxicity in adult patients treated with vancomycin alone and a 16.3% incidence of nephrotoxicity in patients treated with both vancomycin and TZP. Gomes et al7 also investigated the concomitant use of vancomycin and TZP compared to vancomycin plus cefepime. The patients receiving vancomycin and TZP had a 34.8% occurrence of acute kidney injury, while patients receiving vancomycin and cefepime experienced a 12.5% occurrence of nephrotoxicity (p < 0.0001).
Though evidence in adults supports increased nephrotoxicity when TZP is used concomitantly with vancomycin, there are few pediatric data on this subject. This investigation aimed to determine if pediatric patients treated with vancomycin and TZP had a higher incidence of nephrotoxicity than patients treated with vancomycin alone. Secondarily, this study was designed to determine if ICU admission, higher vancomycin serum trough concentrations (>15 mg/L), or receipt of other nephrotoxic agents increased the risk for acute kidney injury in either patient group.
METHODS
Subjects
This was a single-center retrospective cohort evaluation of the development of nephrotoxicity in patients treated with vancomycin alone and patients treated with vancomycin in combination with TZP. The investigation involved patients treated at the Scottish Rite and Egleston campuses of Children's Healthcare of Atlanta, a multicampus not-for-profit institution. Patients were identified by searching electronic pharmacy records for patients who received either vancomycin or vancomycin in conjunction with TZP between November 1, 2012, and January 16, 2013. The population included patients who were ≤19 years of age and who had received either vancomycin or vancomycin plus TZP for ≥48 hours. Patients with underlying renal dysfunction were included, since the protocol at Children's Healthcare of Atlanta is to initiate and adjust doses on the basis of renal function. Demographic information also included notation of patient location: cardiac intensive care, pediatric intensive care, neonatal intensive care, technology-dependent intensive care, or general patient care areas. Patients without baseline laboratory values (i.e., within 24 hours of starting antibiotics) or repeated BUN or serum creatinine were excluded.
Data Collection
The following data were captured from the electronic medical records: age, height, weight, sex, dates and dose of vancomycin and TZP (mg/kg), laboratory data including BUN, serum creatinine values, and vancomycin serum trough concentrations, and whether or not the patient had an ICU stay. Vancomycin troughs were drawn ideally no more than 1 hour before the fourth consecutive dose of intravenous vancomycin, which was presumed to be a steady-state concentration. In the event that a vancomycin trough was not drawn on time, the serum concentration was extrapolated by using population-based pharmacokinetic equations. Additionally, it was documented if the patient was on other nephrotoxic medications, including amphotericin, aminoglycosides, ketorolac, and furosemide. Data were stored in a password-protected Microsoft Excel file (Microsoft, Redmond, WA).
Primary and Secondary Endpoints
The primary endpoint for this investigation was the incidence of nephrotoxicity, which was defined as a 100% increase or greater in baseline serum creatinine or an increase from baseline serum creatinine of at least 0.5 mg/dL on at least 2 readings.6 Secondary endpoints were to determine if ICU stays, elevated serum vancomycin troughs (>15 mg/L), or concomitant administration of other nephrotoxic medications (i.e., amphotericin, aminoglycosides, ketorolac, or furosemide) were related to developing nephrotoxicity.
Statistical Analysis
A sample size of 144 patients (with 72 patients in each group) was estimated to be required. This sample size assumed a 5% incidence of nephrotoxicity in patients receiving vancomycin and 80% power to detect the minimal increase of 15% (5%–20%) in nephrotoxicity rate for patients who received vancomycin in comparison to those who received both vancomycin and TZP. The comparison used Fisher's exact test at a significance (α) level of 0.05.
Baseline demographics were tabulated by frequencies (percentages) for categorical variables and as mean ± SD for continuous variables. Baseline demographics were then analyzed for any statistically significant differences by using a Fisher's exact test for categorical data and Student's ttest for continuous data. To evaluate the incidence of nephrotoxicity in each medication group, a Fisher's exact test was used to assess the incidence of nephrotoxicity with vancomycin alone compared to nephrotoxicity with vancomycin in combination with TZP. For the secondary outcomes of ICU admissions, elevated serum vancomycin troughs (>15 mg/L), and concomitant nephrotoxic medications, relative risk (confidence interval and p value) calculations were completed to determine if any of the secondary outcomes increased the risk of nephrotoxicity for patients treated with vancomycin alone or the combination of vancomycin and TZP. The study design and protocol were approved by the institutional review board at Children's Healthcare of Atlanta.
RESULTS
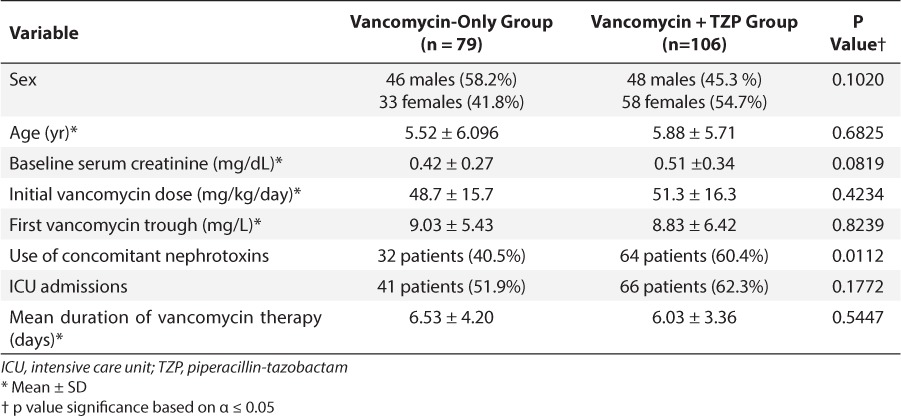
During the study period, there were 130 patients screened for inclusion in the vancomycin group and 162 patients screened for inclusion in the combination group (vancomycin + TZP). Of note, there were 947 patients who underwent treatment with vancomycin in the study period. In an effort to potentially match the number of patients included in each therapy group, 130 patients were randomly selected from the vancomycin group to be screened. Patient screening and enrollment are summarized in the Figure. The most common reason for exclusion was treatment with either vancomycin therapy or combination therapy for less than 48 hours. Patients were also excluded for use of oral rather than intravenous vancomycin, or for having no baseline or follow-up laboratory data. The patients enrolled in each group had similar baseline demographics for sex, age, baseline serum creatinine values, mean initial vancomycin dose (mg/kg/day), mean first serum vancomycin trough, duration of vancomycin treatment, and number of ICU admissions. The combination vancomycin and TZP group had significantly more patients receive concomitant nephrotoxic medications.
Figure.
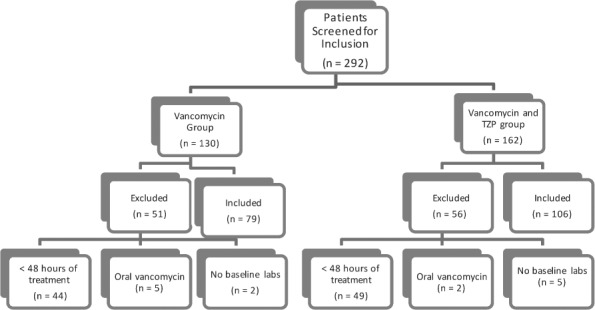
Patient screening and study enrollment.
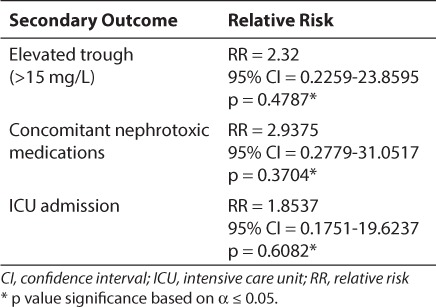
There was a statistically significant difference in the number of patients who developed nephrotoxicity in the vancomycin group versus the vancomycin and TZP group. Of the 79 patients in the vancomycin treatment group, 3 (3.8%) developed nephrotoxicity, while 25 of the 106 patients (23.6%) treated with vancomycin and TZP developed nephrotoxicity (2-sided Fisher's test, p = 0.0001). Furthermore, of the 3 patients with nephrotoxicity in the vancomycin group, 1 of the 3 patients (33.3%) had an elevated serum vancomycin trough (>15 mg/L), 2 of the 3 patients (66.7%) were on other nephrotoxic medications (i.e., amphotericin, aminoglycosides, ketorolac, and/or furosemide), and 2 of the 3 patients (66.7%) were admitted to the ICU for at least 1 day. None of these secondary outcomes had a significant effect on the development of nephrotoxicity in patients receiving vancomycin alone (Table 1).
Table 1.
Influence of Secondary Outcomes on the Incidence of Nephrotoxicity in Patients Receiving Vancomycin Alone
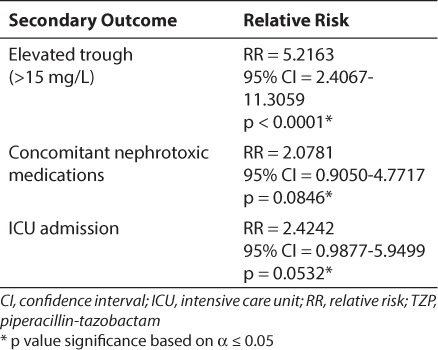
In patients receiving combination therapy, elevated serum vancomycin troughs (≥15 mg/L) appear to be associated with nephrotoxicity (RR 5.2163, CI 2.4067–11.3059, p < 0.0001). Conversely, neither the use of concomitant nephrotoxic medications nor ICU admission significantly increased the risk of nephrotoxicity in these patients. Table 2 captures the analysis of these secondary endpoints.
Table 2.
Influence of Secondary Outcomes on the Incidence of Nephrotoxicity in Patients Receiving Vancomycin in Combination With TZP
DISCUSSION
Vancomycin is often used in conjunction with other antimicrobials, including TZP, for broad empiric coverage. In the past few years, the combination of vancomycin and TZP has received attention owing to a reported increase in nephrotoxicity when the 2 agents are used together. The increase in nephrotoxicity with vancomycin and TZP has been detailed in recent publications, primarily in the adult population.2,6,7 One pediatric review of vancomycin associated-nephrotoxicity specifically states that TZP use in patients with elevated serum vancomycin troughs (≥15 mg/L) may produce rates of nephrotoxicity that are significantly higher than what many clinicians expect from vancomycin alone.9 In our investigation, higher vancomycin troughs (≥15 mg/L) were associated with a greater incidence of nephrotoxicity in the combination therapy group but not in the vancomycin-alone group, thus supporting previous pediatric reviews.
The specific aim of this study was to compare the incidence of nephrotoxicity in patients receiving vancomycin alone and those receiving vancomycin and TZP simultaneously. Initial vancomycin doses were similar between the groups (Table 3), yet the patients receiving combination therapy had a higher rate of nephrotoxicity that was statistically significant (Fisher's test, p = 0.0001). There was a 23.6% rate of nephrotoxicity in patients receiving combination therapy and only a 3.8% incidence of nephrotoxicity in patients receiving vancomycin alone. The low incidence of nephrotoxicity in patients receiving only vancomycin was similar to the consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Disease Pharmacists (5%–7% patients).3 Similarly, the incidence of nephrotoxicity in the combination group (23.6%) fell in between the values that previous studies have cited. According to previous case reports and studies, the incidence of nephrotoxicity for vancomycin and TZP was between 16.3% and 50%.1,2–4,6 We chose to define nephrotoxicity or acute kidney injury as a 100% increase or greater in serum creatinine or an increase of at least 0.5 mg/dL from the baseline serum creatinine that persisted for at least 2 readings. This definition was chosen on the basis of previous literature, particularly the first case report on vancomycin and TZP nephrotoxicity.6 It was also thought that this was an appropriate definition for nephrotoxicity in pediatric patients. Patients with underlying renal dysfunction were not excluded from the evaluation, since patients had to have baseline laboratory values to be included in the investigation. The preliminary laboratory values were used to determine an initial vancomycin dosage regimen for each patient that would be adjusted further on the basis of serum vancomycin trough concentrations. Vancomycin dosage strategies are generally chosen from an institutional protocol. For patients with a post-conception age of ≥44 weeks and normal renal function, the typical starting dose of vancomycin per our hospital policy was 60 mg/kg/day divided every 8 hours with a maximum single dose of 1250 mg. Exceptions to this dosage strategy were patients in the cardiac intensive care units and those with underlying renal dysfunction. Patients in the cardiac ICU were started on 10 mg/kg/dose typically every 8 hours and patients with renal dysfunction were most often treated as “per drug serum concentration.” In patients on a “per drug concentration”–determined regimen, a trough concentration was drawn before the second anticipated dose and the dose was held until a trough value resulted. From the trough concentration, a dosage regimen was selected. There were 4 patients with underlying renal dysfunction in this analysis, 2 in each treatment group. Of these 4 patients, only 1 patient in the combination group experienced nephrotoxicity. That same patient also later required extracorporeal membrane oxygenation. TZP was dosed as 75 to 100 mg/kg/dose, most often given every 8 hours with the mean daily dose of 264 mg/kg/day and a maximum single dose of 4000 mg.
Table 3.
Baseline Patient Characterstics by Treatment Group
Several previous studies have supported that there are many factors that can influence the likelihood of developing nephrotoxicity while receiving intravenous vancomycin. One adult study reported that maintaining a vancomycin serum trough concentration ≥ 15 mg/L was independently associated with renal toxicity.1,10 Previous investigations on this topic have also illustrated that an ICU admission and/or the concurrent use of other nephrotoxic medications may increase the chance of nephrotoxicity for adult patients. These 3 factors—elevated vancomycin troughs (>15 mg/L), ICU admissions, and concomitant use of other nephrotoxic medications—were chosen as secondary outcomes in an effort to determine if any of these factors could increase the risk of developing nephrotoxicity in either patient group. This investigation elucidated that in patients receiving vancomycin alone, there was no increased risk of nephrotoxicity associated with any of these factors. However, there were fewer patients in the vancomycin group who were admitted to the ICU or who were receiving other nephrotoxic medications than in the vancomycin and TZP group. In the patients receiving both vancomycin and TZP, the only factor to significantly increase the chance of nephrotoxicity was having an increased vancomycin trough (>15 mg/L). It would be expected that elevated serum vancomycin troughs would also increase the likelihood for nephrotoxicity in patients receiving vancomycin alone, since previous studies have reported that elevated vancomycin troughs are an independent predictor of nephrotoxicity.
Patients receiving vancomycin and TZP are often assumed to be sicker than those receiving vancomycin alone. In an effort to equalize the treatment groups, the secondary outcome of ICU admissions was chosen. By looking at the effect of ICU admissions on the incidence of nephrotoxicity, we could reduce the concerns that the critical nature of the patients was contributing to nephrotoxicity rates. Since there was no statistically significant increase in nephrotoxicity related to ICU admissions in either patient group, this does not appear to be a significant factor in developing nephrotoxicity.
In the combination group, there were also more patients receiving concomitant nephrotoxic agents than in the vancomycin-alone group (62.3% and 51.9%, respectively). Though this was not an independent contributor to the development of nephrotoxicity in either group, perhaps in combination with the more frequent ICU admissions in the vancomycin and TZP group, it would be a significant factor. One could also speculate that with a larger sample size and more power, a statistically significant difference would be detected for the effect of ICU admissions and concomitant nephrotoxins on the development of nephrotoxicity in the combination therapy group.
This study is not without limitations. In retrospect, it would have been prudent to have considered intravenous contrast agents as nephrotoxins, since we have seen patients who have experienced nephrotoxicity possibly attributable to the combination of vancomycin and an intravenous contrast agent. Though we excluded patients who did not receive at least a 48-hour course of antibiotics with vancomycin and/or TZP, a large percentage of patients had short treatment durations (i.e., 48–96 hours). The mean duration of vancomycin treatment was close to 6 days for each group. If we had more patients with longer treatment courses, perhaps we would have a higher incidence of nephrotoxicity in both treatment groups. In future studies on this topic, it would be valuable to have more patients with longer duration of treatment in order to determine the effect of treatment duration on the development of nephrotoxicity. Lastly, more equal group sizes would be ideal for a more accurate and matched comparison of nephrotoxicity between the 2 treatment groups.
CONCLUSIONS
This investigation supports previous findings that patients are subject to an increased risk of nephrotoxicity when treated with vancomycin and TZP simultaneously compared to vancomycin alone. Additionally, elevated serum vancomycin troughs (>15 mg/L) were a risk factor for developing nephrotoxicity only in the combination therapy group (vancomycin + TZP). Though the investigation did not reveal a statistically significant relationship between ICU admissions or concomitant nephrotoxic medications with the development of nephrotoxicity, these factors cannot be excluded as risk factors, particularly in patients receiving both vancomycin and TZP. In light of this research, increased monitoring of renal function and vancomycin troughs is warranted in patients receiving vancomycin and TZP simultaneously. In addition to observing daily trends in urine output, markers of renal function (i.e., BUN and serum creatinine) and vancomycin troughs should be assessed every 5 to 7 days in this group of patients. Furthermore, when goal trough concentrations are ≥15 mg/L and a patient is also receiving TZP, monitoring should be conducted closer to every 3 to 5 days to ensure no significant accumulation or nephrotoxicity.
ACKNOWLEDGMENTS
A preliminary portion of this manuscript was presented as a poster at the 22nd Annual Pediatric Pharmacy Advocacy Group (PPAG) Meeting Poster Presentations on May 3, 2013, in Indianapolis, Indiana.
Abbreviations
- BUN
blood urea nitrogen
- CI
95% confidence interval
- ICU
intensive care unit
- RR
relative risk
- TZP
piperacillin-tazobactam
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.McKamy S, Hernandez E, Jahng M et al. Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J Pediatr. 2011;158(3):422–426. doi: 10.1016/j.jpeds.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Burgess LD, Drew RH. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy. 2014;34(7):670–676. doi: 10.1002/phar.1442. [DOI] [PubMed] [Google Scholar]
- 3.Rybak M, Lomaestro B, Rotschafer JC et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Disease Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 4.Hazlewood KA, Brouse SD, Pitcher WD et al. Vancomycin-associated nephrotoxicity: grave concern or death by character assassination? Am J Med. 2010;123(2):182.e1–182.e7. doi: 10.1016/j.amjmed.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zosyn [package insert] Philadelphia, PA: Wyeth Pharmaceuticals Inc; 2011. [Google Scholar]
- 6.O'Rourke K. Vancomycin plus piperacillin-tazo may trigger acute kidney injury. Pharm Pract News. 2012;39 http://www.pharmacypracticenews.com/ViewArticle.aspx?d=Clinical&d_id=50&i=June+2012&i_id=853&a_id=20974. Accessed July 19, 2016. [Google Scholar]
- 7.Gomes DM, Smotherman C, Birch A et al. Comparison of acute kidney injury during treatment with vancomycin in combination with piperacillin-tazobactam or cefepime. Pharmacotherapy. 2014;34(7):662–669. doi: 10.1002/phar.1428. [DOI] [PubMed] [Google Scholar]
- 8.Pratt JA, Stricherz MK, Verghese PS, Burke MJ. Suspected piperacillin-tazobactam induced nephrotoxicity in the pediatric oncology population. Pediatr Blood Cancer. 2014;61(2):366–368. doi: 10.1002/pbc.24720. [DOI] [PubMed] [Google Scholar]
- 9.Buck ML. Vancomycin-associated nephrotoxicity and the use of higher trough concentrations in infants and children. Pediatr Pharm. 2014;20(10) [Google Scholar]
- 10.Jeffres MN, Isakow W, Doherty JA et al. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clin Ther. 2007;29(6):1107–1115. doi: 10.1016/j.clinthera.2007.06.014. [DOI] [PubMed] [Google Scholar]


