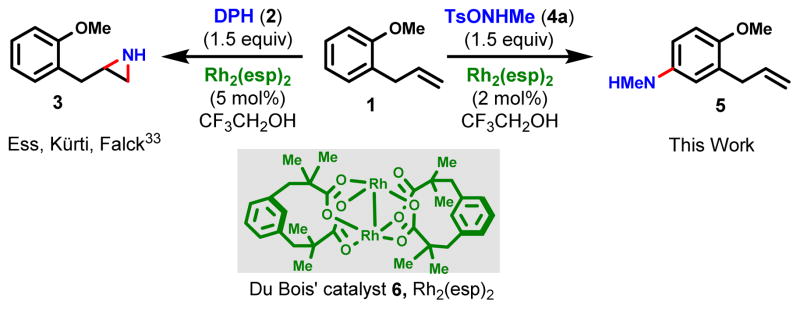
Fig. 1. A dramatic shift in chemoselectivity arises with different aminating agents.
o-Allylanisole (1) undergoes chemoselective olefin aziridination in the presence of catalytic Rh2(esp)2 (6) and aminating agent DPH (2) in 2,2,2-trifluoroethanol (TFE = CF3CH2OH) to give 3. Changing the aminating agent to TsONHMe (4a) furnishes the corresponding N-Me aniline (5).