Abstract
Objective
To report implementation strategies and outcomes of an evidence-based asthma counseling intervention. The Head-off Environmental Asthma in Louisiana (HEAL) intervention integrated asthma counseling (AC) capacity and addressed challenges facing children with asthma in post-disaster New Orleans.
Methods
The HEAL intervention enrolled 182 children (4–12 years) with moderate-to-severe persistent asthma. Recruitment occurred from schools in the Greater New Orleans area for one year. Participants received home environmental assessments and tailored asthma counseling sessions during the study period based on the National Cooperative Inner City Asthma Study and the Inner City Asthma Study. Primary (i.e. asthma symptoms) and secondary outcomes (i.e. healthcare utilization) were captured. During the study, changes were made to meet the demands of a post-hurricane and resource-poor environment which included changes to staffing, training, AC tools, and AC sessions.
Results
After study changes were made, the AC visit rate increased by 92.3%. Significant improvements were observed across several adherence measures (e.g., running out of medications (p=0.009), financial/insurance problems for appointments (p=0.006), worried about medication side-effects (p=0.01), felt medications did not work (p<0.001)). Additionally, an increasing number of AC visits was modestly associated with a greater reduction in symptoms (test-for-trend p=0.059).
Conclusion
By adapting to the needs of the study population and setting, investigators successfully implemented a counseling intervention that improved participant behaviors and clinical outcomes. The strategies for implementing the AC intervention may serve as a guide for managing asthma and other chronic conditions in resource-poor settings.
Keywords: asthma counselors, post-disaster, case management, chronic disease counseling, environmental triggers
1. Introduction
Chronic conditions, such as asthma, disproportionately affect high-risk, resource-poor populations [1]. As such, interventions that are tailored to the uniqueness of these populations and their related settings are needed. The complexities associated with implementing evidence-based interventions in these communities are numerous but can be successfully addressed by utilizing strategies that are realistic and sustainable [2]. Such strategies are needed as the United States continues to encounter disruptions in quality of life, loss of health and social services, hazardous living conditions, environmental changes, and increased psychosocial stressors as a result of natural disasters. Post-Katrina New Orleans is an example of a community that has experienced such changes. Prior to Hurricane Katrina, New Orleans, like other metropolitan cities, was challenged by poverty and high rates of asthma among their inner-city minority children [3]. Following Katrina, children whose asthma was previously under control were now seeking care in emergency departments as they were unable to obtain medication due to lost medical records, disrupted health insurance coverage, and lack of access to a physician [4]. In addition to the closure of several primary care clinics and pharmacies, New Orleans main provider of healthcare for the underinsured, Charity Hospital, also closed permanently.
In order to meet these challenges, an aggressive multi-pronged approach was needed. We responded by implementing an asthma intervention with limited resources that was evidence-based, practical, as well as replicable for this and other chronic conditions requiring a high standard of care. In developing the Head-off Environmental Asthma in Louisiana (HEAL) study, collaborating partners drew upon two NIH multi-center randomized controlled clinical trials. These clinical trials were: 1) The National Cooperative Inner-City Asthma Study (NCICAS), which was a participant-tailored asthma counselor (AC) intervention designed to empower families by focusing on problem solving, asthma education, improving the caretaker’s understanding of the disease and risks, as well as skills in using medications, avoiding triggers, and improving caretaker-provider communication [5], and 2) The Inner-City Asthma Study (ICAS), which was an environmental intervention designed to reduce the specific exposures present in participant homes tailored to each child’s allergen sensitivities [6]. Overall, both interventions were shown to significantly reduce asthma morbidity in earlier studies by: 1. Reducing home allergen levels to which the child was sensitive, 2. Tailoring the intervention to the family’s needs, and 3. Helping the family problem solve to overcome barriers to asthma management [5, 6]. Primary outcomes previously reported for HEAL on this combined asthma intervention showed a 45% reduction in asthma symptoms (p=<0.001) [7]. In this article we describe the unique way in which we combined and adapted these earlier interventions into the hybrid and novel HEAL environmental asthma counselor intervention. We hypothesized that the hybrid intervention could be adapted and successfully implemented in resource poor settings and the outcomes attained would be similar to that of the NCICAS and ICAS interventions.
2. Methods
2.1 Study Design
HEAL was originally designed as a randomized controlled clinical trial requiring 450 participants. However, after six months only 77 children were enrolled necessitating a study redesign which included recruiting only children with known diagnosed asthma, extending the recruitment period, and changing to a pre-post intervention all while retaining the study’s original objectives. Additional details on this change are previously reported [8].
The protocol for the HEAL study was approved by the Institutional Review Boards at the National Institute of Environmental Health Sciences, Tulane University School of Public Health and Tropical Medicine, and Louisiana State University Health Sciences Center. The target population included children ages 4 to 12, with moderate-to-severe asthma who resided in the greater New Orleans area (predominately Orleans and Jefferson Parishes). Participants were recruited primarily through schools, in addition to other venues (e.g., advertisements, flyers) over the course of one year.
The HEAL study design included recurring clinical and home environmental assessments as well as asthma counseling sessions over the course of 1 year. Data was collected during the course of the study from participants (i.e., caretaker and child). Both primary (asthma symptoms) and secondary (utilization) outcome indicators were captured during baseline and 12 month clinic visits and by telephone interview every 3 months (3, 6, 9, and 12 months) by clinic staff. In order to tailor the AC intervention, counseling sessions were initiated after baseline clinic and home assessments were completed.
2.2 Adaptations to the HEAL Study Design
As described above, HEAL was redesigned mid-course to a pre-post intervention in response to lower than expected recruitment. Low recruitment was associated with multiple factors such as families not returning to area after the storm, conflicting family priorities with study requests, difficulty scheduling appointments, staff turnover, and overwhelming response rates (i.e. initial search for undiagnosed asthma and parent concern for child without healthcare led to screening demands that overwhelmed staff).
These challenges also informed a redesign of the asthma counselor intervention. Compared to previous studies, the HEAL AC intervention involved fewer in-person AC visits due to limited resources and conflicting family priorities in the post-disaster setting (Table 1). The original HEAL counseling intervention called for two group AC sessions in addition to four in-person visits with each family. During the study, group sessions were dropped and the number of individual contacts were reduced from six to two in-person visits with a telephone follow-up occurring two weeks after each encounter (Figure 1). HEAL also modified the child attendance requirement for sessions so children could instead attend school after missing so many class days due to the storm. Additional AC sessions could be conducted with the family as needed. Lastly, a final AC close-out session occurred after all other HEAL visits were performed to ensure that the family’s needs had been met.
Table 1.
Intervention Strategies and Activities
| Intervention Strategies | |||
|---|---|---|---|
| Strategies | HEAL | ICAS | NCICAS |
| Staff | AC: Master’s level Diverse backgrounds CHW: Developed rapport with family, established community partnerships Scheduled visits Assisted AC with environmental education intervention |
EC: Environmental Peer Counselor Environmental Interventionist: Performed remediation activities, visit scheduling |
AC: Master’s level Social Worker Clinic staff: Assisted with watching children who came to clinic visit with caregiver |
| Training | 2 day initial central training+ ongoing Investigator shadowing Individual ongoing follow-up |
4 day initial central training with field practice plus 1.5 day central training after study start | Over 3 months 3, 2.5 day sessions 2 wk. clinic observation |
| Staff Evaluation | 1,3,6,9, 12 months evaluation and verbal testing | Yearly site visits with feedback | 2 site visits per year with feedback |
| Environmental Intervention and Supplies | All: Safe sleeping zone, mold and ETS exposure, dust mite covers, cleaning supplies (e.g, dust mops, spray bottles) food storage containers, hypoallergenic bed covers, HEPA unit Tailored: ERAT-recommended Modules-cockroach, rodent, pets, mold, ETS; Focused on doable goals in stressed environment Supplies - HEPA air purifier with filter, dust mop, food storage containers, spray bottles, hypoallergenic bed covers |
All: Safe sleeping zone, dust mite covers, HEPA vacuum, washable blanket, cleaning supplies Tailored a: ERAT-recommended Modules-cockroach, pets, rodent, mold, ETS Supplies - food storage containers, mouse traps, HEPA unit Exterminator for children sensitive and exposed to cockroach |
All: Dust mite covers Pet & ETS exposure Tailored: AC discussions tailored by responses to CARAT Supplies –referrals to community resources for smoking, psychological, and social issues 2 exterminator visits for children sensitive to cockroach |
| Maximum Caseload | 40 at start, 60 midway | 60–75 | 60 families |
| Provider Contact | Communicated with medical providers as needed | NA | NA |
| Intervention Activities | |||
| HEAL | ICAS | NCICAS | |
| Participant Characteristics | |||
| Location | Greater New Orleans, LA | 7 US inner cities | 8 US inner cities |
| Study Design | Pre-Post Intervention | Randomized control | Randomized control |
| No. of Participants | 182 | 937 (469 intervention) | 1033 (515 intervention) |
| Age of Participants | 4 – 12 years old | 5 – 11 years old | 5 – 9 years old |
| Allergens tested b | 23, including 16 molds | 14, including 4 molds | 9, including 4 molds |
| Intervention Characteristics | |||
| Intervention Period | 1 year | 1 year | 1 year |
| Intervention Frequency | 2 group sessionsd, 2 visitsc (1 home), 2 follow-up calls | No group sessions, 5 home visits plus 2 optional visitsc, each followed by telephone calls | 2 group sessions with caretaker and 2 with child, 6 clinic visits with AC alternating with 6 telephone callsc |
| CARAT | Yes | No | Yes |
| ERAT | Yes | Yes | No |
| Outcome Collection Intervals | |||
| Home Environmental Assessments | Baseline, 6, and 12 months | Baseline, 6, and 12 months | None |
| Clinical Outcomes | Every 3 months | Every 2 months | Every 2 months |
Supplies were distributed based on sensitivity and remediation/education refined based on sensitivity and exposure.
ICAS and NCICAS participants had to be sensitive to >1 allergen; HEAL did not require participants to be sensitized.
The frequency varied according to the needs of the participant.
Group sessions dropped
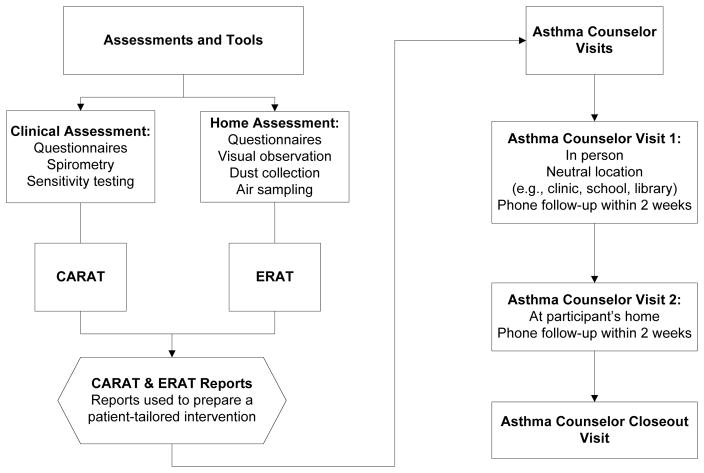
Figure 1. From Clinic and Home Assessments to a Participant-tailored Environmental AC Intervention.
Questionnaires were interviewer-administered at both clinical and home assessments by trained HEAL interviewers (i.e., clinicians, CTRC nurses, home evaluators). Information from the clinical questionnaires was used to feed the Child Asthma Risk Assessment Tool (CARAT). Information gathered from the home assessment was used along with results from allergen skin testing to feed the Environmental Risk Assessment Tool (ERAT). Information gathered from these tools were used by the ACs to construct a participant-tailored intervention.
2.3 Staffing and Training the Asthma Counseling Team
HEAL hired a multidisciplinary team consisting of Masters level ACs of diverse educational backgrounds and community health workers (CHW), based upon the available workforce in the post-disaster community, whereas in NCICAS and ICAS counselors were Masters level social workers and peer level environmental interventionists, respectively (Table 1)[5, 6]. In HEAL, CHWs were paired with ACs in collaborative partnerships that promoted team building and professional career development. We employed ACs who had a foundation in a health-related discipline, experience in asthma, and knowledge of case management.
Retention of intervention staff was needed. The AC and CHW staff turned over twice during the study due to personal challenges resulting from the disaster. The final AC team consisted of three individuals; two recent Masters level public health graduates, and a Masters level mental health counselor. All members of this team had a family history of asthma or personal experience in managing a chronic disease. Based on our rating scale, (ranging from ‘below basic’ to ‘advanced’), their baseline readiness in terms of asthma knowledge and counseling methodology was basic or below basic. In addition to the staff candidates’ ability to learn, their motivation level was considered. The investigators assumed that it was easier to teach principles of case management than to engender enthusiasm, especially during this chaotic time of disruption. Also, time was provided for the staff to relay their Katrina experience to promote team building.
The asthma counselor training curriculum covered asthma physiology, medication, devices, cultural competency, communication skills, environmental control procedures, psychosocial issues, personal safety, and proper case management documentation. The CHWs received a similar but less intensive training regimen compared to the ACs. The training program was revamped after staff turnover to include readiness assessments, and individualized ongoing mentoring and training for the study’s duration. Training sessions included shadowing and “hands on” guidance from the study investigator, with quarterly assessments. Typically within 3 months, an AC or CHW was able to conduct their first session independently from the mentor.
2.4 The Asthma Counseling Intervention
2.4.1 First In-Person Visit
Counselors were assigned 50–60 participant cases each. The initial contact was by phone to establish rapport and schedule the first, in-person visit. Due to a lack of available community clinic sites, this first session was held at a neutral location such as a library or coffee shop. The AC and CHW teams met with both the caregiver and child, if available. During the visit, the AC would review the child’s clinical background, as well as allow the caregiver and child to discuss their experiences with managing asthma. Also, because of post-disaster related crises, oftentimes participants’ social needs (i.e. housing, access to schools, transportation, employment) had to be addressed before addressing asthma management. Counseling was tailored to each child’s clinical and environmental risk profile using assessments developed in the earlier studies. i.e., the Child Asthma Risk Assessment Tool (CARAT) and the Environmental Risk Assessment Tool (ERAT) (Figure 1). The CARAT identified, summarized, scored and ranked the caretaker’s self-report of the child’s risks regarding medical, environmental, adherence, responsibility for asthma care, and attitudes about asthma [5, 9]. The ERAT compiled environmental asthma risks based upon the child’s allergen sensitivities (i.e., allergen skin test results) and exposures (i.e., self-report and home environment assessment results) to provide a summary of intervention topics and actions specific to the child and child’s home (e.g., if a child was allergic to mold and mold was found, the ERAT would recommend the mold intervention module, specifying where and what to clean or fix) [6, 10]. This visit ended with the caregiver developing a set of attainable goals and action steps to work towards before the next session (e.g., refill medications, make appointment with healthcare provider, and remove identified triggers).
2.4.2 In-Person Home Visit
The AC team attempted to conduct the second in-person home visit within one month of the first visit to quickly address home environmental issues that may be impacting the child’s asthma. The child was encouraged to be present along with the caregiver. During the home visit, previous session topics and goals were reviewed. Additional topics identified from the CARAT and the ERAT were then covered if the caretaker was willing and ready. The AC and CHW worked with the family to assist with creating an asthma safe home environment. HEAL took a necessarily practical approach to home remediation that was high in assistance, but low in maintenance. Although every participant received the same supplies at this visit (Table 1), the explanation for using these supplies was tailored to the child’s sensitivities, exposures, and concerns. Caregivers also received counseling about creating a safe place for the child to sleep (i.e., safe-sleeping zone). The counselors demonstrated how to remediate without performing the actual remediation. Emphasis was placed on how each activity could assist in reducing the child’s asthma symptoms.
2.4.3 Documentation, Tools, and Supplies
In addition to the CARAT and ERAT, several communication tools from NCICAS [9, 11] and ICAS [10] were employed to document and guide counseling sessions in HEAL (Table 2). One tool, the AC Checklist, was used by counselors to electronically document every caretaker interaction lasting more than 5 minutes and tracked duration of the session, intervention topics covered, family progress, and barriers. Goal sheets were also adopted from the previous studies to assist participants in establishing short or long-term asthma management goals.
Table 2.
Asthma Counseling Tools
| Tool | Session Used** | Purpose |
|---|---|---|
| A Guide for Helping Children with Asthma [11]* | AC training | Training on NCICAS background, implementation, and AC intervention |
| Asthma Action Plan* | AC visits 1 and 2 | Individualized instructions for daily treatment of asthma and directions for treating worsening symptoms or exacerbation |
| AC Checklist* | All AC visits and calls | Document and track topics, including healthcare obstacles, adherence to medication, recommendations, referrals, symptoms, management, environmental remediation techniques |
| AC Discussion Guide | All AC visits and calls | Guide AC in obtaining understanding of participants’ current symptoms, medications, healthcare utilization, adherence, device technique to identify problem areas to address |
| AC evaluation form | AC training | Quarterly completion to rate AC competency in case management and proficiency in asthma knowledge, included formal feedback from trainers to ACs on current skill/knowledge level and if meeting requirements/expectations for effective counseling |
| Brief Motivational Interviewing [12]* | AC training | Technique to assess participants’ readiness to change/ambivalence, including motivation, barriers, and concerns |
| CARAT* | All AC visits and calls | Tool to identify, score, rank, and summarize participant’s asthma risks |
| Certificate of Completion* | AC Closeout Visit | Formal sealed and signed (by investigators) certificate that child completed study; provided sense of achievement and closure |
| Closeout Form | AC Closeout Visit | Guide AC discussion with participant regarding symptoms, medications, technique, progress made since beginning of study, and future recommendations for management of child’s asthma |
| Education handouts* | AC visits 1 and 2 | Detailed handouts for AC to provide expertise on topics covered in sessions; layman handouts to distribute to literate participants who want more information |
| Environmental supplies* | AC visit 2 (home) | Incentive for caretaker to perform remediation and to permit AC access to home |
| ERAT* | All AC visits and calls | Summarizes home and caretaker environmental assessments with clinic assessment to provide participant-tailored environmental intervention recommendations |
| Goal sheets* | AC visits 1 and 2 | Visual aide to identify actions the participant can choose to perform to decrease exposures in the home to which the child is sensitive |
| Home Visit Checklist* | AC visit 2 (home) | Reminder for AC of participants’ sensitivities and exposures, what to bring to the visit, and any specifics to the home (e.g., unfriendly dog) |
| Peak flow diary | AC visits 1 and 2 | Aid for participant to record peak flow values to increase awareness in changes in airway inflammation |
| Progress notes | All AC visits and calls | Detailed explanation of what occurred pertaining to the participant, including who attended the visit, symptom review, current medications and adherence, healthcare utilization, triggers, device technique, verbal and non-verbal feedback, referrals, and follow-up actions needed |
| Self-assessment pre-post questionnaire | AC training | Assessment of AC knowledge of asthma physiology, medications, recognizing symptoms, device techniques, and more |
| Social learning theory* | AC training | Training emphasizing importance of participant attitudes, expectations, and modeling behavior for change |
| Talk to Your Doctor form | AC visits 1 and 2 | Documented caretaker’s areas of concerns that needed attention to foster communication between participant and healthcare provider |
| Training checklist | AC training | Identified and tracked asthma counseling educational modules and competencies needed in order to perform effective counseling and case management |
| Telephone log* | AC calls | Documented contact with participant and others related to the child’s care |
| Telephone script | AC calls | Standardized telephone interaction with caretakers and provided a guideline of what to say and cover during a call |
Denotes tools used in NCICAS or ICAS
Tools used for AC calls pertain to calls lasting longer than 5 minutes. Tools used for both AC visits 1 and 2 were also used for additional AC in-person visits (which occurred as needed, resources permitting).
In addition to adopted tools, new tools and educational resources were also developed for use during HEAL to, 1.) Improve communication between the AC team, families, and providers, 2.) Enhance training of the AC team, and 3.) Capture and prioritize family’s post-disaster needs (Table 2). One new tool, the AC Discussion Guide, was used at each visit to assist counselors in guiding and eliciting a discussion with participants about their asthma (Tables 2 and 3). Progress notes were utilized to document additional complex problems encountered by families that were not documented in the Discussion Guide or Checklist. A “Talk to Your Doctor Form” was also provided to caregivers who needed assistance communicating or identifying areas of concern to discuss with their healthcare provider. Prior to a counselor speaking with a provider directly, this form could be used to promote patient-provider communication by helping the caretaker communicate concerns to the physician they may have been hesitant to share in the past. When appropriate, ACs went beyond what was provided in the previous studies by actually participating in discussions between the caretaker and healthcare or other service providers if the caregiver identified the need. For participants without primary care providers, the counseling team would offer an updated list of health care providers. Also, a list of community resources were provided to families when needed (e.g., social, financial assistance, educational, housing).
Table 3.
AC Visits and Calls Completed
| Population | N (%) |
|---|---|
| Participants who received ≥ 1 AC in person visit | 165/182 (91%) |
| 1 visit only | 9/165 (5%) |
| 2 visits | 131/165 (79%) |
| 3+ visits | 25/165 (15%) |
| Participants who received ≥1 AC phone follow-up call | 159/182 (87%) |
| 1 phone call | 24/159 (15%) |
| 2 phone calls | 96/159 (60%) |
| 3+ phone calls | 39/159 (25%) |
Educational materials were also provided by the AC such as environmental and asthma self-management device handouts (e.g. spacers, peak flow meters, metered dose inhalers). Asthma Action Plans (AAPs) were provided to assist caregivers in recognizing and treating symptoms. AAPs were participant communication tools written in terms the caregiver could understand and therefore easy to follow at the onset of an asthma exacerbation. Asthma management devices were also provided to families during sessions including spacers, peak flow meters, and nebulizers on an as-needed basis. Environmental supplies for the study were low-cost and low-maintenance- an important consideration when encouraging continuous long-term use in effort to control asthma symptoms (Table 1).
ACs provided individual level support for participants, considering the caretaker and families’ abilities and goals for achieving asthma control. As in the earlier studies, using Brief Motivational Interviewing methods [12], the empowerment model [5, 11],and Social Learning Theory [13], ACs elicited a conversation where the participant could offer their personal experiences living with the condition as well as identify perceived barriers to management.
2.4 Outcome Measures and Statistical Analysis
Primary outcomes were the NCICAS and ICAS measure of maximum symptom days per 2 weeks which was determined by taking the largest value among three indicators: 1) number of days with wheezing, tightness in the chest, or cough; 2) number of nights with disturbed sleep as a result of asthma; and 3) number of days the child had to slow down or discontinued play activities because of asthma [5–7], which was collected at baseline and 12 month clinic visits. Adherence outcomes were taken directly from an adherence questionnaire, captured at baseline and 12 month clinic visits. Medication adherence was defined by the proportion of days with inhaled corticosteroid use in the previous two weeks.
Total time spent on counseling intervention topics was calculated for each participant by summing across all AC session records recorded on the AC Checklist. The total number of in-person asthma counselor visits received by each study participant was determined by summing all visits that occurred prior to the 12 month outcomes collection. Asthma counselor visit activity was quantified by calculating a monthly rate; this was done by dividing the total number of visits that occurred during a given month by the total number of participants who were enrolled in the study during that month. The resulting value was multiplied by 100 to represent the number of visits per 100 enrolled participants.
Linear regression was used to determine the association between the number of in-person AC interventions and the change in symptom days between baseline and 12 months. Clinical characteristics and adherence questions results were compared between baseline and 12 months using McNemar’s tests (categorical outcomes) and paired t-tests (continuous outcomes). Analyses were conducted using SAS version 9.2 (Cary, NC) and R version 2.13.
3. Results
3.1 Delivery of the Asthma Counseling Intervention
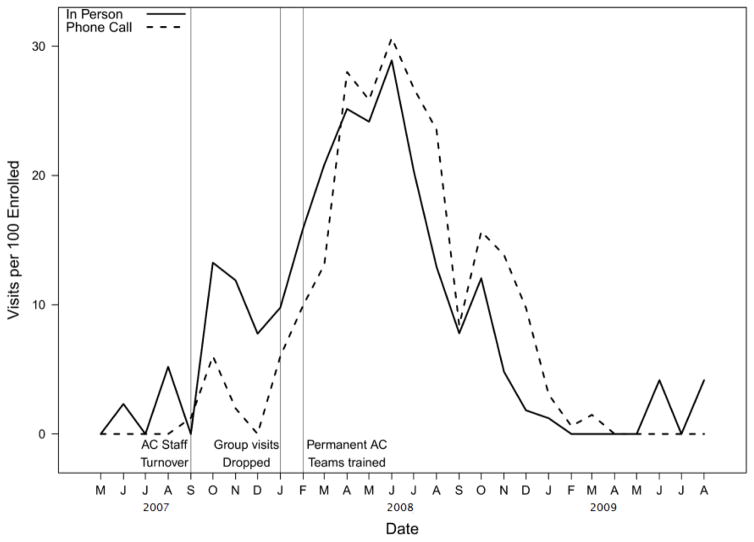
HEAL screened 1864 potential participants via telephone for eligibility. Of those, 210 were screened by the clinic and 182 consented and enrolled in the HEAL intervention. Six months into the study, there were 13 individual visits with an AC per 100 enrolled participants. After dropping the group sessions, child attendance requirements, hiring a new AC/CHW team, and providing ongoing training, by month-12, the visit rate increased by 92.3% (25 in-person visits per 100 enrolled) (Figure 2). By study end, n=165 (91%) had received at least 1 AC visit (Table 3).
Figure 2. AC Visit Activity.
AC visit activity: Dashed and solid lines represent monthly rates (number of visits per 100 enrolled participants) of in-person asthma counselor visits and asthma counselor telephone calls, respectively. Gray vertical lines indicate the transition of changes to AC implementation. The first vertical line represents the first occurrence of staff turnover and dropping of one group session. The second vertical line represents dropping all group sessions. The third vertical line represents the hiring and continuous training of final AC/CHW team.
The majority of participants saw an AC more than once, with 86% (156 of 182) having 2 or more in-person visits and 74% (135 of 182) having 2 or more phone consultations. Children attended 91% (148 of 162) of asthma counseling home visits with their caretaker, while initial visits with the AC were predominantly attended by the caretaker alone (children attended 41%, 64 of 158, of initial visits). Children particated in AC follow-up phone calls with the caretaker 2% or less of the time.
3.2 Asthma Counseling Session Topics Covered
Although, per the HEAL protocol, the frequency of mandatory AC sessions were reduced from the parent studies, participant-tailored AC intervention topics (i.e. medication, adherence, environmental exposures) were completed the majority of time when assigned (>80% HEAL, >95% ICAS). Topics where the counselor documented spending 5 or more minutes (median time spent 20–43 minutes each) included “supplemental,” (reinforcement of previously covered topics (29% of time)), “routine counseling,”(topics on spacer, relationship with clinician, medication plan, adherence, triggers, and symptoms (27% of time)), “environmental,” (covering in depth environmental triggers to which the child was sensitive and exposed (22% of time)), and “adherence,” (covering in-depth medication adherence and potential barriers (15% of time)). The remaining time was split across access to care, school, domestic, financial, house, transportation, mental health, medical, support system, substance abuse, and legal problems (<1.5% of time for each topic).
Sixty-three referrals to other resources were made for 37 participants. Referrals were made for physician (n=11), housing/environmental (n=7), financial/insurance (n=6), smoking cessation (n=5), psychological/behavioral/emotional (n=5), and other reasons (n=28).
3.3 Impact of AC Visits on Asthma Symptoms and Participant Behavior
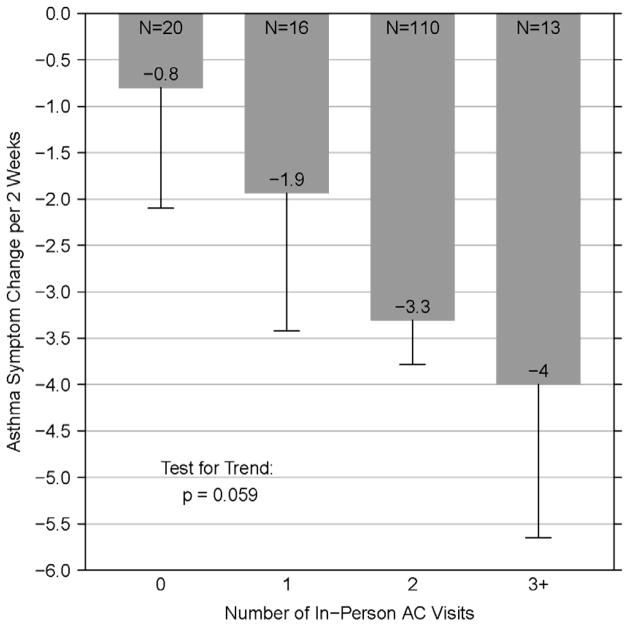
Adherence improved significantly over time as measured by inhaled corticosteroid adherence (adherent to ICS medication ≥7 out of the past 14 days, p=0.003), a reduction in running out of medications (p=0.009), a reduction in worried about medication side effects (p=0.01), and a reduction in the frequency of problems obtaining medications (p=0.005) (Table 4). There was also a significant improvement in caretakers reporting that the medications worked (p<0.001). Asthma symptoms improved over time (p=0.059, test for trend), with participants who met with an AC most often having the largest decrease in symptoms (Figure 3).
Table 4.
Clinical and Adherence Characteristics at Baseline and 12 months
| N | Baseline | 12 months | p-value | |
|---|---|---|---|---|
| Medication | ||||
| Participant is taking ICS and is adherent ≥ 50%* | 127 | 49 (39%) | 67 (53%) | 0.003 |
| Participant has a place for follow-up asthma care | 127 | 113 (89%) | 126 (99%) | <0.001 |
|
| ||||
| Adherence | ||||
| In the last 6 months, answered “yes:” | ||||
| Ever run out of medications | 127 | 30 (24%) | 16 (13%) | 0.009 |
| Used herbal remedies | 127 | 18 (14%) | 9 (7%) | 0.04 |
| Trouble with clinician appointments | 127 | 19 (15%) | 21 (17%) | 0.86 |
| Financial/insurance problems for appointments | 127 | 13 (10%) | 3 (3%) | 0.006 |
| Take any medications for asthma | 127 | 126 (99%) | 125 (98%) | >0.99 |
| Problems obtaining meds from pharmacy | 124 | 25 (20%) | 18 (15%) | 0.23 |
| Financial/insurance problems for medications | 124 | 13 (10%) | 13 (10%) | >0.99 |
| Problems with missing doses | 124 | 24 (19%) | 19 (15%) | 0.44 |
| Worried about medication side effects | 123 | 60 (49%) | 43 (35%) | 0.01 |
| Felt better and stopped taking medications | 123 | 21 (17%) | 14 (11%) | 0.17 |
| Felt the medications do not work | 124 | 28 (23%) | 9 (7%) | <0.001 |
| Medication schedule is hard to follow | 124 | 15 (12%) | 17 (14%) | 0.83 |
| Scale of 1 to 5, with 1 “none” and 5 “a lot/often” | ||||
| Frequency of any problems obtaining medicines | 124 | 1.7 ± 0.9 | 1.4 ± 0.9 | 0.005 |
| Frequency of missing doses | 124 | 2.1 ± 1.0 | 2.2 ± 1.0 | 0.57 |
N includes participants with at least 1 session with an AC prior to the 12 month in person clinic assessment, where adherence measures outcomes were collected by trained data collectors (Tulane GCRC nurses and physicians).
Figure 3. AC Visits and Asthma Symptom Reduction.
AC Visits and Asthma Symptom Reduction: Bars and annotated numbers represent the mean change in maximum symptom days over 12 months. Error bars represent standard error. N represents the number of participants with a 12-month symptoms outcome via phone for the corresponding number of in person AC visit(s). Test for trend p-value is annotated on the plot.
4. Discussion and Conclusion
4.1 Discussion
HEAL introduced study design modifications and strategies that allowed for implementation of an asthma counseling intervention in a post-disaster setting. Within five months of study start HEAL was at risk of ending due to lack of study visit completion and staff turnover. Accordingly, numerous modifications were made that enabled us to implement the study based on the needs of the environment and keeping within study protocol. Fidelity to NCICAS and ICAS combined with flexibility in dealing with obstacles encountered in the post-Katrina environment resulted in an intervention that generated outcomes comparable to these case-controlled studies [7]. We report here that participants who had three or more visits with an AC had their symptoms reduced further than those who saw a counselor only once. This provides support for the effectiveness of the AC intervention but may also reflect that with increased contact, caregivers pay more attention to children’s asthma symptoms and management, which leads to decreased symptoms on its own regardless of the intervention delivered [14]. Families who have children with the poorest control over their asthma are often a mixed group. Some of these families are able to take advantage of the AC resources and have many intervention contacts leading to improved symptoms; while others are experiencing so many difficulties in their lives that they are unable to make appointments and attend visits with the counselor resulting in continued poor control [5, 15]. Nevertheless, multiple visits with an AC provided increased benefit over a single visit.
Medication adherence was one of the topics discussed most frequently in HEAL and corresponds to the improvement seen over time with adherence outcomes. The self-report of challenges with taking medications indicates that although a child may have access to medication, they may not be taking it properly due to misunderstanding of its use or concerns with taking the medication. With proper counseling, any concerns regarding medication use were addressed. It is well established that use of inhaled corticosteroids leads to the reduction of asthma symptoms [16]. Therefore, the time spent on this topic may have contributed to the reduction of asthma symptoms and indicates that asthma management messages on medication can be successfully delivered to caretakers dealing with multiple stressors.
The other topic most frequently covered was demonstration of environmental remediation techniques. ICAS showed that modifying the environment by reducing or eliminating asthma triggers resulted in a 0.8 day reduction in symptom days [6]. The time spent on modifying behavior to reduce or eliminate exposures to which the child is sensitive may have also contributed to the reduced symptom days in HEAL.
Perhaps the most important factor in the success of the HEAL study was its approach of adapting to post-Katrina circumstances and family needs. AC intervention strategies were not only tailored to the participant, as was done in the parent studies, but also adaptive to the unique post-disaster situations not seen before in the US. As was demonstrated in NCICAS, socioeconomically disadvantaged families live in highly challenging environments, where healthcare concerns can be overwhelmed by more pressing problems that distract the family from focusing on and managing the child’s asthma [5]. Given the population movements, environmental devastation, and lack of social and health services, HEAL investigators pushed for study completion by refining the study design, intensively training a unique AC/CHW team to deliver individualized interventions, and refocusing the intervention components to adapt to the specific needs of the study participants and their families. The AC/CHW team was well equipped with up-to-date referral information and guided families in contacting appropriate resources when required to address needs that were beyond what could be provided by HEAL. Also, acknowledging the staff’s Katrina experiences and ongoing post-disaster stress required implementing strategies that promoted the team’s wellbeing. The training simultaneously built individual and team confidence, capacity, and accountability. The combination of an AC with a CHW allowed for greater capacity, understanding and navigation of the community, and increased support system for the families.
To reduce the demands of the study on the staff and population, modifications were made to the AC intervention during study implementation to improve participant attendance. As previously mentioned, this included dropping group sessions that, although were beneficial in NCICAS, proved too difficult to schedule in HEAL. Also, changing the minimum of six in-person to two in-person visits with follow-up calls provided the flexibility needed for the demanding situation. Progress notes were added during the study to provide an additional communication tool to comprehensively capture sessions as well as assist new team members with familiarizing themselves with their cases, which was critical due to the initial staff turnover. By monitoring visit activity throughout the study, investigators were alerted as to when these intervention adjustments were necessary.
In non-controlled pre-post translational studies, limitations must be considered when interpreting findings. The integrity of the intervention may be compromised with necessary adaptations for implementation. Also, there may be study population differences affecting study results. However, because comparable findings in symptom reduction were found between the previous studies and HEAL, the adapted intervention design appeared to be similarly effective [7].
4.2 Conclusion
Post-Katrina New Orleans posed numerous challenges, including water and mold damage, fragmented medical and social services, and chaotic school schedules, among others. The HEAL experience highlights the difficulties encountered in implementing a randomized controlled study in a post-disaster setting. To overcome these difficulties, HEAL investigators introduced strategies in the implementation of HEAL that considered the specific circumstances of participants and scarcely available health and social services. The AC/CHW team assisted participant families by establishing goals that were attainable. Also, the management team was vigilant about team training. In fact, the remaining four ACs trained in HEAL took the national asthma educator certification board exam and passed on first attempt. The exam has a pass rate of 67.7 % [17]. Three certified ACs remained in New Orleans as part of a second phase of HEAL at the Daughters of Charity Services of New Orleans clinics, demonstrating the translatable and sustainable impact of HEAL [18]. The strategies introduced in the implementation of the HEAL AC intervention model supported the reduction of participant symptoms and may be duplicated in managing asthma and other chronic diseases in various resource-poor settings. Overall, the integrity of evidence-based interventions must be balanced with the capacity and needs of the targeted community to ensure success and sustainability.
Acknowledgments
The authors would also like to acknowledge the following funders of HEAL: The National Center of Minority Health and Health Disparities (NCMHD, now the NIMHD); the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health under Contract number NO1-ES-55553; and the Merck Childhood Asthma Network, Inc.(MCAN). Other organizations that contributed include the National Toxicology Program (NIEHS), the U.S. Environmental Protection Agency (Cincinnati, OH), and the de Laski Family Foundation. The Clinical and Translational Research Center of Tulane and Louisiana State Universities Schools of Medicine was supported in whole or in part by funds provided through the Louisiana Board of Regents RC/EEP.
Authors would also like to thank the following institutions, investigators, and staff: Tulane University School of Public Health and Tropical Medicine, Maureen Lichtveld (principal investigator), Faye Grimsley (investigator), LuAnn White (investigator), William Hawkins (program management), Melissa Owsiany (senior program coordinator), Shannon DeGruy, Dorothy Paul, Latasha Barlow, Nicole Bell, Erica Harris (home evaluators); Tulane University Health Sciences Center, Jane El-Dahr (investigator); Tulane University School of Medicine, Maxcie Sikora (physician); Tulane Clinical and Translational Research Center of Tulane and Louisiana State Universities Schools of Medicine, Mary Meyaski-Schluter, Virginia Garrison, Erin Plaia, Annie Stell, Jim Outland, Shanker Japa, Charlotte Marshall (nursing staff); New Orleans Health Department, Kevin Stephens (principal investigator), Mosanda Mvula (investigator), Stacey Denham, Margaret Sanders, Claire Hayes (asthma counselors), Alfreda Porter, Tenaj Hampton, Angela Sarker (community health workers), Mamadou Misbaou Diallo, Shawanda Rogers, David Ali (recruiters), Doryne Sunda-Meya, Ariska Fortenberry (administrative), Florietta M. Stevenson (personnel); Louisiana State University Health Sciences Center School of Nursing, Yvonne Sterling (investigator); Louisiana State University Health Sciences Center, Ken Paris (physician); National Institute of Environmental Health Sciences, Patricia Chulada (health scientist administrator); National Institute of Child Health and Human Development, William Martin II (principal investigator); Visionary Consulting Partners, LLC, Eleanor Thornton (investigator); Constella Group, LLC, Rich Cohn (investigator), Keith Bordelon (study coordinator); Rho, Inc, Herman Mitchell (principal investigator), Suzanne Kennedy (investigator), John Lim (data manager), Gina Allen (research associate), Jeremy Wildfire (statistician), and Rebecca Z. Krouse (statistician). Merck Childhood Asthma Network, Inc, Floyd J. Malveaux (consultant). In addition, David Schwartz (University of Colorado, past Director of NIEHS at time of HEAL) for his innovative input and willingness to support the project early in the process.
Funding:
This project was funded by the National Center of Minority Health and Health Disparities (NCMHD, now the NIMHD); the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health under Contract number NO1-ES-55553; and the Merck Childhood Asthma Network, Inc.(MCAN). Public-private funding provided by the NIH and MCAN was managed under the auspices of the Foundation for the National Institutes of Health. Established by the United States Congress to support the mission of the NIH — improving health through scientific discovery in the search for cures — the Foundation for the NIH is a leader in identifying and addressing complex scientific and health issues. The Foundation is a non-profit, 501(c) (3) charitable organization that raises private-sector funds for a broad portfolio of unique programs that complement and enhance the NIH priorities and activities. [Additional information about the Foundation for the NIH is available online (http://www.fnih.org/).] The Merck Childhood Asthma Network, Inc is a separately incorporated, nonprofit, 501(c) (3) organization funded by the Merck Foundation, the philanthropic arm of Merck & Co., Inc. Other organizations that contributed include the National Toxicology Program (NIEHS), the U.S. Environmental Protection Agency (Cincinnati, OH), and the de Laski Family Foundation. The Clinical and Translational Research Center of Tulane and Louisiana State Universities Schools of Medicine was supported in whole or in part by funds provided through the Louisiana Board of Regents RC/EEP.
Footnotes
Declaration of Interest Section
The authors have no financial and/or personal relationships to disclose that could inappropriately influence (bias) their work.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report. 2011;(32):1–14. [PubMed] [Google Scholar]
- 2.Markus A, Lyon M, Rosenbaum S. Changing pO2licy The Elements for Improving Childhood Asthma Outcomes. 2010 Available from: http://publichealth.gwu.edu/departments/healthpolicy/DHP_Publications/pub_uploads/dhpPublication_1B84EC58-5056-9D20-3D55F16D030BA0BC.pdf.
- 3.Mvula M, et al. Prevalence of asthma and asthma-like symptoms in inner-city schoolchildren. J Asthma. 2005;42(1):9–16. doi: 10.1081/jas-200044746. [DOI] [PubMed] [Google Scholar]
- 4.Abramson D, Garfield R. O.A. Columbia University Mailman School of Public Health, editor. On the Edge: Children and Families Displaced by Hurricanes Katrina and Rita Face a Looming Medical and Mental Health Crisis. Columbia University Mailman School of Public Health, Operation Assist; 2006. pp. 1–5. [Google Scholar]
- 5.Evans R, 3rd, et al. A randomized clinical trial to reduce asthma morbidity among inner-city children: results of the National Cooperative Inner-City Asthma Study. J Pediatr. 1999;135(3):332–8. doi: 10.1016/s0022-3476(99)70130-7. [DOI] [PubMed] [Google Scholar]
- 6.Morgan WJ, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–80. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell H, et al. Implementation of evidence-based asthma interventions in post-Katrina New Orleans: the Head-off Environmental Asthma in Louisiana (HEAL) study. Environ Health Perspect. 2012;120(11):1607–12. doi: 10.1289/ehp.1104242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chulada PC, et al. The Head-off Environmental Asthma in Louisiana (HEAL) study--methods and study population. Environ Health Perspect. 2012;120(11):1592–9. doi: 10.1289/ehp.1104239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell H. For the National Cooperative Inner-City Asthma Study. Child Asthma Risk Assessment Tool. 2000 [cited 2014 May 2]; Available from: http://carat.asthmarisk.org/
- 10.Crain E, et al. Home and allergic characteristics of children with asthma in seven U.S. urban communities and design of an environmental intervention: the Inner-City Asthma Study. Environ Health Perspect. 2002;110(9):939–45. doi: 10.1289/ehp.02110939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mortimer K, Mitchell H. A guide for Helping Children with Asthma (a.k.a. The Grey Book) National Cooperative Inner-City Asthma Study, National Institute of Allergy and Infectious Diseases, Division of Allergy, Immunology and Transplantation; [Google Scholar]
- 12.Rollnick S, et al. Development of a short ‘readiness to change’ questionnaire for use in brief, opportunistic interventions among excessive drinkers. Br J Addict. 1992;87(5):743–54. doi: 10.1111/j.1360-0443.1992.tb02720.x. [DOI] [PubMed] [Google Scholar]
- 13.Bandura A. In: Social Foundations of Thought and Action. Cliffs E, editor. NJ: Prentice-Hall; 1986. [Google Scholar]
- 14.Carter MC, et al. Home intervention in the treatment of asthma among inner-city children. The Journal of allergy and clinical immunology. 2001;108(5):732–7. doi: 10.1067/mai.2001.119155. [DOI] [PubMed] [Google Scholar]
- 15.Wade S, et al. Psychosocial characteristics of inner-city children with asthma: a description of the NCICAS psychosocial protocol. National Cooperative Inner-City Asthma Study. Pediatric pulmonology. 1997;24(4):263–76. doi: 10.1002/(sici)1099-0496(199710)24:4<263::aid-ppul5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 16.National Heart Lung and Blood Institute. [Accessed December 1, 2011];Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma - Full Report 2007. 2007 Aug 28; Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm < http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm>.
- 17.National Asthma Educator Certification Board. Certificants, Certification Corner, Certificant Facts. 2014 [cited 2014 May 2]; Available from: http://www.naecb.com/certificant-corner.php.
- 18.Rapp KI, et al. The HEAL, Phase II Project: enhancing features of an electronic medical record system to improve adherence to asthma guidelines. J Health Care Poor Underserved. 2013;24(1 Suppl):20–8. doi: 10.1353/hpu.2013.0048. [DOI] [PubMed] [Google Scholar]