Abstract
BACKGROUND
Aortic dissection (AoD) is a serious complication of thoracic aortic aneurysm (TAA). Relative risk for AoD in relation to TAA etiology, incidence, and pattern after prophylactic TAA surgery are poorly understood.
OBJECTIVES
This study sought to determine the incidence, pattern, and relative risk for AoD among patients with genetically associated TAA.
METHODS
The population included adult GenTAC participants without AoD at baseline. Standardized core laboratory tests classified TAA etiology and measured aortic size. Follow-up was performed for AoD.
RESULTS
Bicuspid aortic valve (BAV) (39%) and Marfan syndrome (MFS) (22%) were the leading diagnoses in the studied GenTAC participants (n = 1,991). AoD occurred in 1.6% over 3.6 ± 2.0 years; 61% of AoD occurred in patients with MFS. Cumulative AoD incidence was 6-fold higher among patients with MFS (4.5%) versus others (0.7%; p < 0.001). MFS event rates were similarly elevated versus those in patients with BAV (0.3%; p < 0.001). AoD originated in the distal arch or descending aorta in 71%; 52% of affected patients, including 68% with MFS, had previously undergone aortic grafting. In patients with proximal aortic surgery, distal aortic size (descending thoracic, abdominal aorta) was larger among patients with AoD versus those without AoD (both p < 0.05), whereas the ascending aorta size was similar. Conversely, in patients without previous surgery, aortic root size was greater in patients with subsequent AoD (p < 0.05), whereas distal aortic segments were of similar size. MFS (odds ratio: 7.42; 95% confidence interval: 3.43 to 16.82; p < 0.001) and maximal aortic size (1.86 per cm; 95% confidence interval: 1.26 to 2.67; p = 0.001) were independently associated with AoD. Only 4 of 31 (13%) patients with AoD had pre-dissection images that fulfilled size criteria for prophylactic TAA surgery at a subsequent AoD site.
CONCLUSIONS
Among patients with genetically associated TAA, MFS augments risk for AoD even after TAA grafting. Although increased aortic size is a risk factor for subsequent AoD, events typically occur below established thresholds for prophylactic TAA repair.
Keywords: aortic aneurysm, aortic dissection, bicuspid aortic valve, Marfan syndrome
Aortic dissection (AoD) is a devastating complication of thoracic aortic aneurysm (TAA) that confers increased morbidity and mortality (1,2). Patients with genetically associated TAA are at increased risk for AoD, possibly due to structural alterations that impair vascular integrity. Alterations within the aortic wall vary in relation to the genetic etiology of TAA. Although previous research has reported that genetically associated TAA increases the risk of AoD (3–7), the magnitude of differential risk in relation to TAA etiology remains uncertain.
Beyond incidence, the structural predictors of AoD among patients with genetically associated TAA are not fully understood. Increased aortic size, an established risk factor for AoD, is used in consensus clinical guidelines as an indication for prophylactic surgical repair of TAA (8,9). Consistent with increased event risk, consensus guidelines use smaller size–based cutoffs for repair of genetically associated TAA than for sporadic TAA (8). Even when prophylactic surgery is performed, AoD risk may persist (10). Although surgical grafting addresses focal areas of TAA, the vascular substrate for AoD remains in nongrafted aortic segments. Improved understanding of the incidence and pattern of both native and postoperative AoD in the current therapeutic era is important for optimized surveillance and management of at-risk populations.
GenTAC (National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions) is a multicenter prospective registry of participants with genetically associated TAA (11). In accordance with established registry protocol, participants undergo imaging and clinical follow-up for aortopathy-related events. The aims of this study were to determine relative risk for AoD in relation to TAA etiology among a broad cohort that included patients who underwent prophylactic TAA surgery, and then to test the relationship between antecedent aortic size (on pre-dissection imaging) and subsequent AoD.
METHODS
The population included adult (≥18 years of age) GenTAC registry participants without previous AoD in whom clinical follow-up was available. To assure that the population included participants free of AoD and that events were not directly induced by baseline iatrogenic interventions (e.g., surgical manipulation), patients with AoD that was prompted or occurred close to (within 3 months) baseline registry evaluation were excluded from this study.
Full details of the GenTAC protocol, including eligibility criteria and data collection, have been reported (11,12). In brief, the registry is composed of patients with aortic aneurysm and associated genetic conditions, including Marfan Syndrome (MFS), Ehlers-Danlos syndrome, Loeys-Dietz syndrome, Turner syndrome, bicuspid aortic valve (BAV), and familial or premature (age <50 years) TAA. Registry enrollment was conducted at 8 tertiary care centers with expertise in clinical characterization and imaging of aortopathies. At each site, comprehensive clinical indices were collected in a standardized fashion and transferred to a data coordinating center (RTI International, Rockville, Maryland).
Informed consent for study participation was obtained at time of registry enrollment in accordance with Institutional Review Board–approved protocols 190 at each GenTAC participatory site.
IMAGE ANALYSIS
Aortic imaging (timing, modality) was performed at the discretion of local clinicians; images were de-identified at participatory sites and transferred to a central core laboratory (MedStar Health Research Institute, Washington, DC) for analysis. Echocardiography, computed tomography (CT), and magnetic resonance imaging (MRI) were all accepted modalities per the registry protocol.
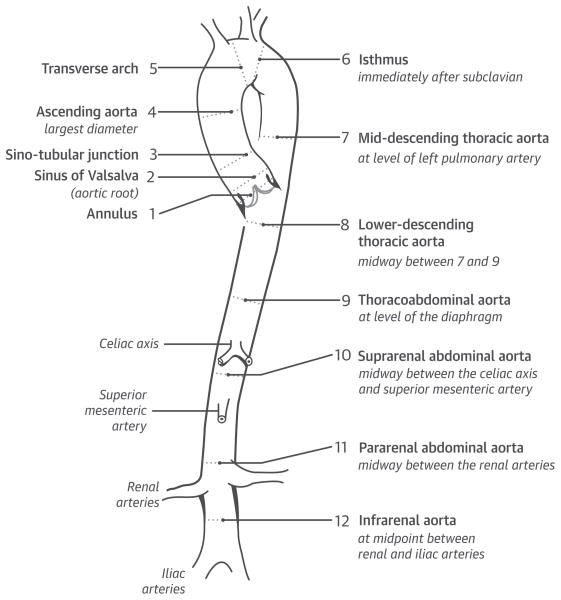
Image analysis was performed blinded to clinical characteristics using a standardized protocol applied to all modalities (13). Aortic dimensions were measured in pre-specified segmental locations (Figure 1) as the largest cross-sectional dimension. Maximal aortic size in each segment was measured perpendicular to the long axis of the aorta. Echocardiographic measurements were performed at mid-systole whenever possible, using an inner-edge-to-inner-edge technique. For MRI and CT, a double oblique technique was used to measure the aorta, which entailed dimensional measurements from inner edge to inner edge (excluding the aortic wall). Only native aortic segments were included in data analysis; grafted aortic segments were excluded.
FIGURE 1. Anatomic Locations of Aortic Segments.
The locations of segments assessed for quantification of aortic size are shown. In each segment, aortic size was quantified based on maximal linear dimension perpendicular to the long axis of the vessel wall.
CLINICAL FOLLOW-UP
Follow-up was performed at pre-specified bi-annual intervals (in accordance with a priori registry design). Standardized data intake forms were used at each follow-up time point to assess incidence and treatment of AoD (e.g., surgery, graft placement, valve implantation) since the last in-person study evaluation. Follow-up was primarily based on primary clinical evaluation at GenTAC sites and supplemented by phone follow-up (for patients unable to undergo in-person clinical evaluation by GenTAC investigators). Data were acquired by dedicated GenTAC research coordinators (who had undergone previous training regarding event ascertainment for AoD and its related complications), and all reported events were verified by GenTAC investigators at each participatory site. Reported AoD was confirmed via review of medical records, including imaging and surgical reports (if performed). Follow-up duration was calculated in relation to baseline evaluation, which was defined for this study as the earliest date of imaging and/or clinical evaluation provided to the GenTAC registry. All AoD cases were confirmed at local GenTAC sites by study investigators with expertise in classification and management of aortopathies and their related complications.
STATISTICAL METHODS
Comparisons between groups with or without AoD were made using Student t test (expressed as mean ± SD) or the Mann-Whitney U test (median and interquartile range [IQR]) as appropriate for continuous variables. Categorical variables were compared using the chi-square test or using the Fisher exact test when there were <5 expected outcomes per cell. Univariable and multivariable logistic regression analyses were performed to evaluate associations between imaging parameters and AoD. Baseline characteristics were evaluated in relation to subsequent AoD events using Kaplan-Meier analysis. For Kaplan-Meier analyses, patients were censored at the date of the last follow-up, which was defined as the last primary clinical evaluation or phone follow-up. A 2-sided p < 0.05 was considered statistically significant. Statistical calculations were performed using SAS version 9.2 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
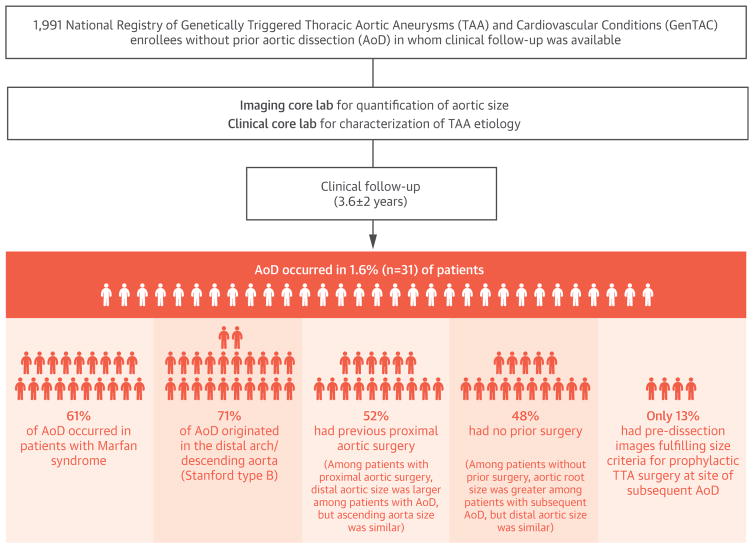
The population comprised 1,991 adult GenTAC registry participants without previous AoD for whom clinical follow-up was available as of December 2015. Follow-up was available in more than 80% of the adult GenTAC registry, including 88% of participants with BAV and 84% with MFS. GenTAC participants with follow-up versus those without follow-up were more commonly men (63% vs. 53%; p < 0.001) and slightly older (45 ± 15 years vs. 41 ± 14 years; p < 0.001). Nearly one-third (31%) of the adult GenTAC registry underwent aortic graft surgery in the proximal ascending aorta (root, ascending, or arch) at time of or before enrollment; the prevalence of proximal aortic graft surgery was similar between participants with and without follow-up (32% vs. 27%; p = 0.08).
Table 1 reports clinical characteristics of the study population, including comparisons between participants with and without follow-up AoD. As shown, AoD occurred in 1.6% (n = 31) of patients. AoD most commonly occurred among patients with MFS (61%; p < 0.001), but did not differ based on the history of aortic graft, valve repair, or replacement (both p = NS). Increased incidence of AoD occurred in patients with MFS despite younger age (38 ± 14 years vs. 46 ± 14 years; p < 0.001) and more common beta-blocker use (62% vs. 47%; p < 0.001) compared with the remainder of the population. Patients who underwent proximal aortic graft surgery at time of or before GenTAC enrollment were older (48 ± 14 years vs. 43 ± 14 years; p < 0.001) and more likely to be diagnosed with MFS (26% vs. 20%; p <0.001) or BAV (51% vs. 33%; p <0.001) compared with patients with nonproximal grafts.
TABLE 1.
Population Characteristics
| Overall (N = 1,991) | AoD+ (n = 31) | AoD − (n = 1,960) | p Value | |
|---|---|---|---|---|
| Age, yrs | 45 ± 15 | 43 ± 13 | 45 ± 15 | 0.5 |
|
| ||||
| Male | 1,251 (63) | 16 (52) | 1,235 (63) | 0.2 |
|
| ||||
| Enrollment diagnosis | ||||
| MFS | 428 (22) | 19 (61) | 409 (21) | |
| BAV | 770 (39) | 2 (6) | 768 (39) | |
| TUR | 101 (5) | 2 (6) | 99 (5) | |
| EDS | 77 (4) | 1 (3) | 76 (4) | <0.001* |
| Loeys-Dietz syndrome | 38 (2) | — | 38 (2) | |
| Other | 577 (29) | 7 (23) | 570 (29) | |
|
| ||||
| Coronary artery disease | 162 (8) | 4 (13) | 158 (8) | 0.3 |
|
| ||||
| Prior coronary intervention (CABG or PCI) | 67 (3) | 2 (6) | 65 (3) | 0.3 |
|
| ||||
| Hypertension | 541 (27) | 10 (32) | 531 (27) | 0.5 |
|
| ||||
| Cardiovascular medications | ||||
| Beta-blocker | 958 (50) | 21 (72) | 937 (50) | 0.02* |
| ACE inhibitor | 312 (16) | 3 (10) | 309 (16) | 0.5 |
| Angiotensin receptor blocker | 344 (17) | 7 (23) | 337 (17) | 0.5 |
| Calcium-channel blockers | 150 (8) | 6 (19) | 144 (7) | 0.03* |
| Statins | 386 (20) | 4 (13) | 382 (20) | 0.5 |
|
| ||||
| Previous aortic surgery | ||||
| Aortic valve repair or replacement | 570 (29) | 11 (35) | 559 (29) | 0.5 |
| Aortic graft implantation | 663 (33) | 12 (39) | 651 (33) | 0.6 |
| Aortic root | 530 (27) | 11 (35) | 519 (26) | 0.2 |
| Ascending aorta | 451 (23) | 8 (26) | 443 (23) | 0.7 |
| Aortic arch | 235 (12) | 2 (6) | 233 (12) | 0.6 |
| Descending aorta | 67 (3) | 1 (3) | 66 (3) | 0.9 |
| Proximal graft implantation† | 629 (32) | 12 (39) | 617 (31) | 0.4 |
Values are mean ± SD or n (%).
p < 0.05.
Aortic graft within root, ascending aorta or aortic arch.
ACE = angiotensin-converting enzyme; AoD = aortic dissection; BAV = bicuspid aortic valve; CABG = coronary artery bypass graft surgery; EDS = Ehlers-Danlos syndrome; MFS = Marfan syndrome; PCI = percutaneous coronary intervention; TUR = Turner syndrome.
TEMPORAL EVENT RISK
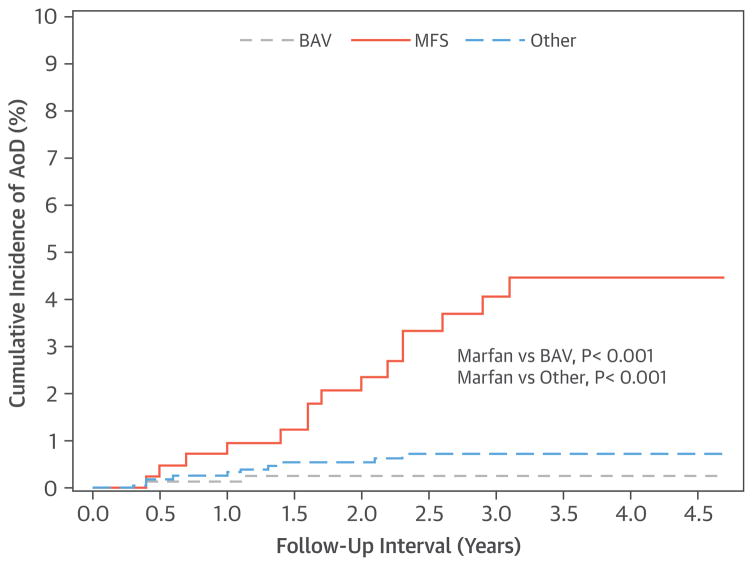
AoD was assessed during a mean follow-up of 3.6 ± 2.0 years from baseline evaluation. After 3 years of follow-up, the cumulative incidence of AoD was 6-fold higher in patients with MFS (4.5%) versus the remainder of the population (0.7%; log-rank p < 0.001). MFS event rates were similarly elevated compared with AoD rates among patients with BAV (0.3%; log-rank p < 0.001). Incidence of AoD was significantly higher among GenTAC participants with MFS compared with participants with BAV and the remainder of the adult GenTAC registry population (both p < 0.001) (Figure 2).
FIGURE 2. AoD Rates in Relation to Diagnostic Etiology of TAA.
Among GenTAC participants, those with Marfan syndrome (MFS) (orange line) demonstrated increased incidence of aortic dissection (AoD) compared with those with bicuspid aortic valve (BAV) (gray line), and the remainder of the adult GenTAC registry (blue line) (both p < 0.001).
Patient with MFS had a similarly high event risk when assessed in relation to a combined endpoint of AoD or death. After 3-year follow-up, cumulative event rates were 5.6% for patients with MFS, which was higher compared with the remainder of the study population (1.6%; log-rank p < 0.001) and the BAV subgroup (0.8%; log-rank p < 0.001). Regarding mortality alone, the cumulative incidence of death was higher for MFS versus BAV (1.9% vs. 0.5%; p = 0.03), with a similar trend for increased mortality in patients with MFS compared with the remainder of GenTAC participants (1.9% vs. 1.0%; p = 0.1). Of the 28 GenTAC participants who died during follow-up, AoD was reported as the cause of death for 2.
DISSECTION LOCATION
Table 2 reports AoD location among affected patients, as well as ancillary data concerning TAA etiology and presence and/or location of previous aortic grafts (for each patient who experienced events). In addition, pre-dissection aortic size (in native segments) is presented for patients with a type A dissection. More than one-half (52%) of patients, including two-thirds (68%) of those with MFS, underwent aortic graft implantation (median: 5 years; IQR: 0.8 to 13 years) before AoD; grafts typically involved the aortic root and/or ascending aorta. Regarding location, AoD was classified as Stanford type B in 22 (71%) cases. Among patients with pre-existing aortic grafts, AoD typically (14 of 16 or 88% of patients) occurred distal to grafted segments. In 1 case, AoD occurred proximal to the grafted segment and in another case, AoD was interposed between the proximal and distal grafted aortic segments.
TABLE 2.
AoD in Relation to Aneurysm Diagnosis and Previous Surgical History
| Subject # | Diagnosis | Dissection Type | Dissection Origin | Pre-Dissection Aortic Size (cm)§ | Imaging Interval (yrs) | Previous Aortic Graft | Graft Location | Previous Aortic Valve Repair or Replacement |
|---|---|---|---|---|---|---|---|---|
| 1 | MFS | A | Root | 4.6 | 1 | No | — | No |
| 2 | MFS | A | Ascending | 3.5 | 2.6 | No | — | No |
| 3 | MFS | A | Ascending | 3.3 | 7 | Yes | Root | Yes |
| 4 | MFS | A | Ascending | 4.2 | 5.6 | No | — | No |
| 5 | TUR | A | Root | 4.0 | 0.4 | No | — | No |
| 6 | BAV | A | Root | 4.2 | 0.4 | No | — | No |
| 7 | BAV | A | Root | 4.9 | 1.1 | No | — | Yes |
| 8 | Other | A | Root | 4.8 | 1.4 | No | — | No |
| 9 | Other | A | Ascending | 5.6 | 1.0 | No | — | No |
| 10 | MFS | B | Arch | 2.3 | Yes | Ascending, root | Yes | |
| 11 | MFS | B | Arch | 2.9 | Yes | Ascending, root, Descending, abdominal | Yes | |
| 12 | MFS | B | Descending | 2.2 | No | — | No | |
| 13 | MFS | B | Descending | 2.8 | Yes | Root | Yes | |
| 14 | MFS | B | Arch | 0.7 | Yes | Descending, abdominal | Yes | |
| 15 | MFS | B | Descending | 0.5 | Yes | Ascending, root | Yes | |
| 16* | MFS | B | Arch | 0.5 | Yes | Ascending | No | |
| 17 | MFS | B | Arch | 0.4 | Yes | Root | Yes | |
| 18 | MFS | B | Descending | 1 | No | — | No | |
| 19*† | MFS | B | Descending | 1.4 | Yes | Ascending, root | Yes | |
| 20 | MFS | B | Arch | 1.4 | No | — | No | |
| 21* | MFS | B | Arch | ‡ | Yes | Ascending, root | No | |
| 22 | MFS | B | Descending | 2.0 | Yes | Root | No | |
| 23 | MFS | B | Descending | 3.9 | Yes | Root | Yes | |
| 24† | MFS | B | Descending | 1.6 | Yes | Ascending, root | Yes | |
| 25 | TUR | B | Descending | 1.4 | No | — | No | |
| 26 | EDS | B | Descending | 0.3 | No | — | No | |
| 27*† | FTAAD | B | Descending | 0.6 | Yes | Ascending, root | Yes | |
| 28 | FTAAD | B | Arch | 0.2 | Yes | Ascending, root | Yes | |
| 29 | FTAAD | B | Arch | 7.4 | No | — | No | |
| 30 | FTAAD | B | Descending | 0.4 | No | — | No | |
| 31 | Other | B | Descending | 5.4 | Yes | Ascending, root | Yes |
Aortic graft implantation performed during interval between baseline evaluation and AoD (n = 4; range: 0.2 to 1.0 years).
Aortic valve replacement or repair performed during interval between baseline evaluation and AoD (n = 3; range: 0.4 to 0.5 years).
No pre-dissection image available.
Pre-dissection aortic size only obtained for patients with type A AoD.
FTAAD = familial thoracic aortic aneurysm and dissection with aortic enlargement; other abbreviations as in Table 1.
ANTECEDENT AORTIC SIZE
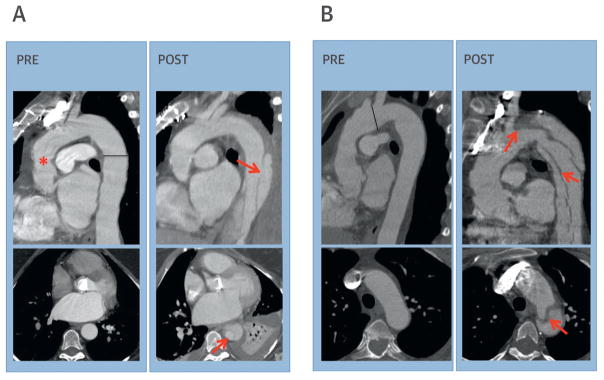
Pre-dissection imaging was available for 30 (97%) patients with subsequent events. Median interval between pre-dissection imaging and subsequent AoD was 1.4 years (IQR: 0.5 to 2.7 years). Echocardiography was the predominant modality used (18 of 30 patients); CT (n = 10) and MRI (n = 2) were less commonly used. Imaging strategies were similar among patients with and without AoD (echocardiography: 60% vs. 64%; CT: 33% vs. 28%; MRI: 7% vs. 8%; p = 0.8). Figure 3 provides representative illustrations of pre- and post-dissection imaging findings among patients with AoD.
FIGURE 3. Pre- and Post-Dissection Imaging.
(A) Pre-AoD (left): chest computed tomography (CT) performed 2 years before AoD in a 47-year-old woman with MFS displays a surgical graft in the ascending aorta (asterisk) and minimal dilation of the descending thoracic aorta (3.0 cm measured at line). Post-AoD (right), note dissection flap (arrow) originates in the descending thoracic aorta, distal to aortic graft. (B) Pre-AoD (left): chest CT performed 1 year before AoD in a 53-year-old woman with MFS demonstrates mild aortic arch dilation (3.1 cm measured at line). Post-AoD (right), note dissection flap originates in the distal aortic arch (arrow), extending throughout the descending thoracic aorta. Abbreviations as in Figure 2.
Among patients with previous proximal aortic graft implantation, 93% of dissections were type B (originating in the descending aorta or aortic arch). Among AoD cases in patients with no history of proximal aortic grafting, 50% were type A (originating in the aortic root or ascending aorta). Baseline aortic size was compared between patients with and without subsequent dissection among the overall GenTAC population (Table 3) and GenTAC patients with MFS (Table 4), inclusive of separate analyses for patients with and without proximal aortic surgery. Both Tables 3 and 4 demonstrate that among patients with proximal aortic surgery, distal aortic size (as measured in the descending thoracic and abdominal aorta) was larger among patients with AoD versus those without AoD (both p < 0.05), whereas ascending aorta size was similar (p = NS). Conversely, among patients without previous surgery, aortic root size was greater among patients with subsequent dissection, whereas distal aortic segments (descending thoracic and abdominal aorta) were of similar size (p = NS). Despite absolute differences in aortic size between groups, patients with AoD rarely had pre-dissection images that fulfilled the conventional criteria for aneurysmal dilation that necessitated prophylactic surgery. Among patients with type A AoD, only 1 of 9 (11%) had an aortic diameter ≥5.0 cm in either the root or ascending aorta; prevalence among participants without AoD was 11.4% (150 of 1,216; p = 0.9). Among patients with type B AoD, only 3 of 22 patients (14%) had a pre-dissection image that demonstrated an aortic diameter ≥5.5 cm in either the arch or descending thoracic aorta, whereas in participants without AoD, the prevalence was 0.3% (4 of 1,248, p < 0.001).
TABLE 3.
Antecedent Aortic Size in Relation to Subsequent AoD: Overall GenTAC Registry
| Overall Population (N = 1,596)
|
History of Proximal Aortic Surgery (n = 711)
|
No History of Proximal Aortic Surgery (n = 885)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AoD− (n = 1,566) | n | AoD+ (n = 30)† | n | p Value | AoD− (n = 696) | n | AoD+ (n = 15) | n | p Value | AoD− (n = 870) | n | AoD+ (n = 15) | n | p Value | |
| Aortic valve annulus | 2.5 (2.2–2.9) | 1,097 | 2.7 (2.5–3.2) | 22 | 0.02* | 2.7 (2.4–3.2) | 344 | 3.1 (2.6–4.2) | 7 | 0.048* | 2.4 (2.2–2.8) | 753 | 2.6 (2.3–3.0) | 15 | 0.12 |
|
| |||||||||||||||
| Sinuses of Valsalva | 3.9 (3.4–4.3) | 1,226 | 4.2 (3.9–4.8) | 21 | 0.01* | 4.1 (3.6–4.6) | 405 | 4.7 (4.3–5.3) | 6 | 0.1 | 3.8 (3.4–4.2) | 821 | 4.2 (3.9–4.6) | 15 | 0.03* |
|
| |||||||||||||||
| Sinotubular junction | 3.2 (2.8–3.7) | 1,141 | 3.5 (2.8–3.9) | 21 | 0.12 | 3.5 (3.0–4.0) | 349 | 3.0 (2.8–5.0) | 6 | 0.6 | 3.1 (2.8–3.6) | 792 | 3.5 (3.2–4.0) | 15 | 0.02* |
|
| |||||||||||||||
| Ascending aorta | 3.6 (3.0–4.2) | 1,135 | 3.7 (3.3–4.3) | 21 | 0.4 | 3.9 (3.2–4.8) | 362 | 3.5 (3.2–4.3) | 7 | 0.6 | 3.5 (2.9–4.0) | 773 | 3.7 (3.3–4.4) | 14 | 0.12 |
|
| |||||||||||||||
| Proximal arch | 3.0 (2.6–3.5) | 1,068 | 3.1 (2.9–3.4) | 21 | 0.08 | 3.1 (2.8–3.6) | 422 | 3.0 (2.9–3.3) | 10 | 0.9 | 2.8 (2.5–3.3) | 646 | 3.2 (3.0–3.5) | 11 | 0.02* |
|
| |||||||||||||||
| Transverse arch | 2.6 (2.2–3.0) | 1,055 | 2.8 (2.5–3.1) | 20 | 0.04* | 2.8 (2.4–3.1) | 434 | 2.6 (2.5–3.2) | 9 | 0.8 | 2.5 (2.1–2.8) | 621 | 2.8 (2.6–3.1) | 11 | 0.01* |
|
| |||||||||||||||
| Isthmus | 2.3 (1.8–2.6) | 872 | 2.5 (2.0–3.0) | 19 | 0.06 | 2.4 (2.2–2.8) | 366 | 2.6 (2.0–3.2) | 9 | 0.4 | 2.1 (1.7–2.4) | 506 | 2.3 (2.1–2.8) | 10 | 0.04* |
|
| |||||||||||||||
| Mid-descending thoracic aorta | 2.3 (2.0–2.7) | 838 | 2.7 (2.4–3.3) | 16 | 0.003* | 2.5 (2.2–2.8) | 362 | 3.3 (2.9–3.6) | 8 | 0.001* | 2.2 (1.9–2.6) | 476 | 2.5 (2.1–2.7) | 8 | 0.2 |
|
| |||||||||||||||
| Thoracoabdominal aorta | 2.2 (2.0–2.5) | 528 | 2.8 (2.3–3.1) | 11 | 0.006* | 2.3 (2.1–2.5) | 281 | 2.9 (2.8–3.1) | 6 | 0.02* | 2.1 (1.9–2.4) | 247 | 2.5 (2.3–2.6) | 5 | 0.13 |
|
| |||||||||||||||
| Suprarenal abdominal aorta | 2.1 (1.9–2.4) | 377 | 2.5 (2.4–2.8) | 9 | 0.004* | 2.2 (1.9–2.4) | 204 | 2.8 (2.6–3.0) | 5 | 0.002* | 2.0 (1.8–2.2) | 173 | 2.3 (2.0–2.4) | 4 | 0.2 |
|
| |||||||||||||||
| Infrarenal aorta | 1.9 (1.6–2.2) | 728 | 1.9 (1.8–2.7) | 14 | 0.03* | 1.9 (1.7–2.3) | 291 | 2.5 (2.2–3.9) | 7 | 0.01* | 1.8 (1.6–2.1) | 437 | 1.8 (1.7–1.9) | 7 | 0.7 |
Values are median (interquartile range [IQR]). All characteristics are measured in centimeters.
p<0.05.
Antecedent aortic size measurement was not available in 1 subject with aortic dissection.
Abbreviations as in Table 1.
TABLE 4.
Antecedent Aortic Size in Relation to Subsequent AoD: MFS Population
| MFS (n = 347)
|
History of Proximal Aortic Surgery (n = 175)
|
No History of Proximal Aortic Surgery (n = 172)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AoD− (n = 329) | n | AoD+ (n = 18) | n | p Value | AoD− (n = 163) | n | AoD+ (n = 12) | n | p Value | AoD− (n = 166) | n | AoD+ (n = 6) | n | p Value | |
| Aortic valve annulus | 2.5 (2.3–2.8) | 240 | 2.8 (2.5–3.5) | 12 | 0.03* | 2.7 (2.4–3.2) | 82 | 3.1 (2.6–4.2) | 6 | 0.14 | 2.4 (2.2–2.6) | 158 | 2.6 (2.3–2.9) | 6 | 0.23 |
|
| |||||||||||||||
| Sinuses of Valsalva | 4.0 (3.6–4.5) | 243 | 4.3 (3.9–4.6) | 11 | 0.13 | 4.2 (3.7–4.9) | 81 | 4.5 (4.3–4.9) | 5 | 0.45 | 4.0 (3.6–4.2) | 162 | 4.2 (3.9–4.3) | 6 | 0.23 |
|
| |||||||||||||||
| Sinotubular junction | 3.1 (2.8–3.6) | 219 | 3.2 (2.8–3.9) | 11 | 0.44 | 3.4 (2.9–3.8) | 63 | 3.0 (2.8–3.2) | 5 | 0.3 | 3.1 (2.8–3.4) | 156 | 3.5 (3.2–3.9) | 6 | 0.04* |
|
| |||||||||||||||
| Ascending aorta | 3.0 (2.8–3.4) | 218 | 3.6 (3.3–3.7) | 12 | 0.003* | 3.1 (2.9–3.5) | 75 | 3.4 (3.1–3.7) | 6 | 0.16 | 3.0 (2.7–3.4) | 143 | 3.7 (3.5–3.7) | 6 | 0.01* |
|
| |||||||||||||||
| Proximal arch | 2.8 (2.5–3.1) | 230 | 3.0 (2.9–3.3) | 13 | 0.01* | 2.9 (2.7–3.3) | 114 | 2.9 (2.8–3.2) | 8 | 0.8 | 2.6 (2.4–2.9) | 116 | 3.1 (3.0–3.5) | 5 | 0.003* |
|
| |||||||||||||||
| Transverse arch | 2.4 (2.2–2.8) | 238 | 2.8 (2.5–3.1) | 12 | 0.008* | 2.7 (2.4–3.0) | 112 | 2.6 (2.4–3.2) | 7 | 0.6 | 2.3 (2.0–2.5) | 126 | 2.8 (2.7–3.1) | 5 | 0.004* |
|
| |||||||||||||||
| Isthmus | 2.1 (1.8–2.4) | 198 | 2.3 (2.0–2.7) | 11 | 0.24 | 2.4 (2.1–2.7) | 94 | 2.5 (1.8–2.7) | 7 | 0.9 | 1.9 (1.6–2.2) | 104 | 2.2 (2.1–2.5) | 4 | 0.07 |
|
| |||||||||||||||
| Mid-descending thoracic aorta | 2.3 (1.9–2.6) | 186 | 2.9 (2.4–3.3) | 8 | 0.008* | 2.5 (2.1–2.8) | 94 | 3.3 (2.6–3.4) | 6 | 0.02* | 2.1 (1.8–2.4) | 92 | 2.4 (2.1–2.6) | 2 | 0.31 |
|
| |||||||||||||||
| Thoracoabdominal aorta | 2.2 (1.9–2.4) | 94 | 2.8 (2.5–2.9) | 6 | 0.04* | 2.2 (2.1–2.5) | 67 | 2.9 (2.8–2.9) | 5 | 0.1 | 2.0 (1.9–2.2) | 27 | 2.5† | 1 | 0.24 |
|
| |||||||||||||||
| Suprarenal abdominal aorta | 2.2 (1.9–2.4) | 75 | 2.6 (2.5–3.0) | 5 | 0.005* | 2.3 (2.0–2.5) | 51 | 2.8 (2.5–3.8) | 4 | 0.02* | 2.0 (1.8–2.2) | 24 | 2.4† | 1 | 0.2 |
|
| |||||||||||||||
| Infrarenal aorta | 1.9 (1.6–2.2) | 200 | 2.2 (1.9–3.0) | 9 | 0.04* | 2.0 (1.7–2.4) | 91 | 3.0 (2.2–3.9) | 5 | 0.03* | 1.8 (1.6–2.1) | 109 | 1.8 (1.7–2.3) | 4 | 0.5 |
Logistic regression analysis (Table 5) demonstrated AoD to be strongly associated with MFS, which conferred a >7-fold increase in relative risk for AoD even after controlling for maximal aortic size on baseline aortic imaging (p < 0.001). Substitution of a binary size–based cutoff in the multivariate model (rather than aortic size as a continuum) demonstrated a maximum aortic diameter of ≥5.0 cm conferred a >3-fold increase in risk for subsequent AoD (odds ratio: 3.73; 95% confidence interval: 1.40 to 9.00; p = 0.005).
TABLE 5.
Multivariable Logistic Regression Model for Prediction of AoD
| Univariable Regression
|
Multivariable Regression†
|
|||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| MFS | 6.00 (2.93–12.82) | <0.001* | 7.42 (3.43–16.82) | <0.001* |
|
| ||||
| Maximum aortic diameter, cm | 1.62 (1.13–2.27) | 0.006* | 1.86 (1.26–2.67) | 0.001* |
|
| ||||
| Follow-up interval, yrs | 0.63 (0.49–0.80) | <0.001* | 0.58 (0.44–0.74) | <0.001* |
p<0.05.
Model chi-square test = 51.8 (p < 0.001); Hosmer and Lemeshow goodness-of-fit test: p = 0.24. CI = confidence interval; OR = odds ratio; other abbreviations as in Table 1.
DISCUSSION
This study demonstrates that patients with genetically associated TAA remain at risk for AoD in the current era (Central Illustration). Among 1,991 adults enrolled in GenTAC, 1.6% experienced AoD over 3.6 ± 2.0 years. AoD occurred despite frequent prophylactic surgical repair of TAA; 52% of affected patients had undergone previous aortic graft implantation that typically involved the aortic root and/or ascending aorta. Consistent with this, AoD most commonly originated in the distal aortic arch or descending thoracic aorta (22 of 31 patients or 71% with Stanford type B). Among patients with pre-existing aortic grafts, AoD typically (14 of 16 patients) occurred distal to grafted segments. Although pre-dissection imaging demonstrated absolute increases in aortic size among patients with AoD, the magnitude of difference was small, as evidenced by the fact that few (4 of 31) of the patients with AoD demonstrated aortic dilation (on pre-dissection imaging at the subsequent site of AoD) that met the criteria for de novo or repeat TAA repair based on current consensus guidelines (1 type A, 3 type B). MFS conferred increased risk for AoD even after controlling for maximal aortic size (odds ratio: 7.42; 95% confidence interval: 3.43 to 16.82; p < 0.001).
CENTRAL ILLUSTRATION. Aortic Dissection With Genetic Aortic Aneurysm.
Longitudinal data from the multicenter GenTAC registry demonstrated that patients with genetically associated thoracic aortic aneurysm (TAA) remained at high risk for aortic dissection (AoD) in the current era, and that risk for AoD persisted even after prophylactic surgical repair of TAA. Although increased aortic size is a risk factor for subsequent AoD, events can occur below established thresholds for prophylactic TAA repair. Marfan syndrome (MFS) conferred increased risk for AoD even after controlling for maximal aortic size.
We believe this is the first study to assess differential risk for AoD in a large-scale population with genetically associated TAA. Our finding that MFS conferred greater relative risk for AoD than did BAV was consistent with previous data; in a population-based cohort in Olmstead County, Minnesota, AoD occurred in 2 of 416 BAV patients, corresponding to an incidence of 3.1 cases per 10,000 patient-years (4). Among patients with MFS, AoD has been reported to occur in approximately 0.17% per year (7). A more recent study reported an increased risk among patients with MFS versus patients with BAV who underwent aortic valve replacement (14). However, this study excluded patients with TAA, leaving a substantial knowledge gap with respect to AoD risk after aortic grafting, as well as uncertainty with regard to diagnostic categorizations and follow-up events due to the fact that analysis was performed via a retrospective review of an administrative database. For our study, diagnostic categories were established via dedicated GenTAC investigators at experienced tertiary care centers, and dedicated follow-up was performed to confirm the incidence and location of AoD.
Despite increased relative risk with MFS, it is important to recognize that BAV is a far more common condition, and that BAV is itself associated with increased risk for AoD compared with the general population (4). Previous studies have also shown that aortic size modifies dissection risk among patients with BAV, as evidenced by longitudinal data by Wojnarski et al. (15), who showed that risk for type A dissection increased stepwise in relation to increases in aortic size in the aortic root and the ascending aorta. In our study, 0.3% of BAV patients developed AoD during a follow-up of 3.6 ± 2.0 years. Applied to the U.S. adult population (in which prevalence of BAV is ~1.37%) (16), our data correspond to a total of approximately 10,000 cases of AoD. In this context, our results highlight the need for dedicated clinical and imaging assessment when AoD is suspected in any patient with genetically associated TAA.
AoD incidence among patients with MFS in our cohort was higher than in previous large-scale population studies. After 3 years of follow-up, cumulative incidence of AoD was 4.5% among adult GenTAC participants with MFS. Previous research by Jondeau et al. (7) reported an annual event rate (AoD/death) of 0.17%, corresponding to a cumulative rate of 0.51% over 3 years (7). As for reasons explaining differential event rates, all patients in our cohort had TAA at enrollment; in addition, 33% had undergone aortic graft surgery, whereas Jondeau et al. (7) excluded such patients. Although this enabled us to assess AoD risk among a broad MFS cohort being treated via established therapeutic interventions, it is possible that patients with MFS referred for TAA surgery represent a high-risk group for whom AoD risk is elevated despite state-of-the-art clinical care. Consistent with this, 68% of the patients with MFS who developed AoD underwent previous aortic graft implantation. Importantly, most of the AoD events in our cohort were nonfatal. Possible explanations for differential rates of AoD and death include: 1) medical therapy and blood pressure lowering in most patients might have reduced rapid progression to aortic rupture; 2) previous proximal aortic grafting in many patients might have shifted the distribution of dissection types away from those with the highest risk; and 3) GenTAC patients in systematic follow-up at major centers were likely to have sought and received state-of-the-art medical and surgical treatment for incident AoD unusually fast. It is also possible that some GenTAC registry participants experienced fatal AoD that went unrecognized due to out-of-hospital events for which death was attributed to other causes (and autopsy was not performed to discern AoD).
Regarding imaging findings, our study adds to the published data that demonstrated that AoD can occur in at-risk patients even when aortic size is normal or minimally dilated (3,17–20). The results demonstrate that risk for AoD persisted even after TAA surgery and that AoD could occur within native aortic segments proximal or distal to prosthetic grafts. Our findings extend upon those of a recent study conducted among patients with MFS, in which previous prophylactic aortic surgery (i.e., graft placement) was associated with risk for subsequent AoD in distal aortic segments even after controlling for aortic size (10). Regarding mechanism, it is likely that patients with TAA undergoing aortic grafting represent a high-risk group in terms of intrinsic vascular properties that predispose them to aortic dilation and AoD; thus, our observed association between surgery and dissection reflects the fact that patients referred for prophylactic grafting are a high-risk phenotype for which risk for dissection (in nongrafted segments) persists after surgical intervention. It is also possible that aortic hemodynamics contributed to AoD risk in nonsurgical or post-operative patients. Prior studies have shown MFS to be associated with decreased distensibility and increased stiffness (21,22), both of which have also been shown to be altered in patients with BAV (23), as has hemodynamic flow (24). Despite well-documented changes in aortic physiology in the setting of native TAA, serial imaging changes in aortic flow and distensibility after graft surgery have yet to be investigated. Although it is possible that altered aortic flow or compliance contributed to AoD risk, dynamic imaging to address this issue was not available.
STUDY LIMITATIONS
First, although data analysis was standardized, imaging was performed using various modalities based on clinical decision-making at GenTAC sites. Although this enabled study of AoD in relation to “real-life” clinical pre-dissection imaging, it is possible that frequent use of echocardiography (64%) may have reduced the utility of imaging to assess for aneurysmal changes and predict AoD in the descending thoracic aorta. Second, timing of imaging was not standardized across GenTAC sites. Our results demonstrated that aortic size was associated with AoD independent of duration between image acquisition and subsequent AoD, supporting the notion that variability in timing of imaging did not systematically bias results. However, it is possible that imaging performed in closer proximity to clinical events might have demonstrated greater aortic dilation among patients with AoD. Finally, results might be biased due to the nature of GenTAC sites (major surgical referral centers) and registry enrollment, which was skewed toward a more severe group of patients with genetically associated aortopathies—adults with TAA, a substantial proportion of whom underwent previous aortic surgery. These considerations might limit generalization of current data across all diagnostic categories. For example, observed low rates of AoD in the Ehlers-Danlos syndrome and Loeys-Dietz syndrome categories are not indicative of diagnosis-specific risks overall and do not encompass risks associated with branch vessel dissection.
CONCLUSIONS
Results from the multicenter GenTAC registry highlight that patients with genetically associated TAA remain at substantial risk for AoD despite state-of-the-art care and conventional imaging at experienced centers. Findings provided further evidence that these patients are at risk for dissection even in the context of minimal aortic dilation and that risk for dissection in native aortic segments persists after prophylactic aortic grafting was performed. Although pre-dissection imaging demonstrated absolute increases in aortic size among patients with subsequent AoD, the magnitude of difference was small, as evidenced by the fact that few patients (13%) with AoD demonstrated aortic dilation at a subsequent site of AoD, meeting criteria for de novo or repeat TAA repair based on current consensus guidelines.
Taken together, these data support the need for new approaches to better predict AoD as may be potentially offered by biomarker-based risk stratification. Further research in the context of GenTAC and other population-based cohorts is warranted to determine whether novel imaging approaches and translational targets, such as genetic mutations or circulating biomarkers, provide incremental utility to predict AoD among at-risk populations.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
In patients with genetically associated TAAs, 1.6% experienced AoD over 3.6 ± 2.0 years. Although pre-dissection imaging identified increases in aortic diameter among those with subsequent dissection, few patients (13%) had dilation at the site of subsequent dissection that met criteria for intervention based on current guidelines. Patients with MFS were at higher risk of dissection even after adjustment for aortic diameter.
TRANSLATIONAL OUTLOOK
Additional research is needed to identify genetic or physiological markers that better stratify patients with genetically associated TAA for risk of dissection.
Acknowledgments
GenTAC supported by National Heart, Lung, and Blood Institute and National Institute of Arthritis, Musculoskeletal and Skin Diseases (HHSN268200648199C, HHSN268201000048C). Dr. LeMaire is a member of the Advisory Board for Baxter Healthcare; the clinical study principal investigator for Vascutek Terumo and Baxter Healthcare; and a clinical trial co-investigator for Medtronic, W.L. Gore & Associates, and GlaxoSmithKline. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- AoD
aortic dissection
- BAV
bicuspid aortic valve
- CT
computed tomography
- GenTAC
National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions
- MFS
Marfan syndrome
- MRI
magnetic resonance imaging
- TAA
thoracic aortic aneurysm
APPENDIX
For a list of the GenTAC Registry Investigators, please see the online version of this article.
References
- 1.Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]
- 2.Mehta RH, Suzuki T, Hagan PG, et al. Predicting death in patients with acute type A aortic dissection. Circulation. 2002;105:200–6. doi: 10.1161/hc0202.102246. [DOI] [PubMed] [Google Scholar]
- 3.Roman MJ, Rosen SE, Kramer-Fox R, Devereux RB. Prognostic significance of the pattern of aortic root dilation in the Marfan syndrome. J Am Coll Cardiol. 1993;22:1470–6. doi: 10.1016/0735-1097(93)90559-j. [DOI] [PubMed] [Google Scholar]
- 4.Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–12. doi: 10.1001/jama.2011.1286. [DOI] [PubMed] [Google Scholar]
- 5.Detaint D, Michelena HI, Nkomo VT, et al. Aortic dilatation patterns and rates in adults with bicuspid aortic valves: a comparative study with Marfan syndrome and degenerative aortopathy. Heart. 2014;100:126–34. doi: 10.1136/heartjnl-2013-304920. [DOI] [PubMed] [Google Scholar]
- 6.Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–98. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- 7.Jondeau G, Detaint D, Tubach F, et al. Aortic event rate in the Marfan population: a cohort study. Circulation. 2012;125:226–32. doi: 10.1161/CIRCULATIONAHA.111.054676. [DOI] [PubMed] [Google Scholar]
- 8.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55:1509–44. doi: 10.1016/j.jacc.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010) Eur Heart J. 2010;31:2915–57. doi: 10.1093/eurheartj/ehq249. [DOI] [PubMed] [Google Scholar]
- 10.den Hartog AW, Franken R, Zwinderman AH, et al. The risk for type B aortic dissection in Narfan syndrome. J Am Coll Cardiol. 2015;65:246–54. doi: 10.1016/j.jacc.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 11.Eagle KA. Rationale and design of the National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (Gen-TAC) Am Heart J. 2009;157:319–26. doi: 10.1016/j.ahj.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroner BL, Tolunay HE, Basson CT, et al. The National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC): results from phase I and scientific opportunities in phase II. Am Heart J. 2011;162:627–32. e1. doi: 10.1016/j.ahj.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asch FM, Yuriditsky E, Prakash SK, et al. The need for standardized methods for measuring the aorta: multimodality core lab experience from the GenTAC Registry. J am Coll Cardiol Img. 2016;9:219–26. doi: 10.1016/j.jcmg.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itagaki S, Chikwe JP, Chiang YP, et al. Long-term risk for aortic complications after aortic valve replacement in patients with bicuspid aortic valve versus Marfan syndrome. J Am Coll Cardiol. 2015;65:2363–9. doi: 10.1016/j.jacc.2015.03.575. [DOI] [PubMed] [Google Scholar]
- 15.Wojnarski CM, Svensson LG, Roselli EE, et al. Aortic dissection in patients with bicuspid aortic valve-associated aneurysms. Ann Thorac Surg. 2015;100:1666–74. doi: 10.1016/j.athoracsur.2015.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 17.Januzzi JL, Marayati F, Mehta RH, et al. Comparison of aortic dissection in patients with and without Marfan’s syndrome (results from the International Registry of Aortic Dissection) Am J Cardiol. 2004;94:400–2. doi: 10.1016/j.amjcard.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 18.Carlson M, Airhart N, Lopez L, Silberbach M. Moderate aortic enlargement and bicuspid aortic valve are associated with aortic dissection in Turner syndrome: report of the International Turner Syndrome Aortic Dissection Registry. Circulation. 2012;126:2220–6. doi: 10.1161/CIRCULATIONAHA.111.088633. [DOI] [PubMed] [Google Scholar]
- 19.Eleid MF, Forde I, Edwards WD, et al. Type A aortic dissection in patients with bicuspid aortic valves: clinical and pathological comparison with tricuspid aortic valves. Heart. 2013;99:1668–74. doi: 10.1136/heartjnl-2013-304606. [DOI] [PubMed] [Google Scholar]
- 20.Mimoun L, Detaint D, Hamroun D, et al. Dissection in Marfan syndrome: the importance of the descending aorta. Eur Heart J. 2011;32:443–9. doi: 10.1093/eurheartj/ehq434. [DOI] [PubMed] [Google Scholar]
- 21.Baumgartner D, Baumgartner C, Schermer E, et al. Different patterns of aortic wall elasticity in patients with Marfan syndrome: a noninvasive follow-up study. J Thorac Cardiovasc Surg. 2006;132:811–9. doi: 10.1016/j.jtcvs.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Groenink M, de Roos A, Mulder BJ, Spaan JA, van der Wall EE. Changes in aortic distensibility and pulse wave velocity assessed with magnetic resonance imaging following beta-blocker therapy in the Marfan syndrome. Am J Cardiol. 1998;82:203–8. doi: 10.1016/s0002-9149(98)00315-4. [DOI] [PubMed] [Google Scholar]
- 23.Nistri S, Grande-Allen J, Noale M, et al. Aortic elasticity and size in bicuspid aortic valve syndrome. Eur Heart J. 2008;29:472–9. doi: 10.1093/eurheartj/ehm528. [DOI] [PubMed] [Google Scholar]
- 24.Bissell MM, Hess AT, Biasiolli L, et al. Aortic dilation in bicuspid aortic valve disease: flow pattern is a major contributor and differs with valve fusion type. Circ Cardiovasc Imaging. 2013;6:499–507. doi: 10.1161/CIRCIMAGING.113.000528. [DOI] [PMC free article] [PubMed] [Google Scholar]