Abstract
OBJECTIVES: To determine if computerized provider order entry (CPOE) implementation impacts the time it takes for preterm neonates to reach their parenteral macronutrient goals.
METHODS: Retrospective review of neonates <1750 g receiving parenteral nutrition (PN) before and after the implementation of CPOE. Primary outcome was the attainment of parenteral macronutrient goals. Secondary outcomes included time to attainment, the frequency of electrolyte abnormalities, and the incidence of required adjustments made to PN orders by verification pharmacists.
RESULTS: Goal PN was achieved by 12/47 (25.5%) intervention vs. 2/44 (4.5%) control group infants (p < 0.05). This goal was attained in 10.8 ± 7.5 days in the intervention group and 10 ± 4.2 days in the control group (p = 0.90). Goal protein was reached by 74.5% of CPOE patients vs. 36.4% of controls, p < 0.05. Lipid goals were achieved by 98% vs. 100% (p = 0.33) of patients and were attained at an average of 1.5 ± 0.8 days vs. 2.0 ± 1.1 days (p < 0.05). Abnormal serum electrolyte values occurred more frequently in the control group (0.79 vs. 1.12/day PN). Adjustments by a verification pharmacist were required in 5.6% of CPOE compared with 30.4% of control group orders (p < 0.05).
CONCLUSIONS: CPOE parenteral nutrition increased the proportion of preterm neonates attaining overall macronutrient goals. With CPOE, protein goals were reached by more patients and goal lipids were achieved faster. This system also decreased the number of pharmacist interventions during verification of PN orders and appeared to positively impact the incidence of serum electrolyte disturbances.
INDEX TERMS: computerized provider order entry, neonatal intensive care unit, parenteral nutrition, premature neonate
INTRODUCTION
Optimization of nutrition is an integral component of the medical management of critically ill neonates. The American Academy of Pediatrics recommends that postnatal growth of premature infants should mimic that of a normal fetus at their corresponding gestational age.1 Data suggest that aggressive nutritional practices are required to overcome energy deficits and adverse outcomes experienced by preterm infants.2,3 Early initiation of parenteral nutrition (PN) is critical to prevent induction of catabolism and growth failure.4
Malnutrition has serious implications for premature neonates, including decreased neuronal growth and myelination, resulting in lower neurocognitive function.5,6 In 124 infants weighing less than 1000 g, protein and energy intakes were found to be significantly and linearly associated with neurodevelopmental outcomes at 18 months corrected for gestational age.7 For every gram increase in weight there was a corresponding 0.03-point increase in Bayley Mental Developmental Index (MDI) scores.7 Each 10 kcal/kg/day provided to an infant was associated with a 4.6-point increase in MDI scores, and each gram per kilogram per day of protein intake was associated with an 8.2-point increase.7 The infants in this study received approximately 98% of their energy from PN.7 This research illustrates the impact that early and aggressive provision of PN has on development in these patients.
The estimated placental transfer of nutrients in the second and third trimester of fetal development is 8 to 10 mg/kg/min of glucose.8 Compared to term infants, preterm infants have higher metabolic demands and less fat and glycogen.8 Achieving a goal glucose infusion rate (GIR) of 10 to 12 mg/kg/min in these infants decreases energy deficits and decreases catabolism.2,8 In infants receiving only glucose, 1% of protein stores are lost each day.3 In utero, protein accretion is 3.5 to 4 g/kg/day.4 Attainment of adequate protein intake with 4 g/kg/day of parenteral amino acids increases anthropometric indices in premature infants and decreases time to regain birth weight.2,3,8,9 While glucose is used as the primary energy sources in utero, fat becomes the primary source of nutrition for an infant post delivery.10 To prevent fatty acid deficiency in neonates, at least 0.5 g/kg/day are needed.4 Early initiation of 3 g/kg/day of lipids has been shown to be safe in premature infants and is supported by current literature to improve weight gain.2,4,8,10
Standardized nutritional practices decrease variability in feeding-related outcomes.11 Implementing enteral nutrition protocols in preterm neonates has been associated with improved feeding outcomes, but little has been published regarding implementation of standardized PN practices in neonates.12,13 A recent publication regarding the development and implementation of computerized physician order entry (CPOE) for PN at a children's hospital demonstrated the ability to eliminate transcription errors and incorporate guideline-based nutritional recommendations.14 Use of nutrition guidelines and CPOE with decision support software for PN orders may help facilitate attainment of adequate nutrition. The purpose of this study is to evaluate the impact of CPOE on the attainment of macro-nutrient goals in preterm infants receiving PN in the neonatal intensive care unit (NICU).
MATERIALS AND METHODS
On April 1, 2014, UF Health Shands Children's Hospital implemented the use of CPOE for PN. Previously, all PN orders were placed by using handwritten order sets on which providers indicated the desired components of the order. The neonatal PN order set within our CPOE system differed from paper orders in several ways (Table 1). Goal amino acids (AAs) and lipids were set to default in the electronic order set, as it is standard of practice at our institution to start at goal lipids and protein rather than starting a defined lower amount and titrating up to goal over a series of days. Additionally, patients with a birth weight less than 1750 g receive an initial premade PN and intravenous lipid product on day of life 0 that is designed to provide a GIR of 4 mg/kg/min, protein of 4 g/kg/day, calcium of 1 mEq/kg/day, and lipids of 3 g/kg/day. Patient-specific PN is ordered on the next day of life, allowing for modifications of dextrose and electrolyte content, based on patient laboratory results and clinical status.
Table 1.
Comparison of Written versus Computerized Entry PN
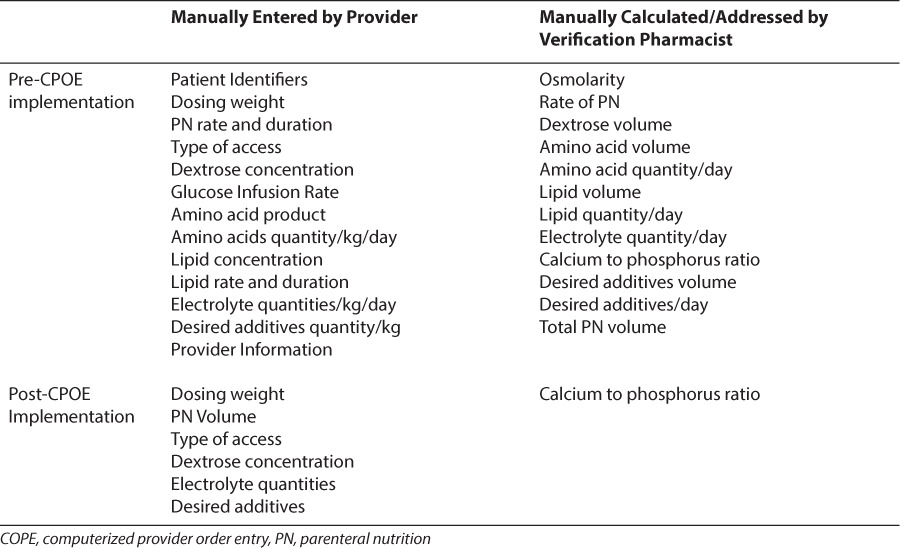
The PN order set contains an order summary panel with information regarding the volume of nutrition ordered (mL/kg/day), the GIR, calories (kcal/kg/day) for all components and individual components of the order (GIR, AA, lipid), and normal dose ranges for electrolytes. Micronutrients such as multivitamins, minerals, and trace elements are defaulted to the appropriate doses. Additives such as heparin are defaulted for central intravenous access. Laboratory values for the previous 48 hours are included in the order entry screen, and a PN report is available that trends the previous 4 days' PN order details (with up and down arrows to denote changes in ordered amounts of PN components). This institution is a tertiary care center with a 60 bed level III NICU. A single-center, retrospective, preimplementation and postimplementation analysis was conducted, comparing the attainment of macronutrient goals in premature neonates receiving PN.
Patients admitted to the NICU between October 1, 2013, and January 1, 2014, for the pre-intervention group, and between June 1, 2014, and September 30, 2014, for the postintervention group, were screened for inclusion into the study. Patients were identified through an electronic medical record report listing all infants in the NICU with an active order for PN on their first day of life. Patients were eligible for inclusion if they had a documented birth weight of less than 1750 g, were initiated on PN within the first 7 days of life, and continued to receive PN for at least 72 hours. Excluded were patients with a documented inborn error of metabolism, patients with delay in PN initiation past 72 hours, and patients who received PN administered through a peripheral intravenous line for greater than 48 hours. Umbilical arterial and venous catheters were considered central lines in this determination. Clinical pharmacist services, PN provider assistance, and a standardized enteral feeding protocol (Table 2) were present and remained consistent throughout both the control and intervention time frames.
Table 2.
Enteral nutrition feeding protocol

Endpoints
The primary objective was proportion of patients who achieved goal PN. Patients were only considered at goal if they met the targets for all 3 macronutrients concurrently. The goal glucose infusion rate, amino acids, and lipids were defined as 10 to 12 mg/kg/min, 4 g/kg/day, and 3 g/kg/day, respectively. The number of days required to achieve all 3 macronutrient goals concurrently was also determined. Other secondary objectives assessed the time to attainment of each of these goals individually, days to regain birth weight, number of electrolyte abnormalities, and number of episodes of hyperglycemia requiring treatment with insulin. The number of required adjustments that a pharmacist made to PN orders, as permitted by the institution's Pharmacy and Therapeutics Committee, was also evaluated during the preintervention and postintervention periods. These adjustments include decreases in amino acid, calcium, and phosphorous contents to allow for appropriate osmolarity and calcium-phosphorous precipitation prevention ratios.
Statistical Analysis
Patient demographics were evaluated by using descriptive statistics. Primary and secondary outcomes that were normally distributed continuous variables were analyzed by using the Student's t test. All categorical data outcomes were analyzed with chi square testing. An a priori level of significance calculation determined a required sample size of 78 total patients to detect a 20% difference within the primary outcome with a power of 0.8 and probability level of 0.05. This study was approved by the University of Florida Institutional Review Board and informed consent was not required.
RESULTS
A total of 193 patients were screened for enrollment, and 102 were excluded for the reasons outlined in the Figure. Ninety-one patients were included in the study (control = 44, intervention = 47). The baseline characteristics are outlined in Table 3. Most patients were born within our institution where it is protocol to give them a standardized bag of PN (initial PN). There were no significant differences with respect to sex, gestational age, birth weight, and APGAR scores. Patients in the intervention group had a smaller average birth length than the control group patients (34.98 vs. 36.97 cm, p = 0.0129). Enteral feeding advancement was completed per protocol and there was not a statistically significant difference in the number of PN days between the groups.
Figure.
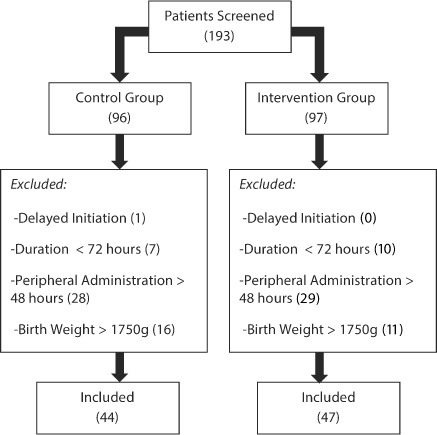
Patient Enrollment
Table 3.
Patient Demographics

Significantly more patients in the intervention achieved the primary outcome of goal PN than in the control group (25.5% vs. 4.5%, p < 0.05). The average time to attainment of this goal was 10 ± 4.2 days in the control group and 10.8 ± 7.5 days in the intervention group (p = 0.90)). Goal GIR was achieved in 29.8% of intervention patients and 20.5% of control patients (p = 0.31) with an average time to attainment of 10.5 ± 7.3 days among intervention group infants and 12.7 ± 5.2 days among controls. Table 4 illustrates that intervention group patients were more likely to attain their AA goal, and while patients in both groups attained their goal lipids, the intervention group achieved this goal sooner. Patients in the intervention group required an average of 5.8 ± 4.4 days to regain their birth weight as compared to 6.2 ± 4.7 days in the control group (p = 0.68).
Table 4.
Secondary Outcomes

Laboratory value analysis showed fewer serum electrolytes outside of normal limits in the intervention group than the control group (0.79 vs. 1.12 abnormalities per day of PN therapy). No statistically significant differences were noted in the type of electrolyte disturbances experienced by patients, in the proportion of patients requiring insulin secondary to hyperglycemia (12.8% vs. 4.5%, p = 0.17), or the percentage of PN orders associated with insulin use. The incidence of adjustments made to PN orders by a verification pharmacist was 5.6% in the intervention group compared with 30.4% in the -control group (p < 0.05) (Table 4).
DISCUSSION
It has been previously reported that standardizing prescribing practices for nutrition in NICU populations increases caloric intake, the provision of macronutrients, and weight gain.15,16 The results of this study show that PN ordering though CPOE with clinical decision support increased the proportion of preterm neonates meeting the primary outcome of goal PN. Goal amino acids were reached by more patients, and while goal lipids were achieved by most patients in both groups, this goal was achieved significantly faster among the post-CPOE population. This process change also decreased the number of interventions required by pharmacists during verification of PN orders and appeared to have a positive impact on the incidence of serum electrolyte disturbances. Time to regain birth weight was not significantly different between the 2 groups, but other long-term growth indices were not evaluated because of the relatively short time frame of data collection for the purposes of the study.
These outcomes are supported by literature from Huston et al,17 concluding that the implementation of a PN calculator support tool helped neonatal providers adhere to goal amino acids and decreased interventions required by a pharmacist to correct PN errors. Modifications required by pharmacists in our study consisted most commonly of decreasing PN components to accommodate volume of PN ordered, osmolarity adjustments, and errors in the ordered amount of calcium and phosphorus, which exceeded the amount allowed per volume of fluid to prevent precipitation. The most notable decrease in required interventions in this study resulted from prompting physicians upon order entry when the total quantity of macronutrients ordered exceeded the total volume ordered. This is most often encountered when PN is being weaned or in volume-restricted patients. While CPOE resolved the need to decrease PN components for volume ordered and osmolarity adjustments, calcium and phosphorous ratios still had to be hand calculated as our current version of CPOE does not do this.
In addition to improved efficiency, similar computerized protocols have shown efficacy in decreasing the number of PN prescribing errors.18–20 While such evaluation was beyond the scope of this trial, these findings further support the implementation of CPOE with clinical decision support for neonatal PN.
Limitations to consider when evaluating the results of this trial include its single-center retrospective design and its small sample size. Additionally, this study used the attainment of intravenous macronutrient goals as surrogate marker for appropriate growth and development, which may not adequately capture all facets leading to optimal growth velocity. Also notable are potential confounders including the education and prior experience of ordering providers, as many of these patients were cared for on academic teaching services by medical residents, and others were cared for by midlevel practitioners. Comorbid conditions or presence of systemic infection were not exclusion criteria but could affect an individual patient's macronutrient needs. Lastly, the provision of enteral nutrition was not accounted for, as the enteral nutrition goals and types of feeds for each patient could vary significantly. It is possible that many of these patients were in fact achieving their overall macronutrient goals but were doing so with a combination of enteral and parenteral nutrition. While this factor helps to explain why some patients in each group did not achieve PN goals, based upon the lack of difference in days of PN between the 2 groups, it still appears the CPOE group yields a higher proportion of patients achieving PN goals with a comparable amount of time receiving PN.
CONCLUSION
CPOE implementation appears to improve some aspects of nutritional provision practices in NICU patients. The increased attainment of macronutrients demonstrated in this trial may have clinically significant long-term influence on the growth and neurodevelopment of these infants. Our system helps to guide clinicians towards evidence-based nutritional advancement while still providing them the freedom to adjust PN intake as necessary. In addition to improved nutritional provision, improved workflow processes were demonstrated by a significant decrease in interventions required by a pharmacist in the post CPOE time frame, which likely helps to justify the value of clinical decision support systems in other institutions.
ACKNOWLEDGMENTS
An abstract of this research was presented on December 6, 2014, at the University HealthSystem Consortium Pharmacy Council Meeting at the ASHP Midyear Clinical Meeting 2014 in Anaheim, California. A poster showing the results of this research was presented at the University HealthSystem Consortium Pharmacy Council Meeting at the ASHP Midyear Clinical Meeting 2015 in New Orleans, Louisiana. The findings of this study were also presented on May 8, 2014, at the Florida Residency Conference at the University of Florida Health Shands Hospital in Gainesville, Florida. The authors of this manuscript would like to acknowledge Ivett Hernandez, PharmD, for her contributions towards this trial.
Abbreviations
- AA
amino acid
- CPOE
computerized provider order entry
- GIR
glucose infusion rate
- MDI
Mental Developmental Index
- NICU
neonatal intensive care unit
- PN
parenteral nutrition
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The primary author, Kyle A. Franco, PharmD, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.American Academy of Pediatrics Committee on Nutrition: nutritional needs of low-birth-weight infants. Pediatrics. 1985;75(5):976–986. [PubMed] [Google Scholar]
- 2.Martin CR, Brown YF, Ehrenkranz RA et al. Nutritional practices and growth velocity in the first month of life in extremely low gestational age newborns. Pediatrics. 2009;124(2):649–657. doi: 10.1542/peds.2008-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thureen P, Melara D, Fennessey PV et al. Effect of low versus high intravenous amino acid intake on very low birth weight infants in the early neonatal period. Pediatr Res. 2003;53(1):24–32. doi: 10.1203/00006450-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Clark RH, Wagner CL, Merritt RJ et al. Nutrition in the neonatal intensive care unit: how do we reduce the incidence of extrauterine growth restriction. J Perinatol. 2003;23(4):337–344. doi: 10.1038/sj.jp.7210937. [DOI] [PubMed] [Google Scholar]
- 5.Ehrenkranz RA, Dusick AM, Vohr BR et al. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117(4):1253–1261. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 6.Cooke R, Foulder-Hughes L. Growth impairment in very preterm and cognitive and motor performance at 7 years. Arch Dis Child. 2003;88(6):482–487. doi: 10.1136/adc.88.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephens BE, Walden RV, Gargus RA et al. First-week protein and energy intakes are associated with 18 month developmental outcomes in extremely low birth weight. Pediatrics. 2009;123(5):1337–1343. doi: 10.1542/peds.2008-0211. [DOI] [PubMed] [Google Scholar]
- 8.Dinerstein A, Nieto RM, Solana CL et al. Early and aggressive nutritional strategy (parenteral and enteral) decreases postnatal growth failure in very low birth weight infants. J Perinatol. 2006;26(7):436–442. doi: 10.1038/sj.jp.7211539. [DOI] [PubMed] [Google Scholar]
- 9.Porcelli PJ, Jr, Sisk PM. Increased parenteral amino acid administration to extremely low-birth-weight infants during early postnatal life. J Pediatr Gastroenterol Nutr. 2002;34(2):174–179. doi: 10.1097/00005176-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Drenckpohl D, McConnell C, Gaffney S et al. Randomized trial of very low birth weight infants receiving higher rates of infusion of intravenous fat emulsions during the first week of life. Pediatrics. 2008;122(4):743–751. doi: 10.1542/peds.2007-2282. [DOI] [PubMed] [Google Scholar]
- 11.Street JL, Montgomery D, Alder SC et al. Implementing feeding guidelines for NICU patients <2000 g results in less variability in nutrition outcomes. J Parenter Enteral Nutr. 2006;30(6):515–518. doi: 10.1177/0148607106030006515. [DOI] [PubMed] [Google Scholar]
- 12.Kuzma-O'Reilly B, Duenas ML, Greecher C et al. Evaluation, development, and implementation of potentially better practices in neonatal intensive care nutrition. Pediatrics. 2003;111(4):e461–e470. [PubMed] [Google Scholar]
- 13.McCallie KR, Lee HC, Mayer O et al. Improved outcomes with a standardized feeding protocol for very low birth weight infants. J Perinatol. 2011;31(suppl):61–67. doi: 10.1038/jp.2010.185. [DOI] [PubMed] [Google Scholar]
- 14.MacKay M, Anderson C, Boehme S Frequency and severity of parenteral nutrition medication errors at a large children's hospital after implementation of electronic ordering and compounding [Epub ahead of print] Nutr Clin Pract. July 24, 2015. [DOI] [PubMed]
- 15.Skouroliakou M, Kouri K, Stathopoulou M et al. Comparison of two types of TPN prescription methods in preterm neonates. Pharm World Sci. 2009;31(2):202–208. doi: 10.1007/s11096-009-9281-4. [DOI] [PubMed] [Google Scholar]
- 16.Lenclen R, Crause-Manicet S, Narcy P et al. Assessment of implementation of a standardized parenteral formulation for early nutritional support of very preterm infants. Eur J Pediatr. 2006;165(8):512–518. doi: 10.1007/s00431-006-0124-1. [DOI] [PubMed] [Google Scholar]
- 17.Huston RK, Markell AM, McCulley EA et al. Computer programming: quality and safety for neonatal parenteral nutrition orders. Nutr Clin Pract. 2013;28(4):515–521. doi: 10.1177/0884533613490741. [DOI] [PubMed] [Google Scholar]
- 18.Lehmann CU, Conner KG, Cox JM. Preventing provider errors: online total parenteral nutrition. Pediatrics. 2004;113(4):748–753. doi: 10.1542/peds.113.4.748. [DOI] [PubMed] [Google Scholar]
- 19.Costakos DT. Of lobsters, electronic medical records, and neonatal total parenteral nutrition. Pediatrics. 2006;117(2):e328–e332. doi: 10.1542/peds.2005-1350. [DOI] [PubMed] [Google Scholar]
- 20.Skouroliakou M, Konsantinou D, Papasaranopoulous P et al. Computer assisted total parenteral nutrition for pre-term and sick term neonates. Pharm World Sci. 2005;27(4):305–310. doi: 10.1007/s11096-005-2462-x. [DOI] [PubMed] [Google Scholar]


