Abstract
BACKGROUND
Breast cancer is one of the most common malignancies in women and is associated with a variety of risk factors. The functional single-nucleotide polymorphism (SNP) C677T in the gene encoding 5,10-methylenetetrahydrofolate reductase (MTHFR) may lead to decreased enzyme activity and affect the chemosensitivity of tumor cells. This study was designed to investigate the association of MTHFR gene polymorphism (SNP) in the pathogenesis of breast cancer among the North Indian women population.
MATERIALS AND METHODS
Genotyping was performed by polymerase chain reaction (PCR) using genomic DNA, extracted from the peripheral blood of subjects with (275 cases) or without (275 controls) breast cancer. Restriction fragment length polymorphism was used to study C677T polymorphism in the study groups.
RESULTS
The distribution of MTHFR (C677T) genotype frequencies, ie, CC, TT, and CT, among the patients was 64.7%, 2.18%, and 33.09%, respectively. In the healthy control group, the CC, TT, and CT frequencies were 78.91%, 1.09%, and 20.1%, respectively. The frequencies of C and T alleles were 81.2% and 18.7%, respectively, in the patient subjects, while they were 88.9% and 11.09%, respectively, among the healthy control group. Frequencies of the CT genotype and the T allele were significantly different (P = 0.007 and P = 0.005, respectively) between the control and the case subjects.
CONCLUSION
This study shows an association of the CT genotype and the T allele of the MTHFR (C667T) gene with increased genetic risk for breast cancer among Indian women.
Keywords: MTHFR, breast cancer, genotype, PCR-RFLP, polymorphism
Introduction
Breast cancer (BC) is the leading cause of death among women worldwide and remains a major public health problem.1,2 Development of BC is a multistep process, arising from genetic alterations, and leads to the transformation of normal mammary epithelial cells into highly malignant derivatives.3 BC originates in the any part of the breast and is caused due to abnormal cell division and growth. Literature reveals that imbalance in folate metabolism may be involved in predisposition to BC.4
The folate metabolism pathway regulates the intracellular folate pool needed for the synthesis and methylation of DNA.5 In the folate metabolism pathway, methylenetetrahydrofolate reductase (MTHFR) is the key enzyme that catalyzes the irreversible reduction of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which is the primary circulating form of folate and the methyl donor in DNA methylation. MTHFR, a critical enzyme in one-carbon metabolism, is of interest because aberrations in DNA synthesis, repair, and methylation have been implicated in BC risk.6 The relationship of folate metabolism with carcinogenesis is based on its involvement in both nucleotide synthesis and DNA methylation. 5-Methyltetrahydrofolate is the methyl donor for MTHFR-mediated remethylation of homocysteine to methionine.7 Methionine is the precursor of S-adenosyl methionine, the universal methyl donor for biological methylation reactions, including DNA methylation.8,9 Involvement of MTHFR in DNA biosynthesis and repair makes it a susceptible candidate gene for BC.10 Recent reports have shown that thymidylate deficiencies may result in the disincorporation of uridylate into DNA, causing an increase in the rates of DNA strand breaks and chromosomal damage.11
Human MTHFR gene is composed of 11 exons encoding a protein of 656 amino acids. It is located on the short arm of Chromosome 1 and has two promoters and isoforms (70 kDa and 77 kDa).12,13 Two common allelic variants of the MTHFR gene [C677T (rs1801133) and A1298C (rs1801131)] have been described for the Ala222Val and Glu429Ala amino acid substitutions, respectively, and this variation plays a role in decreased enzyme activity as well.14–16 The substitution of cytosine (C) with thymine (T) at nucleotide 677 in the MTHFR gene is a common polymorphism (C677T) and is correlated with increased thermolability and reduced MTHFR activity.17 The effect of the 1298C allelic variant on the reduction of enzyme activity remains controversial.18 Aberrant methylation patterns have been found to be associated with the development of BC.19,20 It has been shown that the C677T variant increases the plasma homocysteine concentration in humans and reduces DNA methylation in cancer patients. It leads to reduced synthesis of methionine and a more limited availability of the methyl donor (S-adenosyl methionine) in the presence of the low-activity T allele.21
A clear linkage between MTHFR gene polymorphisms and the risk of developing BC has not been established due to differences among ethnic groups and genetic variability.22 Therefore, this study was designed to investigate the association between the MTHFR (C677T) polymorphism and BC risk among North Indian women in a case–control study.
Material and Methods
Sample selection
A case–control study was performed by comparing the frequencies of the MTHFR C677T genotypes of 550 women subjects, 275 cases with surgically and histopathologically confirmed BC and 275 normal control subjects. All cases were divided into two subgroups according to BC status: a) patients with early cancer stage (including stages I and II); and b) patients with advanced cancer stage (including stages III and IV), according to the American Joint Committee for Cancer Staging and End-Results Reporting manual, 1992.23 Controls were matched to cases with reference to ethnicity, gender, age, and a low-risk working environment. Healthy individuals with a positive history of cancer were excluded from serving as controls as well as those with a former positive history of other types of cancer or with chronic diseases such as diabetes; those with lesions other than due to BC were also excluded.
BC cases and healthy controls blood samples were collected from the Department of Pathology, Era’s Lucknow Medical College and Hospital, Lucknow. Before enrollment in the study, each subject’s written informed consent was obtained in response to a fully written and verbal explanation of the nature of study. Data collection was done for each patient for clinical variables, including age, alcohol consumption, body mass index (BMI), cigarette smoking status, family history of cancer, and so on. Ethical committees’ clearances were obtained from the respective departments, before the recruitment of subjects in this study. The research was conducted in accordance with the principles of the Declaration of Helsinki.
DNA extraction
Genomic DNA extraction from whole blood samples collected from 275 cases and 275 healthy controls was done using a standardized phenol–chloroform extraction method.24 The quantity and quality of DNA were checked by UV spectrophotometry on a NanoDrop spectrophotometer and 0.8% (w/v) agarose gel electrophoresis, respectively. The DNA was stored at −80°C until further analysis.
MTHFR gene C677T polymorphism
The MTHFR C677T polymorphism was analyzed by polymerase chain reaction (PCR) followed by restriction fragment length polymorphism. Genomic DNA was amplified (Veriti™; Applied Biosystems) using the following PCR conditions: 94°C for 4 minutes, 34 cycles at 94°C for 30 seconds, 60.7°C for 45 seconds, 72°C for 45 seconds, and finally 72°C for 12 minutes. The primers used for amplification of the MTHFR C677T gene polymorphisms were as follows: forward primer 5′-TGAAGGAGAAGGTGTCTGCGG GA-3′; and reverse primer 5′-AGGA CGGTGCGGTGAGAGTG-3′.25 Amplification was performed with 25 µL PCR reaction mixture containing 40–100 ng template DNA, 10 pmol of each primer, and 2 × PCR master mix (Fermentas). Amplification success of samples was monitored on 2% agarose gel by electrophoresis and the PCR-amplified product was 198 bp long.
Digestion with restriction enzyme
The PCR products were further digested using Hinf1 enzyme (New England Biolabs) to screen for C677T polymorphism. The enzymatic mixture contained 1 µL restriction enzyme (RE) (Hinf1), 1 µL 10× buffer, 6 µL PCR products, and 2 µL distilled water; the mixture was incubated overnight at 37°C for digestion. The digested product was run on 3% agarose gel at 60 V for 1 hour, and then we observed two bands of 175 bp and 23 bp for homozygous TT, three bands of 198 bp, 175 bp, and 23 bp for heterozygous CT, and one fragment of 198 bp for homozygous CC.
Statistical analysis
The MTHFR (C677T) genotype and allele frequencies in the BC patients were compared to the respective frequencies of the control groups using the chi-square test by using the Statistical Package for the Social Sciences (SPSS) program, version 14. The significance of this study was evaluated by comparing patients and controls, as well as subgroups with controls. Odds ratios (ORs), given with 95% confidence intervals (CIs), were derived to assess the strength of the association between the MTHFR gene polymorphism and risk of BC. P-value was considered significant at <0.05.
Results
Characteristics of the study population
Distribution of the selected demographic characteristics and risk factors in control subjects and BC patients is shown in Table 1. The demographic profile included age, estrogen (positive/negative), BMI, tumor stage/grade, and various habitual risk factors involved in the progression of BC. The mean age of BC patients and healthy controls at the time of diagnosis was 35.5 ± 4.45 years and 35.6 ± 4.45 years, respectively. Estrogen-positive status and tobacco-chewing frequencies were comparatively higher in the case group and were associated with BC (Table 1). BMI was approximately the same in both the study groups. Estrogen-positive individuals were more in the case group and were significantly different from the control subjects (P = 0.0373). The frequencies of various tumor stages, lymph node grades, tumor grades, and metastases among the patients are shown in Table 1. Out of 275 BC patients, 183 (66.54%) had early-stage tumor (stages 1 and 2), and 92 (33.45%) had advanced-stage tumor (stages 3 and 4). About 85.81% patients had N0 grade lymph node, while N1 + N2 grade was found in ≈14.19% case subjects. Twenty-seven (9.82%) patients had intermediate-to-high histological grade (>grade I) disease and 248 (90.18%) case subjects possessed grade I tumor. Out of 275 BC patients, metastasis was confirmed in only 46 (16.72%) subjects (Table 1).
Table 1.
Demographic details of controls and breast cancer patients.
| CHARACTERISTICS | CONTROLS (n = 275) |
CASES (n = 275) |
P-VALUE |
|---|---|---|---|
| Age | 35.6 ± 4.45 | 35.5 ± 4.49 | 0.781 |
| BMI | 24.8 ± 4.90 | 25.4 ± 5.10 | 0.361 |
| Estrogen (+/−) | 150/125 | 175/100 | 0.0373* |
| Tobacco Chewing | 30.54% (n = 84) | 45.09% (n = 124) | 0.0183* |
| **Tumor stage | |||
| 1 and 2 | 66.54% (n = 183) | ||
| 3 and 4 | 33.45% (n = 92) | ||
| **Lymph node grade | |||
| N0 | 85.81% (n = 236) | ||
| N1 + N2 | 14.19% (n = 39) | ||
| **Tumour grade | |||
| Grade 1 | 90.18% (n = 248) | ||
| >Grade 1 | 9.82% (n = 27) | ||
| **Metastasis | |||
| No | 83.27% (n = 229) | ||
| Yes | 16.72% (n = 46) | ||
Note:
Indicates a significant value.
The values for these characteristic are mentioned only in case subjects, as these were absent in control individuals.
MTHFR (C677T) genotype and allele frequencies
The frequencies of the MTHFR (C677T) genotype and alleles in BC patients and control subjects are shown in Table 2 and Figure 1. The frequencies of the CC, TT, and CT genotypes of MTHFR (C677T) were 64.7%, 2.18%, and 33.09% in the patients, and 78.91%, 1.09%, and 20.1%, respectively, in the healthy control group. The CT genotype was significantly different between BC patients and control subjects. The frequencies of C and T alleles were 88.9% and 11.09%, respectively, in the healthy subjects and 81.2% and 18.7%, respectively, among the patients (Table 2 and Fig. 1). Frequency of T allele was increased significantly as compared with that of the C allele (OR: 1.847; 95% CI: 1.312–2.6; P = 0.005) in case subjects (Table 2).
Table 2.
Genotype and allele frequencies of MTHFR (C677T) gene polymorphism in the north Indian women population.
| C677T GENE | CONTROL (n = 275) | PATIENTS (n = 275) | P-VALUE | ODDS RATIO | 95% CI |
|---|---|---|---|---|---|
| Genotype | |||||
| CC | 217 (78.91%) | 178 (64.7%) | – | – | – |
| CT | 55 (20.1%) | 91 (33.09%) | 0.007* | 1.984 | 1.345–2.926 |
| TT | 3 (1.09%) | 6 (2.18%) | 0.794 | 1.209 | 2.904–5.032 |
| Allele | |||||
| C | 489 (88.9%) | 447 (81.2%) | – | – | – |
| T | 61 (11.09%) | 103 (18.7%) | 0.005* | 1.847 | 1.312–2.6 |
Note:
P ≤ 0.05, significant value.
Figure 1.
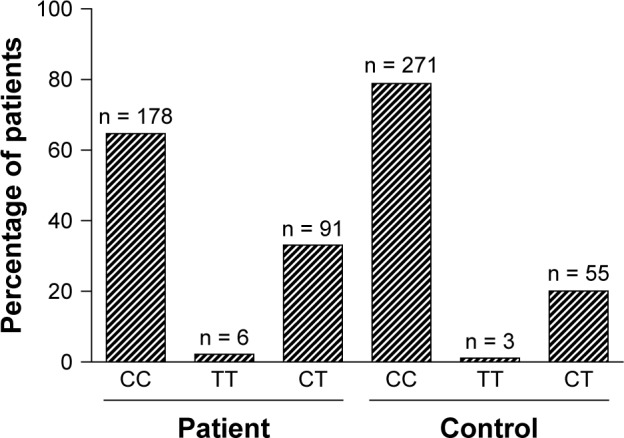
Frequency of C667T genotype in breast cancer cases and control individuals.
Digestion with RE
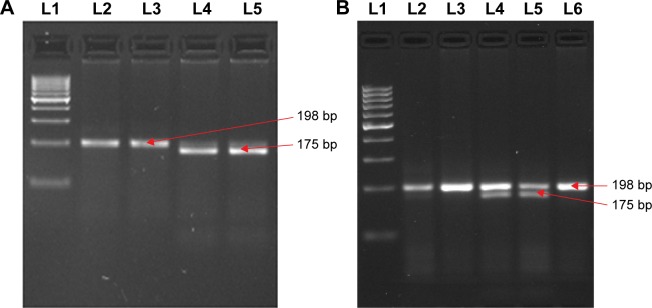
The PCR products of the MTHFR (C677T) gene were digested by REs and the results are shown in Figure 2A and B. The results demonstrate that in the case of C677T polymorphism, an undigested 198 bp band showed the wild-type CC genotype, while two bands of 175 bp and 23 bp confirmed the mutant TT genotype; moreover, three bands of 198 bp, 175 bp, and 23 bp were detected in the heterozygous CT genotype.
Figure 2.
Polymorphism determination of C667T gene by PCR-RFLP.
Notes: Homozygous wild, heterozygous, and homozygous mutant genotypes were identified by the presence and absence of 198 bp, 175 bp, and 23 bp bands, respectively. (A) 100 bp ladder marker (L1), CC (L 2, 3), and TT (L 4 and 5); (B) 100 bp ladder marker (L 1), CC (L 2, 3, 6), and CT (L 4 and 5).
Abbreviations: L, lane; PCR, polymerase chain reaction; RFLP, restriction fragment length polymorphism.
Associations between BC, MTHFR genotypes, and environmental factors
In this study, we also explored the possible association of environmental risk factors with MTHFR (C667T) gene polymorphism on BC susceptibility. The association of the C667T genotype and alleles with environmental factors is shown in Table 3, while smoking and alcoholism data are not shown in the table because these are not significantly associated with MTHFR gene polymorphism. The CT genotype was found to be significant in tobacco-chewing cases as compared to tobacco-chewing controls. The frequencies of the different genotypes and alleles of MTHFR (C677T) gene polymorphism were not significantly associated with tobacco chewing and nonchewing within the control group and the patient group (Table 4). Moreover, we did not find any correlation between the subgroups of cases.
Table 3.
Genotype and allele frequencies of MTHFR (C677T) gene polymorphism in the north Indian women population.
| MTHFR GENOTYPING (C667T) | |||||
|---|---|---|---|---|---|
| TOBACCO CHEWING | CONTROL (n = 84) | PATIENTS (n = 124) | P-VALUE | ODDS RATIO | 95% CI |
| CC | 71 (84.53%) | 88 (70.97%) | – | – | – |
| CT | 12 (14.2%) | 34 (27.5%) | 0.0367* | 2.286 | 1.10–4.73 |
| TT | 1 (1.19%) | 2 (1.62%) | 0.783 | 0.705 | 0.58–8.51 |
| C | 154 (91.6%) | 210 (84.6%) | – | – | – |
| T | 14 (8.3%) | 38 (15.4%) | 0.495 | 1.99 | 1.04–3.80 |
| TUMOR STAGE | 1 AND 2 (n = 183) | 3 AND 4 (n = 92) | P-VALUE | ODDS RATIO | 95% CI |
| CC | 122 (66.6%) | 69 (75.0%) | – | – | – |
| CT | 59 (32.2%) | 22 (23.9%) | 0.162 | 0.65 | 0.37–1.16 |
| TT | 02 (1.09%) | 01 (1.08%) | 1.00 | 0.88 | 0.07–9.93 |
| C | 137 (74.86%) | 66 (71.73%) | – | – | – |
| T | 46 (25.13%) | 26 (28.3%) | 0.66 | 1.17 | 0.66–2.06 |
| LYMPH NODE GRADE | N0 (n = 236) | N1 + N2 (n = 39) | P-VALUE | ODDS RATIO | 95% CI |
| CC | 156 (66.2%) | 26 (66.6%) | – | – | – |
| CT | 79 (33.4%) | 12 (30.8%) | 0.85 | 0.912 | 0.43–1.91 |
| TT | 02 (0.84%) | 01 (2.5%) | 0.37 | 3.20 | 0.26–3.43 |
| C | 181 (76.6%) | 28 (71.7%) | – | – | – |
| T | 55 (23.3%) | 11 (28.3%) | 0.54 | 1.2 | 0.60–2.77 |
| TUMOR GRADE | GRADE 1 (n = 248) | >GRADE 1 (n = 27) | P-VALUE | ODDS RATIO | 95% CI |
| CC | 161 (64.9%) | 20 (74.1%) | – | – | – |
| CT | 84 (33.8%) | 7 (25.9%) | 0.519 | 0.67 | 0.27–1.65 |
| TT | 03 (1.21%) | 00 (0.00%) | 1.00 | 1.12 | 0.056–22.59 |
| C | 205 (82.6%) | 23 (85.1%) | – | – | – |
| T | 43 (17.4%) | 4 (14.8%) | 1.00 | 0.82 | 0.27–2.52 |
| METASTASIS | NO (n = 46) | YES (n = 229) | P-VALUE | ODDS RATIO | 95% CI |
| CC | 28 (60.8%) | 153 (66.9%) | – | – | – |
| CT | 18 (39.1%) | 73 (31.5%) | 0.39 | 0.74 | 0.38–1.42 |
| TT | 00 (0.00%) | 03 (1.52%) | 1.00 | 1.3 | 0.065–5.86 |
| C | 34 (73.9%) | 161 (70.4%) | – | – | – |
| T | 12 (26.1%) | 68 (29.6%) | 0.72 | 1.19 | 0.58–2.45 |
Note:
P ≤ 0.05, significant value.
Table 4.
Genotype and allele frequencies of MTHFR (C677T) gene polymorphism of tobacco chewing and non-tobacco-chewing individuals in the control group and tobacco-chewing and non-tobacco-chewing individuals in the patient group.
| C677T GENE | CONTROL NON-TOBACCO CHEWING (n = 191) | CONTROL TOBACCO CHEWING (n = 84) | P-VALUE | ODDS RATIO | 95% CI |
|---|---|---|---|---|---|
| CC | 146 (76.5%) | 71 (84.53%) | – | – | – |
| CT | 43 (22.5%) | 12 (14.2%) | 0.169 | 0.58 | 0.28 –1.15 |
| TT | 2 (1.047%) | 1 (1.19%) | 0.9 | 1.02 | 0.9–2.34 |
| C | 335 (87.7%) | 154 (91.6%) | – | – | – |
| T | 47 (12.3%) | 14 (8.3%) | 0.22 | 0.64 | 0.34–1.2 |
| C677T GENE | PATIENTS NON-TOBACCO CHEWING (n = 151) | PATIENTS TOBACCO CHEWING (n = 124) | P-VALUE | ODDS RATIO | 95% CI |
| CC | 90 (59.6%) | 88 (70.97%) | – | – | – |
| CT | 57 (37.8%) | 34 (27.5%) | 0.079 | 0.61 | 0.36 –1.02 |
| TT | 4 (2.6%) | 2 (1.62%) | 0.71 | 0.51 | 0.09–2.8 |
| C | 237 (78.5%) | 210 (84.6%) | – | – | – |
| T | 65 (21.5%) | 38 (15.4%) | 0.81 | 0.65 | 0.42–1.02 |
Association of genotypes with pathological conditions
In this section, we explored any possible association between the MTHFR gene polymorphism and clinicopathological features with BC. Patients were divided into two categories as low-risk and high-risk BC groups. The low-risk group comprised patients with tumor stages 1 and 2, grade 1, lymph node N0, and nonmetastasis, whereas the high-risk group contained patients with tumor stages 3 and 4, grade >1, lymph node N1 + N2, and metastasis. Low-risk BC cases were taken as a reference. No significant correlation was observed between this common polymorphism and tumor stage, lymph node, tumor grade, and metastasis in the cases (Table 3)
Discussion
The polymorphism study was conducted on the basis that MTHFR (C667T) gene polymorphism is known to be associated with various cancers and other diseases.26,27 In this study, we tried to determine the correlation between MTHFR (C667T) genotype/allele frequency and BC in subjects from the north Indian population. Our results demonstrated that the CT genotype (heterozygous variant), the TT genotype (homozygous variant), and the T allele have higher frequencies among BC patients as compared to healthy controls. Therefore, the CT genotype and T allele were found to be significantly different between control and BC patients, with P-value = 0.007 and 0.005, respectively. This finding showed the possible role of the C667T gene polymorphism in the pathogenesis of human BC. A meta-analysis showed a significant association between the MTHFR C677T polymorphism (T allele and TT genotype) and BC risk in the Asian population.28 Sihua et al showed the involvement of MTHFR C677T in the prediction of BC subtype and disease progression.29 Several other studies also indicated the association of MTHFR (C677T) with BC risk.30–32 Literature review revealed that our study is in line with these cited studies.
Some studies have emphasized that MTHFR gene polymorphism is not associated with BC pathophysiology.27 Recently, a research group reported nonsignificant distribution of 677C>T genotypes between cases and controls in the north Indian population.33,34 In another study, 243 patients treated with chemotherapy were recruited and followed up for toxicity. Polymorphism analysis of MTHFR C677T showed nonsignificant association with drug-induced toxicity.35 Prasad Chaturvedi et al indicated that MTHFR (C677T) polymorphism is not a risk factor for BC.36,37 Overall, these contradictory conclusions from studies on different Indian populations imply that the role of the CT genotype and T allele in susceptibility to BC might depend on the lifestyle of particular individuals.
A defined etiology and the molecular pathogenesis of BC are largely unknown. Several risk factors, such as height, age, high BMI, obesity, diet, alcohol, smoking, tobacco chewing, underlying genetic difference, geographic variations, hormone/pregnancy-related factors, and environmental exposures (eg, ionizing radiations), and so on, have been identified. These risk factors may play varying roles in BC pathogenicity, progression, and survival in the population.38,39 This study revealed that the CT genotype is significantly associated with tobacco-chewing status (P = 0.0367) in BC individuals. Furthermore, within the control and cancer patient groups, the tobacco-chewing and non-tobacco-chewing subgroups were not significantly associated. It may be inferred that tobacco chewing might be a risk factor for BC. Previous studies showed that regular tobacco chewing is associated with a 35%–45% increase in the risk of BC.40,41 In this study, we found that individuals having higher estrogen levels were significantly more in number among case subjects. Higher estrogen exposure is known to induce aberrant DNA methylation associated with breast carcinogenesis.42 In this study, MTHFR C677T polymorphisms had no statistically significant association with tumor stage, lymph node, metastasis, and tumor grade in patients.
To the best of our knowledge, no study has been performed to assess the association of the MTHFR (C677T) genotype in BC cases of north Indian subjects. Our experimental analysis concludes that there is association of the CT genotype and T allele with the risk of BC in the Indian population. The environmental factor tobacco chewing also plays an important role in BC. Therefore, a further functional study is needed to elucidate the role of MTHFR C677T gene polymorphism in the pathogenesis of BC.
Footnotes
ACADEMIC EDITOR: Barbara Guinn, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 944 words, excluding any confidential comments to the academic editor.
FUNDING: We acknowledge the financial support from the Department of Science and Technology, India. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the study, interpreted the results, drafted the manuscript and carried out the data analyses: SRH, MKA. Contributed to data gathering, and participated in writing and revising the manuscript: MW, SK, CB. Revised and improved the final manuscript: MS, FM, SKS. All authors read and approved the final manuscript.
REFERENCES
- 1.Corrêa SAA, Noronha SMRD, Nogueira-de-Souza NCC, Valletta de Carvalho AMM, Costa JJM. Association between the angiotensin-converting enzyme (insertion/deletion) and angiotensin II type 1 receptor (A1166C) polymorphisms and breast cancer among Brazilian women. J Renin Angiotensin Aldosterone Syst. 2009;10(1):51–58. doi: 10.1177/1470320309102317. [DOI] [PubMed] [Google Scholar]
- 2.Chlebowski RT, Chen Z, Anderson GL. Ethnicity and breast cancer: factors influencing differences in Incidence and outcome. JNCI. 2005;97:439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 3.Perou CM, Sorlie T, Eisen MB, Van De Rijn M, Jeffrey SS, Rees CA. Molecular portraits of human breast tumours. Nature. 2002;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 4.Lewis SJ, Harbord RM, Harris R, Smith GD. Meta-analyses of observational and genetic association studies of folate intakes or levels and breast cancer risk. J Natl Cancer Inst. 2006;98:1607–1622. doi: 10.1093/jnci/djj440. [DOI] [PubMed] [Google Scholar]
- 5.Das PM, Singal R. DNA methylation and cancer. J Clin Oncol. 2004;22:4632–4642. doi: 10.1200/JCO.2004.07.151. [DOI] [PubMed] [Google Scholar]
- 6.Maruti SS, Ulrich CM, Jupe ER, White E. MTHFR C677T and postmenopausal breast cancer risk by intakes of one carbon metabolism nutrients: a nested case control study. Breast Cancer Res. 2009;11:1–7. doi: 10.1186/bcr2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fodinger M, Horl WH, Sunder-Plassmann G. Molecular biology of 5,10- methylenetetrahydrofolate reductase. J Nephrol. 2000;13:20–33. [PubMed] [Google Scholar]
- 8.Choi WS, Mason JB. Folate and carcinogenesis, an integrated scheme. J Nutr. 2000;130:129–132. doi: 10.1093/jn/130.2.129. [DOI] [PubMed] [Google Scholar]
- 9.Stern LL, Bagley PJ, Rosenberg IH, Selhub J. Conversion of 5-formyltetrahydrofolic acid to 5-methyltetrahydrofolic acid is unimpaired in folateadequate persons homozygous for the C677T mutation in the methylenetetrahydrofolate reductase gene. J Nutr. 2000;130:2238–2242. doi: 10.1093/jn/130.9.2238. [DOI] [PubMed] [Google Scholar]
- 10.Shrubsole MJ, Gao YT, Cai Q, et al. MTHFR polymorphisms, dietary folate intake, and breast cancer risk: results from the Shanghai Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2004;13:190–196. doi: 10.1158/1055-9965.epi-03-0273. [DOI] [PubMed] [Google Scholar]
- 11.Blount BCC, Mack MM, Weh CM, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997;94:3290–3295. doi: 10.1073/pnas.94.7.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran P, Leclerc D, Chan M, et al. Multiple transcription start sites and alternative splicing in the methylenetetrahydrofolate reductase gene result in two enzyme isoforms. Mamm Genome. 2002;13:483–492. doi: 10.1007/s00335-002-2167-6. [DOI] [PubMed] [Google Scholar]
- 13.Gaughan DJ, Barbaux S, Kluijitmans LA, Whitehead AS. The human and mouse methylenetetrahydrofolate reductase (MTHFR) genes: genomic organization, mRNA structure and linkage to the CLCN6 gene. Gene. 2000;257:279–289. doi: 10.1016/s0378-1119(00)00392-9. [DOI] [PubMed] [Google Scholar]
- 14.Frosst P, Blom BJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10(10):111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 15.Weisberg IS, Jacques PF, Selhub J, et al. The 1298A–>C polymorphism in methylenetetrahydrofolate reductase (MTHFR): in vitro expression and association with homocysteine. Atherosclerosis. 2001;156:409–415. doi: 10.1016/s0021-9150(00)00671-7. [DOI] [PubMed] [Google Scholar]
- 16.Weisberg I, Tran P, Christensen B, Sibani S, Rozen R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. 1998;64:169–172. doi: 10.1006/mgme.1998.2714. [DOI] [PubMed] [Google Scholar]
- 17.Frosst P, Blom JH, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10(10):111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 18.Yamada K, Chen Z, Rozen R, Matthews RG. Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proc Natl Acad Sci U S A. 2001;98:14853–14858. doi: 10.1073/pnas.261469998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang H, Yan Y, Li T, et al. Methylenetetrahydrofolate reductase polymorphisms and breast cancer risk in Chinese population: a meta-analysis of 22 case-control studies. Tumour Biol. 2014;35:1695–1701. doi: 10.1007/s13277-013-1234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stern LL, Mason JB, Selhub J, Choi SW. Genomic DNA hypomethylation, a characteristic of most cancers, is present in peripheral leukocytes of individuals who are homozygous for the C677T polymorphism in the methylenetetrahydrofolate reductase gene. Cancer Epidemiol Biomarkers Prev. 2000;9:849–853. [PubMed] [Google Scholar]
- 21.Paz MF, Avila S, Fraga MF, et al. Germ-line variants in methyl-group metabolism genes and susceptibility to DNA methylation in normal tissues and human primary tumors. Cancer Res. 2002;62:4519–4524. [PubMed] [Google Scholar]
- 22.Martin DN, Boersma BJ, Howe TM, Goodman JE, Mechanic LE, Chanock SJ. Association of MTHFR gene polymorphisms with breast cancer survival. BMC Cancer. 2006;6:1–10. doi: 10.1186/1471-2407-6-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Joint Committee . American Joint Committee for Cancer Staging and Results Reporting 1977 Manual for Staging of Cancer 1st edn American Joint Committee Chicago American Joint Committee on Cancer 1992 Manual for Staging of Cancer 4th Edn. Philadelphia: Lippincott Company; 1977. [Google Scholar]
- 24.Sambrook J, Frisch EF, Maniatis T. Molecular Cloning; A Laboratory Manual. 2nd ed. New York: Cold Sping Harbor Labortory Press; 1989. pp. 914–919. [Google Scholar]
- 25.AbdRaboh NR, Badr S, Ali S. Prevelance of methylenevtetrahydrofolate reductase C6777T And A1298C polymorphisms in Egyptian patients with 2 daibetes mellitus. Egypt J Genet. 2013;14(1):87–93. [Google Scholar]
- 26.Jakubowska A, Gronwald J, Menkiszak J, et al. Methylenetetrahydrofolate reductase polymorphisms modify BRCA1-associated breast and ovarian cancer risks. Breast Cancer Res Treat. 2007;104:299–308. doi: 10.1007/s10549-006-9417-3. [DOI] [PubMed] [Google Scholar]
- 27.Ajai Singh Sabir Ali, Hussain Syed Rizwan, Kumar Vineet, Mahdi Abbas Ali, Srivastava Rajeshwar Nath. Analysis of correlation of CYR61 and MTHFR Gene Polymorphism in Legg-Calve-Perthes disease. South American Journal of Clinical Research. 2016;3:1–7. [Google Scholar]
- 28.Sihua P, Bingjian L, Wenjing R, Yimin Z, Hongqiang S, Maode L. Genetic polymorphisms and breast cancer risk: evidence from meta-analyses, pooled analyses, and genome-wide association studies. Breast Cancer Res Treat. 2011;127:309–324. doi: 10.1007/s10549-011-1459-5. [DOI] [PubMed] [Google Scholar]
- 29.Rai V. The methylenetetrahydrofolate reductase C677T polymorphism and breast cancer risk in Asian populations. Asian Pac J Cancer Prev. 2014;15(14):5853–5860. doi: 10.7314/apjcp.2014.15.14.5853. [DOI] [PubMed] [Google Scholar]
- 30.Naushad SM, Pavani A, Rupasree Y, Divyya S, Deepti S, Digumarti RR. Association of aberrations in onecarbon metabolism with molecular phenotype and grade of breast cancer. Mol Carcinog. 2012;51(1):E32–E41. doi: 10.1002/mc.21830. [DOI] [PubMed] [Google Scholar]
- 31.Naushad SM, Pavani A, Digumarti RR, Gottumukkala SR, Kutala VK. Epistatic interactions between loci of one carbon metabolism modulate susceptibility to breast cancer. Mol Biol Rep. 2011;38(8):4893–4901. doi: 10.1007/s11033-010-0631-z. [DOI] [PubMed] [Google Scholar]
- 32.Mohammad NS, Yedluri R, Addepalli P, Gottumukkala SR, Digumarti RR, Kutala VK. Aberrations in one carbon metabolism induce oxidative DNA damage in sporadic breast cancer. Mol Cell Biochem. 2011;349(12):159–167. doi: 10.1007/s11010-010-0670-8. [DOI] [PubMed] [Google Scholar]
- 33.Singh V, Parmar D, Singh MP. Do single nucleotide polymorphisms in xenobiotic metabolizing genes determine breast cancer susceptibility and treatment outcomes. Cancer Invest. 2008;26(8):769–783. doi: 10.1080/07357900801953196. [DOI] [PubMed] [Google Scholar]
- 34.Mir MM, Dar JA, Dar NA, Dar MS, Salam I, Lone MM. Combined impact of polymorphism of folate metabolism genes; glutamate carboxypeptidase, methylene tetrahydrofolate reductase and methionine synthase reductase on breast cancer susceptibility in kashmiri women. Int J Health Sci (Qassim) 2008;2(1):314–321. [PMC free article] [PubMed] [Google Scholar]
- 35.Pooja S, Carlus J, Sekhar D, Francis A, Gupta N, Konwar R. MTHFR 677C>T polymorphism and the risk of breast cancer: evidence from an original study and pooled data for 28031 cases and 31880 controls. PLoS One. 2015;10(3):1–16. doi: 10.1371/journal.pone.0120654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaturvedi P, Tulsyan S, Agarwal G, Lal P, Agrawal S, Mittal SRD. Relationship of mthfr and nqo1 pharmacogenetics and chemotherapy clinical outcomes in breast cancer patients. Biochem Genet. 2015;8:209–213. doi: 10.1007/s10528-015-9683-z. [DOI] [PubMed] [Google Scholar]
- 37.Prasad VV, Wilkhoo H. Association of the functional polymorphism C677T in the methylenetetrahydrofolate reductase gene with colorectal, thyroid, breast, ovarian, and cervical cancers. Onkologie. 2011;34(89):4226–4232. doi: 10.1159/000331131. [DOI] [PubMed] [Google Scholar]
- 38.Dumitrescu RG, Cotarla I. Understanding breast cancer risk: where do we stand in 2005? J Cell Mol Med. 2005;9(1):208–222. doi: 10.1111/j.1582-4934.2005.tb00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varela-Rey M, Woodhoo A, Martinez-Chantar M, Mato JM, Lu SC, Alcohol DNA. methylation, and cancer. Acohol Res. 2013;35:25–35. [PMC free article] [PubMed] [Google Scholar]
- 40.Hecht SS. Tobacco smoke carcinogens and breast cancer. Environ Mol Mutagen. 2002;39(2–3):119–126. doi: 10.1002/em.10071. [DOI] [PubMed] [Google Scholar]
- 41.Kaushal M, Mishra AK, Sharma J, et al. Genomic alterations in breast cancer patients in betel quid and non betel quid chewers. PLoS One. 2012;7(8):e43789. doi: 10.1371/journal.pone.0043789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez SV, Russo J. Estrogen and xenoestrogens in breast cancer. Toxicol Pathol. 2010;38(1):110–122. doi: 10.1177/0192623309354108. [DOI] [PMC free article] [PubMed] [Google Scholar]