Abstract
Ossification is a tightly regulated process, performed by specialized cells called osteoblasts. Dysregulation of this process may cause inadequate or excessive mineralization of bones or ectopic calcification, all of which have grave consequences for human health. Understanding osteoblast biology may help to treat diseases such as osteogenesis imperfecta, calcific heart valve disease, osteoporosis, and many others. Osteoblasts are bone-building cells of mesenchymal origin; they differentiate from mesenchymal progenitors, either directly or via an osteochondroprogenitor. The direct pathway is typical for intramembranous ossification of the skull and clavicles, while the latter is a hallmark of endochondral ossification of the axial skeleton and limbs. The pathways merge at the level of preosteoblasts, which progress through 3 stages: proliferation, matrix maturation, and mineralization. Osteoblasts can also differentiate into osteocytes, which are stellate cells populating narrow interconnecting passages within the bone matrix.
The key molecular switch in the commitment of mesenchymal progenitors to osteoblast lineage is the transcription factor cbfa/runx2, which has multiple upstream regulators and a wide variety of targets. Upstream is the Wnt/Notch system, Sox9, Msx2, and hedgehog signaling. Cofactors of Runx2 include Osx, Atf4, and others. A few paracrine and endocrine factors serve as coactivators, in particular, bone morphogenetic proteins and parathyroid hormone. The process is further fine-tuned by vitamin D and histone deacetylases. Osteoblast differentiation is subject to regulation by physical stimuli to ensure the formation of bone adequate for structural and dynamic support of the body. Here, we provide a brief description of the various stimuli that influence osteogenesis: shear stress, compression, stretch, micro- and macrogravity, and ultrasound. A complex understanding of factors necessary for osteoblast differentiation paves a way to introduction of artificial bone matrices.
MeSH Keywords: Core Binding Factor Alpha 1 Subunit, Mechanoreceptors, Osteoblasts, Osteogenesis
Background
Bone is constructed through 3 processes: osteogenesis, modeling, and remodeling. All these processes are mediated by osteoblasts, which work in tight cooperation with bone-resorbing osteoclasts, together constituting a “bone multicellular unit” [1]. The osteoblasts synthetize the bone extracellular matrix (osteogenesis), and the osteoclasts carve out the shape to fit the physical environment (modeling) and adjust it to the demands of the body growth and the changing milieu (remodeling). Fine tuning of this system is crucial for the development of bones, for repairing fractures, and for the correct maintenance of the skeleton throughout life. Sometimes the balance shifts towards resorption of the matrix, as in osteoporosis, thereby predisposing postmenopausal women and older men to fractures and reducing their life expectancy. As many as 22 million women and 5 million men had osteoporosis in the European Union in 2010 [2]. A therapeutic boost to the osteoblast activity could potentially prolong millions of human lives. Attempts have been made to transplant autologous stem cells to bone defects [3], but a deeper understanding is required to turn this into widespread clinical use. Another major clinical problem involving osteoblasts is ectopic osteogenesis, which is a major issue in atherosclerosis and heart valve disease [4]. Clinicians need both the stimulators and the inhibitors of osteoblast differentiation to address the wide spectrum of diseases.
A key aspect of osteogenesis is the interaction of osteoblasts and their precursors with the matrix: tissue engineering, especially with the help of 3D printing, is about to offer scaffolds that are analogous to the natural bone. It is essential to understand how to make the osteoblasts feel at home in a new environment. The current review offers a brief look at the current state of this research.
Definition and Function of Osteoblasts
The term “osteoblast” was coined in the early 20th century [5] as the cells that literally lay bone. Morphologically, osteoblasts are cuboidal cells found on the interface of newly synthesized bone, and strongly basophilic in their cytoplasm. Osteoblasts are present throughout life, but their activity is highest during embryonic skeletal formation and growth. In an adult organism, osteoblasts are activated when there is need to regenerate a defect or when the bone matrix has been depleted [6]. Osteoblasts secrete bone matrix proteins, including collagen type 1 alpha 1 (Col1α1), osteocalcin (OC), and alkaline phosphatase (Alp) [6]. Osteoblasts are not fast responders: from the moment the osteoblast is mature, it takes about 4 months until the synthesis of bone matrix by the cell is detected [7].
Osteoblasts are post-mitotic cells, but they are not terminally differentiated. The osteoblasts that have encircled themselves with the bone matrix eventually differentiate into osteocytes, which are interconnected stellar cells that regulate the turnover of bone material. Osteoblasts that remain on the surface of bone facing the periosteum have an option of becoming inert bone-lining cells or undergo apoptosis. When the mature osteoblast population grows thin (either as a result if a natural turnover or a regenerative process demanding massive recruitment), new osteoblasts are differentiated from mesenchymal progenitor cells; however, their resource is limited [8]. This is a key fact about osteoblasts, and a challenge for researchers. Autologous mesenchymal stem cells may in the future provide an infinite source of new osteoblasts, but osteogenic therapies should be applied with extreme caution in order not to deplete the pool of preosteoblasts.
Embryonic Origin of Osteoblasts
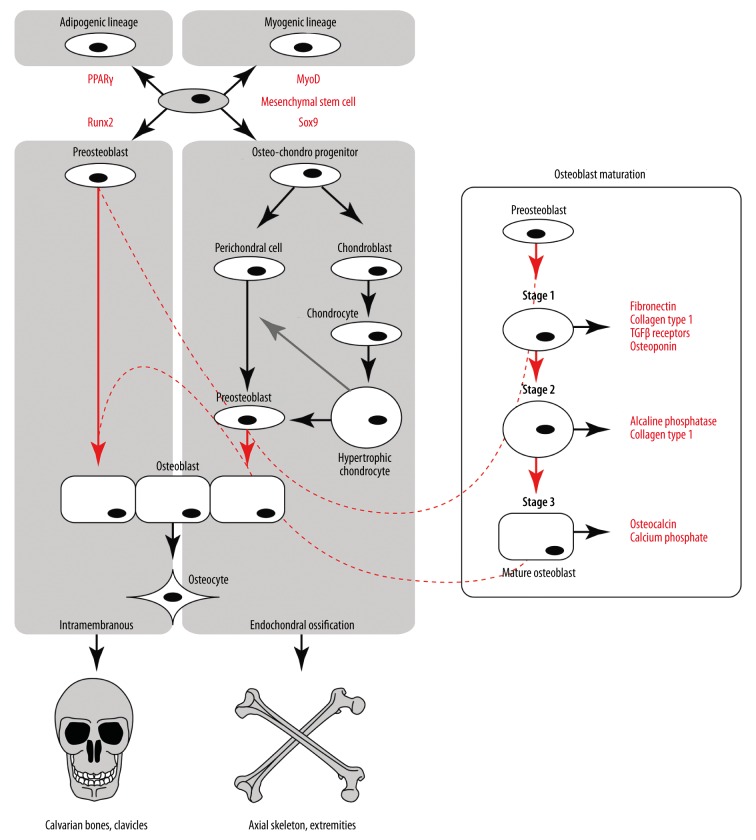
Osteoblasts stem from 2 distinct embryonic populations. One originates from the neural ectoderm [9], and the osteoblasts are formed directly from condensed mesenchymal progenitors without intermediate stages. These osteoblasts are mostly building squamous bones of the calvaria (scull and face) and the part of the clavicle, and the process is termed intramembranous ossification [9] (Figure 1, left). The remaining part of the skeleton is built by endochondral ossification, where osteoblasts stem from an intermediate class of perichondral cells [9] or directly from hypertrophic chondrocytes [10] (Figure 1, middle). The embryonic origin of the axial skeleton osteoblasts is paraxial mesoderm, while the appendicular skeleton cells stem from lateral plate mesoderm. Ossification occurs within the cartilage model, governed by paracrine influence from hypertrophic chondrocytes [8], which also have an ability to differentiate into osteoblasts [10]. In both types of osteogenesis, the cell type preceding osteoblasts is a preosteoblast.
Figure 1.
A flowchart depicting the biogenesis of osteoblasts. Mesenchymal stem cells can give rise to 4 lineages (top left) by expressing corresponding transcriptional regulators: PPARγ for adipogenic, MyoD for myogenic, Runx2 for osteoblastic, and Sox9 for chondrocytic lineages. In intramembranous ossification (osteogenesis in the scull and clavicles), preosteoblasts stem directly from mesenchymal stem cells, while in endochondral (osteogenesis of the axial skeleton and the limbs) a common osteo-chondro progenitor gives rise to both cell types. Hypertrophic chondrocytes in a paracrine manner (gray arrow) regulate transformation of perichondral cells into preosteoblasts, or might itself transform into one. The process of maturation of preosteoblasts is shown in the enlargement on the right.
Stages of Osteoblast Differentiation
The common ancestor for osteoblasts in both the endochondral and the intramembranous ossification are mesenchymal stromal cells (MSCs), also referred to as mesenchymal stem cells due to their multipotency. These cells can give rise either to myoblast, osteoblast, chondrocyte, or adipocyte lineages [7] (Figure 1, top). The primary commitment, and most of the follow-up differentiation, is governed by so-called “master transcriptional regulators” [6]. For the myoblast lineage, it is MyoD, for the chondroblast lineage it is Sox9, for the adipocyte lineage it is PPARγ, and for the osteoblast lineage it is Runx2 (Cbfa1). Activation of Runx2 in the endochondral pathway is preceded by Sox9, limiting the potential of the stem cells to differentiate into osteoblasts and chondroblasts. Once Runx2 is activated, the cells are defined as preosteoblasts, and they undergo a 3-stage differentiation, with each stage characterized by expression of certain molecular markers [11] (Figure 1, right):
In Stage 1 the cells continue to proliferate and express fibronectin, collagen, TGFβ receptor 1, and osteopontin.
In Stage 2 they exit the cell cycle and start differentiating, while maturating the extracellular matrix with Alp and collagen.
In Stage 3 matrix mineralization occurs when the organic scaffold is enriched with osteocalcin, which promotes deposition of mineral substance. Osteocalcin is in fact the second most abundant protein in bone after collagen [12]. At this stage the osteoblast assumes its characteristic cuboidal shape [8].
Regulation of Osteoblast Differentiation (OD)
Model issues
The available data on osteoblast differentiation are fragmented and incomplete. A major source for discrepancies is the lack of a reliable model system and the use of cell lines instead of primary cells. Most key findings were obtained using transgenic mice and morphological analysis. Some experiments have been performed on larger animals, like dogs [13]. Most mechanistic studies involve cell lines, which comes with huge limitations. The most popular cell line is MC3T3-E1 [14,15], which can be defined as a preosteoblast, but is often used to represent osteoblasts and even osteocytes. Other common lines are 2T3 mouse preosteoblasts [16] and rat osteosarcoma cells ROS 17/2.8 [17,18] or UMR106.01 [19]. Primary cells are also used, but they are substantially harder to obtain. Bone marrow stromal cells [20] and primary calvarian osteoblasts COB [19] are isolated from rats and mice [21]. Human material is also used, ranging from fetal osteoblasts HFOB [22] to mature osteoblasts, both available commercially [23], and primary cells isolated from bone explants [24]. Such a diversity in models makes it hard to draw a roadmap of osteoblast differentiation. The current review is no exception from the common rule, and the described pathways should be interpreted with caution. Table 1 shows the models used in the key papers reviewed here.
Table 1.
Major factors implicated in osteoblast differentiation.
| Reference | Factors described | Relevant finding | Model |
|---|---|---|---|
| [12] | Runx2 | Runx2 is necessary to differentiate osteoblasts from precursors | Runx2 knockout mice |
| [38] | Osterix (SP7) | In osterix knockout mice no bone formation occurs. Osterix is downstream of Runx2 | Osterix knockout mice |
| [27] | Msx2 | Bone, tooth, hair anomalies in Msx2 knockout mice | Msx2 knockout mice |
| [28] | Msx2 | Msx2 promotes osteoblastogenesis and inhibits adipogenesis | Primary mouse aortic myofibroblasts; wild type and LDL receptor knockout mice |
| [25] | Sox9 | Sox9 knockout mice lack chondrogenesis | Conditional Sox9 knockout mice |
| [29] | Ihh | Ihh signaling is necessary for endochondral ossification | Mice with ablation of Smo, an ihh receptor; Mesenchymal stem cells from mice |
| [26] | Arid5a | Histone modifications affect chondrocytic differentiation | mouse chondrocytic cell line ATDC5 |
| [33] | Wnt/beta-catenin | Wnt14 promotes osteoblast differentiation through β-catenin | Mice with conditional inactivation of β-catenin, Mesenchymal stem cells from these |
| [34] | Notch | Gain-of-function of Notch causes osteosclerosis | Transgenic mice with gain-of-function and loss-of-function of Notch |
| [35] | Notch | Notch maintains the population of osteoprogenitors, preventing them from differentiating into osteoblasts | Transgenic mice with gain-of-function and loss-of-function of Notch |
| [37] | PTH, Dickopf (Dkk) | PTH suppresses Dkk1 (inhibitor of wnt signaling) expression in OB and activates wnt signaling | Transgenic mice, MC3T3E1 cells |
| [39] | Atf4 | Atf4 makes a complex with Runx2 at the osteocalcin promoter | MC3T3-E1 cells, subclone 4 |
| [31] | NFATc | NFATc represses osteocalcin via HDAC | MC3T3 cells; primary osteoblasts from transgenic mice |
| [43] | Twist | Twist regulates osteoblastogenesis via HDAC | cross-breeding of Runx2 +/− and Twist1 +/− mice |
| [40] | BMP2 | BMP2 stimulates expression of other BMPs and stimulates osteoblast differentiation in vivo and in cell culture | Fetal rat calvarial osteoblasts, in vivo mice |
| [42] | Vitamin D | Vitamin D receptor collaborates with Runx2 in transcriptional activation of target genes | ROS 17/2.8 osteosarcoma cells |
| [56] | Macrogravity | Macrogravity transiently induces expression of c-fos and decreases Osteocalcin | MC3T3-E1a cells |
| [13] | Hydrostatic pressure | Hydrostatic pressure stimulates osteoblastogenesis and “connectivity” of the bone matrix | In vivo mongrel dogs |
| [44] | Bending of the bone | As a result of bending stress, osteoblasts increased in size and spread out in the periosteum | In vivo rat tibia |
| [52] | Shear stress in cell culture | Calibrated the shear stress necessary to induce osteogenic response | hFOB 1.19 (human fetal osteoblastic cells) |
| [55] | Microgravity – spaceflight induced | Microgravity inhibits preosteoblast response to hormonal stimulation and morphogens, preventing differentiation | MC3T3-E1 cells, MG-63 (human osteosarcoma cell line) |
| [24] | Macrogravity | Macrogravity stimulates collagen production by osteoblasts via Erk kinase | Human osteoblast-like cells obtained during hip replacement surgery |
| [57] | Ultrasound | Ultrasound increases number of osteoblasts and expression of TNF-alpha, IL-6 and TGF-beta | Rat primary calvarial osteoblasts. |
| [14] | Compression and vibration in cell culture | Osteoblasts are potentiated by sinusoid vibration combined with noise-type vibration. | MC3T3-E1 cells |
| [23] | Magnetically applied cell strain | Cell strain stimulates osteoblastogenesis via MAP-kinases P38 and Erk | NHO (normal human osteoblasts), commercial |
| [22] | Stretch in 3D collagen type 1 culture | Mechanical strain increased proliferation of cells and expression of CBFA, osteocalcin, ostepontin, alcaline phosphatase, histone H4 | hFOB 1.19 (human fetal osteoblastic cells) |
For ease of reader comprehension, the description of regulatory pathways is divided into the factors committing the precursors to osteoblastic lineage, those that directly mediate differentiation of precursors, and, finally, the paracrine and endocrine factors that fine-tune the process.
Factors mediating commitment to osteoblastic lineage
Sox9 is a master regulator of chondrogenic lineage, but it is also crucial in endochondral bone development. Sox9 mediates the condensation of mesenchymal cells into chondrogenic progenitors (and perichondrial cells that will stimulate osteoblast differentiation), but it is also a necessary step in commitment of the osteoblast precursors. Sox9 knockout mice lack expression of Runx2 in the axial skeleton, and mice are born with heads but without limbs [25]. However, if Sox9 is selectively ablated after mesenchymal condensation, Runx2 expression and osteoblastogenesis take place [25]. Downstream targets of Sox9 are Sox5 and Sox6. Sox9 is also functionally connected with histone modifiers Arid5a and Znf219 [9]. Arid5a acetylates histone H3 at chondrogenesis-important genes, and it is crucial for further differentiation of chondrocytes [26].
Msx2, unlike Sox9, is important in intramembranous osteogenesis. Msx2 knockout mice display remarkable absence of mineralization in the calvarial bones as well as impaired ossification in the axial skeleton [27]. These mice also had neurological deficits and were prone to seizures. Mapping of Msx2 function revealed it was the decision-making factor favoring osteoblastic differentiation over adipogenic. A study in aortic myofibroblasts from mice revealed that Msx2 interacted with C/EBP alpha to inhibit PPARγ transcription factor, which is an adipogenic determinant, just as Runx2 is an osteoblastic determinant [28].
Hedgehog signaling. Hedgehogs were the long-sought “roadmap” factors that establish the three-dimensional architecture in the embryo at large. The hedgehog family has 3 members: Sonic (shh), Indian (ihh), and Desert (dhh) hedgehogs. Ihh is a signal molecule released by perichondrial cells and stimulating osteoblast differentiation in preosteoblasts [29]. Intracellularly, it is conferred by Ptch and Smo receptors and transduced to the Gli transcription factors that stimulate expression of Runx2 [8].
Factors mediating osteoblastic differentiation
Runx2. The osteoblast master regulator Runx2 is predominantly a fetal factor, while in the adult organism its levels are low. Outside the skeleton its expression manifests onset of ectopic calcification [12]. Runx2 target genes include osteopontin, bone sialoprotein, osteocalcin, osteoprotegerin, Rankl, and many others [30]. The key role of Runx2 in osteoblast differentiation was convincingly demonstrated in 1997 by Komori et al. [12]. Heterozygous Runx2 knockout mice had impaired calvarian osteogenesis while homozygous knockouts had no mineralization, the entire skeleton remaining a cartilaginous model. The homozygously knockout animals died at birth since their ribcage could not produce air traction. Apparently, osteoblast differentiation was arrested at the early stage; because osteocalcin was completely absent, osteopontin and alkaline phosphatase were barely detectable. Further research showed that Runx2 role is important at 2 time points: during exit of preosteoblasts from the cell cycle (end of growth – start of matrix maturation) and during late maturation stages of osteoblasts [11]. Therefore, the factors that regulate Runx2 will also regulate osteoblast differentiation (see Figure 2) [8].
Figure 2.
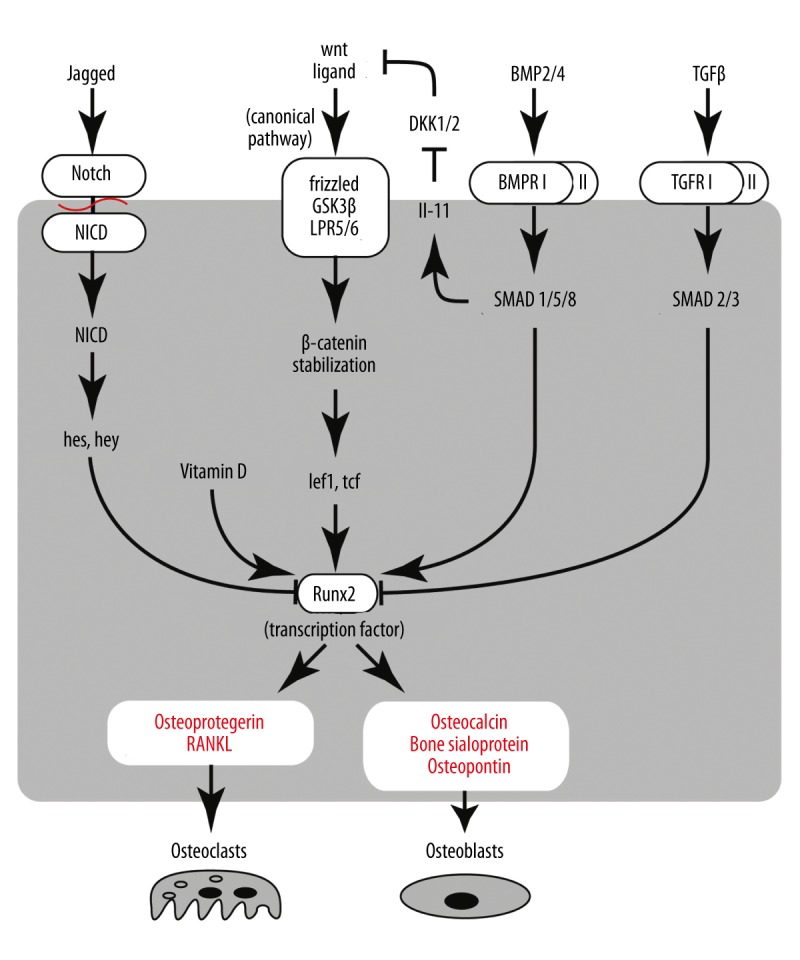
Major regulatory pathways involved in the regulation of Runx2, a chief transcriptional regulator of osteogenesis. Four pathways are elaborated. The Wnt pathway is central in activation of Runx2 via the stabilization of β-catenin. This process in cells other than osteoblasts is inhibited by Dickopf (DKK1/2). This inhibition is removed by BMP signaling via SMADs and Interleukin-11. BMP has other, direct actions on Runx2. TGF-β and Notch signaling is anti-calcific. TGF signal is transmitted into the cell by SMADs (other subsets than BMPs), while Notch relies on the cleavage of its intracellular domain upon activation. Arrows show activation and stump-ends show inhibition.
Wnt/Notch system of Runx2 regulation is perhaps the most studied. Activation of the Wnt/β-catenin pathway and imbalance between the Wnt and Notch pathways leads to ectopic mineralization, such as in aortic valve stenosis and advanced atherosclerosis [4]. Wnt is a secreted paracrine glycoprotein that signals through several possible pathways, one of them termed “canonical” and being important for osteoblast differentiation [7]. The Wnt receptor complex on the cell surface contains lipoprotein receptor (Lpr), frizzled, and GSK3β. It binds to the Wnt ligand and transmits the signal into the cytoplasm. Once inside the cell, the signal is transduced to β-catenin. Normally, β-catenin forms a complex with Axin and Apc, which targets it for degradation. Wnt signal “rescues” β-catenin, and it translocates to the nucleus to bind to its cofactors Tcf and Lef1. Without stimulation, they are also inhibited by binding to Tle and HDAC, both repressors of transcription [31]. Arrival of β-catenin results in their activation and subsequent expression of Runx2, as well as other pro-osteogenic genes [32]. There are a number of Wnt ligands in osteogenic progenitors. Ectopically expressed Wnt14 ligand promotes osteoblast differentiation. Inactivation of beta-catenin during both intramembranous and endochondral ossification leads to ectopic cartilagenesis at the expense of bone formation [33].
Wnt signaling cascade is under the constant balancing influence of Notch. Gain- and loss-of-function transgenic studies revealed a complex role of Notch in osteoblast differentiation. Notch gain-of-function mice paradoxically displayed osteosclerosis, with more mineralization than normal. The early osteoblast markers, Osx, Alp, and Bsp, were increased, but the late ones (osteocalcin and Col1a1) were decreased. Conversely, loss-of-function mutation of Notch led to osteoporosis, but this effect was attributed to inhibition of osteoprotegerin synthesis, which normally inhibits Rank/Rankl signaling in osteoclasts. Osteoprotegerin inhibition promotes osteoclast-mediated resorption of bone [34]. It was further established that Notch maintains osteoblastic progenitors in an undifferentiated stage. Notch knockouts had the same number of osteoblasts as wild types, but much more of them assumed cuboid (i.e., differentiated) shape. In the long run, the mice were depleted of osteogenic potential, causing osteopenia. At the age of 26 weeks, Notch knockout mice had approximately 10% of the normal mass of mineralized bone [35]. Notch is an intracellular signaling protein that binds to its ligand Jagged, and promotes degradation of β-catenin, thereby opposing Wnt. Alternatively, it may first enhance expression of Hes1, Hey1, and HeyL, which inhibit Runx2 [7,35].
Beside Notch, Wnt pathway is opposed by PPARγ master regulator of adipogenesis, via methylation of histones by SETDB1, which is one of the targets of Wnt5 [32[. Wnt is also subject to inhibition by sclerostin and Dickopf (Dkk) 1 and 2 [36,37].
Osterix (Osx or SP7) is a zinc-finger transcription factor downstream of Wnt, plus it can serve as a negative feedback loop, inhibiting Wnt [6]. Studies of Osx knockout mice showed that it mediates both the commitment of MSCs to osteoblastic lineage, and the further differentiation with expression of osteocalcin and Col1α1. Osx knockout mice had normal levels of Runx2, very little Col1α1, and no expression of bone sialoprotein, osteonectin, or osteopontin. The mice were born with short non-mineralized limbs. Mesenchymal stem cells from these mice could be transformed in vitro into both osteoblast and chondroblast phenotypes [38].
Atf4 is also downstream of Runx2, making a complex with it at the promotor of osteocalcin, and both factors are necessary to enable osteocalcin expression. Atf4 is crucial in late stages of osteoblast differentiation. Atf4 knockout mice have reduced osteocalcin and Bsp expression, while overexpression of Atf4 gives a dose-dependent increase of osteocalcin [39]. In addition, Atf4 facilitates import of amino acids into osteoblasts and stimulates Ihh expression in chondrocytes. Atf4 is subject to inhibition by the factor inhibiting atf4-induced transcription FIAT [8].
Paracrine and endocrine factors influencing osteoblastic differentiation
Bone morphogenetic proteins (BMPs) are cytokines especially important for postnatal ossification [8]. BMPs are members of the TGFβ superfamily, and their signaling is typical of it: the ligand binds to a receptor on cell surface, which activates receptor SMADs. They trigger the effector SMADs that translocate to the nucleus and bind to cofactors regulating transcription of target genes. Osteogenic BMPs are BMP2 and 4 [40]. Their effect is dual: they are upstream of Runx2 transcription; and they can team up with Runx2 to regulate transcription of its target genes. The BMP-Runx2 axis is under negative control of several inhibitors: Smurf1 (ubiquitin ligase), which degrades both BMP2 receptor and Runx2, Hey1, which mediates Notch signaling, and TGFβ, which inhibits Runx2 via SMAD3. BMP2 is indispensable for the expression of Osterix and may have a connection with Sox9 [9]. Treatment of myoprogenitor cells with BMP2 leads to increased Alp activity and expression of Msx2 [28]. Mechanical stress also stimulates Alp synthesis via increased expression of BMP2 and -4 and inhibition of Smurf [41].
Besides BMPs, some other growth factors influence osteoblast differentiation: mainly TGFβ, IGF, which activates Osterix [1,20], and FGF. The latter, although being primarily fibroblast-stimulatory, also promotes osteoblast differentiation. Its signaling is believed to be via MAP kinases PKC and PI3K, but the exact mechanisms are not known [8].
Other cytokines have an effect on osteoblast differentiation as well. The best described is by interleukin IL-11. It is activated by FosB/JunD factors dimerizing and binding to its promoter. FosB is in turn stimulated by mechanical stress [36]. Expression of IL-11 can be stimulated by BMP2 via SMADs. They bind to SMAD-binding elements on the IL-11 gene promoter in cooperation with FosB. The function of IL-11 is believed to be inhibition of Dickopf Dkk 1 and 2, which are inhibitors of Wnt signaling, and its decrease in aging may be one of the factors contributing to osteoporosis [36].
Parathyroid hormone (PTH) is one of the upstream regulators of Runx2 [6]. The PTH effect is time-dependent. If the level of this hormone is elevated continuously, bone resorption occurs. If the elevation is intermittent, osteoblast differentiation is stimulated. This is probably because intermittent exposure leads to release of TGFβ from resorbing bone, which in the absence of stimulation causes recruitment of new osteogenic progenitors [8]. PTH works via MAP kinase Erk1/2 and activation of CREB/fos with JunD that leads to expression of IL-11 and suppresses Dickopf (Dkk). Another possible mechanism is via PKC delta that phosphorylates SMAD1 and also dimerizes with JunD [36].
Vitamin D is essential in regulating Runx2 target genes. Vitamin D receptor makes a complex with Runx2 and several other cofactors on the target gene promoter (e.g., osteocalcin) [11,42], and stimulates expression of osteo-specific genes.
HDACs. Runx2 is subject to epigenetic control mechanisms, in particular, by HDAC3-7. Its coactivators are histone transferases p300, CBP, PCAF, MOZ, and MORF. In mice, Runx2 is expressed from embryonic day E10, but its action is not detectable until E13 or even E15. Bialek et al. [43] showed that for several days Runx2 function is suppressed by Twist by binding to its DNA-binding domain and recruitment of HDACs. A combination of heterozygous ablation of Runx2 (cranial bone deficit) and Twist (skull osteopetrosis) results in a normally developed calvarian bone phenotype [43]. HDACs also interfere with transcriptional regulation of Runx2 target genes. Osteocalcin promoter has 2 binding sites – proximal and distal – and Runx2 binds to both of them. To initiate osteocalcin transcription, Runx2 cooperates with cofactors, including p300 and vitamin D receptor, and this process is controlled by histone modifications [11]. For example, osteocalcin transcription and, thus, osteoblast differentiation is blocked by nuclear factor of activated T cells type c (NFATc) (via recruitment of HDAC3) and by TGF-β (via HDAC 4 and 5) [31].
Mechanical Stimulation and Osteoblast Differentiation
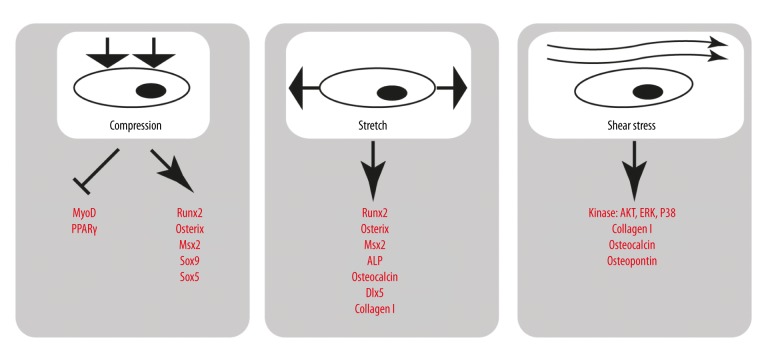
The mechanical influence on osteoblasts is crucial both for the regeneration of broken bones and for the human exploration of space. This is why this topic will gain increased importance in decades to come. The effect of mechanical loading on bone growth is described by Wolff’s law: the structure of bone is formed corresponding to the direction of applied mechanical force. Indeed, pressure applied to a dog bone in vivo results in thickening of the cancellous bone matrix due to accumulation of collagen and mineral deposition [13]. Bending of the bone in vivo, such as exemplified by a bending device on the rat tibia, increases the spreading of osteoblasts over the periosteal surface and the Alp signal [44]. Further effects include activation of cell cycle signaling and proliferation [45]. Conversely, unloading the bone causes osteoporosis in wild types but not in heterozygous Runx2 knockout mice [30]. Different modalities of mechanical stress demonstrate different aspects of bone homeostasis: gravity, vibration, compression, and fluid shear stress are all physiological, but their effects and mechanisms are not similar (see Figure 3).
Figure 3.
A short summary of factors activated in osteoblasts by 3 modalities of mechanical stress. The activated and inhibited factors are shown in red. An arrow symbolizes activation and stump-ends show inhibition.
Mechanical compression and stretch
Cells grown on a 2D surface may be exposed to mechanical stimulation by bending or stretching the matrix underneath. MC3T3 preosteoblasts that were compressed and stretched at high frequency for 3 min per day for a week had a long-term increase in mRNA of osteocalcin and matrix metalloproteinase 9, parallel to reduced cell growth [14]. Compression may even reprogram progenitors of other cell lines. Compression of cultured myoblasts reduced expression of myogenic and adipogenic master regulators, and increased osteogenic regulators. Runx2 activation in this setting was blocked by an inhibitor of MAP kinase p38 [46]. Continuous stretching of cultured myoblasts as a model of distraction osteogenesis activated osteocalcin, Alp, Osx, Runx2, Dlx5, and Col1α1, probably via BMP signaling, and resulted in osteoblast differentiation [47].
A convenient and standardized model is provided by the FlexCell® system, in which cells grown on flexible membranes can be stretched with programmable deformation and frequency. Human primary mesenchymal stem cells respond to FlexCell® by Runx2 and osteocalcin expression, and 3% stretch has a much more pronounced effect than 10% [48]. The 3% stretch also induced calcium deposition, which was blocked by MAP kinase Erk1/2 inhibitors [49]. When even milder stretching regimens were tested (0.8, 1.6, 2.4, and 3.2%), commercial human osteoblasts increased phosphorylation of MAP kinase Erk1/, Col1α1, and osteocalcin in all groups, while Runx2 induction occurred only after 3.2% stretching. This indicates that Runx2 is induced by a very finely tuned stretch signal [50]. Of note, in a 3D culture of human fetal osteoblasts, short (30 min) daily episodes of mechanical stretching (1%) activated Runx2, osteocalcin, Opn, Alp, and Histone H4, at the same time increasing cell proliferation [22].
An exotic way to apply stretch is to draw magnetic micro-particles into cultured human osteoblasts and move them by applying an external magnetic field. A prolonged (3 weeks) treatment led to an increase in osteocalcin and Runx2. The cells formed calcified nodules in culture, which was blocked by an inhibitor of p38 and delayed by the inhibitor of Erk1/2 [23].
Shear stress
Shear stress is probably the most relevant stress modality in normal bone. Mechanical load is transduced inside the bone matrix via the fine network of canaliculi, where it induces fluid flow parallel to the vector of applied force. This liquid shears the cells and also increases hydrostatic pressure in a closed system [51]. Human fetal osteoblasts responded to pulsatile shear stress, but not to steady or oscillatory stress, concurrent with the stresses experienced by bones in vivo. This response constitutes the mechanically induced calcium current in the cells, believed to mediate the mechanosensitive signaling [52]. Shear stress applied to a culture of primary human mesenchymal stem cells induced cuboid morphology, as well as release of Alp, BMP2, Bsp, and osteopontin. Similar effects were attained with the use of the osteogenic medium (dexamethasone, b-glycerophosphate, and ascorbic acid), but the effects of both were not synergistic [53]. A recent study using rat mesenchymal stem cells traced the entire chain of events: rapid rise of intracellular calcium and prostaglandin E (PGE) release activated MAP kinases Erk1/2 and p38, which in turn induced c-fos, vascular endothelial growth factor, and cyclooxygenase 2 (COX2) [20]. Intermittent shear stress was more dependent on PGE, and continuous shear stress was more dependent on MAP kinases. However, both intermittent and continuous shear stress induced endpoint genes: Col1α1, Bsp, osteocalcin, and osteopontin [20].
Shear stress evokes mechanisms distinctly different than stretch. While shear stress induced nitric oxide and PGE signaling, these mechanisms were virtually insensitive to stretch [54]. Besides its effect on osteoblast proliferation, shear stress also reduces osteoblast apoptosis (modeled by addition of TNF alpha) through activation of survival kinase Akt, as shown in an Akt inhibitor study [19].
Gravity
Microgravity, as experienced by humans in space, imposes a great risk on the astronauts by disrupting the natural gravity-regulated homeostatic mechanisms. Astronauts lose 1–2% of their bone mass each month of spaceflight. Rats on board the spacecraft have shown arrest of cortical periosteal growth [55]. Response to microgravity also varies between calvarian bones and the bones of extremities and the axial skeleton. The bones of the scull react with a release of PGE, while the other bones have an increase in cyclic AMP, GSK3, and nitric oxide. In microgravity, the osteoblasts become insensitive to osteogenic stimulation by Vitamin D3 and TGFβ [55]. A systematic array study of mouse osteoblast precursors in microgravity showed a decrease in Runx2, BMP4, and PTH receptor, as well as Alp [16]. Mouse primary calvarian osteoblasts subjected to microgravity showed a similar response: Runx2, osteoglycin, and Sfrp (secreted frizzled-related protein) were downregulated, and a number of factors were induced, such as IL-6, NADPH dehydrogenase, and quinone 1 [21].
Macrogravity, as experienced in spacecraft launch, may in itself constitute a stimulatory signal for osteogenesis and provide temporary protection for the astronauts. A simulated launch of mouse osteoblasts in culture (acceleration up to 3g) led to a transient activation of c-Fos, but also a subsequent decrease of osteocalcin, signifying delayed matrix mineralization [56]. In contrast, prolonged and vigorous hyper-gravity in primary human osteoblast-like cells (13g for 24 hours, lethal) increased signaling by MAP kinases, cAMP, and IP3, and led to enhanced synthesis of collagen, which was abolished by blocking Erk1/2 phosphorylation [24].
Ultrasound
Ultrasound is a relevant stressor because if can affect mineralization and it can be applied non-invasively. Ultrasound stimulation of rat calvarial osteoblasts increases cytokine signaling via TNFα, IL-6 and TGFβ, and increases the number of cells [57]. Ultrasound also increases expression of Runx2, Msx2, Dlx2, Osx, BMP2, and Bsp and leads to calcification in cultured osteosarcoma cells possessing osteoblast properties [17].
Mechanosensitive signaling
The role of mechanosensors is attributed to integrins and stretch-activated calcium channels. The signal is then transmitted to MAPKs, COX2, NO, TNFα, and β-Catenin. Calvarian osteoblasts also respond by PGE2 and IGF-1 release, which in turn promote expression of Runx2 and TGFβ [1]. Two integrins seem to be pivotal in sensing stress by bending the bone: integrin β1 and β5, in which integrin β1 is responsible for eliciting Erk1/2 phosphorylation response leading to proliferation [45]. Mechanically induced Erk1/2 has several targets. It can act as a coactivator of Runx2, binding its runt domain. It is also necessary for acetylation and phosphorylation of histones upstream of the Runx2 binding sites, exposing them for Runx2-mediated activation of transcription. As stated above, it activates transcription of early response genes like c-fos and COX. Beside NO, in shear stress, Erk1/2 can be activated by FAK (focal adhesion kinase) [30]. In osteosarcoma cells, both FlexCell treatment and microgravity increase expression of immediate-early genes egr-1 and NFκB. This induction may also be mediated by activation of MAP kinases Erk1/2 and p38 [18].
3D growth systems for OD
If osteoblasts are to be raised in the lab for a cell replacement therapy, the conditions must maximally resemble their natural environment. There exist a number of systems with 3D structure, where osteoblasts can grow and generate matrix similar to the ordinary bone. They should possess 2 fundamental properties: osteoconductivity (amenability of the surface to the population of seeded precursors) and osteoinductivity (ability to induce differentiation of these cells into mature osteoblasts) [58].
Decellularized donor bone matrix
Original donor bone matrix, but enzymatically treated to extinguish resident cells, is the first choice. Mouse preosteoblasts grow on it, especially after stimulation with the perfusion flow. The regimen with slow speed (and less strain) was more stimulatory for cell proliferation, while higher strain induced expression of Runx2 and osteocalcin, demonstrating that proliferation and differentiation are indeed 2 inversely proportional processes [59].
Natural organic matrices
Matrices made of natural organic compounds like Zein (a protein found in maize) makes a scaffold, which has porosity similar to cancellous bone and osteoconductive to mesenchymal stem cells [60]. Its major drawback is low mechanical strength, which can only be improved when the osteoblasts have deposited sufficient amounts of osteoid. Another example is scaffold composed of shrimp polysaccharide chitosan and pectin. A combination of the 2 with addition of hydroxyapatite is strong, resists hydrolysis, and has sufficient osteoinductive potential for human osteoblasts [61].
Synthetic matrices
Synthetic matrices for osteoblast growth have been available for just about a decade. Seeding preosteoblasts on a 3D mesh of titanium fibers showed good inhabitance by osteoblasts. Furthermore, it was shown that viscosity of the medium imposing shear stress on the attached cells increases their differentiation and mineral deposition in the matrix. The effect was mediated by calcium, NO, and PGE [62]. The model was further enhanced by pre-culturing titanium scaffolds with mesenchymal stem cells that deposited extracellular matrix, promoting attachment and growth of the cells seeded after the first generation was removed [58]. Apart from metal meshes, positive results with human osteoblast precursors have been obtained with polyethylene terephthalate [63], polyurethane [64], and poly-e-caprolactone scaffolds [65].
Conclusions
Osteoblast differentiation is still unclear, but a few basic principles have already been discovered. First, osteoblasts come from multipotent mesenchymal stem cells, with the other alternatives being cartilage, fat, and muscle tissue. Attempts at differentiation of osteoblasts from stem cells should thus both stimulate the osteoblast lineage and inhibit these other ones. Second, the reserve of preosteoblasts is finite, and the depletion of this pool will lead to osteopenia. Third, the function of osteoblasts is balanced by that of osteoclasts, and this balance is crucial for the overall turnover of bone. Fourth, mechanical stimulation is essential for osteoblast differentiation and function, and changes in the normal physical environment, such as in spaceflight, will cause major changes in bone homeostasis.
All these factors need to be considered, the complete sequence of molecular events of osteoblastogenesis needs to be defined, and new matrices with high compatibility need to be developed. Thereafter, we may be able to effectively eliminate osteoporosis, osteogenesis imperfecta, ossification of heart valves and large vessels, and a host of other conditions related to abnormal ossification, as well as providing effective help to orthopedic patients, of which there will continue to be many, even if all medical diseases are cured in years to come.
Footnotes
Source of support: Government of Russian Federation, Grant 074-U01; South-Eastern Norway Regional Health Authority, Norway
References
- 1.Papachroni KK, Karatzas DN, Papavassiliou KA, et al. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol Med. 2009;15(5):208–16. doi: 10.1016/j.molmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Hernlund E, Svedbom A, Ivergard M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344(5):385–86. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 4.Johnson ML, Rajamannan N. Diseases of Wnt signaling. Rev Endocr Metab Disord. 2006;7(1–2):41–49. doi: 10.1007/s11154-006-9003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keith A. Concerning the Origin and Nature of Osteoblasts. Proc R Soc Med. 1927;21(2):301–8. doi: 10.1177/003591572702100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen ED, Gopalakrishnan R, Westendorf JJ. Regulation of gene expression in osteoblasts. Biofactors. 2010;36(1):25–32. doi: 10.1002/biof.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canalis E. Notch signaling in osteoblasts. Sci Signal. 2008;1(17):pe17. doi: 10.1126/stke.117pe17. [DOI] [PubMed] [Google Scholar]
- 8.Long F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2012;13(1):27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura R, Hata K, Matsubara T, et al. Regulation of bone and cartilage development by network between BMP signalling and transcription factors. J Biochem. 2012;151(3):247–54. doi: 10.1093/jb/mvs004. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Tsang KY, Tang HC, et al. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci USA. 2014;111(33):12097–102. doi: 10.1073/pnas.1302703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein GS, Lian JB, van Wijnen AJ, et al. Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene. 2004;23(24):4315–29. doi: 10.1038/sj.onc.1207676. [DOI] [PubMed] [Google Scholar]
- 12.Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–64. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 13.Guldberg RE, Caldwell NJ, Guo XE, et al. Mechanical stimulation of tissue repair in the hydraulic bone chamber. J Bone Miner Res. 1997;12(8):1295–302. doi: 10.1359/jbmr.1997.12.8.1295. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka SM, Li J, Duncan RL, et al. Effects of broad frequency vibration on cultured osteoblasts. J Biomech. 2003;36(1):73–80. doi: 10.1016/s0021-9290(02)00245-2. [DOI] [PubMed] [Google Scholar]
- 15.Lin GL, Hankenson KD. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem. 2011;112(12):3491–501. doi: 10.1002/jcb.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel MJ, Liu W, Sykes MC, et al. Identification of mechanosensitive genes in osteoblasts by comparative microarray studies using the rotating wall vessel and the random positioning machine. J Cell Biochem. 2007;101(3):587–99. doi: 10.1002/jcb.21218. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki A, Takayama T, Suzuki N, et al. Daily low-intensity pulsed ultrasound-mediated osteogenic differentiation in rat osteoblasts. Acta Biochim Biophys Sin (Shanghai) 2009;41(2):108–15. doi: 10.1093/abbs/gmn012. [DOI] [PubMed] [Google Scholar]
- 18.Granet C, Boutahar N, Vico L, et al. MAPK and SRC-kinases control EGR-1 and NF-kappa B inductions by changes in mechanical environment in osteoblasts. Biochem Biophys Res Commun. 2001;284(3):622–31. doi: 10.1006/bbrc.2001.5023. [DOI] [PubMed] [Google Scholar]
- 19.Pavalko FM, Gerard RL, Ponik SM, et al. Fluid shear stress inhibits TNF-alpha-induced apoptosis in osteoblasts: a role for fluid shear stress-induced activation of PI3-kinase and inhibition of caspase-3. J Cell Physiol. 2003;194(2):194–205. doi: 10.1002/jcp.10221. [DOI] [PubMed] [Google Scholar]
- 20.Kreke MR, Sharp LA, Lee YW, Goldstein AS. Effect of intermittent shear stress on mechanotransductive signaling and osteoblastic differentiation of bone marrow stromal cells. Tissue Eng Part A. 2008;14(4):529–37. doi: 10.1089/tea.2007.0068. [DOI] [PubMed] [Google Scholar]
- 21.Capulli M, Rufo A, Teti A, Rucci N. Global transcriptome analysis in mouse calvarial osteoblasts highlights sets of genes regulated by modeled microgravity and identifies a “mechanoresponsive osteoblast gene signature”. J Cell Biochem. 2009;107(2):240–52. doi: 10.1002/jcb.22120. [DOI] [PubMed] [Google Scholar]
- 22.Ignatius A, Blessing H, Liedert A, et al. Tissue engineering of bone: Effects of mechanical strain on osteoblastic cells in type I collagen matrices. Biomaterials. 2005;26(3):311–18. doi: 10.1016/j.biomaterials.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 23.Yuge L, Okubo A, Miyashita T, et al. Physical stress by magnetic force accelerates differentiation of human osteoblasts. Biochem Biophys Res Commun. 2003;311(1):32–38. doi: 10.1016/j.bbrc.2003.09.156. [DOI] [PubMed] [Google Scholar]
- 24.Gebken J, Luders B, Notbohm H, et al. Hypergravity stimulates collagen synthesis in human osteoblast-like cells: Evidence for the involvement of p44/42 MAP-kinases (ERK 1/2) J Biochem. 1999;126(4):676–82. doi: 10.1093/oxfordjournals.jbchem.a022502. [DOI] [PubMed] [Google Scholar]
- 25.Akiyama H, Chaboissier MC, Martin JF, et al. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16(21):2813–28. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amano K, Hata K, Muramatsu S, et al. Arid5a cooperates with Sox9 to stimulate chondrocyte-specific transcription. Mol Biol Cell. 2011;22(8):1300–11. doi: 10.1091/mbc.E10-07-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satokata I, Ma L, Ohshima H, et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24(4):391–95. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- 28.Cheng SL, Shao JS, Charlton-Kachigian N, et al. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem. 2003;278(46):45969–77. doi: 10.1074/jbc.M306972200. [DOI] [PubMed] [Google Scholar]
- 29.Long F, Chung UI, Ohba S, et al. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development. 2004;131(6):1309–18. doi: 10.1242/dev.01006. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Ge C, Long JP, Begun DL, et al. Biomechanical stimulation of osteoblast gene expression requires phosphorylation of the RUNX2 transcription factor. J Bone Miner Res. 2012;27(6):1263–74. doi: 10.1002/jbmr.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choo MK, Yeo H, Zayzafoon M. NFATc1 mediates HDAC-dependent transcriptional repression of osteocalcin expression during osteoblast differentiation. Bone. 2009;45(3):579–89. doi: 10.1016/j.bone.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5(8):442–47. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- 33.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8(5):739–50. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 34.Engin F, Yao Z, Yang T, et al. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med. 2008;14(3):299–305. doi: 10.1038/nm1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilton MJ, Tu X, Wu X, et al. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med. 2008;14(3):306–14. doi: 10.1038/nm1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsumoto T, Kuriwaka-Kido R, Kondo T, et al. Regulation of osteoblast differentiation by interleukin-11 via AP-1 and Smad signaling. Endocr J. 2012;59(2):91–101. doi: 10.1507/endocrj.ej11-0219. [DOI] [PubMed] [Google Scholar]
- 37.Guo J, Liu M, Yang D, et al. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab. 2010;11(2):161–71. doi: 10.1016/j.cmet.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor Osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108(1):17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 39.Xiao G, Jiang D, Ge C, et al. Cooperative interactions between activating transcription factor 4 and Runx2/Cbfa1 stimulate osteoblast-specific osteocalcin gene expression. J Biol Chem. 2005;280(35):30689–96. doi: 10.1074/jbc.M500750200. [DOI] [PubMed] [Google Scholar]
- 40.Chen D, Harris MA, Rossini G, et al. Bone morphogenetic protein 2 (BMP-2) enhances BMP-3, BMP-4, and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in cultures of fetal rat calvarial osteoblasts. Calcif Tissue Int. 1997;60(3):283–90. doi: 10.1007/s002239900230. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Zhang X, Guo Y, et al. Involvement of BMPs/Smad signaling pathway in mechanical response in osteoblasts. Cell Physiol Biochem. 2010;26(6):1093–102. doi: 10.1159/000323987. [DOI] [PubMed] [Google Scholar]
- 42.Montecino M, Lian J, Stein G, Stein J. Changes in chromatin structure support constitutive and developmentally regulated transcription of the bone-specific osteocalcin gene in osteoblastic cells. Biochemistry. 1996;35(15):5093–102. doi: 10.1021/bi952489s. [DOI] [PubMed] [Google Scholar]
- 43.Bialek P, Kern B, Yang X, et al. twist code determines the onset of osteoblast differentiation. Dev Cell. 2004;6(3):423–35. doi: 10.1016/s1534-5807(04)00058-9. [DOI] [PubMed] [Google Scholar]
- 44.Boppart MD, Kimmel DB, Yee JA, Cullen DM. Time course of osteoblast appearance after in vivo mechanical loading. Bone. 1998;23(5):409–15. doi: 10.1016/s8756-3282(98)00119-7. [DOI] [PubMed] [Google Scholar]
- 45.Yan YX, Gong YW, Guo Y, et al. Mechanical strain regulates osteoblast proliferation through integrin-mediated ERK activation. PLoS One. 2012;7(4):e35709. doi: 10.1371/journal.pone.0035709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yanagisawa M, Suzuki N, Mitsui N, et al. Effects of compressive force on the differentiation of pluripotent mesenchymal cells. Life Sci. 2007;81(5):405–12. doi: 10.1016/j.lfs.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Kim IS, Song YM, Cho TH, et al. Synergistic action of static stretching and BMP-2 stimulation in the osteoblast differentiation of C2C12 myoblasts. J Biomech. 2009;42(16):2721–27. doi: 10.1016/j.jbiomech.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Chen YJ, Huang CH, Lee IC, et al. Effects of cyclic mechanical stretching on the mRNA expression of tendon/ligament-related and osteoblast-specific genes in human mesenchymal stem cells. Connect Tissue Res. 2008;49(1):7–14. doi: 10.1080/03008200701818561. [DOI] [PubMed] [Google Scholar]
- 49.Simmons CA, Matlis S, Thornton AJ, et al. Cyclic strain enhances matrix mineralization by adult human mesenchymal stem cells via the extracellular signal-regulated kinase (ERK1/2) signaling pathway. J Biomech. 2003;36(8):1087–96. doi: 10.1016/s0021-9290(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 50.Zhu J, Zhang X, Wang C, Peng X. Different magnitudes of tensile strain induce human osteoblasts differentiation associated with the activation of ERK1/2 phosphorylation. Int J Mol Sci. 2008;9(12):2322–32. doi: 10.3390/ijms9122322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen JH, Liu C, You L, Simmons CA. Boning up on Wolff’s Law: Mechanical regulation of the cells that make and maintain bone. J Biomech. 2010;43(1):108–18. doi: 10.1016/j.jbiomech.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 52.Jacobs CR, Yellowley CE, Davis BR, et al. Differential effect of steady versus oscillating flow on bone cells. J Biomech. 1998;31(11):969–76. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yourek G, McCormick SM, Mao JJ, Reilly GC. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regen Med. 2010;5(5):713–24. doi: 10.2217/rme.10.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smalt R, Mitchell FT, Howard RL, Chambers TJ. Induction of NO and prostaglandin E2 in osteoblasts by wall-shear stress but not mechanical strain. Am J Physiol. 1997;273(4 Pt 1):E751–58. doi: 10.1152/ajpendo.1997.273.4.E751. [DOI] [PubMed] [Google Scholar]
- 55.Carmeliet G, Bouillon R. The effect of microgravity on morphology and gene expression of osteoblasts in vitro. FASEB J. 1999;13(Suppl):S129–34. doi: 10.1096/fasebj.13.9001.s129. [DOI] [PubMed] [Google Scholar]
- 56.Fitzgerald J, Hughes-Fulford M. Gravitational loading of a simulated launch alters mRNA expression in osteoblasts. Exp Cell Res. 1996;228(1):168–71. doi: 10.1006/excr.1996.0313. [DOI] [PubMed] [Google Scholar]
- 57.Li JK, Chang WH, Lin JC, et al. Cytokine release from osteoblasts in response to ultrasound stimulation. Biomaterials. 2003;24(13):2379–85. doi: 10.1016/s0142-9612(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 58.Datta N, Pham QP, Sharma U, et al. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci USA. 2006;103(8):2488–93. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cartmell SH, Porter BD, Garcia AJ, Guldberg RE. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng. 2003;9(6):1197–203. doi: 10.1089/10763270360728107. [DOI] [PubMed] [Google Scholar]
- 60.Gong S, Wang H, Sun Q, et al. Mechanical properties and in vitro biocompatibility of porous zein scaffolds. Biomaterials. 2006;27(20):3793–99. doi: 10.1016/j.biomaterials.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 61.Verma D, Katti KS, Katti DR. Osteoblast adhesion, proliferation and growth on polyelectrolyte complex-hydroxyapatite nanocomposites. Philos Transact A Math Phys Eng Sci. 2010;368(1917):2083–97. doi: 10.1098/rsta.2010.0013. [DOI] [PubMed] [Google Scholar]
- 62.Sikavitsas VI, Bancroft GN, Holtorf HL, et al. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci USA. 2003;100(25):14683–88. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao F, Chella R, Ma T. Effects of shear stress on 3-D human mesenchymal stem cell construct development in a perfusion bioreactor system: Experiments and hydrodynamic modeling. Biotechnol Bioeng. 2007;96(3):584–95. doi: 10.1002/bit.21184. [DOI] [PubMed] [Google Scholar]
- 64.Zanetta M, Quirici N, Demarosi F, et al. Ability of polyurethane foams to support cell proliferation and the differentiation of MSCs into osteoblasts. Acta Biomater. 2009;5(4):1126–36. doi: 10.1016/j.actbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Ergun A, Yu X, Valdevit A, et al. In vitro analysis and mechanical properties of twin screw extruded single-layered and coextruded multilayered poly(caprolactone) scaffolds seeded with human fetal osteoblasts for bone tissue engineering. J Biomed Mater Res A. 2011;99(3):354–66. doi: 10.1002/jbm.a.33190. [DOI] [PubMed] [Google Scholar]