Abstract
Primary and N-alkyl arylamine motifs are key functional groups in pharmaceuticals, agrochemicals and functional materials as well as in bioactive natural products. However, there is a dearth of generally applicable methods for the direct replacement of aryl hydrogens with –NH2/-NH-alkyl moieties. Here, we present a mild dirhodium-catalyzed C-H amination for conversion of structurally diverse monocyclic and fused aromatics to the corresponding primary and N-alkyl arylamines using either NH2/NHalkyl-O-(sulfonyl)hydroxylamines as aminating agents; the relatively weak RSO2O-N bond functions as an internal oxidant. The methodology is operationally simple, scalable, and fast at or below ambient temperature, furnishing arylamines in moderate-to-good yields and with good regioselectivity. It can be readily extended to the synthesis of fused N-heterocycles.
Main Text
Arylamine motifs (1, 2) are prevalent in natural products, pharmaceuticals, agrochemicals, functional materials, and dyestuffs as well as many synthetic reagents and catalysts (3–5). Most traditional methods for their synthesis from a corresponding C(sp2)-H bond involve multi-step sequences and/or, harsh conditions (6–8). In many instances, the initial product (nitro, azide, azo, nitroso, chloronitroso, amide/imide/sulfonamide, or imine) requires an additional step(s) before arriving at a free amine (6). The amination of organic anions is a useful and direct alternative (9), but is generally restricted to the introduction of –NR1R2 (R1,R2 ≠ H) and is not applicable to base sensitive substrates. The seminal studies of Ullman and Goldberg into metal-mediated arene C(sp2)-N bond formation at the beginning of the 20th century were harbingers (10) of more efficient palladium catalyzed cross-couplings between aromatic halides/sulfonates and ammonia or nitrogen surrogates developed independently by Hartwig (11) and Buchwald (12) in the early 1990s. In turn, a steady succession of technical improvements, up to the present time, has been offered with the aim of mitigating the original stringent Hartwig-Buchwald reaction conditions (13–16). All of these procedures, however, are restricted by the need for a pre-functionalized arene.
In more recent years, much attention has been paid to catalytic, direct arene aminations (17–19). Many elegant atom- and step-efficient protocols have been introduced, but virtually all require a directing group while others need an excess of arene, high temperatures, or generate amides, imides, and sulfomamides instead of free amines (20–31). A noteworthy exception is the photoredox driven process of Nicewicz (32) and colleagues that circumvents many of the preceding limitations.
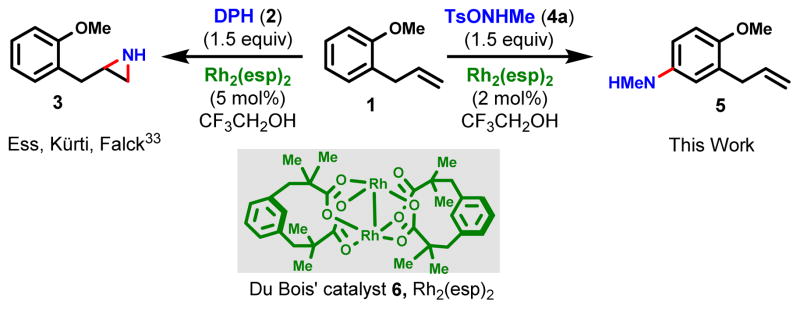
Based upon a combination of experimental observations and theoretical considerations, we anticipated a reaction dichotomy could be achievable between dirhodium-catalyzed aziridination, as reported previously by our laboratories (33), and direct arene amination. Following a systematic investigation, proof-of-principle for arene amination vs. aziridination was demonstrated (Fig. 1). Treatment of 1-allyl-2-methoxybenzene (o-allylanisole, 1) with O-(2,4-dinitrophenyl)-hydroxylamine (2: DPH) (34) and catalytic Rh2(esp)2 (Du Bois’ catalyst, 6: 5 mol%) in 2,2,2-trifluoroethanol (TFE) at 0 °C afforded the corresponding aziridine (3) as the sole product whereas use of N-methyl-O-tosylhydroxylamine (4a) (35) as the aminating agent under otherwise identical conditions furnished exclusively the arene amination adduct 3-allyl-4-methoxy-N-methylaniline (5). Consequently, a more detailed study of the direct arene amination was begun and ultimately led to a versatile, regioselective, operationally simple and mild dirhodium-catalyzed direct arene amination suitable for both intermolecular and intramolecular applications. It should be noted that the relatively weak N-O bond of the aminating agents, many of which are commercial or readily prepared (33–37), functions as an internal oxidant when cleaved during the catalytic amination process, thus obviating the need for addition of an external oxidant to the reaction mixture. Also, the synchronous release of a stoichiometric amount of arylsulfonic acid during the amination is critical since it protonates the arylamines which would otherwise inactivate the Rh catalyst. In some instances, acid sensitive functionality such as epoxides and acetals do not survive.
Fig. 1. A dramatic shift in chemoselectivity arises with different aminating agents.
o-Allylanisole (1) undergoes chemoselective olefin aziridination in the presence of catalytic Rh2(esp)2 (6) and aminating agent DPH (2) in 2,2,2-trifluoroethanol (TFE = CF3CH2OH) to give 3. Changing the aminating agent to TsONHMe (4a) furnishes the corresponding N-Me aniline (5).
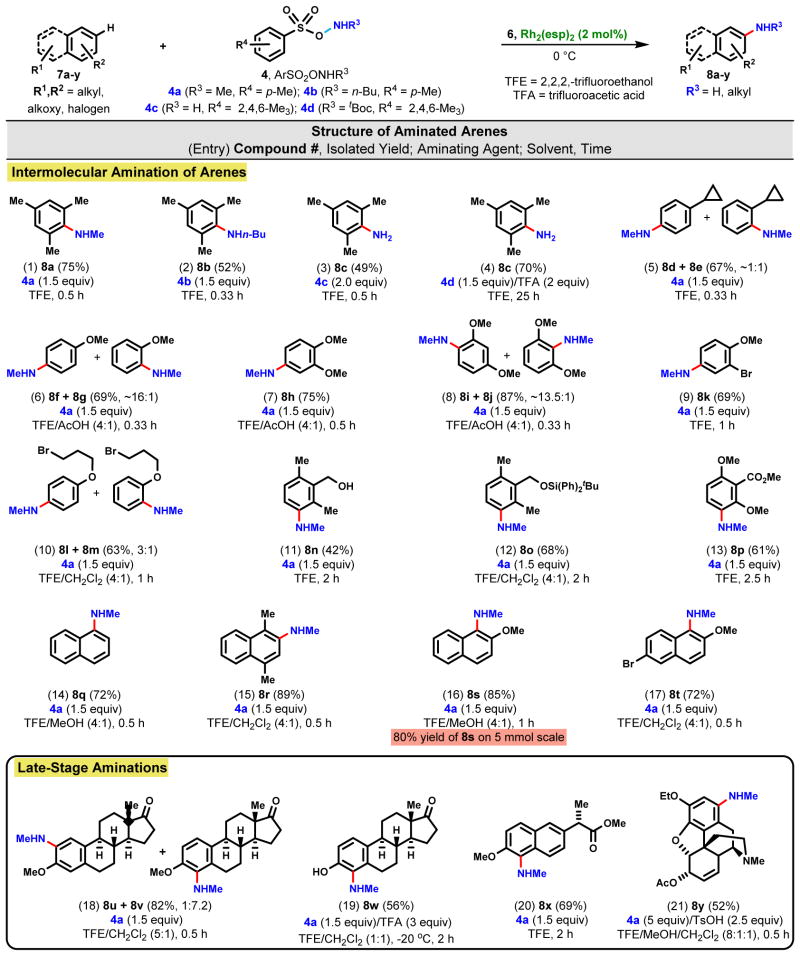
Mesitylene (7a) was selected as a model arene to optimize reaction parameters. Use of 2 mol% Rh2(esp)2 and 1.5 equivalents of aminating agent 4a in TFE as solvent smoothly generated N-methylaniline 8a (75%) in just 30 min at 0 °C (Fig. 2, Entry 1); comparable yields were obtained with 1 mol% and 0.5 mol% catalyst, except the latter required 1 h for complete consumption of starting material. Combinations of TFE with CH2Cl2 or MeOH in ratios up to 1:1 were suitable, but aminations run in EtOH or MeOH as the only solvent delivered poor yields (~10 to 20%) and those run in only DMF, CH3CN, THF, toluene, and dioxane failed. Amongst alternative Rh-catalysts, Rh2(OAc)2 and Rh2(C8H15O2)4 were the next best with moderate yields (~40 to 45%) while the performance by Rh2(TFA)4 was poor. Results from other catalysts are summarized in Table S1. Generally, reactions were quenched as soon as the substrate was completely consumed to minimize by-product formation. Air and water (5% v/v) were well tolerated making this methodology operationally convenient.
Fig. 2. Direct intermolecular amination of arenes.
Reactions were conducted on a 0.1–0.5 mmole scale at 0.1 M using 2,2,2-trifluoroethanol (TFE = CF3CH2OH) as solvent or using mixtures of TFE and other solvents as indicated.
The successful transfer of a nBuNH- unit to 7a from TsONHnBu (4b) affording 8b in 52% yield (Fig. 2, Entry 2) suggested more complex amino moieties should also be suitable. For the introduction of -NH2 into 7a, we used 2,4,6-Me3C6H3S(O)2ONH2 (36) (4c, 2 equiv) as aminating agent because of its greater stability at room temperature compared with TsONH2 (37). Although all of 4c was consumed, a considerable amount of unreacted 7a was recovered and a modest yield (49%) of 8c was realized (Entry 3). On the other hand, the simple and expedient generation of the aminating agent in situ from 2,4,6-Me3C6H3S(O)2ONHtBoc (4d, 1.5 equiv) and CF3CO2H (2 equiv) proved quite effective and boosted the yield of 8c to 70% (Entry 4), although the reaction was much slower than in the case of aminating agent 4c (Entry 3) and appeared to be dependent upon the rate of tBoc cleavage.
The results from other representative arenes are also summarized in Fig. 2. Despite having only a single aliphatic substituent, cyclopropylbenzene (7d) was well behaved, affording a 1:1 p-/o-mixture (8d & 8e) in 20 min at 0 ºC with no evidence of addition to the strained three-membered ring (Entry 5). The directing effect in favor of the para-isomer was more pronounced with anisole (7f) and led to a 16:1 distribution of 8f and 8g (Entry 6). This effect completely dominated in veratrole (7h) that gave 8h as the predominant regioisomer (Entry 7) and was equally evident in the conversion of 1,3-dimethyoxybenzene (7i) into 8i, although a minor amount (6%) of the ortho-isomer 8j was found despite the increased steric congestion at this site (Entry 8). Addition of acetic acid to the reaction solvent for the latter three examples (i.e., Entries 6–8) improved the yields and suppressed the formation of mauve-colored by-products that we assume arose from further oxidation of the aminated adducts. The ortho/para-directing effect of electron donating substituents is typical for electrophilic aromatic additions; the preference for the para-isomer vs. ortho might reflect the steric demand of the Rh-nitrenoid complex. Consistent with these results, aromatics with only electron-withdrawing substituents, e.g., -CF3 and –CN, fail to aminate under these reaction conditions.
Aryl bromide 7k (Entry 9) and terminal alkyl bromide 7l (Entry 10) endured to deliver 8k and a mixture of 8l/8m (3:1), respectively, leaving the halogens available for further manipulation, if desired. The presence of some functional groups, however, partially retard amination, e.g., exposure of benzyl alcohol 7n to the standard amination conditions generated a modest amount of 8n (42%) after 2 h (Entry 11); on the other hand, protection as silyl ether 7o resulted in a much improved yield of 8o (68%) (Entry 12). Fortunately, a synthetically useful yield of amine could be obtained even in the presence of an electron withdrawing substituent, e.g., 7p→8p (Entry 13). The amination process was also extended to fused aromatics: naphthalene (7q) and 1,4-dimethylnapthalene (7r) gave rise to N-methyl-1-naphthylamine (8q, Entry 14) and N-methyl-1,4-dimethyl-2-naphthylamine (8r, Entry 15), respectively, while 2-methoxynaphthalene (7s) readily underwent amination to give 8s in 85% yield on a 0.5 mmol scale and in 80% yield on a 5 mmol scale (Entry 16). The smooth amination of 2-methoxy-6-bromonaphthalene (7t) into N-methyl-2-methoxy-6-bromo-1-naphthylamine (8t, Entry 17) succinctly illustrates the chemo- and regio-selectivities of this methodology.
To validate the suitability of this methodology for late stage functionalization of complex molecules, O-methylestrone (7u) was smoothly converted to a mixture of 8u and 8v (1:7.2, Entry 18) in very good combined yield while only one regioisomer 8w (Entry 19) was obtained from the parent phenol, estrone (7w), thus demonstrating its applicability to substrates containing potentially sensitive benzylic, tertiary, and α-keto hydrogens. The scope was further defined with the amination of methyl naproxen 7x to 8x (Entry 20) and morphine derivative 7y to 8y (Entry 21). The latter required the addition of p-toluenesulfonic acid (2.5 equiv) to ensure the teriary amine was fully protonated to avoid passification of the catalyst.
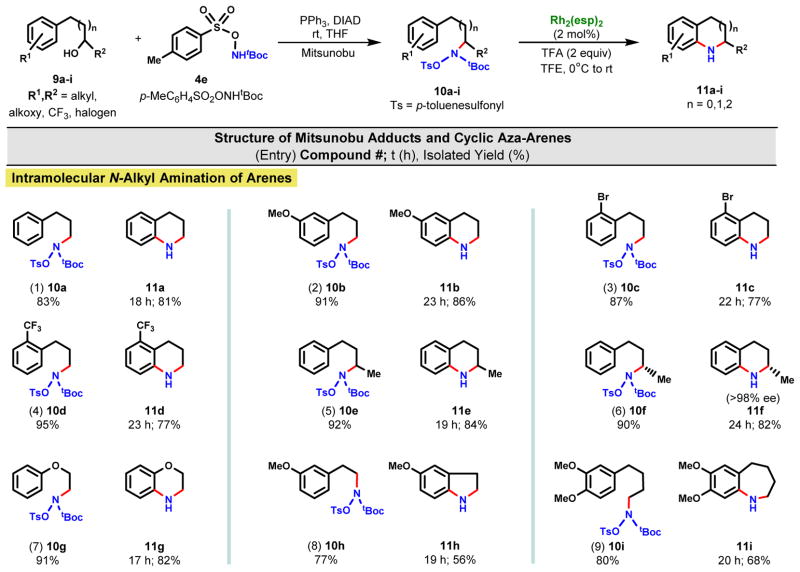
The intramolecular version of the amination proved quite facile and represents one of the mildest and most efficient aza-annulations currently available to the practitioner (Fig. 3) (38, 39). The enabling amination functionality was introduced in uniformly good yields via the Mitsunobu inversion under standard conditions from the corresponding alcohols. Acidic cleavage of the tBoc and subsequent in situ amination proceeded at 0 °C. Fused aza-annulated bicycles were created in good yields irrespective of additional ring substituents, 10a→11a (Entry 1), electron donating, 10b→11b (Entry 2), or electron withdrawing functionality, 10c→11c (Entry 3) and 10d→11d (Entry 4). Control studies of the reaction of 10b confirmed that there was less than 2% of the ortho-annulation product, 8-methoxy-1,2,3,4-tetrahydroquinoline, based upon 13C NMR analysis of the crude reaction product (Fig. S5–S6). Secondary alcohols likewise participated readily in the Mitsunobu and aza-annulation reactions, 9e→10e→11e (Entry 5). This 2-step sequence, when conducted using the chiral alcohol 9f, showed no loss of stereochemical integrity (Entry 6). Incorporation of a heteroatom into the ring closure, e.g., 9g→10g→11g (Entry 7), did not perturb the chemistry and provided easy access to the dihydrobenzoxazine class of heterocycles. The yield declined somewhat for making the 5-membered dihydroindole 11h from alcohol 9h (Entry 8), but improved for the 7-membered tetrahydrobenzazepine 11i from 9i (Entry 9).
Fig. 3. Direct intramolecular cyclization (aza-annulation) of arenes.
Cyclizations were conducted at 0.1 M using 2,2,2-trifluoroethanol (TFE = CF3CH2OH) as the solvent on a 0.1–0.3 mmol scale unless otherwise indicated.
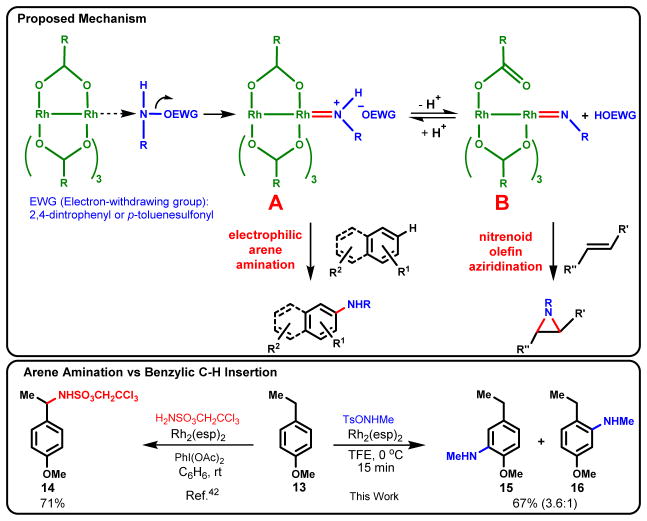
As a beginning towards gaining insight into the mechanism of the amination, a 1:1 mixture of naphthalene (7q) and 7q-d8 was treated with a limited amount of amination reagent (4a, 0.5 equiv) under otherwise standard reaction conditions. Samples were taken and quenched at 10, 20, 30 and 40 min. Analysis via SIM-LC/MS revealed the product ratios remained constant at ~1:1, a ratio inconsistent with an organometallic C-H activation pathway which are typically ~3:1 or higher (40, 41). Based on density functional theory calculations, we previously suggested that aziridination of alkenes involves the dirhodium-nitrenoid intermediate B shown in Fig. 4 that arises from overall NH transfer from the DPH-aminating reagent to the dirhodium catalyst (33). In contrast, reaction of O-tosylhydroxylamine reagents with the dirhodium catalyst favor intermediate A because TsO− is weakly basic and the equilibrium with intermediates B lies far to the left. The chemoselectivity might be explained by the more electrophilic nature of intermediate A versus nitrenoid B. This preliminary hypothesis is consistent with the observation that moderate to strong bases such as K2CO3, Et3N, and pyridine completely inhibit amination, but not aziridination. Moreover, addition of TsOH (1.5 equiv) to the reaction of 1 with 2,4-DNPONHMe (12) produced only the arene amination adduct 5 and no aziridine. As an additional control, it was shown that the presence of 2,4-DNP-OH (1.5 equiv) did not alter the reaction manifold in favor of aziridination when 4a was utilized as the aminating reagent and only 5 was observed.
Fig. 4.
Proposed intermediates leading to amination versus aziridination and control study of arene amination vs benzylic C-H insertion.
It was also instructive to compare our methodology with the intermolecular Rh-catalyzed amination procedure of Du Bois to gain a perspective of their respective complementary chemoselectivities (Fig. 4) (42). Both have similar efficiency using p-ethylanisole (13), but the Du Bois procedure leads to benzylic C-H insertion only whereas our methodology gives arene amination exclusively providing 15 and 16 in a combined 67% yield.
The influence of ligands and counter ions on the reactivity of organometallics is well precedented (43, 44). However, examples of such dramatic bifurcation of the reaction manifold are rare and warrant closer study to understand the energetics and full synthetic potential of this metalloid-nitrogen umpolung for direct arene aminations.
Supplementary Material
Acknowledgments
J.R.F. thanks NIH (HL034300, HL111392, DK038226) and the Robert A. Welch Foundation (Grant I-0011) for funding. L.K. gratefully acknowledges the generous financial support of Rice University, National Institutes of Health (R01 GM-114609-01), National Science Foundation (CAREER:SusChEM CHE-1455335), the Robert A. Welch Foundation (Grant C-1764), ACS-PRF (Grant 51707-DNI1), Amgen (2014 Young Investigators’ Award for LK) and Biotage (2015 Young Principal Investigator Award) that are greatly appreciated.
Footnotes
References
- 1.Hili R, Yudin AK. Making carbon-nitrogen bonds in biological and chemical synthesis. Nat Chem Biol. 2006;2:284–287. doi: 10.1038/nchembio0606-284. [DOI] [PubMed] [Google Scholar]
- 2.Ricci A, editor. Amino Group Chemistry: From Synthesis to the Life Sciences. Wiley-VCH; 2008. p. 394. [Google Scholar]
- 3.Angelici RJ. Reagents for Transition Metal Complex and Organometallic Synthesis. Vol. 28 Wiley-Interscience; New York: 1990. [Google Scholar]
- 4.Dalla Cort A, Gasparrini F, Lunazzi L, Mandolini L, Mazzanti A, Pasquini C, Pierini M, Rompietti R, Schiaffino L. Stereomutations of atropisomers of sterically hindered Salophen ligands. J Org Chem. 2005;70:8877–8883. doi: 10.1021/jo051367v. [DOI] [PubMed] [Google Scholar]
- 5.Candeias NR, Branco LC, Gois PMP, Afonso CAM, Trindade AF. More sustainable approaches for the synthesis of N-based heterocycles. Chem Rev. 2009;109:2703–2802. doi: 10.1021/cr800462w. [DOI] [PubMed] [Google Scholar]
- 6.Hartwig JF, Shekhar S, Shen Q, Barrios-Landeros F. Synthesis of anilines. Chem Anilines. 2007;1:455–536. [Google Scholar]
- 7.Xie YS, Vijaykumar BVD, Jang K, Shin HH, Zuo H, Shin DS. One-pot conversion of phenols to anilines via Smiles rearrangement. Tetrahedron Lett. 2013;54:5151–5154. [Google Scholar]
- 8.Yu J, Zhang P, Wu J, Shang Z. Metal-free C-N bond-forming reaction: Straightforward synthesis of anilines, through cleavage of aryl C-O bond and amide C-N bond. Tetrahedron Lett. 2013;54:3167–3170. [Google Scholar]
- 9.Daskapan T. Synthesis of amines by the electrophilic amination of organomagnesium, -zinc, -copper, and -lithium reagents. ARKIVOC. 2011:230–262. [Google Scholar]
- 10.Kunz K, Scholz U, Ganzer D. Renaissance of Ullmann and Goldberg reactions - progress in copper catalyzed C-N-, C-O- and C-S-coupling. Synlett. 2003:2428–2439. [Google Scholar]
- 11.Hartwig JF. Evolution of a fourth generation catalyst for the amination and thioetherification of aryl halides. Acc Chem Res. 2008;41:1534–1544. doi: 10.1021/ar800098p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surry DS, Buchwald SL. Biaryl phosphane ligands in palladium-catalyzed amination. Angew Chem, Int Ed. 2008;47:6338–6361. doi: 10.1002/anie.200800497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia N, Taillefer M. A very simple copper-catalyzed synthesis of anilines by employing aqueous ammonia. Angew Chem, Int Ed. 2009;48:337–339. doi: 10.1002/anie.200802569. [DOI] [PubMed] [Google Scholar]
- 14.Zeng X, Huang W, Qiu Y, Jiang S. An efficient copper-catalyzed synthesis of anilines by employing aqueous ammonia. Org Biomol Chem. 2011;9:8224–8227. doi: 10.1039/c1ob06208e. [DOI] [PubMed] [Google Scholar]
- 15.Lundgren RJ, Stradiotto M. Recent advances in the Buchwald-Hartwig amination reaction enabled by the application of sterically demanding phosphine ancillary ligands. Aldrichimica Acta. 2012;45:59–65. [Google Scholar]
- 16.Maejima T, Shimoda Y, Nozaki K, Mori S, Sawama Y, Monguchi Y, Sajiki H. One-pot aromatic amination based on carbon-nitrogen coupling reaction between aryl halides and azido compounds. Tetrahedron. 2012;68:1712–1722. [Google Scholar]
- 17.Davies HML, Dai X. Synthetic reactions via C-H bond activation: Carbene and nitrene C-H insertion. Compr Organomet Chem III. 2007;10:167–212. [Google Scholar]
- 18.Jiao J, Murakami K, Itami K. Catalytic methods for aromatic C-H amination: An ideal strategy for nitrogen-based functional molecules. ACS Catal. 2016;6:610–633. [Google Scholar]
- 19.Starkov P, Jamison TF, Marek I. Electrophilic amination: The case of nitrenoids. Chem - Eur J. 2015;21:5278–5300. doi: 10.1002/chem.201405779. [DOI] [PubMed] [Google Scholar]
- 20.Tsang WCP, Zheng N, Buchwald SL. Combined C-H functionalization/C-N bond formation route to carbazoles. J Am Chem Soc. 2005;127:14560–14561. doi: 10.1021/ja055353i. [DOI] [PubMed] [Google Scholar]
- 21.Inamoto K, Saito T, Katsuno M, Sakamoto T, Hiroya K. Palladium-Catalyzed C-H activation/intramolecular amination reaction: A new route to 3-aryl/alkylindazoles. Org Lett. 2007;9:2931–2934. doi: 10.1021/ol0711117. [DOI] [PubMed] [Google Scholar]
- 22.Brasche G, Buchwald SL. C-H functionalization/C-N bond formation: Copper-catalyzed synthesis of benzimidazoles from amidines. Angew Chem, Int Ed. 2008;47:1932–1934. doi: 10.1002/anie.200705420. [DOI] [PubMed] [Google Scholar]
- 23.Inamoto K, Saito T, Hiroya K, Doi T. Palladium-catalyzed intramolecular amidation of C(sp2)-H bonds: Synthesis of 4-aryl-2-quinolinones. J Org Chem. 2010;75:3900–3903. doi: 10.1021/jo100557s. [DOI] [PubMed] [Google Scholar]
- 24.Shrestha R, Mukherjee P, Tan Y, Litman ZC, Hartwig JF. Sterically controlled, palladium-catalyzed intermolecular amination of arenes. J Am Chem Soc. 2013;135:8480–8483. doi: 10.1021/ja4032677. [DOI] [PubMed] [Google Scholar]
- 25.Xue Y, Fan Z, Jiang X, Wu K, Wang M, Ding C, Yao Q, Zhang A. RhIII-Catalysed hydrazine-directed C(sp2)-H amination of phenidones with N-alkyl-O-benzoyl-hydroxylamines. Eur J Org Chem. 2014;2014:7481–7488. [Google Scholar]
- 26.Chen Z, Wang B, Zhang J, Yu W, Liu Z, Zhang Y. Transition metal-catalyzed C-H bond functionalizations by the use of diverse directing groups. Org Chem Front. 2015;2:1107–1295. [Google Scholar]
- 27.Park Y, Park KT, Kim JG, Chang S. Mechanistic studies on the Rh(III)-mediated amido transfer process leading to robust C-H amination with a new type of amidating reagent. J Am Chem Soc. 2015;137:4534–4542. doi: 10.1021/jacs.5b01324. [DOI] [PubMed] [Google Scholar]
- 28.Shin K, Kim H, Chang S. Transition-Metal-Catalyzed C-N bond forming reactions using organic azides as the nitrogen source: A journey for the mild and versatile C-H amination. Acc Chem Res. 2015;48:1040–1052. doi: 10.1021/acs.accounts.5b00020. [DOI] [PubMed] [Google Scholar]
- 29.Sun F, Gu Z. Decarboxylative alkynyl termination of palladium-catalyzed Catellani reaction: A facile synthesis of α-alkynyl anilines via ortho C-H amination and alkynylation. Org Lett. 2015;17:2222–2225. doi: 10.1021/acs.orglett.5b00830. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki C, Hirano K, Satoh T, Miura M. Direct synthesis of N-H carbazoles via iridium(III)-catalyzed intramolecular C-H amination. Org Lett. 2015;17:1597–1600. doi: 10.1021/acs.orglett.5b00502. [DOI] [PubMed] [Google Scholar]
- 31.Takamatsu K, Hirano K, Satoh T, Miura M. Synthesis of indolines by copper-mediated intramolecular aromatic C-H amination. J Org Chem. 2015;80:3242–3249. doi: 10.1021/acs.joc.5b00307. [DOI] [PubMed] [Google Scholar]
- 32.Romero NA, Margrey KA, Tay NE, Nicewicz DA. Site-selective arene C-H amination via photoredox catalysis. Science. 2015;349:1326–1330. doi: 10.1126/science.aac9895. [DOI] [PubMed] [Google Scholar]
- 33.Jat JL, Paudyal MP, Gao H, Xu QL, Yousufuddin M, Devarajan D, Ess DH, Kürti L, Falck JR. Direct stereospecific synthesis of unprotected N-H and N-Me aziridines from olefins. Science. 2014;343:61–65. doi: 10.1126/science.1245727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Z. O-(2,4-dinitrophenyl)hydroxylamine. Synlett. 2014;25:1186–1187. [Google Scholar]
- 35.John ORS, Killeen NM, Knowles DA, Yau SC, Bagley MC, Tomkinson NCO. Direct α-oxytosylation of carbonyl compounds: One-Pot synthesis of heterocycles. Org Lett. 2007;9:4009–4012. doi: 10.1021/ol701774y. [DOI] [PubMed] [Google Scholar]
- 36.Mendiola J, Rincon JA, Mateos C, Soriano JF, de Frutos O, Niemeier JK, Davis EM. Preparation, use, and safety of O-mesitylenesulfonylhydroxylamine. Org Process Res Dev. 2009;13:263–267. [Google Scholar]
- 37.Kawase M, Kikugawa Y. Intramolecular cyclization of alkylhydroxylamines in acid. Chem Pharm Bull. 1981;29:1615–1623. [Google Scholar]
- 38.Cherest M, Lusinchi X. A novel electrophilic N-amination via electron deficient complexes: Action of ferric chloride on N-acetyloxyamides. Tetrahedron Lett. 1989;30:715–718. [Google Scholar]
- 39.Carpino LA. O-Acylhydroxylamines. II. O-mesitylenesulfonyl-, O-(p-toluenesulfonyl)-, and O-mesitoylhydroxylamine. J Am Chem Soc. 1960;82:3133–3135. [Google Scholar]
- 40.Jones WD. Isotope effects in C-H bond activation reactions by transition metals. Acc Chem Res. 2003;36:140–146. doi: 10.1021/ar020148i. [DOI] [PubMed] [Google Scholar]
- 41.Simmons EM, Hartwig JF. On the interpretation of deuterium kinetic isotope effects in CH bond functionalizations by transition metal complexes. Angew Chem Int Ed. 2012;51:3066–3072. doi: 10.1002/anie.201107334. [DOI] [PubMed] [Google Scholar]
- 42.Espino CG, Fiori KW, Kim M, Du Bois J. Expanding the scope of C-H aminations through catalysts design. J Am Chem Soc. 2004;126:15378–15379. doi: 10.1021/ja0446294. [DOI] [PubMed] [Google Scholar]
- 43.Hegedus LS. Transition Metals in the Synthesis of Complex Organic Molecules. Chap 2. University science Books; 1994. pp. 16–22. [Google Scholar]
- 44.Espino CG, Du Bois J. A Rh-catalyzed C-H insertion reaction for the oxidative conversion of carbamates to oxazolidinones. Angew Chem Int Ed. 2001;40:598–600. doi: 10.1002/1521-3773(20010202)40:3<598::AID-ANIE598>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 45.Zhu C, Li G, Ess DH, Falck JR, Kurti L. Elusive metal-free primary amination of arylboronic acids: Synthetic studies and mechanism by density functional theory. J Am Chem Soc. 2012;134:18253–18256. doi: 10.1021/ja309637r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.González I, Mosquera J, Guerrero C, Rodríguez R, Cruces J. Selective monomethylation of anilines by Cu(OAc)2-promoted cross-coupling with MeB(OH)2. Org Lett. 2009;11:1677–1680. doi: 10.1021/ol802882k. [DOI] [PubMed] [Google Scholar]
- 47.Dang TT, Ramalingam B, Seayad AM. Efficient ruthenium-catalyzed N-methylation of amines using methanol. ACS Catal. 2015;5:4082–4088. [Google Scholar]
- 48.Maiti A, Reddy PVN, Sturdy M, Marler L, Pegan SD, Mesecar AD, Pezzuto JM, Cushman M. Synthesis of casimiroin and optimization of Its quinone reductase 2 and aromatase inhibitory activities. J Med Chem. 2009;52:1873–1884. doi: 10.1021/jm801335z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kung AC, Falvey DE. Photogenerated N-methyl-N-1-naphthylnitrenium ion: Laser flash photolysis, trapping rates, and product study. J Org Chem. 2005;70:3127–3132. doi: 10.1021/jo0477749. [DOI] [PubMed] [Google Scholar]
- 50.Ortiz-Marciales M, Rivera LD, Jesús MD, Espinosa S, Benjamin JA, Casanova OE, Figueroa IG, Rodríguez S, Correa W. Facile rearrangement of O-silylated oximes on reduction with boron trifluoride/borane. J Org Chem. 2005;70:10132–10134. doi: 10.1021/jo0516178. [DOI] [PubMed] [Google Scholar]
- 51.Manas ARB, Smith RAJ. Aromatic annulation with bis-phenylthionium ions. Tetrahedron. 1987;43:1847–1856. [Google Scholar]
- 52.Chen F, Surkus AE, He L, Pohl MM, Radnik J, Topf C, Junge K, Beller M. Selective catalytic hydrogenation of heteroarenes with N-graphene-modified cobalt nanoparticles (Co3O4–Co/NGr@α-Al2O3) J Am Chem Soc. 2015;137:11718–11724. doi: 10.1021/jacs.5b06496. [DOI] [PubMed] [Google Scholar]
- 53.Cooke MP, Jr, Widene RK. Lithium-Halogen exchange-initiated cyclization reactions. 3. Intramolecular conjugate addition reactions of unsaturated acylphosphoranes. J Org Chem. 1987;52:1381–1396. [Google Scholar]
- 54.de Haan R, de Zwart EW, Cornelisse J. Intramolecular photocycloaddition of a vinyl ether to CF3-substituted 2-methoxy-5-phenyipent-1-enes. J Photochem Photobiol A: Chem. 1997;102:179–188. [Google Scholar]
- 55.Sridharan V, Avendaño C, Menéndez JC. CAN-catalyzed three-component reaction between anilines and alkyl vinyl ethers: Stereoselective synthesis of 2-methyl-1,2,3,4- tetrahydroquinolines and studies on their aromatization. Tetrahedron. 2007;63:673–681. [Google Scholar]
- 56.Wang WB, Lu SM, Yang PY, Han XW, Zhou YG. Highly enantioselective iridium-catalyzed hydrogenation of heteroaromatic compounds, quinolines. J Am Chem Soc. 2003;125:10536–10537. doi: 10.1021/ja0353762. [DOI] [PubMed] [Google Scholar]
- 57.Dugar S, Sharma A, Kuila B, Mahajan D, Dwivedi S, Tripathi V. A concise and efficient synthesis of substituted morpholines. Synthesis. 2015;47:712–720. [Google Scholar]
- 58.Gómez C, Maciá B, Lillo VJ, Yus M. [1,2]-Wittig rearrangement from chloromethyl ethers. Tetrahedron. 2006;62:9832–9839. [Google Scholar]
- 59.Kulkarni A, Zhou W, Török B. Heterogeneous catalytic hydrogenation of unprotected indoles in water: A green solution to a long-standing challenge. Org Lett. 2011;13:5124–5127. doi: 10.1021/ol2019899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Asiamah I, Hodgson HL, Maloney K, Allen KJH, Krol ES. Ring substitution influences oxidative cyclisation and reactive metabolite formation of nordihydroguaiaretic acid analogues. Bioorg Med Chem. 2015;23:7007–7014. doi: 10.1016/j.bmc.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 61.Fan KH, Lever JR, Lever SZ. Effect of structural modification in the amine portion of substituted aminobutyl-benzamides as ligands for binding r1 and r2 receptors. Bioorg Med Chem. 2011;19:1852–1859. doi: 10.1016/j.bmc.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.