Abstract
Schizophrenic patients show altered sensory perception as well as changes in electrical and magnetic brain responses to sustained, frequency-modulated sensory stimulation. Both the amplitude and temporal precision of the neural responses differ in patients as compared to control subjects, and these changes are most pronounced for stimulation at gamma frequencies (20–40 Hz). In addition, patients display enhanced spontaneous gamma oscillations, which has been interpreted as ‘neural noise’ that may interfere with normal stimulus processing. To investigate electrophysiological markers of aberrant sensory processing in a model of schizophrenia, we recorded neuronal activity in primary somatosensory cortex of mice heterozygous for the schizophrenia susceptibility gene Neuregulin 1. Sensory responses to sustained 20–70 Hz whisker stimulation were analyzed with respect to firing rates, spike precision (phase locking) and gamma oscillations, and compared to baseline conditions. The mutants displayed elevated spontaneous firing rates, a reduced gain in sensory-evoked spiking and gamma activity, and reduced spike precision of 20–40 Hz responses. These findings present the first in vivo evidence of the linkage between a genetic marker and altered stimulus encoding, thus suggesting a novel electrophysiological endophenotype of schizophrenia.
Keywords: Schizophrenia, Neuregulin 1, Signal-to-noise ratio, Gamma oscillations, Somatosensory cortex, Endophenotypes
Introduction
The neuropathophysiological mechanisms underlying perceptual deficits in schizophrenia have been a subject of intense research during the past decades. Using electroencephalography (EEG) or magnetoencephalography (MEG), several studies have measured neuronal activity in response to sustained visual, auditory and somatosensory stimuli in patients. Schizophrenic subjects displayed reduced amplitudes of steady-state oscillations during sustained 20–40 Hz sensory stimulation, and a decreased temporal precision of responses (Edgar et al. 2013; Ikuta et al. 2007; Krishnan et al. 2005; Kwon et al. 1999; Light et al. 2006; Mulert et al. 2011; Spencer et al. 2008; Teale et al. 2013; Tsuchimoto et al. 2011). In addition to these stimulus-related deficits, an aberrant increase in oscillatory power (20–50 Hz) during baseline conditions has been observed in schizophrenic subjects (Brockhaus-Dumke et al. 2008; Itil et al. 1972; Kikuchi et al. 2011; Spencer 2012; Venables et al. 2009). It has been suggested that these changes reflect a reduced signal-to-noise ratio (SNR) of cortical responses (Flynn et al. 2008; Krishnan et al. 2005; Vogels and Abbott 2007; Winterer et al. 2000), where the ‘signal’ is related to aberrant stimulus-evoked activity, and the ‘noise’ is related to increased baseline activity (for review, see Gandal et al. 2012a).
Some of these neurophysiological markers of schizophrenia have also been observed in various animal models of schizophrenia. These models were generated by pharmacological or genetic interventions to reflect certain aspects of the schizophrenia pathology. Research has shown that interfering with N-Methyl-d-aspartate (NMDA) receptor function and related pathways results in neurophysiological changes similar to those seen in patients. Describing the baseline activity in freely moving or anesthetized animals, studies found enhancements in oscillatory 30–80 Hz power (Carlen et al. 2012; Del Pino et al. 2013; Gandal et al. 2012b; Hakami et al. 2009; Kittelberger et al. 2012; Kocsis 2012; Kulikova et al. 2012; Ma and Leung 2007; Marquis et al. 1989; Molina et al. 2014; Pinault 2008) and in spiking activity (Belforte et al. 2010; Carlen et al. 2012; Jackson et al. 2004; Molina et al. 2014; Wood et al. 2012; Zhang et al. 2012) in different cortical and subcortical regions. In addition, reduced temporal precision of spiking and oscillatory activity has been reported in these models, in particular in the hippocampus and prefrontal cortex (Carlson et al. 2011; Del Pino et al. 2013; Featherstone et al. 2013; Molina et al. 2014; Nason et al. 2011; Sigurdsson et al. 2010; Zhang et al. 2012).
Despite the progress in illuminating the neuronal mechanisms of schizophrenia, little is known about changes in sensory information processing in primary sensory cortices in schizophrenia models. In response to auditory stimulation under awake conditions, reduced oscillatory power has been described in various disease models (Carlson et al. 2011; Featherstone et al. 2013; Vohs et al. 2012). However, an exact location of these deficits could not be defined due to the low spatial resolution of the EEG recordings. In addition, optogenetic activation of the primary somatosensory cortex of anesthetized mice with a deletion of the NR1 subunit of the NMDA receptor resulted in neuronal firing that was less temporally precise than in control mice (Carlen et al. 2012). However, responses to direct whisker stimulation have not been investigated. So far only one study has assessed sensory-evoked gamma activity in a schizophrenia model. Rats treated with the NMDA receptor antagonist ketamine displayed a reduction in stimulus-locked gamma oscillations in response to a single whisker deflection (Kulikova et al. 2012). Whether induced, non-locked gamma components are also altered, and whether the gamma deficits are stimulus-specific, is currently not known.
The Neuregulin 1 (NRG1) gene has been robustly associated with an increased susceptibility for schizophrenia (Agim et al. 2013; Li et al. 2006; Munafo et al. 2006), and may be linked in particular to the non-deficit subtype of the disorder (Bakker et al. 2004). The gene ranks 16th among more than 500 reported risk genes (Sun et al. 2008) and shows a strong association with behavioral and electrophysiological endophenotypes of schizophrenia in humans (Greenwood et al. 2011; Greenwood et al. 2012). Mice heterozygous for NRG1 (Meyer and Birchmeier 1995) also display various endophenotypes, including deficits in auditory novelty detection, contextual fear conditioning and social interaction (Ehrlichman et al. 2009), as well as hyperlocomotion and impaired sensory gating after pharmacological challenge (Duffy et al. 2008).
Here we investigated sensory-related spiking and gamma activity in the primary somatosensory cortex in the Neuregulin 1 model of schizophrenia. To examine frequency-specific changes in sensory responses, we stimulated whiskers at frequencies ranging from 20 to 70 Hz. This range is important for the whisking behavior in mice (Jin et al. 2004; Ritt et al. 2008; Voigts et al. 2008) and also encompasses frequencies at which deficits have previously been reported in patients with schizophrenia (e.g., Krishnan et al. 2005; Kwon et al. 1999; Teale et al. 2013). Based on prior work in other schizophrenia models, we hypothesized changes in spiking and oscillatory activity during sensory stimulation relative to baseline conditions. The findings were expected to help elucidate the cellular basis of schizophrenia-related deficits in sensory processing.
Materials and methods
Animals
Animal procedures adhered to the ethical guidelines of the NIH and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Animals were obtained from S. Siegel and T. Brodkin (University of Pennsylvania) and bred on a C57BL/6/129 hybrid background (Ehrlichman et al. 2009). Heterozygous NRG1 mutants were originally created by fusing β-galactosidase to exon 6 of the NRG1 allele, which is the N-terminal of the EGF-like domain that is present in all NRG1 isoforms (Meyer and Birchmeier 1995). Thus, the mutation affects all types of NRG1 transcripts (Meyer et al. 1997). A total of 19 adult male mice aged 4–6 months were used for the procedures, of which 12 were NRG 1 +/− mice, and 7 were wild-type (WT) littermates (NRG1 +/+). Mice were genotyped twice using tail clips both after birth and after death. Polymerase chain reaction (PCR; 35 cycles, annealing at 60 °C) was performed with the REDExtract-NAmp Tissue PCR Kit (Sigma–Aldrich) and the following primers: Primer 1 (NDF−): 5′-TGC TGC TTT CTT CGC TCT TCA GAA GC-3′. Primer 2 (NDF+): 5′-GAG ATG GTC ATG TCC TTG TCA CTA AC-3′. Primer 3 (NDFneo): 5′-CGA ATT CGC CAA TGA CAA GAC GCT G-3′.
Preparation
Mice were initially sedated with xylazine (13 mg/kg i.p.; Butler Schein Animal Health, Dublin, Ohio) and after 15 min, anesthetized with 3.5 % isoflurane (Abbott, North Chicago, Illinois). All surgical procedures were carried out under isoflurane maintained between 1 and 1.5 %. After performing a tracheotomy, animals were artificially ventilated with the mouse ventilator MiniVent 845 (Harvard Apparatus; 140–170 strokes/min, 180–220 μl/stroke) and placed in the stereotaxic frame (model 1730, David Kopf Instruments, Tujunga, California). Mice received a single dose of glycopyrrolate (0.02 mg/kg i.p.; American Regent, Shirley, New York) and dexamethasone (4 mg/kg i.p.; American Regent) to reduce brain swelling and secretions, respectively. A craniotomy was performed over the barrel cortex (3.2–4.3 mm lateral, 0.6–1.6 mm posterior to Bregma) and the dura was carefully resected. After the probe was inserted, the cortex was left to recover for about 1 h before recordings began. Throughout the recordings, isoflurane was replaced by fentanyl (10–40 μg/kg/h i.p.) to maintain the animal in a sedated state. If the animals presented any sign of discomfort, isoflurane 0.1–0.5 % was added to supply this anesthesia regime. Body temperature was maintained at 35–37 °C with a heating pad and a thermometer.
Electrophysiology
Recordings (Fig. 1) were performed with 16-channel silicon probes configured as 2 × 2 tetrodes (Neuronexus, Ann Arbor, Michigan). The probe consisted of two shanks, spaced 150 μm apart, with two tetrodes each, also spaced 150 μm apart. Each tetrode was made of four electrodes placed in a diamond shape. Electrodes had a diameter of 11 μm and were spaced 25 μm apart. Probes were carefully inserted into the brain perpendicular to the surface and lowered to layers II/III and IV under visual guidance and based on readings from the micromanipulator. Signals were amplified (×5,000) and filtered (local field potential = 0.1–300 Hz; spikes = 600–6 kHz), and digitized at 33.657 kHz (Cheetah, Neuralynx). Waveforms crossing set thresholds (100–120 μV) were captured via the A/D card and analyzed off-line. Multi-unit activity (MUA) was defined as all spikes recorded by one tetrode and reflects the activity of a local neuronal population. Single unit activity (SUA), which presumably reflects the firing of single neurons, was first identified using automated clustering software utilizing peak and trough feature sets (KlustaKwik). These clusters were then examined manually for waveform shape to discard non-cell clusters, and combine clusters that captured waveforms from the same cell. These clusters were further refined by hand upon examination of the interspike intervals (SpikeSort3D, Neuralynx). Off-line re-evaluation of clusters was essential to avoid false clusters or extra clusters typically added by the automatic clustering routine. Most tetrodes had one to three separable cells. However, their spontaneous and stimulus-evoked firing rates were too low (<2 Hz) to perform statistical comparisons. Therefore, the analysis was restricted to MUAs.
Fig. 1.
Recordings of sensory-evoked multi-unit activity in the mouse barrel cortex. a Recording and stimulation setup. Tetrode recordings were acquired in lightly anesthetized mice. Air puff stimuli were applied as 1 s long trains at different frequencies (20–70 Hz, repeated every 5 s) to 2–5 whiskers at a time. Contralateral responses were recorded in layers II/III and IV in the barrel field of the primary somatosensory cortex. b Example response. Raw traces from the four wires of a tetrode during 70 Hz stimulation (bottom trace). c Filtering procedure. The raw data was low-pass filtered (0.1–300 Hz) to obtain local field potentials (top panel), and high-pass filtered (0.6–6 kHz; middle panel) to obtain the local spiking activity (bottom panel). Here spontaneous activity is shown. d Spike analysis. The MUA was further analyzed off-line with SpikeSort3D to isolate single unit activity (SUA). The left column shows that for every SUA, four spike shapes recorded at four tetrode channels are available for feature analysis. Features such as the amplitude of the peak and trough were used to cluster similar spike shapes that are thought to emerge from the same neuron. In the cluster display (top left), every dot represents one spike recorded from the same tetrode in 3D feature space. Spikes with similar characteristics tend to be in closer proximity and can thus be identified as a distinct SUA. As an additional criterion, SUAs comprise less than 2 % of the spikes that occur at rates faster than every 2 ms. The interspike interval histograms of the three example SUAs, showing absence of spikes at or less than 1 ms, are displayed in the right column. e Left panel. Example of sensory-evoked MUA response in WT mice. Top-to-bottom: Responses to 20–70 Hz stimulation and example histogram (70 Hz). Left-to-right: Peri-stimulus raster plots, phase raster plots and vector strength (VS) for each stimulus frequency (every line represents one trial and every dot represents one spike). Black box indicates stimulation onset and offset (duration: 1 s). All VS values are significant at p < 0.001. Right panel. Example of sensory-evoked MUA responses from NRG1 (+/−) mice (same conventions as in WT panel)
Stimulation
Whiskers were trimmed to 0.7 cm and stimulated with air puffs delivered with Picospritzer III (General Valve, Fairfield, New Jersey; 70 psi at the source). Air puffs were applied with a glass capillary for precise stimulation of 2–5 whisker tips (usually whiskers in rows C/D, arcs 3/4). Stimulation was targeted to evoke the largest response in local field potentials and multi-unit spiking with the shortest latency (Histogram software, Neuralynx). Air puff stimuli were controlled by Igor software (WaveMetrics Inc., Lake Oswego, Oregon). Stimuli consisted of 1 s trains of square pulses (width: 2 ms) repeated every 5 s, delivered at frequencies ranging from 10 to 70 Hz in steps of 10 Hz. Each stimulus train was presented 50 times in a pseudo-randomized order.
Analysis
Off-line analysis was performed using Spike2 software (Cambridge Electronic Design, Cambridge, England) and statistical tests were assessed using Igor Pro (Wavemetrics, Lake Oswego, Oregon), SPSS (version 20.0, SPSS Inc., Chicago, Illinois) or Xlstat (Addinsoft, Brooklyn, New York). Responses to 20–70 Hz stimuli were analyzed.
Firing rates and signal-to-noise ratio
Peri-stimulus time histograms (PSTHs) of MUAs were constructed by first counting the number of spikes in each bin (bin width: 1 ms) across all trials and dividing by the total number of trials to determine the mean firing rate (MFR). The MFR was then divided by the bin size to express neuronal responses in Hz. The analysis of stimulus-evoked activity was restricted to the sustained part of the neural response, measured as the MFR 0.05–1 s poststimulus onset. Baseline firing was defined as spontaneous firing in the absence of stimulation and was calculated 0.95 s before stimulus onset across all trials in each condition. The signal-to-noise ratio (SNR) of the sensory responses was calculated by dividing the MFR during the response to stimulation by the MFR during baseline. The gain in spike SNR across the stimulation conditions was calculated by fitting a linear function to the individual MUA responses.
Response precision
Temporal fidelity of neuronal responses was measured in terms of phase locking. First, phase histograms were generated for sustained responses during the stimulus train. Then stimulus-evoked spikes were counted for each stimulation condition across all trials and plotted on a 0–360° axis (100 bins), corresponding to the time between two successive stimuli in a stimulus train. To determine the degree of phase locking of sustained responses to the stimulus, vector strength r was calculated according to the following formula:
L is the vector length, n is the total number of spikes in the phase histogram for a given stimulation frequency, θ is the phase angle at which a spike occurs: θ = 2π(t/T). T is the period of the stimulation frequency and t is the time at which the spike occurs. To assess the statistical significance p of the vector strength, the Rayleigh statistic was used: p = exp(−nr2). Values of vector strength were included in the population analysis if phase histograms contained >45 spikes and p ≤ 0.001.
Inclusion criteria
MUAs were included in the analysis if the responses were large and showed frequency modulation and a short onset latency (14 ± 1.5 ms). To test whether MUA responses were significant, PSTHs were computed for each MUA across all trials (bin size: 50 ms). MUA responses were compared to a Poisson distribution with μ equal to the mean number of spikes per bin during baseline activity measured for 1.5 s before stimulus presentation across all trials. If the probability of observing an equal or greater number of spikes was less than 0.1 %, the response was considered significant:
where x is the first bin after stimulus presentation and μ is the average of 1.5 s of baseline spiking (30 bins) before stimulus presentation.
Gamma oscillations
Changes in gamma power (20–40 Hz) of the local field potentials (LFPs) were measured during 50–70 Hz stimulation. These stimulation parameters were chosen based on a previous study on gamma oscillations induced by high frequency whisker stimulation in rat barrel cortex (Ewert et al. 2008). LFPs included in the power analysis were derived from tetrodes at which MUA responses with short latencies (14 ± 1.5 ms) and clear stimulus responses (PSTH analysis) were simultaneously recorded. Power spectral analysis was performed with a 8,192 point Hanning window on single-trial LFPs, thus revealing non-stimulus-locked (induced) components. Power was measured for sustained responses 0.15–1 s after stimulus onset, thus excluding onset responses (high frequency experiment). Baseline power was measured during a 0.85 s window, 1 s before stimulus onset. Power measures quantified during stimulation and baseline conditions included absolute gamma in the 20–40 Hz range (19.7–39.7 Hz), and total power in the 5–100 Hz range (5.3–99.3 Hz). In addition, relative gamma was calculated by dividing absolute gamma by the total power spectrum, thus accounting for possible influences in the low frequency range on gamma power. From 16 LFPs recorded in each trial, four LFPs that showed maximal relative gamma values were included in the analysis of gamma SNR. Gamma SNR was measured by dividing relative gamma during stimulation by relative gamma during baseline. The gain in gamma SNR across stimulation frequencies was assessed by fitting a linear function to the average relative gamma power of individual LFPs in each condition (least squares method).
Results
To characterize the influence of NRG1 alterations on cortical sensory-evoked responses, we performed extracellular recordings using tetrodes in the barrel cortex of wild-type (WT) and NRG1 heterozygous mice [NRG1 (+/−)]. We compared the responses to whisker deflections with brief air puffs at increasing frequencies (20–70 Hz) as well as spontaneous activity (Fig. 1a). The example in Fig. 1b shows raw traces from the four wires in a tetrode during responses to a train of whisker stimulation at 70 Hz. The raw traces (before filtering) mostly represent local field potentials (LFPs) given their much higher amplitude compared to spikes. An initial large phasic response is followed by sustained high frequency activity for the duration of the stimulus train. To extract spikes, the traces were filtered (0.6–6 kHz), thresholded and single units separated by cluster cutting analysis (Fig. 1c, d). Clustering extracellular spike waveforms from the different wires in the tetrode typically enabled discrimination of 1–3 units from a single tetrode (Fig. 1d). However, their spontaneous and evoked firing rates were too low (<2 Hz) to allow robust statistical comparisons. Thus, data analysis was instead performed on multi-unit activity (MUA). Quantitative data were derived from 28 to 25 MUAs for WT and NRG1 (+/−) mice, respectively.
Elevated spontaneous and stimulus-evoked firing rates in NRG1 (+/−) mice
All MUAs were significantly activated by repetitive whisker deflections at all stimulation frequencies (Fig. 1e), as assessed by Poisson statistics (see “Materials and methods”). The example recordings in Fig. 1e illustrate the main difference between WT (left panel) and the NRG1 (+/−) mice (right panel). In both cases, the dot rastergrams showed a robust response to all frequencies (20–70 Hz from top to bottom, each line is a trial). However, both the baseline and the response firing rates were higher in the NRG1 (+/−) mouse than the control animal (Fig. 1e, rasters). Spike output during train stimulation maintained consistent phase relationships with the stimulus cycle (Fig. 1e, phase) but the vector strength (VS; discussed below) was smaller in the NRG1 (+/−) mouse for frequencies at and below 40 Hz (Fig. 1e, VS).
Outside periods of stimulation, the mean firing rate was 3.84 ± 0.95 Hz (mean ± SEM) for WT and 22.61 ± 4.39 Hz for NRG1 (+/−) animals (Mann–Whitney, p < 0.0001; Fig. 2a). Thus, on average NRG1 (+/−) mice displayed a 5.9-fold increase in baseline mean firing rates compared to WT controls.
Fig. 2.
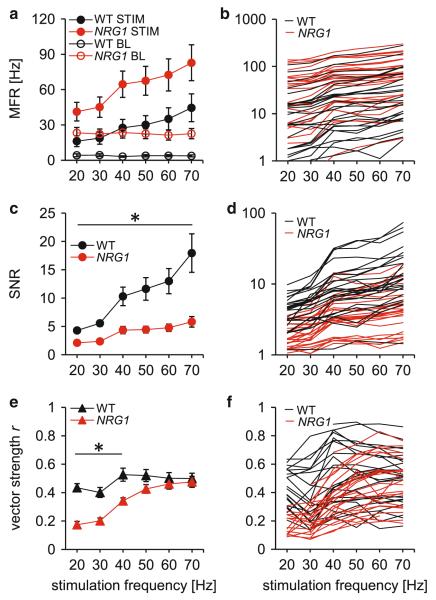
Differences in stimulus-evoked responses between WT and NRG1 (+/−) mice. a, b Mean firing rate (MFR) for WT MUAs (n = 28) and NRG1 (+/−) MUAs (n = 25) during 20–70 Hz stimulation (plain circles) and baseline conditions (open circles). c, d Signal-to-noise ratio (SNR) for WT and NRG1 (+/−) MUAs during 20–70 Hz stimulations. e, f Vector strengths for WT and NRG1 (+/−) MUAs during 20–70 Hz stimulation. Average MUA results are displayed in the left column (a, c and e), and individual MUA trends are displayed in the right column (b, d and f). Error bars denote SEM; star (asterisk) indicates significant difference between groups (see Tables 1, 2 for details)
We next characterized neuronal responses to repetitive whisker stimulation. For both strains, MUA mean firing rate increased with increasing stimulation frequencies (Fig. 2a, b). On average, WT mice increased their mean firing rate by 5.54 ± 1.46 Hz per 10 Hz increase in stimulation frequency, while NRG1 (+/−) mice increased their mean firing rate by 8.37 ± 1.71 Hz per 10 Hz increase in stimulation frequency (Mann–Whitney, p > 0.05). Those values were calculated from the equation of the linear fit of the tuning curves of individual MUAs (Fig. 2b).
When comparing the absolute stimulus-evoked activity, NRG1 (+/−) animals displayed a significantly elevated mean firing rate in response to all stimulation frequencies below 60 Hz (Mann–Whitney, Bonferroni-corrected; Table 1).
Table 1.
Between-group comparison of stimulus-evoked firing rates
| Frequency (Hz) | WT MFR (Hz) | NRG1 MFR (Hz) | U | p |
|---|---|---|---|---|
| 20 | 16.20 ± 4.63 | 41.28 ± 7.92 | 192 | 0.004 |
| 30 | 18.77 ± 5.13 | 45.11 ± 8.66 | 199 | 0.007 |
| 40 | 27.52 ± 7.16 | 64.58 ± 11.18 | 183 | 0.003 |
| 50 | 30.11 ± 7.78 | 67.52 ± 12.27 | 190 | 0.004 |
| 60 | 35.19 ± 9.40 | 72.52 ± 13.64 | 204 | 0.009 |
| 70 | 44.58 ± 11.82 | 82.84 ± 15.25 | 215 | 0.016 |
Mean firing rates (MFR) of WT (n = 28) and NRG1 (+/−) (n = 25) sustained MUA responses were compared per condition (Mann–Whitney). The table lists the mean ± SEM of WT and NRG1 responses, the Mann–Whitney U and the corresponding p value (highlighted in bold to indicate significance). Bonferroni-corrected significance level: p = 0.008
Diminished signal-to-noise ratio and gain in NRG1 (+/−) mice
Since NRG1 (+/−) MUAs showed higher mean firing rates both during spontaneous and sensory-evoked activity compared to WT animals, we calculated the signal-to-noise ratio (SNR) for the two strains. SNR was calculated as the ratio of the mean firing rate during whisker stimulation divided by that during baseline.
Despite the increased firing rate, SNR was reduced in NRG1 (+/−) mice compared to WT mice at all stimulation frequencies tested (Mann–Whitney, Bonferroni-corrected; Table 2; Fig. 2c, d).
Table 2.
Between-group comparison of signal-to noise ratios
| Frequency (Hz) | WT SNR | NRG1 SNR | U | p |
|---|---|---|---|---|
| 20 | 4.30 ± 0.40 | 2.12 ± 0.20 | 576 | <0.0001 |
| 30 | 5.57 ± 0.54 | 2.37 ± 0.22 | 604 | <0.0001 |
| 40 | 10.31 ± 1.63 | 4.35 ± 0.64 | 552 | <0.001 |
| 50 | 11.63 ± 1.99 | 4.44 ± 0.61 | 553 | <0.001 |
| 60 | 13.01 ± 2.22 | 4.78 ± 0.66 | 567 | <0.0001 |
| 70 | 17.96 ± 3.40 | 5.82 ± 0.94 | 571 | <0.0001 |
Signal-to-noise ratios (SNRs) of WT (n = 28) and NRG1 (+/−) (n = 25) sustained responses were compared per condition (Mann–Whitney). Displayed are the mean ± SEM of WT and NRG1 (+/−) responses, the Mann–Whitney U and the corresponding p value (highlighted in bold if significant). Bonferroni-corrected significance level: p = 0.008
In both groups, the SNR increased monotonically with increasing stimulation frequency, but the increase was smaller in NRG1 (+/−) mice than in WT animals. While the relative response magnitude increased by factor 2.63 ± 0.62 per 10 Hz increase in stimulation frequency in WT mice, the increase was only 0.74 ± 0.16 in NRG1 (+/−) mice (Mann–Whitney, p < 0.001). Those values were extracted from the equation of the linear fit of the tuning curves of individual MUAs (Fig. 2d).
Reduced precision of sensory-evoked responses in NRG1 (+/−) mice
Since precise spike timing might have important implications for the representation of sensory stimuli in primary cortical areas, we assessed whether the reduction in the SNR observed in NRG1 (+/−) animals was associated with a decrease in the temporal precision of the sensory-evoked responses. We calculated the phase locking of MUA to the stimulus cycles for each stimulation frequency. The degree to which responses were phase-locked to the stimuli was assessed using a vector strength analysis (see “Materials and methods”), where higher values of vector strength r denote better phase locking (a vector strength value of 1 indicates perfect phase locking to the stimulus, whereas a vector strength of 0 indicates absence of a consistent phase relationship of the spikes to the stimulus cycles).
For all stimulation conditions, MUAs from WT and NRG1 (+/−) animals displayed significant phase locking to the stimulus cycles (Fig. 2e, f; see raw data from one experiment in Fig. 1e). In WT mice, the frequency of the stimulation did not substantially affect the temporal fidelity of the MUA responses. For NRG1 (+/−) mice, MUAs showed a reduction in their phase locking to the stimulus cycles for frequencies below 50 Hz (Bonferroni-corrected t tests; Table 3). Thus, when the sensory-evoked firing rate is low, the decrease in the SNR observed in NRG1 (+/−) animals occurs concomitantly with a decrease in the temporal precision.
Table 3.
Between-group differences in vector strength
| Frequency (Hz) | WT VS | NRG1 VS | WT (n) | NRG1 (n) | t (df) | p |
|---|---|---|---|---|---|---|
| 20 | 0.43 ± 0.03 | 0.17 ± 0.02 | 22 | 14 | 6.6 (34) | <0.0001 |
| 30 | 0.40 ± 0.04 | 0.18 ± 0.03 | 26 | 22 | 4.9 (46) | <0.0001 |
| 40 | 0.53 ± 0.05 | 0.34 ± 0.03 | 24 | 23 | 3.3 (45) | 0.002 |
| 50 | 0.52 ± 0.04 | 0.43 ± 0.04 | 24 | 23 | 1.7 (45) | 0.098 |
| 60 | 0.50 ± 0.04 | 0.46 ± 0.04 | 25 | 23 | 0.7 (46) | 0.463 |
| 70 | 0.50 ± 0.04 | 0.36 ± 0.05 | 24 | 22 | 2.2 (44) | 0.037 |
Group differences in vector strength (VS) were assessed per condition using unpaired t tests. The mean VS ± SEM of WT and NRG1 (+/−) responses are shown as well as the number of MUAs (n), the t statistic, the degrees of freedom (df) and the corresponding p value (in bold for significant differences). Bonferroni-corrected significance level: p = 0.008
Reduced sensory-induced gamma oscillations in NRG1 (+/−) mice
The LFP from barrel cortex demonstrated robust gamma (20–40 Hz) oscillations during the response to trains of stimulation (Fig. 3a). Gamma oscillations were already visible in the raw LFP (top row) but were made clear by filtering the LFP between 10 and 50 Hz (Fig. 3a, middle row, filtered).
Fig. 3.
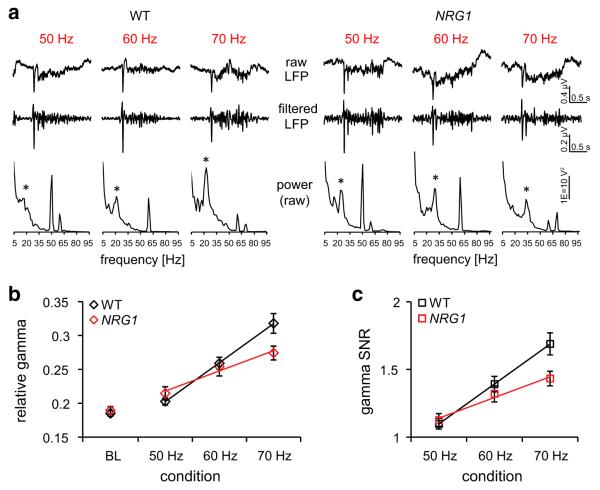
Reduced sensory gamma oscillations in NRG1 (+/−) mice. a Example LFP response and power during high frequency (50–70 Hz) stimulation in a WT mouse (left panel) and an NRG1 (+/−) mouse (right panel). Top-to-bottom: Single-trial raw LFP trace, filtered LFP trace (10–50 Hz) and power spectrum of the raw LFP. The power was measured across all stimulus trials and thus reflects induced, non-locked components. Power peaks appear in the gamma range (20–40 Hz; indicated by asterisk), at the stimulation frequency (reflecting stimulus-evoked activity) and at 60 Hz (reflecting electrical noise). b Gamma power during baseline (BL) and stimulation (50–70 Hz) conditions. Relative gamma power was calculated as the ratio of absolute gamma (20–40 Hz) and total power (5–100 Hz). Shown are the average gamma values for 36 WT LFPs (black diamonds) and 32 NRG1 (+/−) LFPs (red diamonds), with additional trend lines reflecting average differences in gain between WT (black line) and NRG1 (+/−) responses (red line) across the stimulation conditions. The increase in relative gamma power across 50–70 Hz was measured for each LFP with a linear fit using the least squares method. WT mice displayed a higher gain in relative gamma power than NRG1 (+/−) mice (Mann–Whitney, U = 889, p < 0.001). c Gamma SNR across 50–70 Hz. Gamma SNR was quantified by dividing relative gamma power during stimulation by relative gamma power during baseline for each LFP response (shown in b). Displayed are average gamma SNR values for WT mice (black squares) and NRG1 (+/−) mice (red squares). Trend lines indicate the increase, or gain, in gamma SNR across frequencies for WT (black line) and NRG1 (+/−) mice (red line). The gain was calculated by fitting a linear function to the gamma SNR during 50–70 Hz stimulation. The gain in gamma SNR was reduced in NRG1 (+/−) animals relative to WT controls (Mann–Whitney, U = 862, p < 0.0001). Error bars indicate SEM
The power spectrum of the unfiltered LFP responses showed a 1/f profile with a clear peak at around 20–25 Hz in the WT example, and around 30–35 Hz in the NRG1 (+/−) example (Fig. 3a, bottom row, peak indicated by asterisk). We observed some variability in the gamma peak across individual animals, but not across groups (WT: 23.8 ± 0.8 Hz, n = 36; NRG1 (+/−): 23.7 ± 0.8 Hz, n = 32; Mann–Whitney, p > 0.05).
We divided the total power within the gamma frequency band (between 20 and 40 Hz) by the total power between 5 and 100 Hz to obtain relative gamma power at each stimulation frequency (Fig. 3b). In addition, we divided relative gamma power during whisker responses by that during baseline to obtain the SNR of sensory-induced gamma oscillations (Fig. 3c). Both parameters increased as a function of stimulation frequency in both strains (Fig. 3b, c). Individual measurements were well fit by a linear function and the average of all linear fits for each strain is superimposed to the mean power at each frequency. However, relative gamma and gamma SNR increased significantly more in WT than NRG1 (+/) mice (Mann–Whitney; p < 0.001 for relative gamma and p < 0.0001 for SNR).
Discussion
EEG and MEG studies in schizophrenic patients revealed changes in baseline neural activity, and in the amplitude and temporal precision of the neural response to sustained sensory stimulation (e.g., Krishnan et al. 2009; Spencer 2012). To investigate these electrophysiological markers in a model of schizophrenia, we compared cellular responses during baseline conditions and during sustained whisker stimulation in WT and NRG1 (+/−) mice. The results revealed schizophrenia-like changes in spontaneous activity as well as alterations in firing rates, spike precision and gamma oscillations during stimulation. As far as we are aware, this is the first in vivo report of impaired encoding of frequency-modulated sensory information in a mouse with a genetic mutation associated with schizophrenia.
Elevated baseline firing in NRG1 (+/−) mice
Our finding of enhanced baseline spiking activity is in line with previous reports in other animal models expressing various endophenotypes of schizophrenia. An in vitro study showed that the deletion of the NRG1 receptor ErbB4 enhances excitatory and inhibitory activity in hippocampal slices, as suggested by an increased number of EPSCs in both excitatory and inhibitory neurons, and increased spontaneous firing rates of inhibitory neurons (Del Pino et al. 2013). In addition, various in vivo studies have linked reduced NMDA receptor activity (either via genetic deletion of the NR1 subunit or by NMDA receptor block) to schizophrenia-like behavioral and cognitive disturbances (Belforte et al. 2010; Jackson et al. 2004), as well as enhanced spontaneous firing rates in different cortical and subcortical areas, including the primary somatosensory cortex (Belforte et al. 2010; Carlen et al. 2012; Jackson et al. 2004; Molina et al. 2014; Wood et al. 2012; Zhang et al. 2012). Increased cortical excitation after NMDA receptor blockage has been shown to result from reduced activity of inhibitory neurons, followed by disinhibition (and elevated firing) of excitatory cells (Homayoun and Moghaddam 2007). In line with this finding, modeling studies revealed that increased spontaneous cellular activity is due to increased excitation and disinhibition in a cortical neuronal network (Murray et al. 2012; Vogels and Abbott 2007).
Together, the results obtained in genetic, pharmacological and computational studies indicate that increased excitation and reduced inhibition are associated with enhanced spontaneous neuronal activity and schizophrenia-related cognitive impairments. Our results demonstrating increased baseline activity in NRG1 (+/−) mutants are in agreement with these findings, since NRG1 is an important regulator of the excitatory-inhibitory neuronal microcircuitry. Loss of NRG1 function is associated with a reduction in inhibition (less excitatory drive to inhibitory cells; Ting et al. 2011), and an increase in excitation (less inhibitory drive to excitatory cells, i.e., disinhibition; Fazzari et al. 2010). In addition, NRG1 (+/−) mutants display various cognitive endophenotypes of schizophrenia (Ehrlichman et al. 2009).
Some studies on NRG1-related effects on baseline responses are at variance with our findings, however. In hippocampal slices, there were no changes in basal synaptic transmission in mice overexpressing NRG1 type I (Deakin et al. 2012) and in heterozygous NRG1 EGF-like domain knockout mice (Bjarnadottir et al. 2007). Similarly, other studies reported that NRG1 treatment of hippocampal slices had no effects on basal synaptic transmission (Bjarnadottir et al. 2007; Huang et al. 2000; Kwon et al. 2005), although one study reported increased firing rates of inhibitory cells in hippocampal and cortical slices after NRG1 treatment (Li et al. 2011). Moreover, acute extracellular recordings of single cells and LFPs in hippocampus and nucleus accumbens in urethane anesthetized NRG1 type III knockouts revealed no difference in mean firing rates between mutant and WT neurons (Nason et al. 2011). The contradiction with our results may be explained by differences in recording methodology (in vitro versus in vivo), anesthesia type and depth, or differences in the brain region and NRG1 isoform/mutation under investigation. With regard to anesthesia, baseline firing rates are generally decreased under deep anesthesia such as under urethane, which may occlude potential NRG1-related changes in firing rate in the nucleus accumbens (Nason et al. 2011). More generally, it is conceivable that NRG1-related effects on baseline firing rates are dependent on the precise excitatory–inhibitory tuning of the network, which might differ according to the type of NRG1 mutation or isoform, the connectivity in the brain region being studied, and the brain state (awake versus anesthetized). In support of this hypothesis, brain state-dependent effects have been shown after NMDA antagonist treatment, with decreased and increased baseline firing rates in urethane-anesthetized animals (Gratton et al. 1987) and awake animals (Jackson et al. 2004), respectively. In addition, modeling studies showed that increases in baseline firing rates depend on the functional connectivity of the network (i.e., responsiveness of sending and target neurons) and on the combination of hyperexcitability and disinhibition (Vogels and Abbott 2007).
Increased neuronal responsiveness to sensory stimulation in NRG1 (+/−) mice
We further demonstrated enhanced mean firing rates in response to 20–50 Hz whisker stimulation in NRG1 (+/−) mice. To the best of our knowledge, this is the first report of changes in sensory-evoked neuronal firing rates in an animal model with a genetic mutation associated with schizophrenia in vivo. In agreement with our results, recent patch-clamp experiments in hippocampal slices of ErbB4 knockout mice revealed that the firing rate of inhibitory neurons is enhanced after ramp stimulation compared to firing rates in normal mice (Del Pino et al. 2013). In addition, hippocampal stimulation in anesthetized ErbB4 mutants was shown to facilitate hippocampal LFP responses (Del Pino et al. 2013). Similarly, enhanced firing rates were found during electrical stimulation of pyramidal neurons in hippocampal slices of mice deficient for the NR1 subunit of the NMDA receptor (Gandal et al. 2012b).
Modeling studies suggested that increased stimulus-evoked firing as well as increased baseline firing result from elevated levels of excitation and reduced inhibition in neuronal networks (Vogels and Abbott 2007). In particular, the level of background activity or ‘noise’ can affect the integrative properties of the neuron (Destexhe and Pare 1999) and its responsiveness to a stimulus, the ‘gain’ (Chance et al. 2002). In pyramidal cells, increasing background synaptic activity results in a more depolarized membrane potential and larger fluctuations in membrane potential (Destexhe and Pare 1999). Consequently, pyramidal cells show an enhanced responsiveness, also to inputs that would normally be subthreshold (Ho and Destexhe 2000). In NRG1 (+/−) mutants, a similar mechanism might explain enhanced response amplitudes that are independent of the stimulation frequency.
Reduced signal-to-noise ratio and gain of spiking activity in NRG1 (+/−) mutants
We observed a reduction in spike SNR and in SNR gain across stimulation frequencies in NRG1 (+/−) mutants, while mean firing rates (not normalized to baseline firing) had similar gain functions in mutants and WT mice. This pattern suggests that decreased SNR gain in mutants is primarily due to enhanced baseline firing rates. The findings indicate that elevated baseline firing rates significantly alter cortical processing of stimulus-related information. Such mechanism provides a possible link between the previously reported enhanced firing rates in the prefrontal and somatosensory cortices in schizophrenia models based on NMDA receptor hypofunction, and the associated deficits in cognitive functioning (Belforte et al. 2010; Jackson et al. 2004).
Impaired phase locking of spikes in NRG1 (+/−) mice
We further demonstrated reductions in sensory-evoked phase locking of firing rates for stimulation frequencies between 20 and 40 Hz in NRG1 mutants. Previous research has investigated changes in phase relationships between oscillatory brain activity (i.e., LFPs) in various schizophrenia models, revealing alterations in phase locking between brain areas (phase coherence; Del Pino et al. 2013; Nason et al. 2011; Nicolás et al. 2011; Sigurdsson et al. 2010; Zhang et al. 2012) and between trials (intertrial coherence; Carlson et al. 2011; Featherstone et al. 2013; Vohs et al. 2012). To the best of our knowledge, only two studies have examined the timing of action potentials in rodent models of schizophrenia. One study recorded activity from regular spiking cells in the prefrontal cortex of freely behaving rats and found a reduction in pair-wise spike correlations after MK801 administration (Molina et al. 2014). Another study examined the reliability of stimulus-related spike timing in mice with an interneuronspecific deletion of the NR1 subunit of the NMDA receptor. The mutants showed less precise responses to optogenetic activation of the somatosensory cortex under anesthesia (Carlen et al. 2012). However, frequency-specific effects were not investigated. Extending these results, our study provides the first evidence for frequency-specific impairments in phase locking of spikes in response to a sensory stimulus in a mouse model with genetic alterations associated with schizophrenia. Our findings in NRG1 (+/−) mice indicate that enhanced neural noise reduces temporal precision of neuronal firing to a somatosensory stimulus. Along with the decreased spike SNR, deficient phase locking may provide a mechanistic explanation for the adverse impact of increased baseline firing on cognition as previously reported (Belforte et al. 2010; Jackson et al. 2004).
Decreased stimulus-induced gamma oscillations in NRG1 (+/−) mice
Another novel finding of our study is that NRG1 (+/−) mutants show a smaller gain in relative gamma and gamma SNR with increasing stimulation frequency compared to WT mice. Together with the finding of impaired phase locking to 20–40 Hz stimulation, these results suggest a reduced ability of the neuronal circuitry in NRG1 (+/−) mutants to entrain both evoked and induced gamma activity. These results represent the first demonstration of deficits in sensory-evoked gamma oscillations during sustained, frequency-modulated whisker stimulation in a schizophrenia-related model.
Our findings concur with previous reports of gamma deficits in primary somatosensory cortex during transient whisker deflection in ketamine-treated rats, both under awake and lightly anesthetized conditions (Kulikova et al. 2012). Our findings also support results in mice with an interneuron-specific deletion of the NR1 subunit of the NMDA receptor. Upon optogenetic stimulation of the primary somatosensory cortex at frequencies ranging from 8 to 200 Hz, the gamma SNR (30–60 Hz) was reduced during 30–60 Hz stimulation in the anesthetized mutants (Carlen et al. 2012). Furthermore, our findings extend previous research on changes in auditory-evoked gamma power in different schizophrenia animal models. These studies recorded neural activity across the whole brain, and found reductions in auditory-evoked gamma oscillations and gamma SNR in Dysbindin-1 knockouts (Carlson et al. 2011), in NR1 knockouts (Gandal et al. 2012b), and in mice treated with the NMDA antagonists MK801 or ketamine (Saunders et al. 2012). Changes in auditory-evoked responses have also been demonstrated in rats treated with ketamine or muscimol, both of which enhanced oscillatory power in response to 30–40 Hz stimulation (Vohs et al. 2012). In addition, reduced gamma oscillations in response to auditory stimuli have been reported in rats treated with methylazoxymethanol acetate (Lodge et al. 2009). Furthermore, our results support the role of NRG1 in regulating gamma oscillations, as previously demonstrated in hippocampal slices (Andersson et al. 2012; Deakin et al. 2012; Fisahn et al. 2009).
The generation of gamma oscillations depends on fast-spiking, parvalbumin-positive interneurons, in particular basket cells, which synchronize the activity of pyramidal cells (Bartos et al. 2007; Cardin et al. 2009; Sohal et al. 2009; Volman et al. 2011). The fact that the NRG1 receptor ErbB4 is primarily expressed by parvalbumin-positive chandelier and basket cells (Fazzari et al. 2010) suggests that NRG1-mediated effects on gamma rhythms are due to impaired inhibitory signaling. The exact mechanisms underlying such a deficit are currently not clear, however.
Possible anesthesia effects
One limitation of our study is that recordings were made under isoflurane/fentanyl anesthesia. While offering the advantage of controlling the state of arousal of the animals and avoiding an interference of the stimulus response by active whisking, anesthesia might change excitatory and inhibitory signaling pathways and thus affect the response properties. In addition, an interaction between the anesthesia regimen and the NRG1 mutation is possible.
While we cannot fully rule out these confounding effects, we argue that their impact is probably small. First, the level of the isoflurane was kept at a minimum during the recordings (0.1–0.5 %)—well below the concentration at which effects on somatosensory responses detected in previous studies in rats (Detsch et al. 1999, 2002a; Vahle-Hinz et al. 2007) and humans (Hume and Durkin 1986). Second, we observed sustained sensory responses that were modulated by the stimulation frequency in both WT and NRG1 (+/−) mice, including robust gamma oscillations in both strains, which indicate that the level of isoflurane used did not alter response properties in either group. Third, isoflurane should decrease network excitability (Becker et al. 2012; Detsch et al. 2002b; Vahle-Hinz et al. 2007), rather than enhance it, as seen in the NRG1 (+/−) mutants. Finally, fentanyl may have an impact on signal transmission in nucleus accumbens (Hirose et al. 1998; Yoshida et al. 1999) and hippocampus (Horita et al. 1989; Kouvaras et al. 2008); however, the only study investigating somatosensory processing did not find an effect (Hume and Durkin 1986).
Moreover, previous research suggests that the observed changes in neural activity in our mutants as well as in other animal models of schizophrenia are at least to some extent independent of anesthetic conditions. For example, augmented spontaneous firing rates have been described in NR1-deficient mice during active exploration (Belforte et al. 2010) and under anesthesia (Carlen et al. 2012), in awake rats treated with the NMDA inhibitor MK801 (Jackson et al. 2004), and in hippocampal slices of ErbB4 knockout mice (Del Pino et al. 2013). Furthermore, stimulation-induced changes such as whisker-evoked gamma oscillations were shown to be impaired in ketamine-treated, awake and anesthetized rats (Kulikova et al. 2012). In accordance with the role of NRG1 in ErbB4 signaling and NMDA receptor expression (Bjarnadottir et al. 2007; Mei and Xiong 2008; Newell et al. 2013), interfering with either NRG1, ErbB4 or NMDA receptor signaling should change gamma power and spiking activity in a similar way. Thus, anesthesia-independent neuronal alterations in ErbB4 knockout mice and NMDA models of schizophrenia strongly suggest that the presented findings in NRG1 (+/−) mice are not simply an artifact of the anesthesia use.
Relevance to findings in schizophrenic patients
The results demonstrate for the first time that a mutation of the schizophrenia-risk gene NRG1 is associated with impaired encoding of perceptual information in the somatosensory cortex. Previous EEG and MEG studies revealed that in response to somatosensory stimulation, schizophrenic subjects display enhanced ERP amplitudes (Ikuta et al. 2007; Shagass 1977), decreased phase locking (Teale et al. 2013), reduced phase coherence and decreased power in the 20–40 Hz band (Arnfred 2012; Arnfred et al. 2006; Arnfred et al. 2011). Our findings in NRG1 (+/−) mutants parallel these reports, suggesting that NRG1 (+/−) mice display an electrophysiological endophenotype of schizophrenia linked to deficient somatosensory information processing. In addition, it is conceivable that the described endophenotype reflects more general schizophrenia-related deficits in sensory processing. In schizophrenia patients, many of the reported abnormalities in the somatosensory domain have also been found with auditory and visual stimuli in patients, including reductions in temporal precision and in 20–40 Hz power (Krishnan et al. 2005; Kwon et al. 1999; Light et al. 2006).
Furthermore, our results showed enhanced spiking activity during baseline conditions in NRG1 (+/−) mutants. Enhanced baseline activity has previously been observed in schizophrenic patients in the 20–50 Hz band of the spontaneous EEG activity (Brockhaus-Dumke et al. 2008; Itil et al. 1972; Kikuchi et al. 2011; Spencer 2012; Venables et al. 2009), and increased 40–85 Hz power in healthy subjects after treatment with the NMDA inhibitor ketamine (Hong et al. 2010). Increased spiking activity in animal models of schizophrenia and enhanced gamma oscillations in patients have been interpreted to reflect abnormally elevated cortical noise that interferes with stimulus processing (Gandal et al. 2012a; Hakami et al. 2009). Supporting this notion, our study described reductions in spike SNR during somatosensory stimulation. In schizophrenic patients, reductions in SNR have been demonstrated during auditory stimulation and cognitive tasks (Krishnan et al. 2005; Winterer et al. 2000), and may be particularly pronounced in the gamma range (Gandal et al. 2012a). Deficits in SNR have been linked to sensory and cognitive processing deficits in schizophrenia patients (Winterer et al. 2004; Winterer and Weinberger 2004; Winterer et al. 2000). Whether patients might display SNR reductions in the somatosensory domain, as suggested by our study, is open for future investigation.
Acknowledgments
We would like to thank Steve Siegel and Ted Brodkin (University of Pennsylvania) for kindly providing NRG1 (+/−) mutants and WT mice. This work was supported by the IRTG 1328 Schizophrenia and Autism of the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG; C. Barz), the Interdisciplinary Center for Clinical Research (IZKF Aachen) within the Faculty of Medicine at the RWTH Aachen University (C. Barz), the postdoctoral fellowship of the Fondation pour la Recherche Medicale SPE20070709864 (T. Bessaih), the Barrel Cortex Function (BaCo-Fun) research group of the DFG (D. Feldmeyer), the Helmholtz association (D. Feldmeyer), as well as the National Institute of Health (NIH) grants MH064045 (T. Abel), P50-MH096891 (T. Abel and R. Gur, PI) and R01 EY020765 (D. Contreras).
Contributor Information
Claudia S. Barz, Department of Psychiatry, Psychotherapy and Psychosomatics, Medical School, RWTH Aachen University, Aachen, Germany; Department of Neuropathology, Medical School, RWTH Aachen University, Aachen, Germany; Department of Ophthalmology, Medical School, RWTH Aachen University, Aachen, Germany; IZKF Aachen, Medical School, RWTH Aachen University, Aachen, Germany
Thomas Bessaih, Sorbonne Universités, UPMC Univ Paris 06, UM 119, Neuroscience Paris Seine (NPS), Paris 75005, France; CNRS, UMR 8246, NPS, Paris 75005, France; INSERM, U1130, NPS, Paris 75005, France.
Ted Abel, Department of Biology, University of Pennsylvania, Philadelphia, USA; Smilow Center for Translational Research, Philadelphia, USA.
Dirk Feldmeyer, Department of Psychiatry, Psychotherapy and Psychosomatics, Medical School, RWTH Aachen University, Aachen, Germany; Jülich Aachen Research Alliance (JARA) – Translational Brain Medicine, Aachen, Germany.
Diego Contreras, Department of Neuroscience, School of Medicine, University of Pennsylvania, Philadelphia, USA.
References
- Agim ZS, et al. Discovery, validation and characterization of Erbb4 and Nrg1 haplotypes using data from three genome-wide association studies of schizophrenia. PLoS One. 2013;8:3. doi: 10.1371/journal.pone.0053042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson RH, et al. Neuregulin and dopamine modulation of hippocampal gamma oscillations is dependent on dopamine D4 receptors. Proc Natl Acad Sci USA. 2012;109:13118–13123. doi: 10.1073/pnas.1201011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnfred SM. Proprioceptive information processing in schizophrenia. Dan Med J. 2012:59. [PubMed] [Google Scholar]
- Arnfred SM, Hemmingsen RP, Parnas J. Delayed early proprioceptive information processing in schizophrenia. Br J Psychiatry. 2006;189:558–559. doi: 10.1192/bjp.bp.105.017087. [DOI] [PubMed] [Google Scholar]
- Arnfred SM, Morup M, Thalbitzer J, Jansson L, Parnas J. Attenuation of beta and gamma oscillations in schizophrenia spectrum patients following hand posture perturbation. Psychiatry Res. 2011;185:215–224. doi: 10.1016/j.psychres.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Bakker SC, Hoogendoorn MLC, Selten JP, Verduijn W, Pearson PL, Sinke RJ, Kahn RS. Neuregulin 1: genetic support for schizophrenia subtypes. Mol Psychiatry. 2004;9:1061–1063. doi: 10.1038/sj.mp.4001564. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Becker K, Eder M, Ranft A, von Meyer L, Zieglgansberger W, Kochs E, Dodt HU. Low dose isoflurane exerts opposing effects on neuronal network excitability in neocortex and hippocampus. PLoS One. 2012;7:18. doi: 10.1371/journal.pone.0039346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belforte JE, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnadottir M, et al. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1 +/− knock-outs compared with wild-type mice. J Neurosci. 2007;27:4519–4529. doi: 10.1523/JNEUROSCI.4314-06.2007. doi: 10.1523/JNEUROSCI.4314-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Mueller R, Faigle U, Klosterkoetter J. Sensory gating revisited: relation between brain oscillations and auditory evoked potentials in schizophrenia. Schizophr Res. 2008;99:238–249. doi: 10.1016/j.schres.2007.10.034. doi: 10.1016/j.schres.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Cardin JA, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen M, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2012;17:537–548. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GC, et al. Dysbindin-1 mutant mice implicate reduced fast-phasic inhibition as a final common disease mechanism in schizophrenia. Proc Natl Acad Sci USA. 2011;108:3. doi: 10.1073/pnas.1109625108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance FS, Abbott L, Reyes AD. Gain modulation from background synaptic input. Neuron. 2002;35:773–782. doi: 10.1016/s0896-6273(02)00820-6. [DOI] [PubMed] [Google Scholar]
- Deakin IH, et al. Transgenic overexpression of the type I isoform of neuregulin 1 affects working memory and hippocampal oscillations but not long-term potentiation. Cereb Cortex. 2012;22:1520–1529. doi: 10.1093/cercor/bhr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pino I, et al. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79:1152–1168. doi: 10.1016/j.neuron.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Pare D. Impact of network activity on the integrative properties of neocortical pyramidal neurons in vivo. J Neurophysiol. 1999;81:1531–1547. doi: 10.1152/jn.1999.81.4.1531. [DOI] [PubMed] [Google Scholar]
- Detsch O, Vahle-Hinz C, Kochs E, Siemers M, Bromm B. Isoflurane induces dose-dependent changes of thalamic somatosensory information transfer. Brain Res. 1999;829:77–89. doi: 10.1016/s0006-8993(99)01341-4. [DOI] [PubMed] [Google Scholar]
- Detsch O, Kochs E, Siemers M, Bromm B, Vahle-Hinz C. Differential effects of isoflurane on excitatory and inhibitory synaptic inputs to thalamic neurones in vivo. Br J Anaesth. 2002a;89:294–300. doi: 10.1093/bja/aef170. [DOI] [PubMed] [Google Scholar]
- Detsch O, Kochs E, Siemers M, Bromm B, Vahle-Hinz C. Increased responsiveness of cortical neurons in contrast to thalamic neurons during isoflurane-induced EEG bursts in rats. Neurosci Lett. 2002b;317:9–12. doi: 10.1016/s0304-3940(01)02419-3. [DOI] [PubMed] [Google Scholar]
- Duffy L, Cappas E, Scimone A, Schofield PR, Karl T. Behavioral profile of a heterozygous mutant mouse model for EGF-like domain neuregulin 1. Behav Neurosci. 2008;122:748–759. doi: 10.1037/0735-7044.122.4.748. [DOI] [PubMed] [Google Scholar]
- Edgar JC, et al. Cortical thickness as a contributor to abnormal oscillations in schizophrenia? Neuroimage Clin. 2013;4:122–129. doi: 10.1016/j.nicl.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlichman RS, et al. Neuregulin 1 transgenic mice display reduced mismatch negativity, contextual fear conditioning and social interactions. Brain Res. 2009;1294:116–127. doi: 10.1016/j.brainres.2009.07.065. doi: 10.1016/j.brainres.2009.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewert TA, Vahle-Hinz C, Engel AK. High-frequency whisker vibration is encoded by phase-locked responses of neurons in the rat’s barrel cortex. J Neurosci. 2008;28:5359–5368. doi: 10.1523/JNEUROSCI.0089-08.2008. doi: 10.1523/JNEUROSCI.0089-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzari P, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, MT-L V, Suh JD, Lin R, Lucki I, Siegel SJ. Electrophysiological and behavioral responses to ketamine in mice with reduced Akt1 expression. Psychopharmacology. 2013;227:639–649. doi: 10.1007/s00213-013-2997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A, Neddens J, Yan L, Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb Cortex. 2009;19:612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn G, et al. Increased absolute magnitude of gamma synchrony in first-episode psychosis. Schizophr Res. 2008;105:262–271. doi: 10.1016/j.schres.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Klook K, Siegel SJ. Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology. 2012a;62:1504–1518. doi: 10.1016/j.neuropharm.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, et al. GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction Transl. Psychiatry. 2012b;17:69. doi: 10.1038/tp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton A, Hoffer BJ, Freedman R. Electrophysiological effects of phencyclidine in the medial prefrontal cortex of the rat. Neuropharmacology. 1987;26:1275–1283. doi: 10.1016/0028-3908(87)90087-6. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, et al. Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia AJ. Psychiatry. 2011;168:930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Light GA, Swerdlow NR, Radant AD, Braff DL. Association analysis of 94 candidate genes and schizophrenia-related endophenotypes. PLoS One. 2012;7:13. doi: 10.1371/journal.pone.0029630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakami T, et al. NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PLoS One. 2009;4:0006755. doi: 10.1371/journal.pone.0006755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose N, Yoshida Y, Koide S, Takada K, Saigusa T, Koshikawa N. Effects of propofol and fentanyl on extracellular levels of gamma-aminobutyric acid in the rat nucleus accumbens: an in vivo microdialysis study. J Oral Sci. 1998;40:165–170. doi: 10.2334/josnusd.40.165. [DOI] [PubMed] [Google Scholar]
- Ho N, Destexhe A. Synaptic background activity enhances the responsiveness of neocortical pyramidal neurons. J Neurophysiol. 2000;84:1488–1496. doi: 10.1152/jn.2000.84.3.1488. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Buchanan RW, O’Donnell P, Thaker GK, Weiler MA, Lahti AC. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology. 2010;35:632–640. doi: 10.1038/npp.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horita A, Carino MA, Chinn C. Fentanyl produces cholinergically-mediated analeptic and EEG arousal effects in rats. Neuropharmacology. 1989;28:481–486. doi: 10.1016/0028-3908(89)90083-x. doi: 10.1016/0028-3908(89)90083-X. [DOI] [PubMed] [Google Scholar]
- Huang YZ, et al. Regulation of Neuregulin Signaling by PSD-95 Interacting with ErbB4 at CNS Synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. doi: 10.1016/S0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- Hume AL, Durkin MA. Central and spinal somatosensory conduction times during hypothermic cardiopulmonary bypass and some observations on the effects of fentanyl and isoflurane anesthesia. Electroencephal Clin Neurophysiol Evoked Potential Sect. 1986;65:46–58. doi: 10.1016/0168-5597(86)90036-5. doi: 10.1016/0168-5597(86)90036-5. [DOI] [PubMed] [Google Scholar]
- Ikuta T, et al. Differences in waveforms of cerebral evoked potentials among healthy subjects, schizophrenics, manicdepressives and epileptics. J Med Invest. 2007;54:303–315. doi: 10.2152/jmi.54.303. [DOI] [PubMed] [Google Scholar]
- Itil TM, Saletu B, Davis S. EEG findings in chronic schizophrenics based on digital computer period analysis and analog power spectra. Biol Psychiatry. 1972;5:1–13. [PubMed] [Google Scholar]
- Jackson ME, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci USA. 2004;101:8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin TE, Witzemann V, Brecht M. Fiber types of the intrinsic whisker muscle and whisking behavior. J Neurosci. 2004;24:3386–3393. doi: 10.1523/JNEUROSCI.5151-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi M, et al. Frontal areas contribute to reduced global coordination of resting-state gamma activities in drug-naive patients with schizophrenia. Schizophr Res. 2011;130:187–194. doi: 10.1016/j.schres.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Kittelberger K, Hur EE, Sazegar S, Keshavan V, Kocsis B. Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations: relevance for the NMDA receptor hypofunction model of schizophrenia. Brain Struct Funct. 2012;217:395–409. doi: 10.1007/s00429-011-0351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B. Differential Role of NR2A and NR2B Subunits in N-Methyl-D-Aspartate Receptor Antagonist-Induced Aberrant Cortical Gamma Oscillations. Biol Psychiatry. 2012;71:987–995. doi: 10.1016/j.biopsych.2011.10.002. doi: 10.1016/j.biopsych.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouvaras E, et al. Fentanyl treatment reduces GABAergic inhibition in the CA1 area of the hippocampus 24 h after acute exposure to the drug. Neuropharmacology. 2008;55:1172–1182. doi: 10.1016/j.neuropharm.2008.07.025. doi: 10.1016/j.neuropharm.2008.07.025. [DOI] [PubMed] [Google Scholar]
- Krishnan GP, Vohs JL, Hetrick WP, Carroll CA, Shekhar A, Bockbrader MA, O’Donnell BF. Steady state visual evoked potential abnormalities in schizophrenia. Clin Neurophysiol. 2005;116:614–624. doi: 10.1016/j.clinph.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Krishnan G, Hetrick W, Brenner C, Shekhar A, Steffen A, O’Donnell B. Steady state and induced auditory gamma deficits in schizophrenia. Neuroimage. 2009;47:1711. doi: 10.1016/j.neuroimage.2009.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulikova SP, Tolmacheva EA, Anderson P, Gaudias J, Adams BE, Zheng T, Pinault D. Opposite effects of ketamine and deep brain stimulation on rat thalamocortical information processing. Eur J Neurosci. 2012;36:3407–3419. doi: 10.1111/j.1460-9568.2012.08263.x. doi: 10.1111/j.1460-9568.2012.08263.x. [DOI] [PubMed] [Google Scholar]
- Kwon JS, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25:9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Collier DA, He L. Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet. 2006;15:1995–2002. doi: 10.1093/hmg/ddl122. doi: 10.1093/hmg/ddl122. [DOI] [PubMed] [Google Scholar]
- Li KX, et al. Neuregulin 1 regulates excitability of fast-spiking neurons through Kv1.1 and acts in epilepsy. Nat Neurosci. 2011;15:267–273. doi: 10.1038/nn.3006. [DOI] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, Braff DL. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Leung LS. The supramammillo-septal-hippocampal pathway mediates sensorimotor gating impairment and hyperlocomotion induced by MK-801 and ketamine in rats. Psychopharmacology. 2007;191:961–974. doi: 10.1007/s00213-006-0667-x. [DOI] [PubMed] [Google Scholar]
- Marquis KL, Paquette NC, Gussio RP, Moreton JE. Comparative electroencephalographic and behavioral effects of phencyclidine, (+)-SKF-10,047 and MK-801 in rats. J Pharmacol Exp Ther. 1989;251:1104–1112. [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- Meyer D, Yamaai T, Garratt A, Riethmacher-Sonnenberg E, Kane D, Theill LE, Birchmeier C. Isoform-specific expression and function of neuregulin. Development. 1997;124:3575–3586. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- Molina LA, Skelin I, Gruber AJ. Acute NMDA receptor antagonism disrupts synchronization of action potential firing in rat prefrontal cortex. PLoS One. 2014:9. doi: 10.1371/journal.pone.0085842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulert C, Kirsch V, Pascual-Marqui R, McCarley RW, Spencer KM. Long-range synchrony of gamma oscillations and auditory hallucination symptoms in schizophrenia. Int J Psychophysiol. 2011;79:55–63. doi: 10.1016/j.ijpsycho.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Thiselton DL, Clark TG, Flint J. Association of the NRG1 gene and schizophrenia: a meta-analysis. Mol Psychiatry. 2006;11:539–546. doi: 10.1038/sj.mp.4001817. doi: 10.1038/sj.mp.4001817. [DOI] [PubMed] [Google Scholar]
- Murray JD, Anticevic A, Gancsos M, Ichinose M, Corlett PR, Krystal JH, Wang XJ. Linking Microcircuit Dysfunction to Cognitive Impairment: effects of disinhibition associated with Schizophrenia in a cortical working memory model. Cereb Cortex. 2012;29:29. doi: 10.1093/cercor/bhs370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nason MW, Jr, Adhikari A, Bozinoski M, Gordon JA, Role LW. Disrupted activity in the hippocampal-accumbens circuit of type III neuregulin 1 mutant mice. Neuropsychopharmacology. 2011;36:488–496. doi: 10.1038/npp.2010.180. doi: 10.1038/npp.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell KA, Karl T, Huang XF. A neuregulin 1 transmembrane domain mutation causes imbalanced glutamatergic and dopaminergic receptor expression in mice. Neuroscience. 2013;248:670–680. doi: 10.1016/j.neuroscience.2013.06.037. [DOI] [PubMed] [Google Scholar]
- Nicolás MJ, López-Azcárate J, Valencia M, Alegre M, Pérez-Alcázar M, Iriarte J, Artieda J. Ketamine-Induced Oscillations in the Motor Circuit of the Rat Basal Ganglia. PLoS One. 2011;6:e21814. doi: 10.1371/journal.pone.0021814. doi: 10.1371/journal.pone.0021814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D. N-methyl D-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008;63:730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Ritt JT, Andermann ML, Moore CI. Embodied information processing: vibrissa mechanics and texture features shape micromotions in actively sensing rats. Neuron. 2008;57:599–613. doi: 10.1016/j.neuron.2007.12.024. doi: 10.1016/j.neuron.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JA, Gandal MJ, Siegel SJ. NMDA antagonists recreate signal-to-noise ratio and timing perturbations present in schizophrenia. Neurobiol Dis. 2012;46:93–100. doi: 10.1016/j.nbd.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shagass C. Early evoked potentials. Schizophr Bull. 1977;3:80–92. doi: 10.1093/schbul/3.1.80. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM. Baseline gamma power during auditory steady-state stimulation in schizophrenia. Front Hum Neurosci. 2012:5. doi: 10.3389/fnhum.2011.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64:369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Kuo PH, Riley BP, Kendler KS, Zhao Z. Candidate genes for schizophrenia: a survey of association studies and gene ranking. Am J Med Genet B Neuropsychiatr Genet. 2008;5:1173–1181. doi: 10.1002/ajmg.b.30743. [DOI] [PubMed] [Google Scholar]
- Teale P, Pasko B, Collins D, Rojas D, Reite M. Somatosensory timing deficits in schizophrenia. Psychiatry Res. 2013;212:73–78. doi: 10.1016/j.pscychresns.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting AK, et al. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci. 2011;31:15–25. doi: 10.1523/JNEUROSCI.2538-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimoto R, et al. Reduced high and low frequency gamma synchronization in patients with chronic schizophrenia. Schizophr Res. 2011;133:99–105. doi: 10.1016/j.schres.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Vahle-Hinz C, Detsch O, Siemers M, Kochs E. Contributions of GABAergic and glutamatergic mechanisms to isoflurane-induced suppression of thalamic somatosensory information transfer. Exp Brain Res. 2007;176:159–172. doi: 10.1007/s00221-006-0604-6. [DOI] [PubMed] [Google Scholar]
- Venables NC, Bernat EM, Sponheim SR. Genetic and disorder-specific aspects of resting state EEG abnormalities in schizophrenia. Schizophr Bull. 2009;35:826–839. doi: 10.1093/schbul/sbn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels TP, Abbott LF. Gating deficits in model networks: a path to schizophrenia? Pharmacopsychiatry. 2007;40:S73–S77. doi: 10.1055/s-2007-992130. [DOI] [PubMed] [Google Scholar]
- Vohs JL, Chambers RA, O’Donnell BF, Krishnan GP, Morzorati SL. Auditory steady state responses in a schizophrenia rat model probed by excitatory/inhibitory receptor manipulation. Int J Psychophysiol. 2012;86:136–142. doi: 10.1016/j.ijpsycho.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigts J, Sakmann B, Celikel T. Unsupervised whisker tracking in unrestrained behaving animals. J Neurophysiol. 2008;100:504–515. doi: 10.1152/jn.00012.2008. [DOI] [PubMed] [Google Scholar]
- Volman V, Behrens MM, Sejnowski TJ. Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. J Neurosci. 2011;31:18137–18148. doi: 10.1523/JNEUROSCI.3041-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Winterer G, et al. Schizophrenia: reduced signal-to-noise ratio and impaired phase-locking during information processing. Clin Neurophysiol. 2000;111:837–849. doi: 10.1016/s1388-2457(99)00322-3. [DOI] [PubMed] [Google Scholar]
- Winterer G, Coppola R, Goldberg TE, Egan MF, Jones DW, Sanchez CE, Weinberger DR. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia AJ. Psychiatry. 2004;161:490–500. doi: 10.1176/appi.ajp.161.3.490. [DOI] [PubMed] [Google Scholar]
- Wood J, Kim Y, Moghaddam B. Disruption of prefrontal cortex large scale neuronal activity by different classes of psychotomimetic drugs. J Neurosci. 2012;32:3022–3031. doi: 10.1523/JNEUROSCI.6377-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Koide S, Hirose N, Takada K, Tomiyama K, Koshikawa N, Cools AR. Fentanyl increases dopamine release in rat nucleus accumbens: involvement of mesolimbic mu- and delta-2-opioid receptors. Neuroscience. 1999;92:1357–1365. doi: 10.1016/s0306-4522(99)00046-9. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yoshida T, Katz DB, Lisman JE. NMDAR antagonist action in thalamus imposes delta oscillations on the hippocampus. J Neurophysiol. 2012;107:3181–3189. doi: 10.1152/jn.00072.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]