Abstract
Background
Coagulation factor XIII (FXIII) plays an important role in wound healing by stabilizing fibrin clots and crosslinking extracellular matrix proteins. FXIII is expressed in cells of the monocyte/macrophage and dendritic cell lineages in response to type-2 cytokines.
Objective
We sought to determine the association between FXIII and asthma pathobiology.
Methods
We analyzed the expression of FXIII mRNA and protein level in bronchoalveolar lavages obtained before and after segmental allergen challenge from mild asthma subjects, and in induced sputum samples collected from subjects with mild-moderate and severe asthma.
Results
FXIII mRNA and protein were highly upregulated in bronchoalveolar cells and fluid after allergen challenge and mRNA level correlated with protein amount. In sputum of asthmatic subjects, FXIII was positively correlated with type-2 immune response and markers of the dendritic cells (CD209 and CD207). FXIII expression was also associated with increased airflow limitation (FEV1/FVC and RV/TLC) and greater reversibility to β-agonist.
Conclusions
FXIII was upregulated in the airway of asthma subjects after allergen exposure. Its expression in the sputum of asthma patients correlated with the type-2 immune response and airflow limitation. Excessive activity of FXIII could contribute to the pathophysiology of airway obstruction in asthma.
Keywords: Factor XIII, asthma, severe asthma, allergy, pulmonary function, airway obstruction, air trapping, inflammation, eosinophils, IL-13
Introduction
Asthma is characterized by both airway inflammation and remodeling. Airway remodeling is reflected by thickening of the reticular basement membrane, mucus gland hypertrophy, deposition of extracellular matrix, increased smooth muscle mass and angiogenesis 1–4. Collectively, these changes contribute to persistent airflow limitation and asthma severity.
Airway inflammation is associated with plasma extravasation, and the exposure of plasma to tissue factor triggers a cascade of coagulation factors that leads to thrombin activation and fibrin clotting [reviewed in 5]. We and others have reported increased thrombin activity in airways of patients with asthma 6, 7. Wagers et al. have observed fibrin deposition in the airway of a patient who died in status asthmaticus 8. Mechanistically, the study by Wagers et al. suggested that fibrin reduces surfactant function which then ultimately leads to airway closure and hyperresponsiveness. In severe asthma, the pro-fibrinogenic pathway is increased compared with less severe disease 9, 10.
Factor XIII (FXIII) covalently cross-links fibrin at the end of the coagulation cascade. FXIII is a transglutaminase present extracellularly as plasma FXIII (pFXIII), and intracellularly as cellular FXIII (cFXIII). pFXIII is activated by thrombin and possesses a multitude of substrates that participate in the stability of the fibrin clot during the wound healing process 11, 12. These substrates include fibronectin, thrombospondin-1, α2-antiplasmin, thrombin-activatable fibrinolysis inhibitor, actin, von Willebrand factor, and plasminogen activator inhibitor-2. Because of its function to stabilize fibrin clots, FXIII plays a major role in acute thrombotic events such as myocardial infarction, ischemic stroke, deep vein thrombosis, and pulmonary embolism 13. Furthermore, fibrin plugs with or without eosinophilic inflammation are found in the life-threatening plastic bronchitis, which causes implicate several pulmonary diseases including asthma14, 15. However, the presence and role of FXIII in the airway of asthmatic subjects have yet to be analyzed. In vitro, allergen-activated peripheral blood mononuclear cells (PBMC) from asthmatic patients express more FXIII mRNA than untreated cells 16. Also, FXIII protein level was augmented in bronchoalveolar lavage fluid (BAL) from children with bronchoalveolar inflammation compared with lavage fluid from normal children 17. Recently, expression quantitative trait locus mappings identified cis-acting expression-associated variants in FXIII in relationship with asthma pathogenesis in children 18. In addition recently, accumulation of FXIII+ dendritic cells was reported in the lung tissue from individuals who died from asthma19.
FXIII expression is upregulated in IL-4- and IL-13-activated macrophages 20, 21 suggesting FXIII is a marker of alternatively activated macrophages. Production of FXIII in alternatively activated macrophages was further confirmed in nasal polyps from patients with chronic rhinosinusitis 22 where FXIII is thought to contribute to fibrin deposition. Using whole human genome expression microarrays, we have previously shown that FXIII was one of the transcripts upregulated in BAL cells following an in vivo segmental allergen challenge (SBP-Ag) in asthma 23.
Based on these observations we hypothesized that FXIII production by airway cells may be associated with type-2 immune characteristics and a loss of pulmonary function in asthma. To test this hypothesis, we analyzed FXIII in both BAL fluids after a SBP-Ag, and induced sputum samples from a group of asthmatic subjects enrolled in the Severe Asthma Research Program (SARP) at the University of Wisconsin.
Methods
Subjects, BAL cell preparations and study designs
The study protocol was approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board (IRB). Informed written consent was obtained from subjects prior to participation.
All 7 subjects undergoing the SBP-Ag and BAL were atopic, with at least one positive skin prick test. These subjects had a history of mild asthma with airway reversibility to albuterol. None of the subjects were using inhaled or oral corticosteroids. Detailed methods for bronchoscopy, SBP-Ag, and BAL cell preparation have previously been described 24. Blood eosinophils (EOS) were purified by negative selection as previously described 25. More details are provided in the online supplement.
Induced sputum was obtained by standard methods 26, 27 from 56 subjects enrolled in the Severe Asthma Research Program (SARP) at the University of Wisconsin. Additional details are provided in the online supplement. The subjects had severe (n=22) or non-severe asthma (n=34) as defined by the American Thoracic Society criteria. Sputum samples were processed in 2 sets. Set 1 (n=23) includes sputum samples obtained over a period of 12 months (2010 to 2011). Set 1 was used for association analyses between transcripts. 33 sputum samples (set 2) obtained between 2007 and 2010 were added to samples from set 1 for association analyses between FXIII with asthma characteristics. The subject characteristics were obtained as described in the online supplement.
RNA, real-time qPCR and ELISA
Total RNA was extracted from unfractionated BAL cells or purified BAL EOS using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Total RNA from sputum samples was extracted according to the Trizol Reagent manufacturer’s recommendations. Typically, 400–800 ng of total RNA were recovered from the sputum samples. The reverse transcription reaction was performed using the Superscript III system (Invitrogen/Life Technologies, Grand Island, NY, USA). Expression of mRNA was determined by qPCR using SYBR Green Master Mix (SABiosciences, Frederick, MD, USA). Data are expressed as fold change using the comparative cycle threshold (ΔΔCt) method as described previously 28. The values presented and used for correlations are fold change = (2ΔΔCt) compared to the lowest expression among the asthmatic subjects, which was fixed at 1. Sputum samples with housekeeping gene (GUSB) Ct values >25 were excluded of the study. More details are provided in the online supplement.
FXIII protein was measured in the BAL fluid using the Zymutest Factor XIII-A kit from Hyphen Biomed, France. Free FXIII or FXIII complexed with FXIIIB were both measurable by the ELISA kit. Samples were processed as recommended by the manufacturer. The assay sensitivity was ~1ng/ml.
Statistical analysis
To compare expression of genes in total BAL cells and purified BAL EOS by RT-qPCR or ELISA, data were analyzed using the Wilcoxon signed-rank test. Demographic factors were compared between non-severe and severe asthma using the Wilcoxon rank sum test and the chi square test for association. In the sputum, FXIII expression levels were compared among groups using the Wilcoxon rank sum test and to continuous measurements using the Spearman rank correlation coefficient. A trend test for an association between increasing ICS dose and FXIII expression was obtained by regressing log FXIII on ICS dose where dose levels none through high were re-coded as 0 through 3. Because FXIII expression tended to increase with age, all analyses were also performed using age-adjusted FXIII expression levels, which were calculated by regressing log FXIII on age and obtaining the standardized residuals. A two-sided p-value less than 0.05 was regarded as statistically significant.
Results
FXIII is increased in BAL after a SBP-Ag
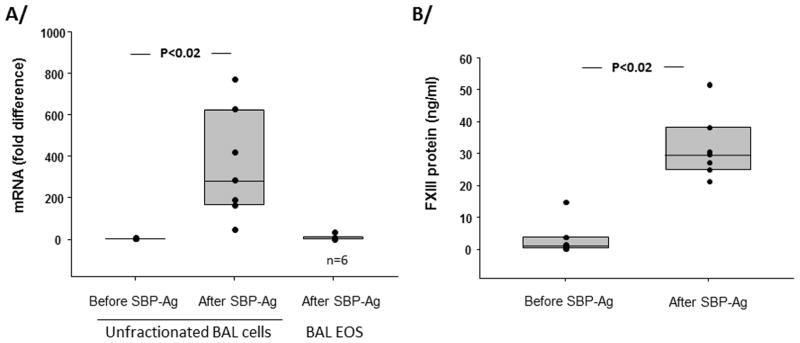
FXIII mRNA level was assessed by RT-qPCR of unfractionated BAL cells before and 48 h after SBP-Ag and BAL EOS purified 48 h after SBP-Ag (Figure 1). FXIII mRNA level increased 300-fold in BAL cells after SBP-Ag compared to BAL cells obtained from the same subjects at baseline (before allergen challenge). The main cell population (74 % of the total) recruited into the airway 48 h after SBP-Ag was EOS (Supplementary Table 2). FXIII mRNA was low in purified BAL EOS compared to unfractionated BAL cells (Figure 1) demonstrating that EOS are not the main source of FXIII mRNA. FXIII protein amount in BAL fluid was close to the sensitivity of the ELISA before SBP-Ag and increased to ~30 ng/ml after allergen challenge (Figure 1). In BAL fluid obtained 48 h after SPB-Ag, FXIII protein amount was correlated to mRNA levels (Supplementary Figure 1). The ratio of albumin to total protein was similar in BAL fluid and plasma (Supplementary Figure 1). In contrast, the ratio of FXIII to albumin in BAL fluid diverged from the ratio in plasma, suggesting FXIII-expressing cells rather than blood provided FXIII protein in the BAL fluid.
Figure 1. FXIII transcript and protein are increased in BAL after segmental bronchoprovocation with an allergen (SBP-Ag).
A/ FXIII mRNA was analyzed by RT-qPCR in unfractionated BAL cells before and 48h after SBP-Ag and purified EOS after SBP-Ag. Symbols indicate data from individual subjects, box plots are composite data from 7 subjects among whom 6 had highly purified EOS (>95%). B/ FXIII protein in BAL fluids before and after SBP-Ag was analyzed by ELISA. Box plots depict the median and the interquartile range between the 25th and 75th percentiles for 7 subjects.
FXIII mRNA level correlates with expression of markers of the type-2 immune response and expression of markers of the dendritic cell family
In a first set of the sputum samples (set 1), 23 asthmatic subjects were analyzed for FXIII expression vis-à-vis expression of several transcripts coding for type-2, type-1 or type-17 immune response, as well as cellular markers. The characteristics of these subjects are described in the Supplementary Table 3. Table 1 shows that FXIII mRNA level correlates with expression of a type-2 cytokine (IL-13) and chemokine (CCL17/TARC), and with the type-2 cytokine (IL-4 and IL-13)-induced membrane protein, CD23β/FcεRII.
Table 1.
Sputum FXIII mRNA level correlates with expression of markers of both the type-2 immune response and the dendritic cell family, n=23 (set 1)
| r | P value | |
|---|---|---|
| IL-13 mRNA | 0.48 | 0.02 |
| CCL17 mRNA | 0.68 | 0.0005 |
| IFN-γ mRNA | 0.23 | 0.30 |
| IL-17 mRNA | 0.21 | 0.35 |
| CD23β mRNA | 0.79 | <0.0001 |
| PF4 mRNA | − 0.04 | 0.84 |
| CD64 mRNA | 0.15 | 0.49 |
| CD163 mRNA | − 0.08 | 0.72 |
| CCL18 mRNA | 0.15 | 0.51 |
| CD209 mRNA | 0.68 | 0.0005 |
| CD207 mRNA | 0.55 | 0.008 |
FXIII is expressed by platelets and the monocyte/macrophage/dendritic cell family 29, 30. Therefore, expression of markers of these cell types was analyzed for association with FXIII. Platelet factor 4 (PF4) a marker of platelets, did not correlate with FXIII expression (Table 1). CD64, the high affinity immunoglobulin gamma Fc receptor 1 and CD163, which is induced by anti-inflammatory mediators (glucocorticoids or IL-10) and suppressed by pro-inflammatory cytokines (IFN-γ and TNF) 31, 32, are monocyte/macrophage specific markers. CD64 and CD163 did not relate to FXIII expression (Table 1). These findings led to an assessment of whether a restricted and specific monocyte/macrophage phenotype, such as alternatively activated (M2) macrophages, was the source of FXIII. Thus, in addition to CD163, CCL18 expression was measured and used as a marker of M2 macrophages; CCL18 did not show association with FXIII (Table 1). However, both CD209 (DC-SIGN, CLEC4L) a marker of immature dendritic cells (DC) and CD207 (CLEC4K) expressed by Langerhans cells highly correlated with FXIII expression levels (Table 1), suggesting that the DC family could be an important source of FXIII in sputum.
FXIII expression in sputum samples from non-severe versus severe asthmatic individuals
For further analysis of FXIII mRNA levels in relation to asthma severity and characteristics of asthma including pulmonary functions, 33 more asthmatic subjects (set 2) were added to set 1. The characteristics of the 56 asthmatic patients (34 non-severe and 22 severe) are described in Table 2. Patients with severe asthma were older (p=0.0002) and had reduced pulmonary functions compared to the non-severe asthma group (FEV1 % predicted, p=0.008). Markers of atopy (IgE and skin prick test) and sputum inflammation (EOS and neutrophils) were similar between severe and non-severe asthma. Also, of 56, 23 subjects were using no or low level of corticoids while 33 subjects were taking medium or high levels of corticoids, with 22 of 33 belonging to the severe population.
Table 2.
Subject characteristics, n=56
| Non-severe n=34 | Severe n= 22 | p | |
|---|---|---|---|
| Age | 24 [21, 36] | 46 [39, 53] | 0.0002 |
| Gender (female/male) | 15/19 | 11/11 | 0.67 |
| Caucasian race | 79% (3AA, 2H, 1AsA, 1NtA) | 82% (4AA) | 0.82 |
| FEV1 PP | 86 [75, 99] | 71 [61, 94] | 0.008 |
| % reversibility FEV1 PP | 11 [7, 20] | 14 [6, 26] | 0.48 |
| FEV1/FVC PP | 90 [81, 98] | 85 [74, 94] | 0.15 |
| Level of corticoid intake (none/low/medium/high) | 15/8/10/1 | 0/0/1/21 | <0.0001 |
| IgE (IU/ml) | 100 [38, 288] | 117 [36, 405] | 0.97 |
| Skin prick test + | 91% | 76% | 0.13 |
| Sputum eosinophils % | 0.8 [0.1, 2.4], n=31 | 0.7 [0.0, 5.3], n=18 | 0.92 |
| Sputum neutrophils % | 44 [29, 60], n=31 | 58 [43, 76], n=18 | 0.11 |
Median [25th, 75th]
AA, African American; H, Hispanic; AsA. Asian American; NtA, Native American; FEV1, force expiratory volume in 1 second; FVC, force vital capacity; % reversibility, reversibility after β-agonist; PP, % predicted.
P values (Wilcoxon rank sum test or chi square test for association) <0.05 indicates statistical significant differences between the non-severe and the severe groups.
The median of FXIII expression level was ~3.5 fold greater in the severe compared to the non-severe asthma population (45 [13, 80] vs. 13 [7, 27], p=0.02; Supplementary Figure 2A); though part of this difference may be explained by the severe group being older on average, as age-adjusted FXIII did not differ significantly between groups (p=0.23). Given that a primary difference between severe and non-severe asthma was the dose of inhaled corticosteroids, we analyzed whether FXIII levels were dependent on the daily corticosteroid doses. The subjects were divided in 4 groups composed of individuals taking either no (n=15), low (n=8), medium (n=11) or high (n=22) levels of corticoids (Supplementary Figure 2B). No significant association between FXIII and corticosteroid dose was seen (p=0.23).
FXIII expression and measures of pulmonary functions
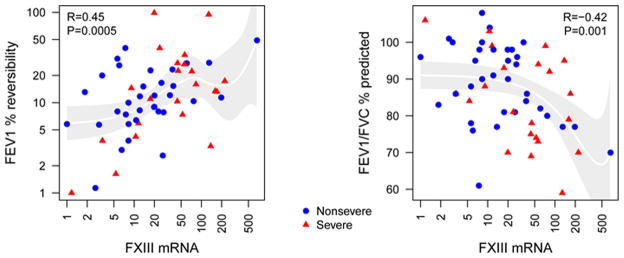
Because fibrin deposition is found in the lung tissue of severe asthmatic subjects and FXIII substrates include fibrin and fibronectin, the latter of which is a major factor in airway remodeling 33, we speculated that FXIII expression level would associate with decreased lung function. Table 3 shows inverse correlation between FXIII with the ratio FEV1/FVC % predicted (r= −0.42, p=0.001, n=56), and to a lesser extent with FEV1 or FEV1/FVC % predicted after β-agonist (FEV1/FVCMX PP). FXIII also correlated with reversibility of FEV1 to β-agonist (r= 0.45, p=0.005), and to RV/TLC % predicted (r=0.37, p=0.008) (Table 3). The association between FXIII and FEV1/FVC % predicted or reversibility of FEV1 to β-agonist were graphed and shown in Figure 2 where values from each independent subjects were plotted.
Table 3.
Sputum FXIII mRNA level correlates with pulmonary functions in asthma, n=56
| n | FXIII no adjustment | FXIII age-adjusted | |||
|---|---|---|---|---|---|
| r | p-value | r | p-value | ||
| % reversibility FEV1 PP | 56 | 0.45 | 0.0005 | 0.39 | 0.003 |
| FEV1/FVC PP | 56 | − 0.42 | 0.001 | − 0.35 | 0.008 |
| RV/TLC PP | 51 | 0.37 | 0.008 | 0.36 | 0.01 |
| FEV1/FVCMX PP | 56 | − 0.30 | 0.02 | − 0.21 | 0.11 |
| FEV1 PP | 56 | − 0.30 | 0.03 | − 0.20 | 0.15 |
% reversibility; reversibility after β-agonist; FEV1, force expiratory volume in 1 second; FVC, force vital capacity; MX, maximum after β-agonist; PP, percentage predicted; RV, residual volume; TLC, total lung capacity.
Figure 2. FXIII mRNA expression correlates with % reversibility of FEV1 % predicted to β-agonist, and with FEV1/FVC % predicted.
Some of the correlations described in Table 3 were graphed with non-severe (n=34) shown in blue circles and severe subjects (n=22) shown in red triangles.
The predicted pulmonary functions take into consideration ethnicity, height, sex, and age 34, 35. However, due to the difference of age in the severe versus non-severe population (Table 2), FXIII was also analyzed for correlation to lung physiology characteristics after an adjustment to age. Age-adjusted FXIII still correlated with FEV1/FVC % predicted, % reversibility of FEV1 to β-agonist and RV/TLC % predicted (Table 3). Collectively, these data suggest that higher airway FXIII expression may lead to increased airflow limitation and air-trapping.
Sputum FXIII expression and relationship with a type-2 environment
The majority of the asthmatic subjects included in this study were allergic (Table 2) as indicated by allergen sensitization (47 positive skin prick test of the 55 tested individuals). Also, 46 of the 56 asthmatic subjects had total blood IgE levels above 30 IU/ml, and 20 of the 51 sputum samples had ≥ 1.5% EOS.
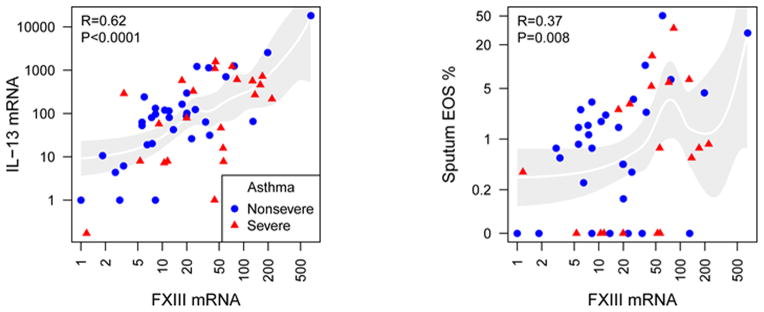
In accordance to Table 1 (set 1), FXIII was positively associated to IL-13 expression in sputum samples (Table 4). Also, FXIII correlated with total blood IgE, and sputum or blood EOS (Table 4). By contrast, macrophages inversely correlated with FXIII (Table 4). The association between FXIII and IL-13 mRNA level or the percentage of EOS in sputum samples were graphed and shown in Figure 3 where values from each independent subject were plotted.
Table 4.
FXIII mRNA level associates with characteristics of a type-2 environment, n=56
| n | FXIII no adjustment | FXIII age-adjusted | |||
|---|---|---|---|---|---|
| r | p-value | r | p-value | ||
| Total blood IgE | 56 | 0.28 | 0.04 | 0.35 | 0.009 |
| Sputum IL-13 mRNA | 55 | 0.62 | <0.0001 | 0.56 | <0.0001 |
| Sputum EOS % | 49 | 0.37 | 0.008 | 0.24 | 0.10 |
| Sputum PMN % | 49 | 0.24 | 0.10 | 0.22 | 0.12 |
| Sputum MAC % | 49 | − 0.44 | 0.001 | − 0.40 | 0.005 |
| Blood EOS % | 56 | 0.29 | 0.03 | 0.24 | 0.08 |
EOS, eosinophils; MAC, macrophages; PMN, neutrophils
Figure 3. FXIII mRNA expression correlates with IL-13 mRNA levels, and the percentage of EOS in sputum.
Some of the correlations described in Table 4 were graphed with non-severe (n=34) shown in blue circles and severe subjects (n=22) shown in red triangles.
After age adjustment, FXIII remained significantly associated with IgE and IL-13 with a trend for sputum and blood EOS (Table 4). These data further demonstrate a significant association between FXIII expression levels in airway cells and a type-2 environment in asthma.
Discussion
The role of the coagulation cascade proteins, particularly FXIII, is largely unknown in asthma. We found that FXIII was upregulated in the airway of mild asthmatic subjects after allergen challenge. Furthermore, FXIII expression in the sputum of mild-to-severe asthma subjects correlated with type-2 immune response and airway obstruction. Our results indicate FXIII may be a component of the molecular mechanisms that enhance airway obstruction in asthma. FXIII stabilizes fibrin clots, binds and activates fibroblasts, and increases collagen stabilization 36–40, all of which are mechanisms implicated in wound healing, tissue repair and remodeling. In addition, our study supports the hypothesis that FXIII expression is associated with the type-2 immune response, particularly with a type-2 cytokine (IL-13), chemokine (CCL17) and a type-2-cytokine-responsive gene (CD23β). These results are in accordance with reports showing upregulation of FXIII in IL-4- or IL-13-activated monocytes/macrophages 20, 21.
In addition to IL-4- or IL-13-activated monocytes/macrophages, differentiation of DC by IL-4 plus GM-CSF also induces cellular FXIII 41. FXIII protein has been found in the intracellular compartment of dermal DC, which express CD209 (DC-SIGN) and CD11b (ITGAM) [30 and reviewed in 42] and in leishmaniasis skin lesions, where they functioned as antigen-presenting cells similar to CD207+ Langerhans cells 43. We found that the expression of FXIII correlates with transcript levels of CD209 and CD207, suggesting that the DC may be an important source of FXIII in the airway of asthmatic subjects. Three main DC subsets have been reported in human lung 44, 45 but expression of CD209 or FXIII by these DC subsets has not been reported. The lack of association between FXIII expression and the presence of macrophages or markers of macrophages (CD163 or CCL18), suggests that M2 or type-2-cytokine-activated macrophages might not be the main source of FXIII in the airways of asthma patients. These data are in contrast of the reported findings in nasal polyps in patients with rhinosinusitis where FXIII expression was highly correlated with markers of M2 macrophages 22. However, our data agree with the work of Jayo et al, which found increased FXIII expression during differentiation of monocytes into DC using GM-CSF and the type-2 cytokine, IL-4 41. Nevertheless, our study suggests that, in vivo, and in asthma, type-2 cytokines might further favor differentiation and/or recruitment of FXIII+ DC rather than FXIII+ M2-macrophages. A recent work by Cagnoni et al 19 also supports that DC are the source of FXIII in the large airways of fatal asthma cases. However, we cannot rule out that other airway cells such as epithelial cells, which are known as fibrinogen producer cells, may also produce FXIII. The exact source of FXIII and the presence of FXIII in airway DC from asthmatic subjects will require further investigations.
In addition to its function as an extracellular protein, FXIII is also functional intracellularly. Intracellular FXIII (cFXIII) is activated by low Ca2+ concentrations in the absence of thrombin 46. cFXIII has many known intracellular substrates including, actin, myosin, vinculin, filamin, the type-1 angiotensin II receptor-associated protein and tubulin 11. Because of its known targets and its association with microfilaments 47, cellular FXIII function in cytoskeletal remodeling appears evident. Particularly, cFXIII has been implicated in the Fcγ and complement-mediated phagocytic activities of macrophages 48, and regulation of the migration of monocyte-derived DC 41. This latter function is important because DC migrate from tissue to lymph nodes, which is a crucial step in the development of the adaptive immune response. Although Cagnoni et al propose that FXIII+ DC remain resident in the lung tissue where they reactivate primed lymphoid cells19. Therefore, a high type-2 environment leading to higher FXIII expression by DC may induce or enhance the immune response to a specific allergen.
FXIII levels in the airway cells of asthmatic individuals undergoing an exacerbation were not evaluated. However, recruitment of FXIII+ cells and/or upregulation of FXIII expression in sputum correlated with airway obstruction (FEV1/FVC and RV/TLC) and reversibility indicating that FXIII expression level in sputum may change over time with asthma symptoms and exacerbation. Importantly, the patterns of airflow limitation, premature airway closure/air-trapping, and incomplete reversibility with bronchodilator are the characteristics of severe asthma 34, and indicate substantial dysfunction in peripheral airways. FXIII may prove to be a biomarker of the corticosteroid-refractory processes that underlie severe asthma.
The measurement of FXIII in various fluids from human subjects could be a challenge due to the numerous FXIII substrates and its presence in complex protein aggregates. Brims et al 9 expressed concerns regarding the measurement of FXIII in sputum from asthmatic subjects and suggested that FXIII was either trapped by fibrin in the tissue or degraded by neutrophil elastase. This dilemma demonstrates the suitability of measuring transcripts rather than protein in sputum samples, but it also indicates the need to develop antibodies that recognize substrate-bound FXIII. Despite this challenge, we were able to use ELISA to quantify FXIII protein in BAL fluid after SBP-Ag, while the levels in sputum samples were too close to the level of detection to make conclusive measurements. The inability to measure FXIII in the sputum may indicate that it is bound to other proteins, or that sufficient accumulation of FXIII in sputum samples might require asthma symptoms or exacerbations.
In conclusion, we analyzed FXIII expression in asthmatic subjects and demonstrated that airway FXIII production is upregulated by an in vivo allergen challenge in mild asthmatic patients. In addition, in mild-to-severe asthma, FXIII expression in sputum samples correlated with DC markers, type-2 environment and airflow limitation. These findings suggest a potential contribution of FXIII to asthma pathology and disease severity.
Supplementary Material
Supplemental Table 1: Primer sequences used for real-time PCR
Supplemental Table 2. Inflammatory cell populations in BAL before and 48h after segmental bronchoprovocation with an allergen (SBP-Ag).
Supplementary Table 3. Subject characteristics: Set 1
Key Messages.
Airway FXIII is allergen-induced in asthma
Airway FXIII associates with airflow limitation and characteristics of severe asthma
FXIII, a cross-linker of fibrin and fibronectin could contribute to pathophysiology in asthma
Acknowledgments
Funding: This work was supported by the Severe Asthma Research Program Grant R01 HL069116, U10 HL109168, Program Project Grant P01 HL088594, and Clinical and Translational Research Center Grant UL1 RR025011 and UL1 TR000427 from the National Institutes of Health.
The authors thank Erin Billmeyer, Michele Wolff and Holly Eversoll for patient recruitment, screening, and assessments; Gina Crisafi, Katie Popp and Helen Werner for preparing sputum samples; Gina Crisafi for protocol coordination; Larissa DeLain for laboratory technical support. We thank Mats Johansson for his inputs on the manuscript. We acknowledge contributions from all investigators, staff, and participants in the SARP study.
Abbreviations
- BAL
bronchoalveolar lavage
- DC
dendritic cells
- EOS
eosinophils
- FXIII
coagulation factor XIII
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- ICS
Inhaled corticosteroids
- RV
residual volume
- SARP
Severe Asthma Research Program
- SBP-Ag
segmental bronchoprovocation with an allergen
- TLC
total lung capacity
Footnotes
Conflicts of interest: none
This article has online Supporting Information
References
- 1.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma From bronchoconstriction to airways inflammation and remodeling. Am J Resp Crit Care Med. 2000;161:1720–45. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 2.Shifren A, Witt C, Christie C, Castro M. Mechanisms of remodeling in asthmatic airways. J Allergy (Cairo) 2012;2012:316049. doi: 10.1155/2012/316049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest. 1999;104:1001–6. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol. 2011;128:451–62. doi: 10.1016/j.jaci.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 5.Chambers RC. Procoagulant signalling mechanisms in lung inflammation and fibrosis: novel opportunities for pharmacological intervention? Br J Pharmacol. 2008;153(Suppl 1):S367–78. doi: 10.1038/sj.bjp.0707603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabazza EC, Taguchi O, Tamaki S, Takeya H, Kobayashi H, Yasui H, et al. Thrombin in the airways of asthmatic patients. Lung. 1999;177:253–62. doi: 10.1007/pl00007645. [DOI] [PubMed] [Google Scholar]
- 7.Terada M, Kelly EA, Jarjour NN. Increased thrombin activity after allergen challenge: a potential link to airway remodeling? Am J Respir Crit Care Med. 2004;169:373–7. doi: 10.1164/rccm.200308-1156OC. [DOI] [PubMed] [Google Scholar]
- 8.Wagers SS, Norton RJ, Rinaldi LM, Bates JHT, Sobel BE, Irvin CG. Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness. J Clinical Invest. 2004;114:104–11. doi: 10.1172/JCI19569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brims FJ, Chauhan AJ, Higgins B, Shute JK. Coagulation factors in the airways in moderate and severe asthma and the effect of inhaled steroids. Thorax. 2009;64:1037–43. doi: 10.1136/thx.2009.114439. [DOI] [PubMed] [Google Scholar]
- 10.de Boer JD, Majoor CJ, van ‘t Veer C, Bel EH, van der Poll T. Asthma and coagulation. Blood. 2012;119:3236–44. doi: 10.1182/blood-2011-11-391532. [DOI] [PubMed] [Google Scholar]
- 11.Richardson VR, Cordell P, Standeven KF, Carter AM. Substrates of Factor XIII-A: roles in thrombosis and wound healing. Clin Sci (Lond) 2013;124:123–37. doi: 10.1042/CS20120233. [DOI] [PubMed] [Google Scholar]
- 12.Muszbek L, Bereczky Z, Bagoly Z, Komaromi I, Katona E. Factor XIII: a coagulation factor with multiple plasmatic and cellular functions. Physiol Rev. 2011;91:931–72. doi: 10.1152/physrev.00016.2010. [DOI] [PubMed] [Google Scholar]
- 13.Muszbek L, Bereczky Z, Bagoly Z, Shemirani AH, Katona E. Factor XIII and atherothrombotic diseases. Semin Thromb Hemost. 2010;36:18–33. doi: 10.1055/s-0030-1248721. [DOI] [PubMed] [Google Scholar]
- 14.Seear M, Hui H, Magee F, Bohn D, Cutz E. Bronchial casts in children: a proposed classification based on nine cases and a review of the literature. Am J Respir Crit Care Med. 1997;155:364–70. doi: 10.1164/ajrccm.155.1.9001337. [DOI] [PubMed] [Google Scholar]
- 15.Brogan TV, Finn LS, Pyskaty DJ, Jr, Redding GJ, Ricker D, Inglis A, et al. Plastic bronchitis in children: a case series and review of the medical literature. Pediatr Pulmonol. 2002;34:482–7. doi: 10.1002/ppul.10179. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda T, Mochida S, Fukushima Y, Makino S. Detection of allergen-induced genes in peripheral blood mononuclear cells of patients with allergic asthma using subtractive hybridization. J Allergy Clin Immunol. 1995;96:1076–82. doi: 10.1016/s0091-6749(95)70193-1. [DOI] [PubMed] [Google Scholar]
- 17.Katona E, Nagy B, Kappelmayer J, Baktai G, Kovacs L, Marialigeti T, et al. Factor XIII in bronchoalveolar lavage fluid from children with chronic bronchoalveolar inflammation. J Thromb Haemost. 2005;3:1407–13. doi: 10.1111/j.1538-7836.2005.01353.x. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S, Zhou X, Thibault DM, Himes BE, Liu A, Szefler SJ, et al. A genome-wide survey of CD4 lymphocyte regulatory genetic variants identifies novel asthma genes. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cagnoni EF, Ferreira DS, Ferraz da Silva LF, Nicoletti Carvalho Petry AL, Gomes Dos Santos AB, Rodrigues Medeiros MC, et al. Bronchopulmonary lymph nodes and large airway cell trafficking in patients with fatal asthma. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Chaitidis P, O’Donnell V, Kuban RJ, Bermudez-Fajardo A, Ungethuem U, Kuhn H. Gene expression alterations of human peripheral blood monocytes induced by medium-term treatment with the TH2-cytokines interleukin-4 and -13. Cytokine. 2005;30:366–77. doi: 10.1016/j.cyto.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Torocsik D, Bardos H, Nagy L, Adany R. Identification of factor XIII-A as a marker of alternative macrophage activation. Cell Mol Life Sci. 2005;62:2132–9. doi: 10.1007/s00018-005-5242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R, et al. Increased expression of factor XIII-A in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2013;132:584–92. e4. doi: 10.1016/j.jaci.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esnault S, Kelly EA, Schwantes EA, Liu LY, Delain LP, Hauer JA, et al. Identification of genes expressed by human airway eosinophils after an in vivo allergen challenge. PLoS One. 2013;8:e67560. doi: 10.1371/journal.pone.0067560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, et al. Decreased expression of membrane IL-5R alpha on human eosinophils: I. Loss of membrane IL-5 alpha on eosinophils and increased soluble IL-5R alpha in the airway after antigen challenge. J Immunol. 2002;169:6452–8. doi: 10.4049/jimmunol.169.11.6452. [DOI] [PubMed] [Google Scholar]
- 25.Liu LY, Mathur SK, Sedgwick JB, Jarjour NN, Busse WW, Kelly EAB. Human airway and peripheral blood eosinophils enhance Th1 and Th2 cytokine secretion. Allergy. 2006;61:589–98. doi: 10.1111/j.1398-9995.2006.01060.x. [DOI] [PubMed] [Google Scholar]
- 26.Gern JE, Vrtis R, Grindle KS, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. American Journal of Respiratory and Critical Care Medicine. 2000;162:2226–31. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 27.Denlinger LC, Sorkness RL, Lee WM, Evans MD, Wolff MJ, Mathur SK, et al. Lower airway rhinovirus burden and the seasonal risk of asthma exacerbation. Am J Respir Crit Care Med. 2011;184:1007–14. doi: 10.1164/rccm.201103-0585OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esnault S, Kelly EA, Nettenstrom LM, Cook EB, Seroogy CM, Jarjour NN. Human eosinophils release IL-1β and increase expression of IL-17A in activated CD4(+) T lymphocytes. Clin Exp Allergy. 2012;42:1756–64. doi: 10.1111/j.1365-2222.2012.04060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muszbek L, Adany R, Szegedi G, Polgar J, Kavai M. Factor XIII of blood coagulation in human monocytes. Thromb Res. 1985;37:401–10. doi: 10.1016/0049-3848(85)90069-6. [DOI] [PubMed] [Google Scholar]
- 30.Nestle FO, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells in psoriasis. Autostimulation of T lymphocytes and induction of Th1 type cytokines. J Clin Invest. 1994;94:202–9. doi: 10.1172/JCI117308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van den Heuvel MM, Tensen CP, van As JH, Van den Berg TK, Fluitsma DM, Dijkstra CD, et al. Regulation of CD163 on human macrophages: cross-linking of CD163 induces signaling and activation. J Leukoc Biol. 1999;66:858–66. doi: 10.1002/jlb.66.5.858. [DOI] [PubMed] [Google Scholar]
- 32.Buechler C, Ritter M, Orso E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67:97–103. [PubMed] [Google Scholar]
- 33.Meerschaert JA, Kelly EA, Mosher DF, Busse WW, Jarjour NN. Segmental antigen challenge increases fibronectin in bronchoalveolar lavage fluid. American Journal of Respiratory and Critical Care Medicine. 1999;159:619–25. doi: 10.1164/ajrccm.159.2.9806053. [DOI] [PubMed] [Google Scholar]
- 34.Sorkness RL, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Chung KF, et al. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. Journal of Applied Physiology. 2008;104:394–403. doi: 10.1152/japplphysiol.00329.2007. [DOI] [PubMed] [Google Scholar]
- 35.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 36.Nemeth AJ, Penneys NS, Bernstein HB. Fibrous papule: a tumor of fibrohistiocytic cells that contain factor XIIIa. J Am Acad Dermatol. 1988;19:1102–6. doi: 10.1016/s0190-9622(88)70279-0. [DOI] [PubMed] [Google Scholar]
- 37.Barry EL, Mosher DF. Binding and degradation of blood coagulation factor XIII by cultured fibroblasts. J Biol Chem. 1990;265:9302–7. [PubMed] [Google Scholar]
- 38.Dardik R, Krapp T, Rosenthal E, Loscalzo J, Inbal A. Effect of FXIII on monocyte and fibroblast function. Cell Physiol Biochem. 2007;19:113–20. doi: 10.1159/000099199. [DOI] [PubMed] [Google Scholar]
- 39.Greenberg CS, Birckbichler PJ, Rice RH. Transglutaminases: multifunctional cross-linking enzymes that stabilize tissues. FASEB J. 1991;5:3071–7. doi: 10.1096/fasebj.5.15.1683845. [DOI] [PubMed] [Google Scholar]
- 40.Paye M, Nusgens B, Lapiere CM. Factor XIII of blood coagulation decreases the susceptibility of collagen precursors to proteolysis. Biochim Biophys Acta. 1991;1073:437–41. doi: 10.1016/0304-4165(91)90212-y. [DOI] [PubMed] [Google Scholar]
- 41.Jayo A, Conde I, Lastres P, Jimenez-Yuste V, Gonzalez-Manchon C. Possible role for cellular FXIII in monocyte-derived dendritic cell motility. Eur J Cell Biol. 2009;88:423–31. doi: 10.1016/j.ejcb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, et al. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–42. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 43.Sotto MN, Halpern I, Kauffman MR, Pagliari C. Factor XIIIa+ dermal dendrocyte parasitism in American tegumentary leishmaniasis skin lesions. Am J Dermatopathol. 2010;32:15–8. doi: 10.1097/DAD.0b013e3181ab4695. [DOI] [PubMed] [Google Scholar]
- 44.Gill MA. The role of dendritic cells in asthma. J Allergy Clin Immunol. 2012;129:889–901. doi: 10.1016/j.jaci.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 45.Bratke K, Lommatzsch M, Julius P, Kuepper M, Kleine HD, Luttmann W, et al. Dendritic cell subsets in human bronchoalveolar lavage fluid after segmental allergen challenge. Thorax. 2007;62:168–75. doi: 10.1136/thx.2006.067793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polgar J, Hidasi V, Muszbek L. Non-proteolytic activation of cellular protransglutaminase (placenta macrophage factor XIII) Biochem J. 1990;267:557–60. doi: 10.1042/bj2670557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adany R, Antal M. Three different cell types can synthesize factor XIII subunit A in the human liver. Thromb Haemost. 1996;76:74–9. [PubMed] [Google Scholar]
- 48.Sarvary A, Szucs S, Balogh I, Becsky A, Bardos H, Kavai M, et al. Possible role of factor XIII subunit A in Fcgamma and complement receptor-mediated phagocytosis. Cell Immunol. 2004;228:81–90. doi: 10.1016/j.cellimm.2004.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Primer sequences used for real-time PCR
Supplemental Table 2. Inflammatory cell populations in BAL before and 48h after segmental bronchoprovocation with an allergen (SBP-Ag).
Supplementary Table 3. Subject characteristics: Set 1