Abstract
Patients with gluteus minimus and medius tears that fail nonoperative management may be indicated for surgical repair; however, structural failure after gluteal tendon repair remains unacceptably high. This is likely related to the limited healing potential of tendinous tissue, which is poorly vascular and heals by formation of fibrocartilaginous scar tissue rather than histologically normal tendon. An emerging option to augment tendon healing is the use of a bioinductive implant that is designed to amplify the host healing response and induce the formation of healthy tendon tissue. Though it is rapidly being adopted for partial- and full-thickness rotator cuff tears, this implant has not yet been used in the hip. A detailed technical description and a discussion of the advantages and disadvantages of the technique are provided.
Though historically lateral hip pain has been attributed to trochanteric bursitis, recent studies have recognized tears of the gluteus medius and minimus as an important cause of lateral hip pain.1, 2 Gluteal tears are analogous to tears of the rotator cuff—pathophysiologically and clinically. Both injuries most often result from a chronic, degenerative process, with or without a proceeding traumatic event,1 and are classified as either full- or partial-thickness tears (full-thickness gluteus tears often lead to significant weakness in hip abduction).1
The first step in management of gluteal medius tears is nonoperative, including physical therapy, activity modification, and nonsteroidal anti-inflammatory drugs.3 Patients that fail nonoperative management may be indicated for surgery. Open and endoscopic repair approaches have been described, with studies indicating equal symptomatic relief, and fewer postoperative complications for endoscopic techniques.3, 4
An emerging option to augment tendon healing is the use of a bioinductive implant.3, 4 Bovine Achilles tendon is used to construct the implant as it is an abundant source of type I collagen containing minimal of the unwanted impurities (e.g., hair follicles, elastin) commonly found in the dermis—this enables a more pure construct. Because the implant is not intended to provide immediate strength, the source tissue can be broken down to the collagen fiber level, and then reconstituted into a highly porous structure designed to induce formation of new tendinous tissue. Because the implant is composed of purified collagen, it is absorbed over time (about 6 months) without inducing an inflammatory response.
Though it is rapidly being adopted for partial- and full-thickness rotator cuff tears,5, 6 this implant has not yet been used in the hip. The only contraindication is a known hypersensitivity to bovine collagen (source material for the implant). This article describes the technique of endoscopic repair of a full-thickness gluteus medius tendon tear augmented with a collagen implant.
Surgical Technique
Patient Setup
A step-by-step video of the procedure can be seen in Video 1. The patient is brought into the operating room, where general anesthesia is administered. The patient is placed supine on the operating table with her feet held in the hip distraction apparatus. No distraction is performed. All body prominences are well padded. The operative extremity is adducted to approximately 30° from midline to allow the greater trochanter to be perpendicular to the pelvis and to take tension off the abductor tendons. The left hip should then be prepped and draped in the usual sterile fashion.
Portal Placement
C-arm fluoroscopy is used to confirm the placement of a 17-gauge spinal needle at the midpoint of the greater trochanter in both the superior/inferior and anterior/posterior planes. Two portals are created in line with the greater trochanter—one proximally and one distally. The first portal is created approximately 3 cm proximal to the most proximal aspect of the greater trochanter. The distal portal is created in line with this at a point 5 cm from the spinal needle. The spinal needle piercing the iliotibial band is then identified and removed.
Approach
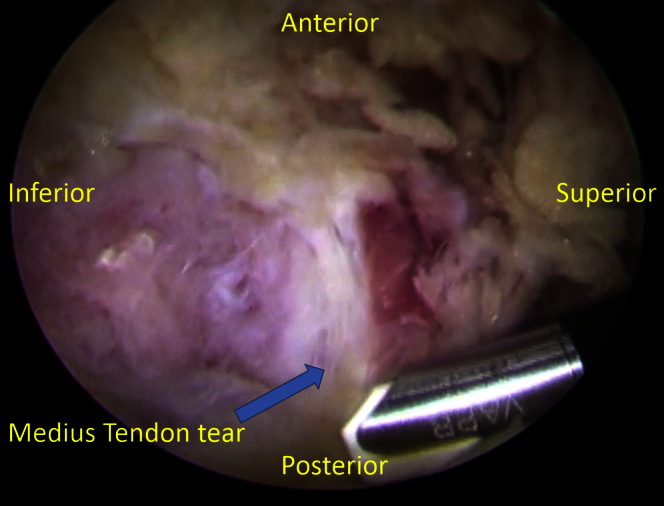
Using the radiofrequency probe (VAPR; Mitek, Raynham, MA), the iliotibial band is incised proximally and distally, exposing the trochanteric bursa (Fig 1). The overlying bursa is then resected with a 4.5-mm shaver (Smith & Nephew, London, United Kingdom), allowing identification of the full-thickness gluteus medius tendon with minimal retraction. The free edge of the tendon is gently debrided. The native foot print of the gluteus medius insertion is then identified at the greater trochanter. The area is debrided to remove any overlying soft tissue, and expose bleeding bone to aid in healing.
Fig 1.
The patient is placed supine with feet held in the hip distraction apparatus. The operative extremity is adducted to approximately 30° from midline. Looking through the more distal of the 2 portals created (both in line with the greater trochanter), the iliotibial band and is incised proximally with the radiofrequency probe (VAPR; Mitek), exposing the trochanteric bursa. The overlying bursa is then resected with a 4.5-mm shaver allowing identification of the full-thickness gluteus medius tendon with minimal retraction.
Tendon Repair
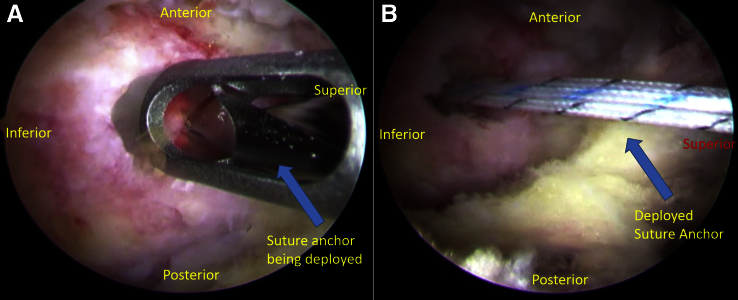
Using a 7-mm twist-in cannula, an all-suture, double-loaded anchor (Smith & Nephew) is placed into the greater trochanter at the footprint of the gluteus medius tendon insertion (Fig 2). An accessory anterior portal in line with the previously placed needle is then placed and used to retrieve the sutures through the gluteus medius tendon via a suture-grasping device (Fig 3). The sutures are tied down sequentially via the cannula to achieve an airtight repair of the tendon down to bone.
Fig 2.
The patient is placed supine with feet held in the hip distraction apparatus. The operative extremity is adducted to approximately 30° from midline. Looking through the more distal of the 2 portals created (both in line with the greater trochanter), (A) a 7-mm twist-in cannula is used to deploy an all-suture, double-loaded anchor. This is placed into the greater trochanter at the footprint of the gluteus medius tendon insertion. (B) Here the deployed anchor can be observed.
Fig 3.
The patient is placed supine with feet held in the hip distraction apparatus. The operative extremity is adducted to approximately 30° from midline. Looking through the more distal of the 2 portals created (both in line with the greater trochanter), a suture-grasping device is used (through an accessory portal in line with the previously placed needle) to retrieve the sutures through the gluteus medius tendon. The sutures are tied down sequentially via the cannula to achieve an airtight repair of the tendon down to the bone.
Implant Delivery
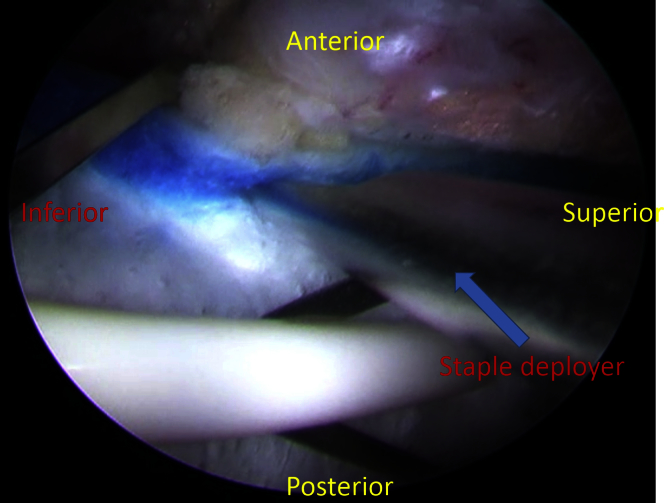
A 20 × 25–mm “bioinductive implant” (Rotation Medical, Plymouth, MN) is delivered endoscopically from the distal portal, and positioned over the repaired tendon, with the distal end of the implant overlapping onto the bone of the greater trochanter (Fig 4). The delivery instrument (Rotation Medical) holds the implant in this position while the implant is stapled to the underlying gluteus medius tendon. A new midline portal is established to introduce the tendon-stapling instrument approximately perpendicular to the gluteus medius tendon. Custom-designed tendon staples, made from a polylactic acid copolymer and designed to absorb in approximately 12 months (Rotation Medical), are used to affix the implant around its perimeter to the underlying tendon (Fig 5). Three tendon staples are placed along the proximal end of the implant, and then 2 tendon staples are placed along each side. The tendon staples are positioned approximately 3 mm in from the edge of the implant and are spaced about 5 mm apart. The delivery instrument holding the implant in place is then removed after placement of the first 5 tendon staples, after which the final tendon staples are delivered (Fig 5).
Fig 4.
The patient is placed supine with feet held in the hip distraction apparatus. The operative extremity is adducted to approximately 30° from midline. Looking through the accessory portal, a 20 × 25–mm bioinductive implant is delivered endoscopically from the distal portal, and positioned over the repaired tendon, with the distal end of the implant overlapping onto the bone of the greater trochanter. The delivery instrument holds the implant in this position while the implant is stapled to the underlying gluteus medius tendon.
Fig 5.
The patient is placed supine with feet held in the hip distraction apparatus. The operative extremity is adducted to approximately 30° from midline. Looking through the accessory portal, staples are used around its perimeter to affix the implant to the underlying tendon. Three tendon staples are placed along the proximal end of the implant, and then 2 tendon staples are placed along each side. The tendon staples are positioned approximately 3 mm in from the edge of the implant and are spaced about 5 mm apart.
Staple Fixation
Finally, custom-designed PEEK (polyether ether ketone) bone staples (Rotation Medical) are inserted to anchor the distal end of the implant to the greater trochanter bone. The Rotation Medical Bone Staple Introducer system (Rotation Medical), with a bone punch, is used to create holes in the bone at the distal end of the implant into the greater trochanter. The bone punch is then removed from the shaft of the instrument while keeping the instrument in place to maintain alignment with the holes in the bone. A bone staple is then delivered through the shaft of the instrument into the created holes (Fig 6)—the instrument is then removed. When delivering staples, slight tension should be applied to the implant to ensure good contact with the underlying gluteus medius tendon. Two bone staples are placed at the distal end of the implant, approximately 3 mm from the edge of the implant and spaced about 10 mm apart. The stability of the implant is confirmed endoscopically. Care should be taken to ensure that the tendon and bone staples did not interfere with the underlying sutures and anchor used in the repair. Pearls and pitfalls can be seen in Table 1.
Fig 6.
The patient is placed supine with feet held in the hip distraction apparatus. The operative extremity is adducted to approximately 30° from midline. Looking through the accessory portal, 2 bone staples are placed at the distal end of the implant, approximately 3 mm in from the edge of the implant, and spaced about 10 mm apart. Care should be taken to ensure that the tendon and bone staples does not interfere with the underlying sutures and anchor used in the repair.
Table 1.
Pearls and Pitfalls for Implant Procedure
| Pearls | Pitfalls | Complications |
|---|---|---|
| Debride native footprint of medius to expose bleeding bone and aid healing. | Be sure to resect sufficient bursa to allow visualization of the tendon. | Implant may tear during implantation, requiring additional staples for fixation. |
| Place 3 staples along proximal end, and 2 placed on the sides. | Make sure staples are at least 3 mm in from the border, and 5 mm apart. | |
| Apply slight tension to implant when stapling to bone to ensure good contact with tendon. | Keep instrument in place after removing bone punch to maintain hole alignment. |
After copious irrigation, all portals are closed with No. 3-0 nylon suture. Sterile dressing is applied to the operative site and the patient is transferred to the recovery room in stable condition. A brace is not required.
Postoperatively, the patient is protected with crutches for at least 8 weeks. The patient is transitioned from toe-touch weight-bear to partial weight-bearing by postoperative day 2. Protective partial weight-bearing is maintained for 6 weeks, at which point full weight-bearing is allowed. Range of motion exercises are instituted with no resistive abduction exercises for at least 3 months from surgery. This is similar to a protective rotator cuff protocol.
Discussion
Although a paucity of literature exists on surgical repair of gluteal tendon tears, many case series report good outcomes and significant improvement in clinical symptoms.1, 3, 4, 7 Meta-analyses of the existing case series have shown that endoscopic repair leads to similar improvement in pain and function, albeit with fewer complications compared with open techniques.3, 4 Thus, endoscopic repair has become the favored method.
Much like surgical repair of the rotator cuff, structural failure after gluteal tendon repair (assessed by postoperative magnetic resonance imaging) remains unacceptably high, with reports ranging from 16% to 35%.8, 9 The high rate of re-tears is likely related to the limited healing potential of tendinous tissue, which is poorly vascular and heals by formation of fibrocartilaginous scar tissue rather than histologically normal tendon.10
Unlike a dermal graft, which provides immediate strength to repair, the bioinductive implant is intended to provide long-term improvement and reduced re-tear rates through amplification of the host healing response and induction of healthy tendon tissue. Augmentation is based on the increase in tendon thickness of 2 to 2.5 mm generated by the collagen implant.5, 11 Finite-element analysis has demonstrated that the addition of a 2-mm layer of new tissue will reduce peak strain in partial-thickness tears by 40% for an articular-sided tear and by 47% for a bursal-sided tear.5 The reduced strain at the tear permits it to fill in with new physiologic tissue. Formation of new tendon tissue is thought to augment the strength of the degenerated tendon, ultimately shifting the balance from progressively worse tearing (possibly to complete tear12) to healing and improved outcomes.
The “bioinductive” designation (i.e., its ability to stimulate the formation of new tendinous tissue) is based on the finding that the extracellular matrix that grows into the implant remodels into linearly arranged collagen fibers. The highly porous nature of the implant encourages fibroblast growth and replication. The low tensile modulus of the implant allows the migrated fibroblasts to experience the same physiologic strain as the underlying tendon, enabling production of functional connective tissue—leading to physiologic repair and ultimately structural stability. In sham operated animals (which had the same surgical procedure but no implant placement), no new tissue formed over the tendon; only when the implant was placed on the tendon was the formation of new tendinous tissue induced.11
The advantages of the bioinductive implant are thus 2-fold: first, its structural properties enable the induction of new tendinous tissue. Second, its purity enables tissue induction and absorption of the implant without any inflammatory response. The main limitation is that because of its low strength, it cannot be used to bridge a gap in situations where the rotator cuff cannot be repaired in direct contact with the humeral head (Table 2).
Table 2.
Advantages and Disadvantages of Using the Bioinductive Implant
| Advantages | Disadvantages |
|---|---|
| Induces formation of healthy physiologic tendon tissue | Does not provide immediate structural stability |
| Composed of purified collagen, which absorbs without an inflammatory response | Cannot be used if patient has bovine collagen hypersensitivity |
| Increases tendon thickness, allowing for tear to be filled in | Implant may tear during implantation requiring additional staples for fixation |
Regarding complications, no data exist regarding this procedure in the hip, but more than 1,200 similar procedures have been performed for rotator cuff repair, and no adverse events have been reported. The main caution that must be taken is care during initial stapling of the implant, when a risk of tearing exists. This would require additional staples for fixation.
Again, although no studies have yet been completed on outcomes of the procedure in the hip, in a submitted study of partial-thickness rotator cuff tears, all patients treated with the implant in the shoulder have had new tissue formation,5 which is similar to that seen in a preclinical animal study.11 Seven of 13 patients had complete filling of the rotator cuff defect, and the remaining 6 had progressive improvement in tendon quality. There was no tear progression in any patient for the 24 months of follow-up, and American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form (ASES) scores significantly improved in all patients.
Although more work needs to be done in the field, it appears that the bioinductive implant is a good option for rotator cuff tears. Because of the remarkable similarly between the gluteus medius/minimus tendons and rotator cuff, the implant was used here to help augment a gluteus medius tear. The procedure is simple, easily reproducible, and may be a good option for gluteus medius tears.
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article.
Supplementary Data
Video demonstration of an endoscopic gluteus medius tendon rupture repair with subsequent placement of a bioinductive implant. The implant is composed of highly purified bovine collagen, which ultimately breaks down over 6 months. The highly porous, low-strength, and low-modulus design induces the migrating fibroblasts to produce physiologic tendon, ultimately augmenting the repair.
References
- 1.Domb B.G., Carreira D.S. Endoscopic repair of full-thickness gluteus medius tears. Arthrosc Tech. 2013;2:e77–e81. doi: 10.1016/j.eats.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domb B.G., Nasser R.M., Botser I.B. Partial-thickness tears of the gluteus medius: Rationale and technique for trans-tendinous endoscopic repair. Arthroscopy. 2010;26:1697–1705. doi: 10.1016/j.arthro.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Chandrasekaran S., Lodhia P., Gui C., Vemula S.P., Martin T.J., Domb B.G. Outcomes of open versus endoscopic repair of abductor muscle tears of the hip: A systematic review. Arthroscopy. 2015;31:2057–2067.e2. doi: 10.1016/j.arthro.2015.03.042. [DOI] [PubMed] [Google Scholar]
- 4.Alpaugh K., Chilelli B.J., Xu S., Martin S.D. Outcomes after primary open or endoscopic abductor tendon repair in the hip: A systematic review of the literature. Arthroscopy. 2015;31:530–540. doi: 10.1016/j.arthro.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Bokor D.J., Sonnabend D.H., Deady L., Cass B., Young A.A., Van Kampen C.L. Evidence of healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a collagen implant: A 2-year MRI follow-up. Muscles Ligaments Tendons J. 2015;5:144–150. doi: 10.11138/mltj/2016.6.1.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bokor D.J., Sonnabend D.H., Deady L., Cass B., Young A.A., Arnoczky S. Preliminary investigation of a biological augmentation of reotator cuff repairs using a collagen implant: A 2-year MRI follow-up. Muscles Ligaments Tendons J. 2015;5:144–150. doi: 10.11138/mltj/2015.5.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandrasekharan S., Gui C., Hutchinson M.R., Lodhia P., Suarez-Ahedo C., Domb B.G. Outcomes of endoscopic gluteus medius repair: Study of thirty-four patients with minimum two-year follow-up. J Bone Joint Surg Am. 2012;16:1340–1347. doi: 10.2106/JBJS.N.01229. [DOI] [PubMed] [Google Scholar]
- 8.McGonagle L., Haebich S., Breidahl W., Fick D.P. MRI and clinical analysis of hip abductor repair. Hip Int. 2015;25:24–27. doi: 10.5301/hipint.5000194. [DOI] [PubMed] [Google Scholar]
- 9.Makridis K.G., Lequesne M., Bard H., Djian P. Clinical and MRI results in 67 patients operated for gluteus medius and minimus tendon tears with a median follow-up of 4.6 years. Orthop Traumatol Surg Res. 2014;100:849–853. doi: 10.1016/j.otsr.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad Z., Wardale J., Brooks R., Henson F., Noorani A., Rushton N. Exploring the application of stem cells in tendon repair and regeneration. Arthroscopy. 2012;28:1018–1029. doi: 10.1016/j.arthro.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Van Kampen C., Arnoczky S., Parks P. Tissue-engineered augmentation of a rotator cuff tendon using a reconstituted collagen scaffold: A histological evaluation in sheep. Muscles Ligaments Tendons J. 2013;3:229–235. [PMC free article] [PubMed] [Google Scholar]
- 12.Strauss E.J., Salata M.J., Kercher J. The arthroscopic management of partial-thickness rotator cuff tears: A systematic review of the literature. Arthroscopy. 2011;27:568–580. doi: 10.1016/j.arthro.2010.09.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video demonstration of an endoscopic gluteus medius tendon rupture repair with subsequent placement of a bioinductive implant. The implant is composed of highly purified bovine collagen, which ultimately breaks down over 6 months. The highly porous, low-strength, and low-modulus design induces the migrating fibroblasts to produce physiologic tendon, ultimately augmenting the repair.