Abstract
Background
Neuregulin-1 and ErbB4 are genetically associated with schizophrenia, and detailed knowledge of the cellular and subcellular localization of ErbB4 is important for understanding how neuregulin-1 regulates neuronal network activity and behavior. Expression of ErbB4 is restricted to interneurons in the rodent hippocampus and cortex. However, controversy remains about the cellular expression pattern in primate brain and its subcellular distribution in postsynaptic somatodendritic locations versus presynaptic terminals.
Methods
ErbB4 expression was analyzed in pyramidal cells and interneurons in the frontal cortex of five species: C57BL6 mice (n = 3), ErbB4−/− mice (n = 2), Sprague-Dawley rats (n = 3), two macaque species (n = 3 + 2), and humans (normal control subjects, n = 2). We investigated 1) messenger RNA in mice, macaques, and humans; 2) protein expression in all species using highly specific monoclonal antibodies; and 3) specificity tests of several ErbB4 antibodies on brain samples (mouse, macaque, human).
Results
ErbB4 RNA is restricted to interneurons in the frontal cortex of mice. ErbB4 protein is undetectable in pyramidal cells of rodents, macaques, and human frontal cortex, whereas most interneurons positive for parvalbumin, calretinin, or cholecystokinin, but only a minority of calbindin-positive cells, co-express ErbB4 in macaques. Importantly, no presynaptic ErbB4 expression was detected in any species.
Conclusions
The interneuron-selective somatodendritic expression of ErbB4 is consistent with a primary role of neuregulin-ErbB4 signaling in the postsynaptic modulation of gamma-aminobutyric acidergic function in rodents and primates. Our data validate the use of rodents to analyze effects of abnormal ErbB4 function as a means to model endophenotypes of psychiatric disorders.
Keywords: Chandelier cell, gamma-aminobutyric acid (GABA), human, inhibition, neuregulin, parvalbumin, primate, schizophrenia
The neuregulin-1 (NRG1)-ErbB4 signaling pathway has emerged as a genetically and biologically plausible candidate contributing to the pathogenesis of schizophrenia (1–4). Changes in the relative expression of different ErbB4 splice variants (5,6) and in the biological activity of NRG1-ErbB4 signaling (7) have been reported in schizophrenia. Recent findings suggest that this pathway can functionally link dopaminergic and glutamatergic neurotransmitter systems to regulate network activity and synaptic plasticity (8). The observations that ErbB4 activation strongly augments hippocampal gamma oscillations and that ErbB4-deficient mice exhibit reduced oscillatory power (9) implicate this pathway in the synchronization of local neuron ensembles, likely via a primary effect on parvalbumin (PV)-positive interneurons (9). Moreover, NRG1 acutely depotentiates hippocampal early-phase long-term potentiation (10) via a cascade of events that include increase of extracellular dopamine, activation of dopamine D4 receptors, and the subsequent internalization of GluA1-containing a-amino-3-hydroxyl-5-isoxazolepropionate (AMPA) receptors (8).
Because of the recent development and characterization of highly specific monoclonal antibodies and through the use of single-cell reverse transcriptase-polymerase chain reaction (RT-PCR), it has become evident that in the hippocampus of adult rodents, ErbB4 protein and messenger RNA (mRNA) expression is limited to gamma-aminobutyric acid (GABA)ergic interneurons (11,12). Developmentally, ErbB4 is expressed in migrating interneuron precursor cells (13–15), and lack of ErbB4 results in reduced numbers of cortical interneurons in adults (12–14) and in impaired connectivity of GABAergic neurons (16,17). Thus, in addition to the modulation of dopaminergic transmission and glutamatergic synaptic plasticity, regulation of the maturation and maintenance of cortical interneurons must be added to the effects of neuregulin (NRG)-ErbB4 signaling that have strong implications for schizophrenia (18,19). Parvalbumin-positive interneurons are of particular interest, because they appear to play a prominent role in mediating the modulatory effects of NRG1 on hippocampal network oscillations (9) and the regulation of neuronal inhibition in the frontal cortex of mice (20,21).
Some studies have recently proposed the existence of a population of ErbB4 receptors on GABAergic presynaptic terminals in the cortex (16,21,22) but not in the hippocampus of mice (11). The question regarding the major site of action of ErbB4 is highly important for a thorough understanding of NRG-ErbB4 signaling (12,18). Because morphological changes in chandelier cell cartridges innervating the axon initial segment of cortical pyramidal cells have been implicated in schizophrenia (23) and because presynaptic expression of ErbB4 has been reported in these terminals in mice (16), it is imperative to further investigate the possibility of presynaptic expression in rodents and, more importantly, in primates.
Despite this recent progress in rodents, our understanding of the cellular expression and subcellular localization of ErbB4 in the human brain is still rudimentary. Only a few studies have examined the expression of ErbB4 protein in the human brain (7,24,25), and their findings of widespread ErbB4 expression in cortical neurons and glial cells are at variance with the receptor distribution in rodents. To understand the functional role of the NRG1-ErbB4 pathway in humans, it is therefore important to further investigate the cellular and subcellular distribution of ErbB4 receptors in the primate brain. Here, we present a detailed analysis of cellular ErbB4 expression in the frontal cortex of rodents and primates, including humans. Our results are important for the ongoing investigation into the possible contribution of ErbB4 signaling to neural processes altered in schizophrenia, as they validate the use of mouse models with altered NRG-ErbB4 signaling properties to investigate endophenotypes believed to be affected in the disorder.
Methods and Materials
A detailed description of methods and materials is provided in Supplement 1. Please note that we use the new nomenclature (26) for ligand-gated ion channels (e.g., glutamate receptor subunits) throughout the article.
Animals and Human Brain Samples
Animal and human brain samples were included as follows: 1) electrophysiology and single-cell RT-PCR (Figure 1): four Nkx2-1Cre: RCE Swiss Webster mice (28) (ages p15–p21; in-house breeding); 2) ErbB4 protein expression in pyramidal cells and interneurons (Figures 2–5 and 7, see also Figures S1–S3 in Supplement 1): three male C57Bl/6J mice (ages p70–p90) (Jackson Laboratories, Bar Harbour, Maine), three male Sprague-Dawley rats (ages p85–p110) (Jackson Laboratories), and two male rhesus monkeys (Macaca mulatta, ages 7 years and 12.5 years); 3) tests for specificity of antibodies by Western blot analysis (Figure 3 and Figures S2 and S3 in Supplement 1): two male rhesus monkeys (see above), two male C57Bl/6J mice (age > 3 months) (Jackson Laboratories), and two male ErbB4−/− HER4heart mice (27) (age > 3 months, in-house breeding); and 4) ErbB4 co-expression analysis in subtypes of interneurons (Figure 6): three male long-tailed macaque monkeys (Macaca fascicularis, 3–4 years of age).
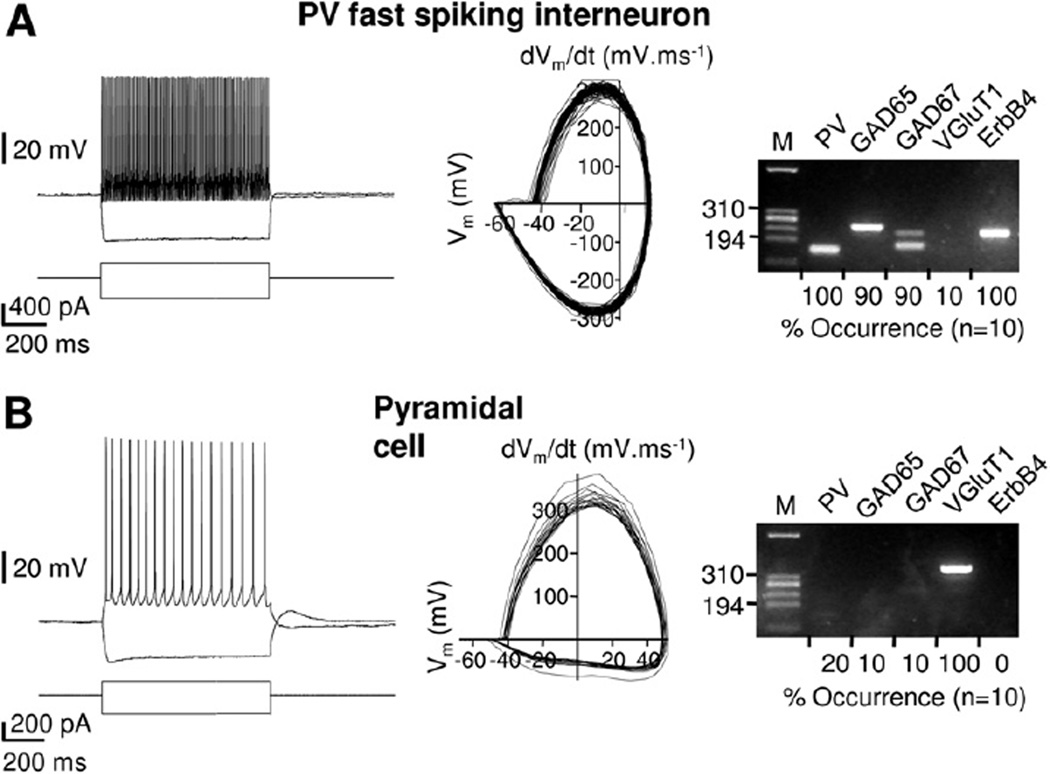
Figure 1.
ErbB4 messenger RNA(mRNA) expression is restricted to frontal cortical interneurons in mice. Left: representative examples of action potential discharges from a fast-spiking interneuron (A) and a pyramidal cell (B) in response to 300 pA and 150 pA depolarizing current injections, respectively. Elicited action potential trains serve to illustrate differences between the high-frequency firing patterns of fast-spiking interneurons and the more regular lower-frequency firing commonly observed in pyramidal cells. Membrane holding potentials were adjusted to −60 mV. Center: corresponding phase plots of the voltage responses shown on the left. The plots illustrate the waveform properties of the action potentials such as spike and after hyperpolarization amplitudes, as well as maximum slope during the rising and repolarizing phases. Right: results of corresponding single-cell reverse transcriptase-polymerase chain reaction analyses. HaeIII-digested ΦX174 DNA was used as size reference (M, 310 and 194 base pair fragments indicated). Summary data from the respective population analyses are shown below each gel image. ErbB4 mRNA was detected in all parvalbumin-positive interneurons but not in any of the 10 pyramidal cells analyzed. GAD, glutamic acid decarboxylase; M, size marker; PV, parvalbumin; VGluT1, vesicular glutamate transporter 1.
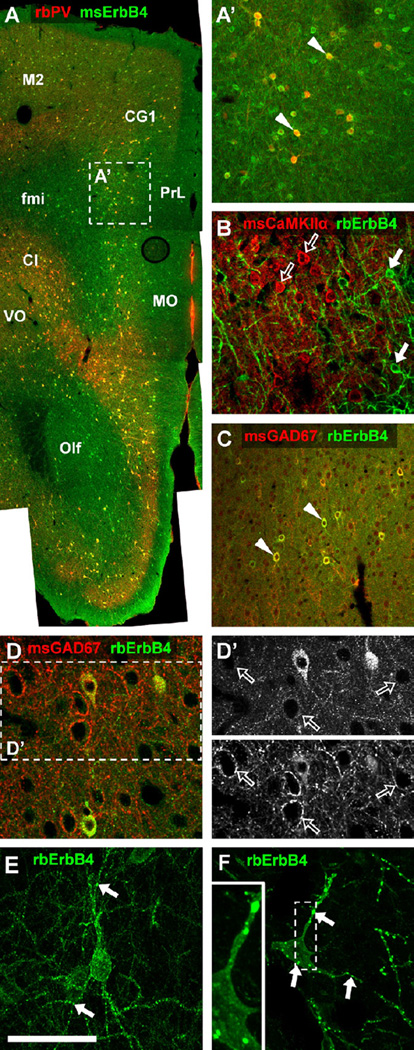
Figure 2.
Cellular and subcellular expression of ErbB4 protein in the medial frontal cortex of rodents. (A) The overview image shows the expression of ErbB4 protein (green channel) throughout layers II to VI in the medial frontal lobe. ErbB4-positive somata are of round to oval shape and are evenly distributed in dorsomedial cortical areas (secondary motor cortex [M2], cingulate cortex area 1 [CG1]) but accumulate at high density in the claustrum and deep layers V to VI of ventromedial cortices (prelimbic cortex [PrL], medial orbital cortex [MO], ventral orbital cortex [VO]). A high density of ErbB4-positive cells is also evident in the olfactory bulb, whereas only few interstitial cells express ErbB4 in white matter forceps minor. Please note that nearly all interneurons detected by rabbit anti-parvalbumin antibody (rbPV) (red channel) express ErbB4 (arrowheads in A′) regardless of their location in the frontal lobe. (B) Calcium/calmodulin-dependent protein kinase II alpha-expressing pyramidal cells (open arrows) detected with mouse antibody (msCaMKIIα) show no double-labeling and are clearly separated from ErbB4-positive neurons (arrows). (C) Co-immunodetection with mouse GAD67 antibody (msGAD67) shows that many interneurons express ErbB4 in the somatodendritic compartment (arrowheads). (D, D′) In contrast, gamma-aminobutyric acidergic terminals surrounding neuronal somata are immunonegative for ErbB4 (open arrows), as signal in the green channel is not different from background. (E, F) Z-stack confocal imaging (63×, five planes .27 µm apart) reveals dendritic ErbB4 accumulations (arrows) in the mouse (E) and rat (F), consistent with receptor targeting to glutamatergic postsynaptic sites as previously reported. In the rat, the puncta on somata (F) are clearly within the cell (see insert); however, their exact subcellular location is yet to be determined. ErbB4 was detected in mice (n = 3) and rats (n = 3) using mouse mAb-77 (msErbB4) (A,A′) or rabbit mAb-10 (rbErbB4) (B–F). Scale bar = 640 µm (A), 200 µm (A′, C), 100µm (B, D, D′), 50 µm (E, F). Cl, claustrum; fmi, forceps minor; Olf, olfactory bulb; other abbreviations as in Figure 1.
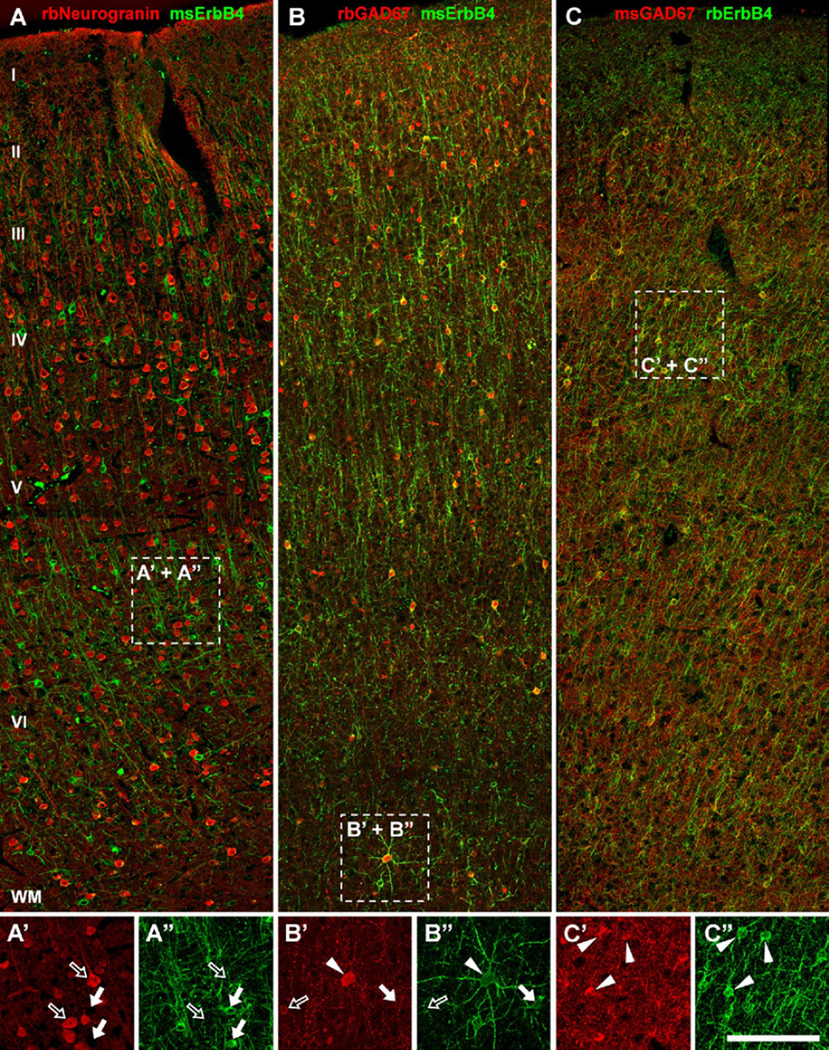
Figure 5.
ErbB4 is expressed in a subset of gamma-aminobutyric acidergic interneurons in monkey frontal cortex. (A, A″) ErbB4 immunoreactivity (arrows) is absent from pyramidal cells labeled with rabbit anti-neurogranin antibody (rbNeurogranin) (open arrows). (B, C) However, ErbB4 is strongly expressed in many GAD67-positive interneurons in layers II to VI, similar to rodent and human frontal cortex. Note that the observed pattern of cellular co-expression of GAD67 and ErbB4 is very similar between experiments, despite using two different antibodies against each protein (ErbB4: mAb-77 in A–B″, mAb-10 in C–C″; n = 2). Cells labeled only in red are marked by open arrows, cells only labeled in green are marked by arrows, and co-expressing cells are marked by arrowheads. Scale bar = 150 µm (A, B, C) or 100 µm (A′, A″, B′, B″, C′, C″). WM, white matter. Other abbreviations as in Figure 4.
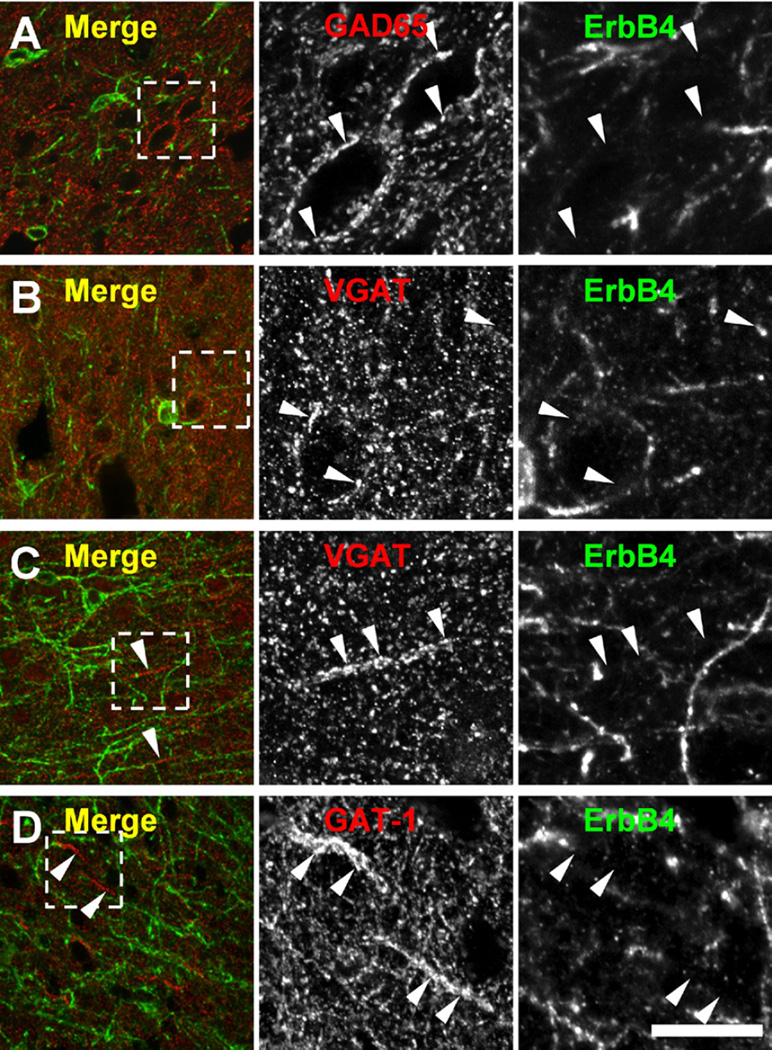
Figure 7.
ErbB4 is not detected on terminals of gamma-aminobutyric acidergic interneurons in the frontal cortex of rats and rhesus monkeys. Putative terminals were visualized by immunofluorescence against GAD65, vesicular gamma-aminobutyric acidergic transporter (VGAT), and GABAtransporter-1 (GAT-1) in the frontal cortex of rats (A, B) (n = 3) and rhesus monkeys (C, D) (n = 2). In the rat, VGAT and GAD65 (arrowheads) immunoreactive puncta juxtapose somatodendritic segments of target cells and are consistently immunonegative in the frontal cortex. Similarly, GAT-1 and VGAT immunoreactive chandelier cell cartridges (arrowheads) in the monkey frontal cortex are immunonegative for ErbB4 mAb-10. Scale bar = 100 µm (overlays) or 30 µm (single channels). Other abbreviations as in Figure 4.
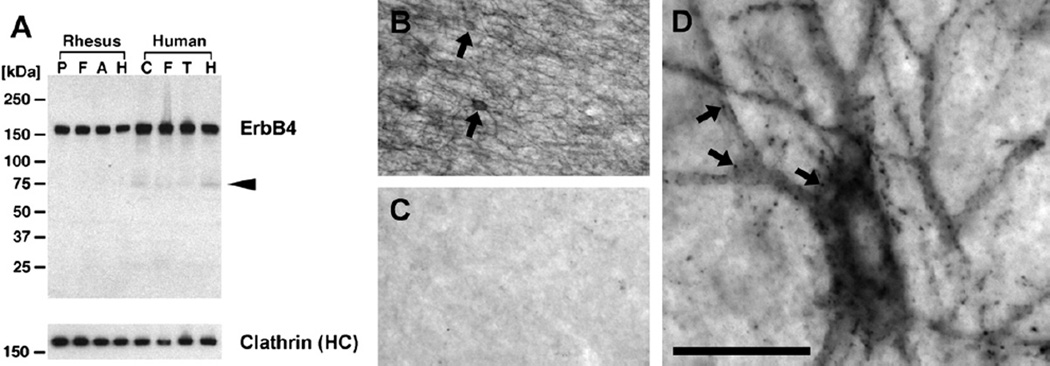
Figure 3.
ErbB4 protein is strongly expressed in the forebrain of primates. (A) ErbB4 Western blot in various primate cortical areas analyzed with rabbit mAb-10 (1 µg/mL). Protein lysates from rhesus monkey (42 µg/lane) and human (75 µg/lane) were normalized to yield similar signal intensities for the reference protein clathrin heavy chain (HC) (see Methods and Materials). Overwhelmingly, full-length ErbB4 (180 kDa) is detected, and the arrowhead points to a weak band likely corresponding to the processed 75 kDa intracellular domain of ErbB4. Molecular masses of reference proteins are as indicated. (B) Immunohistochemical detection of ErbB4 with mAb-10 (n = 2) labels many small round to oval multipolar cells (arrows) in the frontal cortex of rhesus monkeys, resembling the size, shape, and distribution of interneurons. (C) The staining is entirely absent when mAb-10 is omitted. (D) High-magnification imaging reveals ErbB4 clusters on soma and dendrites (arrows), consistent with our findings in rodents (cf. Figure 2E,F). Scale bar = 160 µm (B, C), 24 µm (D). A, anterior cingulate cortex; C, cerebral cortex; F, dorsal frontal cortex; H, hippocampus; P, dorsolateral prefrontal cortex; T, temporal lobe.
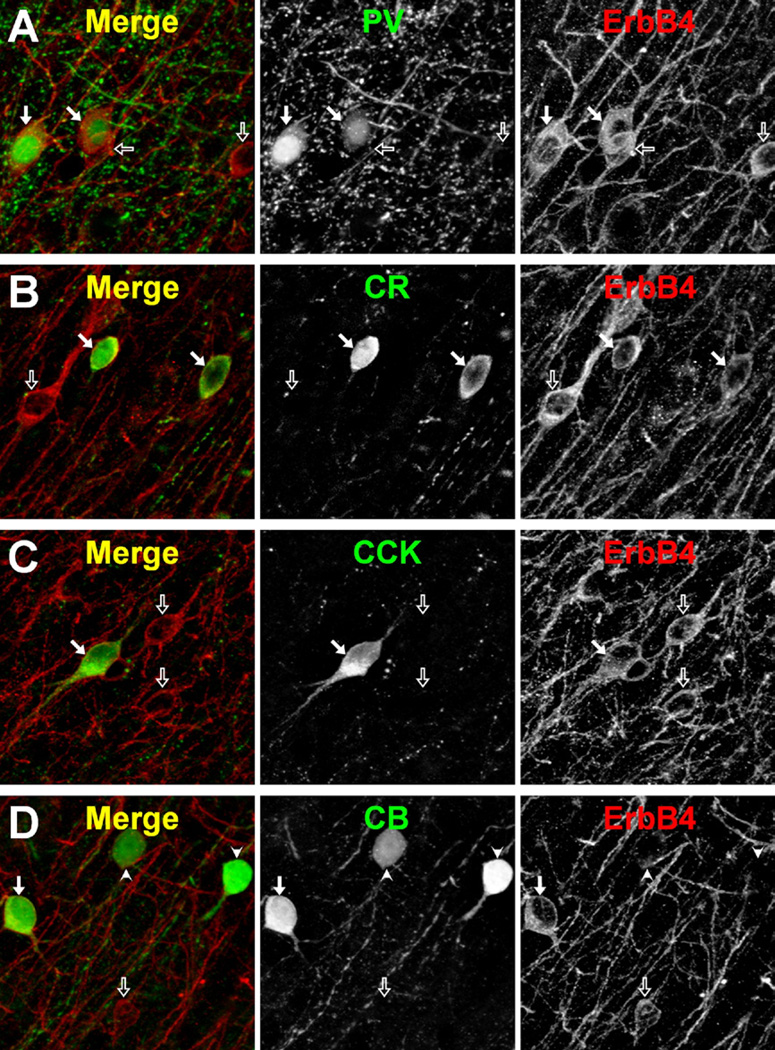
Figure 6.
ErbB4 co-localization with different interneuron markers in monkey prefrontal cortex. Cryostat sections double-labeled for ErbB4 and either (A) parvalbumin (PV), (B) calretinin (CR), (C) cholecystokinin (CCK), or (D) calbindin D-28K (CB); n = 3. Single channel (black and white panels) and merged projection images (five Z-planes taken .5 µm apart) of deconvolved image stacks are shown. Arrows point to double-labeled cells and open arrows point to ErbB4-only labeled cells: in panel (D) arrowheads point to CB-only labeled cells. Scale bar = 10 µm.
All animals were raised under a 12-hour light/12-hour dark cycle with food and water provided ad libitum. Human brain tissues from two male adult normal control individuals were from the Stanley Medical Research Institute (Chevy Chase, Maryland) and were used in Figures 3 and 4 and Figure S2 in Supplement 1. Human RNA samples were purchased from Ambion (Austin, Texas) and Stratagene (La Jolla, California) (Figures S4 and S5 in Supplement 1). All procedures were approved and followed the appropriate National Institutes of Health Guidelines for the Care and Use of Laboratory Animals or Use of Human Tissue.
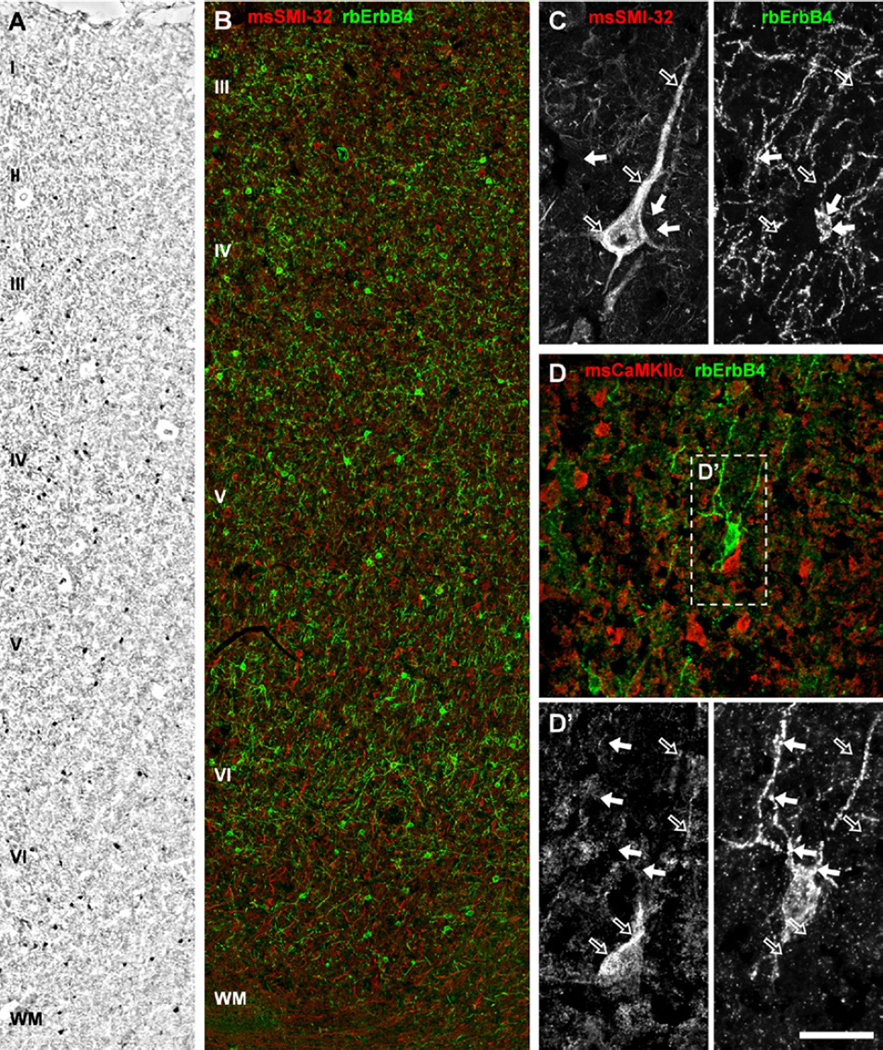
Figure 4.
ErbB4 expression is restricted to nonpyramidal cells of the human frontal cortex. (A) Immunohistology and (B) double immunofluorescence show ErbB4-positive multipolar cells detected by rabbit mAb-10 (rbErbB4) in all layers except layer I and also in a few interstitial cells in white matter (WM) (n = 2). Note that only layers III to WM are shown in (B) because of the deterioration of immersion-fixed human tissue. (B–D) ErbB4 (arrows) is not detected in pyramidal cells (open arrows) labeled with mouse monoclonal antibodies against either neurofilament-H (msSMI-32) (B, C) or calcium/calmodulin-dependent protein kinase II α (msCaMKII) (D, D’). Note that dendritic clusters of ErbB4 immunoreactivity (right hand panel, green channel in D) are evident on human neurons (arrows in D′), similar to those seen in rodents (cf. Figure 2) and rhesus monkey (cf. Figure 4). Scale bar = 200 µm (A), 140 µm (B), 75 µm (D) or 50 µm (C), 32 µm (D′). Other abbreviations as in Figure 3.
Results
Interneuron-Specific Expression of ErbB4 mRNA in Frontal Cortex of Mice
To analyze the cellular expression of ErbB4 transcripts in the frontal cortex of mice, we tested individual neurons by patch-clamp electrophysiology and single-cell RT-PCR, with a focus on PV-positive interneurons. Parvalbumin interneurons were identified by their nonpyramidal morphology and by high-frequency spike discharges (> 100 Hz), little accommodation, short action potentials, and large after hyperpolarizations (29,30). These properties are presented as phase plots of membrane potential (31). Interneuron identity was confirmed by single-cell RT-PCR for PV, glutamic acid decarboxylase 65 (GAD65), and glutamic acid decarboxylase 67 (GAD67) and the absence of the vesicular glutamate transporter 1 (VGluT1) (Figure 1A). By contrast, frontal cortical pyramidal neurons displayed accommodating low-frequency spike discharges, small after hyperpolarizations, and longer action potentials and tested positive for VGluT1 but very rarely for transcripts for GAD65, GAD67, and PV (Figure 1B). ErbB4 mRNA was detected in all PV fast-spiking interneurons (n = 10 of 10) but not in pyramidal cells (n = 0 of 10; Table S1 in Supplement 1). We additionally included epidermal growth factor receptor (EGFR or ErbB1) and ErbB3 to obtain a better idea of overall ErbB-family expression but found transcripts for EGFR to be entirely absent and for ErbB3 to be mostly absent (Table S1 in Supplement 1). These findings are consistent with ErbB3 expression being largely restricted to glial cells (32) and with the downregulation of hippocampal EGFR expression after birth (33).
Interneuron-Specific Expression of ErbB4 Protein in Rodent Frontal Cortex
A low-magnification overview shows that most (> 90%) PV-immunoreactive interneurons co-express ErbB4 in the mouse frontal cortex (Figure 2A, A′). The highest densities of ErbB4- immunoreactive cells are found throughout layers II to VI, and some are also located in white matter. In contrast, using calcium/calmodulin-dependent protein kinase II alpha (CaMKIIα) as a marker for principal neurons, we found no evidence for ErbB4 expression in pyramidal cells (Figure 2B). Double immunofluorescence with GAD67 shows that many interneurons express ErbB4 in the somatodendritic compartment (Figure 2C), whereas signals on GABAergic terminals were indistinguishable from background (Figure 2D, D′). Similar expression patterns were found in the cerebral cortex of rats (not shown). Using high-magnification Z-stacks, we found high-level expression of ErbB4 in puncta on somata and dendrites of both mice (Figure 2E) and rats (Figure 2F). This pattern is largely consistent with the targeting of ErbB4 to glutamatergic postsynaptic densities in interneurons (11,34,35). The very large immunoreactive clusters in Figure 2F appeared in a subset of ErbB4-positive cortical and hippocampal interneurons of the rat but were rarely seen in the mouse, suggesting subtle species differences in subcellular targeting of ErbB4.
Specificity of ErbB4 Protein Detection in Monkey and Human Frontal Cortex
The monoclonal antibody (mAb)-10 detects a single band of ~180 kDa in Western blots of mouse hippocampus (11), consistent with the size of full-length ErbB4. To validate this antibody for use in primates, we first analyzed tissue extracts from adult rhesus monkey and human cortex using Western blot analysis. mAb-10 strongly and selectively detected ErbB4 in all samples (Figure 3A), with similar signal intensities between different cortical areas of the same species. No other bands were visible, except for a very weak signal in all human samples at ~75 to 80 kDa apparent molecular mass, indicative of the presence of low levels of the cleaved intracellular domain of ErbB4 (36).
Next, we tested whether mAb-10 specifically detects ErbB4 on sections from monkey frontal cortex. Immunohistochemistry revealed expression in small multipolar cells scattered throughout the cortex, surrounded by a dense network of neurites (Figure 3B). Omission of primary antibody resulted in complete absence of labeling (Figure 3C). High-magnification microscopy shows a pattern of immunoreactive puncta in the somatodendritic compartment (Figure 3D), similar to our findings in rodents (Figure 2E,F). To further validate labeling specificity, we used rabbit serum HL5941, which is directed against the intracellular domain of ErbB4 (11), together with mouse mAb-77, which binds an epitope in the extracellular domain. Both antibodies consistently co-labeled multipolar cells throughout the cortex, without evidence for expression in pyramidal cells (Figure S1 in Supplement 1). This result is consistent with our findings in rodents but differs from previous studies that reported ErbB4 expression mostly in cortical pyramidal cells (24,25).
To resolve this apparent contradiction, we revisited the specificity of the monoclonal antibody HFR-1, which was used in Bernstein et al. (24), in Western blotting of mouse, rhesus monkey, and human frontal cortex. Monoclonal antibody HFR-1 detected the 180 kDa band in both primate species (Figure S2A in Supplement 1). However, on monkey and especially on human samples, HFR-1 reacted strongly with multiple additional bands in the ~35 to 50 kDa range. Using HFR-1 on sections of rhesus monkey and mouse, we found co-labeling with serum HL5941 on interneurons but also additional binding (Figure S2B,C in Supplement 1). These results suggest that the reported extensive labeling of pyramidal cells by HFR-1 in the human cortex (24) may be due to nonspecific binding. We did not perform any experiments with the ErbB4 antibody sc-283, used in Thompson et al. (25), because we demonstrated recently that sc-283 also exhibits major nonspecific labeling (11).
Interneuron-Specific Expression of ErbB4 in Human and Monkey Brain
Based on the specificity of mAb-10 in Western blotting of human brain lysates (Figure 3A), we analyzed the expression of ErbB4 by immunofluorescence on immersion-fixed sections of human dorsolateral prefrontal cortex. ErbB4-positive neurons were scattered throughout cortical layers II to VI. Immunoreactive cell bodies were small and multipolar, and most had a round to oval shape indicative of interneurons (Figure 4A). We next used double immunofluorescence of ErbB4 and markers of pyramidal cells, neurofilament H (Figure 4B,C), and CaMKIIα (Figure 4D) and consistently found non-overlapping expression patterns. For technical reasons, we were unable to do the converse experiment (double immunofluorescence of ErbB4 with inhibitory markers), because in our hands, mouse antibodies against different markers of interneurons and mouse mAb-77 did not work on immersion-fixed human tissue. Because mAb-77 was generated against human ErbB4 (37) and exclusively binds the native folded protein, the lack of immunoreactivity may be due to rapid deterioration of the extracellular epitope’s tertiary structure in postmortem immersion-fixed human tissue.
To positively identify ErbB4-expressing neurons in the primate brain, we next used perfusion-fixed rhesus monkey prefrontal cortex sections. ErbB4-positive cells were labeled with mAb-77 and identified as interneurons based on lack of neurogranin immunoreactivity (Figure 5A–A″) and by co-expression of GAD67 (Figure 5B–B″). This result was verified by double immunofluorescence with mAb-10 and a different antibody against GAD67 (Figure 5C–C″) or either neurofilament H or CaMKIIα (not shown). Our findings in monkey are thus consistent with the results from mouse, rat, and human frontal cortex, demonstrating that the interneuron-selective pattern of cortical ErbB4 expression is similar across rodent and primate species.
Of note, we found in preliminary experiments that heat-induced antigen retrieval increased the immunodetection of ErbB4 on dendrites by both mAb-10 and HFR-1 in human and monkey sections and to a lesser degree also in rodents. This result suggests that the intracellular domain of ErbB4 might be partly masked by protein-protein interactions both in rodents and primates, consistent with its conserved sequence.
ErbB4 Is Preferentially Expressed in Subtypes of Interneurons in Monkey Brain
To quantitatively define the cell types that express ErbB4 in area 46 of the monkey dorsolateral prefrontal cortex, we used double immunofluorescence with the neuronal marker NeuN. Cells in the superficial and middle layers (97.7% and 91.6%, respectively) co-expressed both proteins (Table 1), indicating that ErbB4 immunoreactivity is associated with neurons. Consistent with these observations and with our results obtained in mouse hippocampus (12), most ErbB4-positive neurons (79.0% and 80.6% in the superficial and middle layers, respectively) were also immunoreactive for GABA.
Table 1.
Mean (SD)a Percentage of Neurons Containing ErbB4 and the Indicated Cell Type Marker for Both Superficial and Middle Zones of Monkey Area 46
| Percentage of Cells with Listed Marker That Also Express ErbB4 |
Percentage of ErbB4 Cells That Also Express the Listed Marker |
|||
|---|---|---|---|---|
| Marker | Superficial | Middle | Superficial | Middle |
| NeuN | 31.4 (3.5) | 17.7 (3.4) | 97.7 (2.3) | 91.6 (3.8) |
| GABA | 80.3 (11.2) | 79.3 (19.0) | 79.0 (16.1) | 80.6 (24.9) |
| PV | 100 (.0) | 98.9 (1.9) | 12.3 (11.3) | 48.2 (12.7) |
| CR | 94.8 (2.7) | 88.9 (6.7) | 47.9 (5.0) | 26.4 (4.6) |
| CCK | 93.3 (5.8) | NA | 8.1 (3.4) | NA |
| CB | 29.7 (9.2) | 10.5 (4.0) | 9.4 (4.2) | 5.3 (1.4) |
CB, calbindin D-28K; CCK, cholecystokinin; CR, calretinin; GABA, gamma-aminobutyric acid; NA, not applicable; NeuN, neuronal nuclei; PV, parvalbumin.
As indicated by the small standard deviations, the proportions of dual-labeling were quite similar across all three macaque monkeys.
We next determined the degree of co-expression of ErbB4 and the calcium-binding proteins PV, calretinin (CR), calbindin D-28K (CB), or the neuropeptide cholecystokinin (CCK) (Figure 6, Table 1). In the superficial layers, virtually all PV (100%), CR (94.8%), and CCK (93.3%) immunoreactive neurons expressed ErbB4, compared with only 29.7% of CB-positive interneurons. Similarly, in the middle layers of area 46, nearly all PV (98.9%) and CR (88.9%) immunoreactive neurons, but only 10.5% of CB-labeled neurons, co-expressed ErbB4. The percentage of CCK cells expressing ErbB4 was not determined in the middle layers because the somata of CCK-expressing neurons are mainly localized to the superficial layers (38). Consistent with the preferential localization of CR cells in the superficial layers and PV cells in the middle layers of area 46 (39), approximately half of ErbB4-positive cells in the superficial layers expressed CR, whereas approximately half of ErbB4-positive cells in the middle layers expressed PV (Table 1).
No Evidence for Presynaptic ErbB4 Expression in the Frontal Cortex of Monkey and Rat
Recently, immunoreactivity of the postsynaptic marker ankyrin-G was shown to be reduced at chandelier cell inputs to the axon initial segment of pyramidal cells in superficial layers of prefrontal area 46 of persons diagnosed with schizophrenia (23). These findings prompted us to test whether ErbB4 is present on GABAergic terminals in the adult frontal cortex of rhesus monkeys and rats (Figure 7). The axon initial segment was identified by expression of ankyrin-G, and chandelier cell terminals were labeled by immunoreactivity for GABA transporter 1 or vesicular GABA transporter. In contrast, antibodies against GAD65 or GAD67 labeled mostly somatodendritic-targeting axons and terminals. Using several different immunofluorescence protocols and both mouse mAb-77 and rabbit mAb-10 to visualize ErbB4, we were unable to detect ErbB4 immunoreactivity on any type of GABAergic terminals. In recent studies performed with a polyclonal antiserum raised against a large intracellular antigen (40), presynaptic GABAergic terminals in mouse cortex were reported to be immunoreactive for ErbB4 (16,22). We therefore tested the specificity of this reagent (rabbit polyclonal serum 0618) on mouse brain lysates and tissue. Figure S3 in Supplement 1 shows a predominant reactivity with the 180 kDa full-length receptor but additional binding to numerous weaker bands that are persistent in ErbB4 knockouts. Our results using highly selective monoclonal antibodies against both extracellular and intracellular epitopes thus do not support the notion of presynaptic ErbB4 expression in the frontal cortex and are consistent instead with accumulation of the receptor in the somatodendritic region of interneurons in the cortex of rodents and primates.
Semiquantitative Analysis of ErbB4 Splice Variants in Monkey and Human Frontal Cortex
Finally, we analyzed the relative amounts of alternatively spliced ErbB4 transcripts harboring the cytoplasmic 1 (CYT1) exon that couples receptor activation to the phosphoinositide-3 kinase/Akt signaling pathway (41). Differences in CYT1-containing transcripts have previously been reported in studies comparing ErbB4 expression in subjects with schizophrenia and normal control subjects (5,6). Using semiquantitative RT-PCR of monkey and human cortical RNA samples, we observed no significant difference in the expression of pan-ErbB4 mRNA (frontal cortex vs. prefrontal cortex; 1.8 ± .31 vs. 1.6 ± .34 arbitrary units ± SD). However, cytoplasmic 2 (CYT2) isoform levels were twofold higher compared with CYT1 variants in human and monkey frontal cortex (Figure S4 in Supplement 1), suggesting that the CYT2 splice variant is the most common isoform and that the relative amounts are similar between primate species. These data are also in agreement with the relatively low levels of CYT1 isoforms in the mouse cerebral cortex (42).
Discussion
This study presents two major findings. First, ErbB4 expression is evident in a subset of GABAergic interneurons and undetectable in pyramidal neurons of the frontal cortex. This cell type-specific pattern is conserved from rodents to primates and likely also in humans and thus validates the use of mice with targeted mutations of NRG or ErbB4 to analyze the possible role of this signaling pathway in schizophrenia. Second, ErbB4 is undetectable by immunofluorescence in frontal cortical GABAergic terminals in rodents and primates, suggesting that the NRG1-ErbB4 pathway influences interneuron activity predominantly at somatodendritic sites, notably at glutamatergic postsynapses. These conclusions are based on experiments using molecular techniques, as well as biochemical and histological approaches with antibodies of demonstrated high specificity for ErbB4.
Interneuron-Specific ErbB4 mRNA Expression
Using single cell analysis of electrophysiologically characterized neurons, we have demonstrated that ErbB4 mRNA expression is restricted to interneurons in the frontal neocortex of mice. This result is consistent with findings in allocortical structures, the hippocampus (11) and the piriform cortex (data not shown), suggesting that this pattern may be representative for the entire cerebral cortex of mice. Of functional importance, at least four different variants of ErbB4 are generated by differential splicing of mRNA, including two different types of the intracellular domain of ErbB4, termed CYT1 and CYT2 (41). Only the CYT1-containing variant can directly activate phosphoinositide-3 kinase/Akt (42,43). Moreover, a PPXY motif within the CYT1 domain promotes the ubiquitination, endocytosis, and degradation of ErbB4 (44). Our semiquantitative analysis of ErbB4 splice variants shows that in the frontal cortex of rhesus monkey and healthy humans, the CYT2 variant is more common than CYT1, consistent with relative abundance of both isoforms in the mouse cerebral cortex (42). Recent studies provided evidence for elevated expression of ErbB4-CYT1 mRNA in postmortem brains from persons diagnosed with schizophrenia that harbor schizophrenia-associated single nucleotide polymorphisms and a core risk haplotype (5,6). However, it is currently not known if and to what extent CYT1-containing ErbB4 receptors account for the reported effects of NRG/ErbB4 signaling on GABAergic transmission, dopamine release, and glutamatergic synaptic plasticity, in particular given that ErbB4-CYT1 appears to be targeted for degradation (see above).
Interneuron-Specific ErbB4 Protein Expression
We report here that ErbB4 receptor protein is selectively expressed in a subpopulation of interneurons in the frontal cortex of mice, similar to expression in the hippocampus (11,12). Moreover, our results suggest that this pattern of interneuron-specific cortical expression of ErbB4 is essentially identical in rodents and monkeys and likely to be at least similar in humans. With respect to the validity of our data, it is important to point out that the pattern of ErbB4 immunoreactivity was indistinguishable between two monoclonal antibodies raised against the extracellular domain (37) and an intracellular epitope (11). The difference between our results and two prior reports on ErbB4 expression in pyramidal neurons in human (24) and monkey cerebral cortex (25) could be due to use of reagents with limited specificity in the prior studies, as shown here and in an earlier study (11).
The expression of ErbB4 in different GABAergic neurons may vary regionally in the brain. The percentage of ErbB4-positive PV cells is significantly higher in the frontal cortex (>98%) compared with the hippocampus (42%) (12). This result is similar to data from the frontal cortex of rats (15) and the somatosensory cortex of mice (16), suggesting that almost all PV-positive interneurons in the neocortex may be subject to modulation by NRG-ErbB4 signaling. These results have important implications because of the significance of PV-positive interneurons for proper functioning of the cortex and their assumed role in the pathophysiology of schizophrenia (45).
Subcellular Distribution of ErbB4
Recent studies have reported presynaptic ErbB4 expression in mice using electron microscopy (16) or immunofluorescence (22); of note, both studies used the same antiserum that detects ErbB4 but shows additional nonspecific binding. In contrast to these reports, even using antigen unmasking procedures combined with tyramide signal amplification, we could not detect ErbB4 on GABAergic terminals in the monkey and rat frontal cortex using four different markers for GABAergic axons and terminals (this study) and expression in just a low percentage of CCK-positive, but not PV-positive, terminals in the mouse (11). Although we can currently not exclude the possibility that an epitope is masked and therefore eludes detection with a monoclonal antibody, this seems to be an unlikely explanation to account for the lack of ErbB4 detection at presynapses because we utilized two different mAbs that target either the extracellular or intracellular domains of the receptor. In any case, the question of subcellular targeting of ErbB4 within neurites of interneurons is critically important when considering the modulatory role of NRG1 in neural network activity and requires further investigation.
Regulation of the Excitation of Inhibitory Neurons and Its Importance for Neuronal Network Activity
Based on our finding of absence of ErbB4 immunofluorescence in axon initial segment-targeting terminals in the frontal cortex of monkeys and rodents, we favor the hypothesis that acute activation of the NRG-ErbB4 pathway is likely to modulate GABAergic transmission onto target neurons predominantly via a postsynaptic mechanism at glutamatergic synapses on interneurons (12,18).
Several studies have shown the importance of glutamatergic drive at asymmetric synapses on GABAergic and specifically PV-positive interneurons for neuronal network activity and behavior, and ErbB4 receptors are well poised to regulate these functions (8–10,34,35). Selective ablation of the AMPA receptor GluA1 subunit at glutamatergic postsynaptic sites of PV-positive interneurons, as well as mutation of GluA4 that is selectively expressed by GABAergic neurons in the hippocampus, result in the reduction of kainate-induced gamma oscillation power (46); similar reductions in gamma power were observed in ErbB4 null mice (9). In addition, GluA1 and GluA4 mutant mice, the former restricted to PV-positive interneurons, exhibited behavioral impairments that suggested deficits in working and episodic memory (46). With respect to N-methyl-D-aspartate (NMDA) receptors, elimination of the obligatory GluN1 subunit selectively in PV neurons of mice resulted in impairments of gamma rhythms and specific cognitive behaviors (47). In another study that focused on the role of NMDA receptor transmission at glutamatergic synapses onto GABAergic neurons, ablation of GluN1 in approximately 50% of cortical and hippocampal GABAergic interneurons resulted in reduced neuronal synchrony, disinhibition of cortical excitatory output, and emergence of schizophrenia-like behaviors in mutant mice (48). Consistent with a modulatory role of ErbB4 receptors at these asymmetric synapses, surface expression of GluA1-containing AMPA receptors is regulated by NRG1 (10) and dopamine D4 receptors, which act as downstream mediators of NRG-ErbB4 signaling (8). Mice lacking ErbB4 receptors in PV-positive neurons exhibit behavioral deficits that are similar to other rodent models for schizophrenia (21) (Shamir et al., unpublished data, May 2011). Taken together, these studies suggest that changes in the activity of AMPA, NMDA, or ErbB4 receptors, all of which are co-localized at glutamatergic post-synapses on GABAergic fast-spiking interneurons, may account for many of the neuronal network and cognitive behavioral deficits associated with schizophrenia.
In conclusion, our data provide evidence for conserved expression patterns of ErbB4 across different rodent and primate species, both at the cellular and subcellular level. This result promotes the notion that, as of yet, no fundamental discrepancy inherent to ErbB4 expression has been proven between humans and other mammals. Therefore, we propose to revise the widely used working hypothesis of widespread ErbB4 expression in human pyramidal cells, as it fundamentally affects the experimental design and data interpretation and thus could misdirect future research efforts aimed at understanding the involvement of NRG-ErbB4 signaling during development and maturation of the brain, its functions in healthy humans, and its possible importance for the etiology underlying schizophrenia.
Acknowledgments
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (AB, CJM), and National Institutes of Health Grants MH085108 (KNF) and MH051234 (DAL). JN is currently affiliated with the Department of Histology, JSW Life Sciences GmbH, Graz-Grambach, Austria. LT is currently affiliated with the Laboratoire de Neurobiologie des processus adaptatifs, Université Pierre et Marie Curie, Paris, France.
We thank Dr. Richard Saunders and David Yu of the National Institute of Mental Health for kindly providing the brain samples of Macaca mulatta and the Stanley Foundation for providing human brain tissue. Confocal microscopy imaging was performed at the Microscopy and Imaging Core of National Institutes of Child Health and Human Development with the assistance of Dr. Vincent Schram and Chip Dye.
Dr. Neddens is an employee of JSW Life Sciences GmbH, a preclinical and clinical contract research organization. Dr. Lewis currently receives investigator-initiated research support from the Bristol-Myers Squibb Foundation, Bristol-Myers Squibb, Curridium Ltd, and Pfizer, and in 2007 to 2010 served as a consultant in the areas of target identification and validation and new compound development to AstraZeneca, BioLineRx, Bristol-Myers Squibb, Hoffman-Roche, Lilly, Merck, Neurogen, and SK Life Science.
Footnotes
All other authors reported no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: Understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32:485–495. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Strat Y, Ramoz N, Gorwood P. The role of genes involved in neuroplasticity and neurogenesis in the observation of a gene-environment interaction (GxE) in schizophrenia. Curr Mol Med. 2009;9:506–518. doi: 10.2174/156652409788167104. [DOI] [PubMed] [Google Scholar]
- 3.Li D, Collier DA, He L. Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet. 2006;15:1995–2002. doi: 10.1093/hmg/ddl122. [DOI] [PubMed] [Google Scholar]
- 4.Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 5.Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- 6.Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: Association and expression studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141:142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- 7.Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 8.Kwon OB, Paredes D, Gonzalez CM, Neddens J, Hernandez L, Vullhorst D, et al. Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proc Natl Acad Sci U S A. 2008;105:15587–15592. doi: 10.1073/pnas.0805722105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisahn A, Neddens J, Yan L, Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: Implications for schizophrenia. Cereb Cortex. 2009;19:612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25:9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vullhorst D, Neddens J, Karavanova I, Tricoire L, Petralia RS, McBain CJ, et al. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci. 2009;29:12255–12264. doi: 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neddens J, Buonanno A. Selective populations of hippocampal interneurons express ErbB4 and their number and distribution is altered in ErbB4 knockout mice. Hippocampus. 2010;20:724–744. doi: 10.1002/hipo.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, et al. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- 14.Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, et al. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Yau HJ, Wang HF, Lai C, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: Preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb Cortex. 2003;13:252–264. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]
- 16.Fazzari P, Paternain AV, Valiente M, Pla R, Luján R, Lloyd K, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- 17.Krivosheya D, Tapia L, Levinson JN, Huang K, Kang Y, Hines R, et al. ErbB4-neuregulin signaling modulates synapse development and dendritic arborization through distinct mechanisms. J Biol Chem. 2008;283:32944–32956. doi: 10.1074/jbc.M800073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buonanno A. The neuregulin signaling pathway and schizophrenia: From genes to synapses and neural circuits. Brain Res Bull. 2010;83:122–131. doi: 10.1016/j.brainresbull.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis DA, Hashimoto T. Deciphering the disease process of schizophrenia: The contribution of cortical GABA neurons. Int Rev Neurobiol. 2007;78:109–131. doi: 10.1016/S0074-7742(06)78004-7. [DOI] [PubMed] [Google Scholar]
- 20.Chen YJ, Zhang M, Yin DM, Wen L, Ting A, Wang P, et al. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc Natl Acad Sci U S A. 2010;107:21818–21823. doi: 10.1073/pnas.1010669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen L, Lu YS, Zhu XH, Li XM, Woo RS, Chen YJ, et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2010;107:1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Cruz DA, Weaver CL, Lovallo EM, Melchitzky DS, Lewis DA. Selective alterations in postsynaptic markers of chandelier cell inputs to cortical pyramidal neurons in subjects with schizophrenia. Neuropsychopharmacology. 2009;34:2112–2124. doi: 10.1038/npp.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernstein HG, Lendeckel U, Bertram I, Bukowska A, Kanakis D, Dobrowolny H, et al. Localization of neuregulin-1alpha (heregulin-alpha) and one of its receptors, ErbB-4 tyrosine kinase, in developing and adult human brain. Brain Res Bull. 2006;69:546–559. doi: 10.1016/j.brainresbull.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Thompson M, Lauderdale S, Webster MJ, Chong VZ, McClintock B, Saunders R, et al. Widespread expression of ErbB2, ErbB3 and ErbB4 in non-human primate brain. Brain Res. 2007;1139:95–109. doi: 10.1016/j.brainres.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 26.Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tidcombe H, Jackson-Fisher A, Mathers K, Stern DF, Gassmann M, Golding JP. Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc Natl Acad Sci U S A. 2003;100:8281–8286. doi: 10.1073/pnas.1436402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- 29.Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, et al. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci. 1997;17:3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- 31.Pinsker HM, Bell J. Phase plane description of endogenous neuronal oscillators in Aplysia. Biol Cybern. 1981;39:211–221. doi: 10.1007/BF00342773. [DOI] [PubMed] [Google Scholar]
- 32.Gerecke KM, Wyss JM, Karavanova I, Buonanno A, Carroll SL. ErbB transmembrane tyrosine kinase receptors are differentially expressed throughout the adult rat central nervous system. J Comp Neurol. 2001;433:86–100. doi: 10.1002/cne.1127. [DOI] [PubMed] [Google Scholar]
- 33.Fox IJ, Kornblum HI. Developmental profile of ErbB receptors in murine central nervous system: Implications for functional interactions. J Neurosci Res. 2005;79:584–597. doi: 10.1002/jnr.20381. [DOI] [PubMed] [Google Scholar]
- 34.Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci U S A. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, et al. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- 36.Elenius K, Corfas G, Paul S, Choi CJ, Rio C, Plowman GD, et al. A novel juxtamembrane domain isoform of HER4/ErbB4. Isoform-specific tissue distribution and differential processing in response to phorbol ester. J Biol Chem. 1997;272:26761–26768. doi: 10.1074/jbc.272.42.26761. [DOI] [PubMed] [Google Scholar]
- 37.Chen X, Levkowitz G, Tzahar E, Karunagaran D, Lavi S, Ben-Baruch N, et al. An immunological approach reveals biological differences between the two NDF/heregulin receptors, ErbB-3 and ErbB-4. J Biol Chem. 1996;271:7620–7629. [PubMed] [Google Scholar]
- 38.Oeth KM, Lewis DA. Postnatal development of the cholecystokinin innervation of monkey prefrontal cortex. J Comp Neurol. 1993;336:400–418. doi: 10.1002/cne.903360307. [DOI] [PubMed] [Google Scholar]
- 39.Condé F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: Distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- 40.Zhu X, Lai C, Thomas S, Burden SJ. Neuregulin receptors, erbB3 and erbB4, are localized at neuromuscular synapses. EMBO J. 1995;14:5842–5848. doi: 10.1002/j.1460-2075.1995.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carpenter G. ErbB-4: Mechanism of action and biology. Exp Cell Res. 2003;284:66–77. doi: 10.1016/s0014-4827(02)00100-3. [DOI] [PubMed] [Google Scholar]
- 42.Elenius K, Choi CJ, Paul S, Santiestevan E, Nishi E, Klagsbrun M. Characterization of a naturally occurring ErbB4 isoform that does not bind or activate phosphatidyl inositol 3-kinase. Oncogene. 1999;18:2607–2615. doi: 10.1038/sj.onc.1202612. [DOI] [PubMed] [Google Scholar]
- 43.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carraway KL., 3rd E3 ubiquitin ligases in ErbB receptor quantity control. Semin Cell Dev Biol. 2010;21:936–943. doi: 10.1016/j.semcdb.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 47.Carlén M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.31. [published online ahead of print April 5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]