Abstract
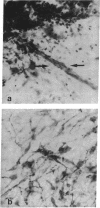
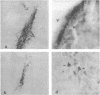
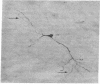
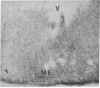
GT1 is an immortalized cell line that synthesizes and secretes the neurohormone gonadotropin-releasing hormone (GnRH). We have placed these cells into the brains of adult mutant hypogonadal (hpg) mice, which lack a functional GnRH gene, to determine whether such cells could differentiate in situ and support gonadal development. Immunocytochemical detection of GnRH revealed that these cells migrated widely in the central nervous system and elaborated axonal processes which on rare occasion projected to the normal target, the median eminence. Using a battery of antibodies, we demonstrated that these cells could cleave the GnRH precursor and that the amidated decapeptide as well as other cleavage products were present. The presence of biologically active material and its appropriate secretion were further documented by gonadal growth in both males and females. The morphological differentiation of the GT1 cells correlated with the density of cells injected. Those remaining within the injection site and/or forming a tumor retained a simple, rounded or fibroblastic appearance. Those cells that migrated into the host away from such tumors assumed the simple fusiform shape of normal GnRH neurons with dendrites extending from one or both poles. When cell density was drastically reduced a much more complex dendritic arbor was elaborated. These data suggest that such cell lines can be useful in reversing genetic defects and in studying such processes as GnRH neuronal migration, axonal targeting, and cytological differentiation.
Full text
PDF
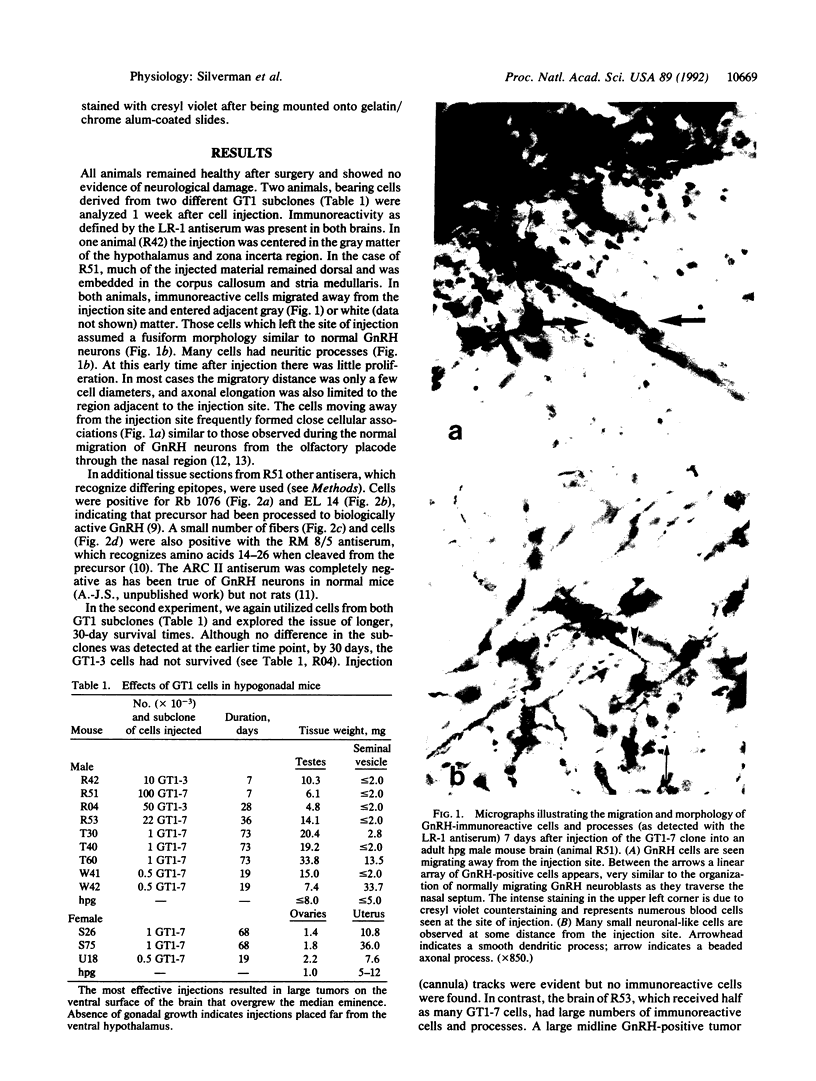
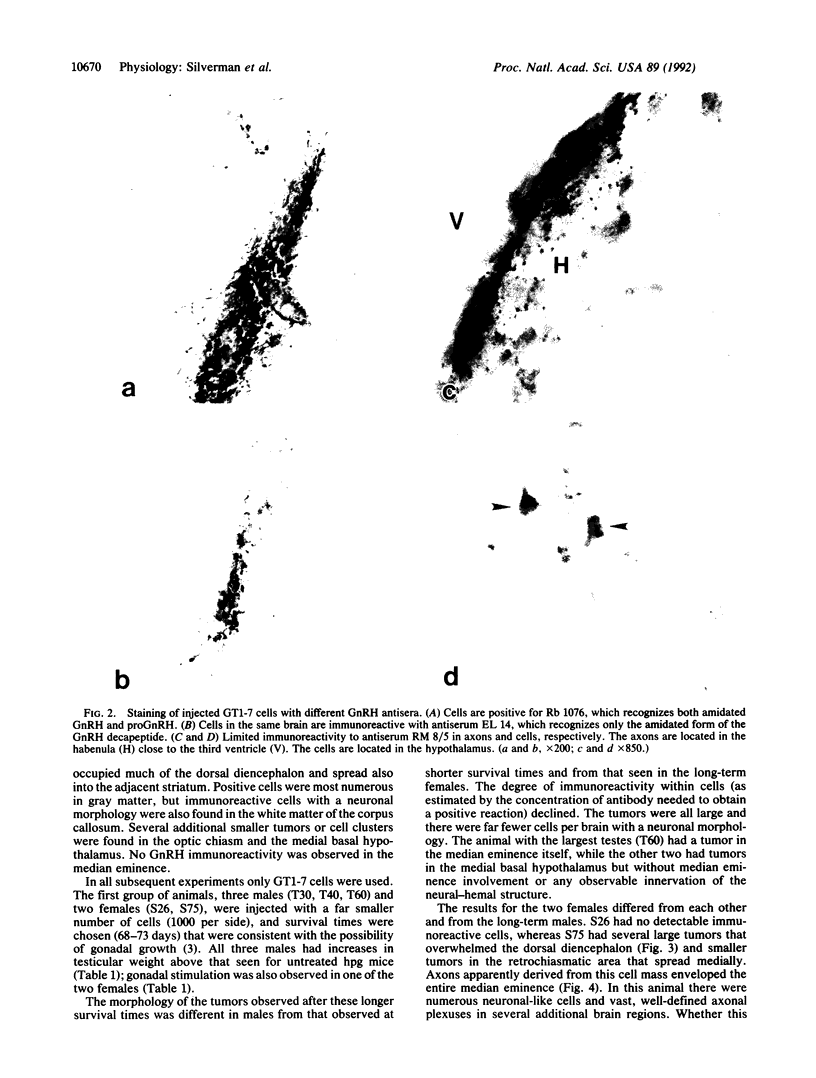
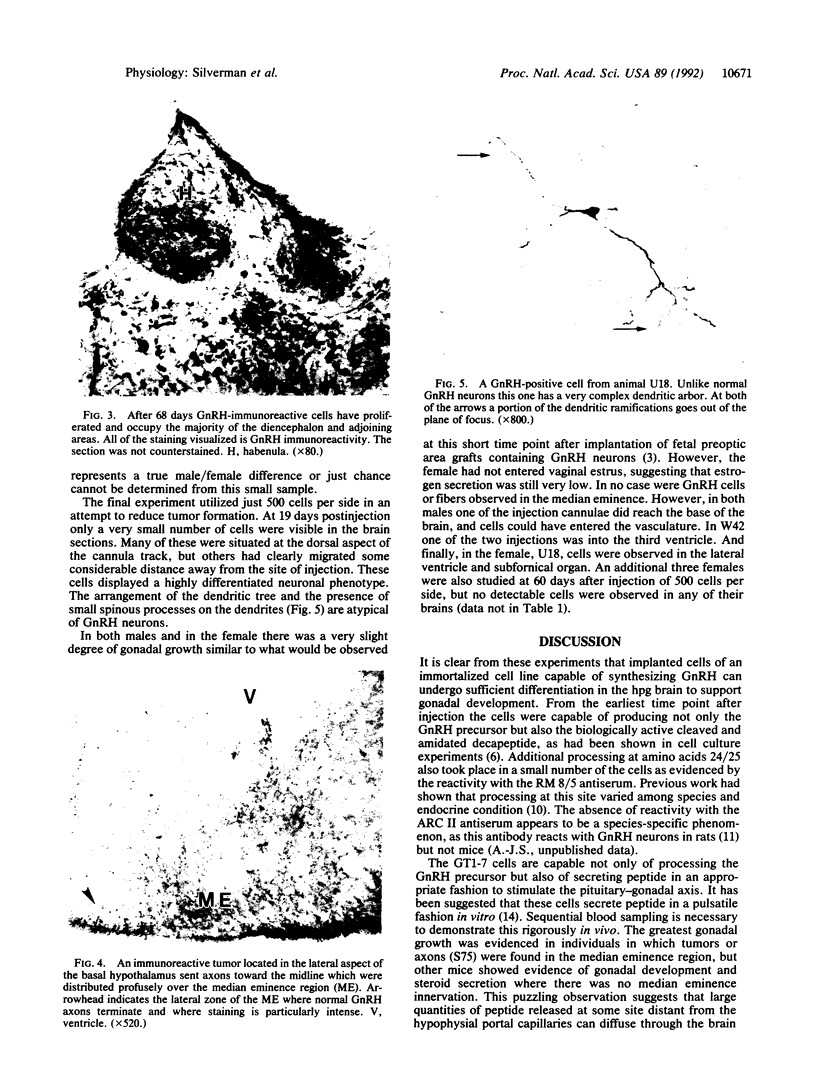

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ellinwood W. E., Ronnekleiv O. K., Kelly M. J., Resko J. A. A new antiserum with conformational specificity for LHRH: usefulness for radioimmunoassay and immunocytochemistry. Peptides. 1985 Jan-Feb;6(1):45–52. doi: 10.1016/0196-9781(85)90075-0. [DOI] [PubMed] [Google Scholar]
- Krieger D. T., Perlow M. J., Gibson M. J., Davies T. F., Zimmerman E. A., Ferin M., Charlton H. M. Brain grafts reverse hypogonadism of gonadotropin releasing hormone deficiency. Nature. 1982 Jul 29;298(5873):468–471. doi: 10.1038/298468a0. [DOI] [PubMed] [Google Scholar]
- Liposits Z., Merchenthaler I., Wetsel W. C., Reid J. J., Mellon P. L., Weiner R. I., Negro-Vilar A. Morphological characterization of immortalized hypothalamic neurons synthesizing luteinizing hormone-releasing hormone. Endocrinology. 1991 Sep;129(3):1575–1583. doi: 10.1210/endo-129-3-1575. [DOI] [PubMed] [Google Scholar]
- Martínez de la Escalera G., Choi A. L., Weiner R. I. Generation and synchronization of gonadotropin-releasing hormone (GnRH) pulses: intrinsic properties of the GT1-1 GnRH neuronal cell line. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1852–1855. doi: 10.1073/pnas.89.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A. J., Hayflick J. S., Zoeller R. T., Young W. S., 3rd, Phillips H. S., Nikolics K., Seeburg P. H. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science. 1986 Dec 12;234(4782):1366–1371. doi: 10.1126/science.3024317. [DOI] [PubMed] [Google Scholar]
- Mellon P. L., Windle J. J., Goldsmith P. C., Padula C. A., Roberts J. L., Weiner R. I. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990 Jul;5(1):1–10. doi: 10.1016/0896-6273(90)90028-e. [DOI] [PubMed] [Google Scholar]
- Ronnekleiv O. K., Naylor B. R., Bond C. T., Adelman J. P. Combined immunohistochemistry for gonadotropin-releasing hormone (GnRH) and pro-GnRH, and in situ hybridization for GnRH messenger ribonucleic acid in rat brain. Mol Endocrinol. 1989 Feb;3(2):363–371. doi: 10.1210/mend-3-2-363. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M., Pfaff D. W. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989 Mar 9;338(6211):161–164. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- Silverman A. J., Silverman R., Lehman M. N., Witkin J. W., Millar R. P. Localization of a peptide sequence contained in the precursor to gonadotropin releasing hormone (GnRH). Brain Res. 1987 Feb 3;402(2):346–350. doi: 10.1016/0006-8993(87)90042-4. [DOI] [PubMed] [Google Scholar]
- Silverman A. J., Witkin J. W., Millar R. P. Light and electron microscopic immunocytochemical analysis of antibodies directed against GnRH and its precursor in hypothalamic neurons. J Histochem Cytochem. 1990 Jun;38(6):803–813. doi: 10.1177/38.6.2186087. [DOI] [PubMed] [Google Scholar]
- Silverman A. J., Zimmerman E. A., Gibson M. J., Perlow M. J., Charlton H. M., Kokoris G. J., Krieger D. T. Implantation of normal fetal preoptic area into hypogonadal mutant mice: temporal relationships of the growth of gonadotropin-releasing hormone neurons and the development of the pituitary/testicular axis. Neuroscience. 1985 Sep;16(1):69–84. doi: 10.1016/0306-4522(85)90048-x. [DOI] [PubMed] [Google Scholar]
- Wetsel W. C., Mellon P. L., Weiner R. I., Negro-Vilar A. Metabolism of pro-luteinizing hormone-releasing hormone in immortalized hypothalamic neurons. Endocrinology. 1991 Sep;129(3):1584–1595. doi: 10.1210/endo-129-3-1584. [DOI] [PubMed] [Google Scholar]
- Wray S., Nieburgs A., Elkabes S. Spatiotemporal cell expression of luteinizing hormone-releasing hormone in the prenatal mouse: evidence for an embryonic origin in the olfactory placode. Brain Res Dev Brain Res. 1989 Apr 1;46(2):309–318. doi: 10.1016/0165-3806(89)90295-2. [DOI] [PubMed] [Google Scholar]