The human IPS has been subdivided according to multiple different organizational schemes. Here, we determined the localization of two visual short-term memory (VSTM) intraparietal sulcus (IPS) regions with respect to IPS topographic regions and the role of different IPS regions in location- and feature-based processing. We show that understanding the multiplex nature of IPS in visual cognition may only be achieved by examining how regions identified by different tasks and methods may colocalize with each other.
Keywords: fMRI, IPS, vision
Abstract
Based on different cognitive tasks and mapping methods, the human intraparietal sulcus (IPS) has been subdivided according to multiple different organizational schemes. The presence of topographically organized regions throughout IPS indicates a strong location-based processing in this brain region. However, visual short-term memory (VSTM) studies have shown that while a region in the inferior IPS region (inferior IPS) is involved in object individuation and selection based on location, a region in the superior IPS (superior IPS) primarily encodes and stores object featural information. Here, we determined the localization of these two VSTM IPS regions with respect to the topographic IPS regions in individual participants and the role of different IPS regions in location- and feature-based processing. Anatomically, inferior IPS showed an 85.2% overlap with topographic IPS regions, with the greatest overlap seen in V3A and V3B, and superior IPS showed a 73.6% overall overlap, with the greatest overlap seen in IPS0-2. Functionally, there appeared to be a partial overlap between IPS regions involved in location- and feature-based processing, with more inferior and medial regions showing a stronger location-based processing and more superior and lateral regions showing a stronger feature-based processing. Together, these results suggest that understanding the multiplex nature of IPS in visual cognition may not be reduced to examining the functions of the different IPS topographic regions, but rather, it can only be accomplished by understanding how regions identified by different tasks and methods may colocalize with each other.
NEW & NOTEWORTHY
The human IPS has been subdivided according to multiple different organizational schemes. Here, we determined the localization of two visual short-term memory (VSTM) intraparietal sulcus (IPS) regions with respect to IPS topographic regions and the role of different IPS regions in location- and feature-based processing. We show that understanding the multiplex nature of IPS in visual cognition may only be achieved by examining how regions identified by different tasks and methods may colocalize with each other.
historically, the parietal cortex has been thought to be involved in location-based processing (Mishkin et al. 1983). Consistent with this idea, multiple distinct topographical regions along the intraparietal sulcus (IPS) have been identified (Schluppeck et al. 2005; Silver et al. 2005; Swisher et al. 2007; Konen and Kastner 2008a; Silver and Kastner 2009; see also Sereno et al. 2001).
Meanwhile the parietal cortex, IPS in particular, has been shown to be involved in a variety of cognitive processes and has been parcellated into numerous subdivisions based on these processes, such as attention, motor processing, shape representation, three-dimensional shape processing, visual short-term memory (VSTM) encoding and storage, episodic memory encoding and retrieval, number representation, and decision making (e.g., Corbetta and Shulman 2002; Culham and Valyear 2006; Dehaene et al. 2003; Georgieva et al. 2009; Gottlieb and Balan 2010; Harvey et al. 2013; Konen and Kastner 2008b; Wagner et al. 2005; Xu and Chun 2009). For example, research examining the neural mechanisms supporting the encoding and storage of multiple objects in VSTM has identified two separate regions within IPS. One of these regions is located in the inferior IPS region (hence forward referred to as inferior IPS for simplicity) and is involved in selecting and individuating objects through their spatial location. The other region is located in the superior IPS region (hence forward referred to as superior IPS for simplicity) whose response tracks VSTM capacity and participates in the detailed encoding and storage of the features of the selected objects (Xu and Chun 2006, 2007, 2009; Xu 2008, 2009; see also Todd and Marois 2004, 2005; Bettencourt and Xu 2016).
Some of the parietal regions identified by the different cognitive tasks are likely related and reflect the same underlying processing, such as the encoding and storage of visual information in superior IPS. For example, in motor tasks participants were often required to make a motor movement to a location stored in VSTM (e.g., Vesia et al. 2010). Similarly, parietal activation corresponding to the representation of three-dimensional shapes may be driven in part by the fact that the three-dimensional shapes used were notably more complex and contain more shape information than the two-dimensional control shapes (Georgieva et al. 2009).
One may argue that perhaps the IPS regions defined by spatial topographic mapping are the most fundamental way of characterizing IPS functions because they are obtained in independent mapping studies that are reproducible across laboratories, and that other ways of delineating IPS functions are based on somewhat arbitrary cognitive tasks and may be considered obsolete in light of these topographic maps. However, this view is rather limited in a number of ways.
First, although location processing is an important aspect of vision, object perception and VSTM are also fundamental and important components of visual cognition, with VSTM being an integral part of perception (see a discussion in Xu 2002). Thus the tasks that have been used to identify inferior and superior IPS in their differential roles in object selection, encoding, and storage should not be considered as arbitrary cognitive tasks, but rather, they tap into some fundamental aspects of visual cognition that may not be captured by location processing. The same could be said for all the other cognitive tasks that have been associated with the parietal cortex. Before we understand how parietal regions activated by different cognitive tasks are related to each other, it is somewhat premature and prejudiced to argue that IPS topographic maps are the most principled way of understanding parietal function.
Second, IPS topographic maps are defined by functional tasks by drawing observers′ attention to specific locations of the visual display in a systematic manner. This is just one instance of how regions in IPS may be defined functionally, no different from how other functional regions have been defined there, apart from the usage of different tasks and procedures.
Third, in a macaque monkey functional (f)MRI study, it was found that IPS topographic maps colocalized quite poorly with the IPS regions defined by Lewis and Van Essen (2000a,b) according to architectonic subdivisions, such that the lateral intraparietal area (LIP) only partially overlapped with one of the topographic maps (Arcaro et al. 2011). In other words, IPS maps do not correspond to architectonically defined macaque IPS regions. In contrast, topographic areas defined in early visual areas such as V1, V2, V3, and V3a showed a high degree of agreement with architectonically defined areas. To the extent that architectonic defined areas signal structural changes between brain regions that usually correspond to a change in function, without fully understanding the implications of the disagreement between IPS topographic maps and architectonically defined regions in Macaques, using these maps as principled landmarks to assess other functional subdivisions within the human IPS is not fully justified at this point and can be potentially misleading.
Fourth, and most significantly, although the discovery of the IPS topographic regions has generated the excitement that perhaps we could finally characterize parietal function through the existence of these distinctive regions, similar to how ventral visual cortex has been characterized, in reality, however, we are far from achieving this goal. Unlike the topographic regions in occipital cortex, whose distinctive roles in visual processing have been well-documented, functional distinctions among the IPS topographic regions remain largely unknown (e.g., Konen and Kastner 2008b). This could partially be due to the difficulty in localizing these regions, especially in higher IPS regions. Although this could reflect the need for further methodological improvement in localizing these regions, given the overall strength of the signal, this could also suggest that IPS topographic regions may not be the ultimate way of characterizing all IPS functions.
Consistent with this latter view, in a recent fMRI multivoxel pattern decoding study (Bettencourt and Xu 2016), we found that VSTM decoding from none of the IPS topographic regions consistently correlated with behavioral VSTM performance. In contrast, superior IPS, as defined by its correlation with behavioral VSTM capacity measures from an independent localizer, showed successful VSTM decoding that was correlated with behavioral VSTM performance. Thus the IPS topographic regions were not sufficient in capturing the VSTM function associated with parietal cortex. If we had only examined decoding responses from these topographic regions, we would have falsely concluded that none of the IPS regions contributed significantly to VSTM information storage. In a separate line of research, it has also been shown that the IPS topographic maps exhibited partial and inconsistent overlap with number and size defined parietal regions across participants (Harvey et al. 2013, 2015). Again, IPS topographic regions by themselves were insufficient in capturing IPS's role in number and size processing.
Thus there seems to exist partially overlapping functional regions involved in different aspect of visual cognition within IPS. Besides spatial representation, presently there is little experimental evidence showing that the boundaries delineated by the IPS topographic maps correspond to other distinctive cognitive functions. This suggests that IPS topographic organization may not serve as a fundamental principle to study other cognitive functions associated with IPS. Nevertheless, it would be important to understand how regions defined by different tasks and techniques colocalize with each other. Only by doing so will we form a complete understanding of the multiplex nature of IPS and its role in visual cognition.
In this study, we investigated the degree to which inferior and superior IPS colocalize with the IPS topographic regions. This would inform us about the extent to which visual information is represented in a topographic manner in inferior and superior IPS during object selection and encoding. This, in turn, would enable us to clarify possible functional dissociations among the different IPS topographical regions in visual cognition.
The strong and weak location-based representations in inferior and superior IPS, respectively (Xu and Chun 2006), along with the report that VSTM evoked parietal activity overlaps only with IPS0-2 (Sheremata et al 2010), suggest that inferior IPS would overlap extensively with the IPS topographic regions but that superior IPS may, at best, show only a partial overlap. Swisher et al. (2007) attempted to directly colocalize these brain regions at the group level across studies using Talairach space. However, while inferior IPS appeared to be most highly localized to IPS0, due to strong individual variations in the exact location of the IPS topographic regions, superior IPS was located within one standard deviation away from several topographic regions, making it difficult to assess its overlap with any IPS topographic region.
Here we examined the anatomical and functional relationship between inferior and superior IPS regions and the IPS topographic regions within the same set of participants. We found that both inferior IPS and superior IPS showed substantial overlap with the IPS topographic regions (inferior IPS: 85%; superior IPS: 74%). Inferior IPS overlapped primarily with V3A/B and superior IPS with IPS0-2. Additionally, in a VSTM task, we found that instead of a strict location vs. feature processing distinction within IPS, there appears to be a continuum of processing that varies across regions, with more inferior and medial regions showing stronger location based processing, while superior and lateral regions are more involved in the encoding and storage of features.
MATERIALS AND METHODS
Participants
Twelve paid participants (7 female) completed the anatomical overlap experiment. Ten of those 12 participants (6 female) also completed the VSTM experiment. All participants were recruited from the Harvard University community and gave written informed consent for participation in accordance with and the approval of the Institutional Review Board of Harvard University. Participants were between 21 and 34 yr old (mean age = 28.1). All had normal or corrected to normal visual acuity and all were right-handed.
Visual Stimuli and Experimental Paradigm
Stimuli were generated by Macintosh MacBook Pro using either VisionEgg (Straw 2008) or Matlab with Psychtoolbox extensions (Brainard 1997; Pelli 1997) and presented by liquid crystal display projected onto a screen mounted inside the scanner bore.
Localizer paradigms.
Participants completed three separate tasks to localize the topographic IPS regions, inferior IPS, and superior IPS.
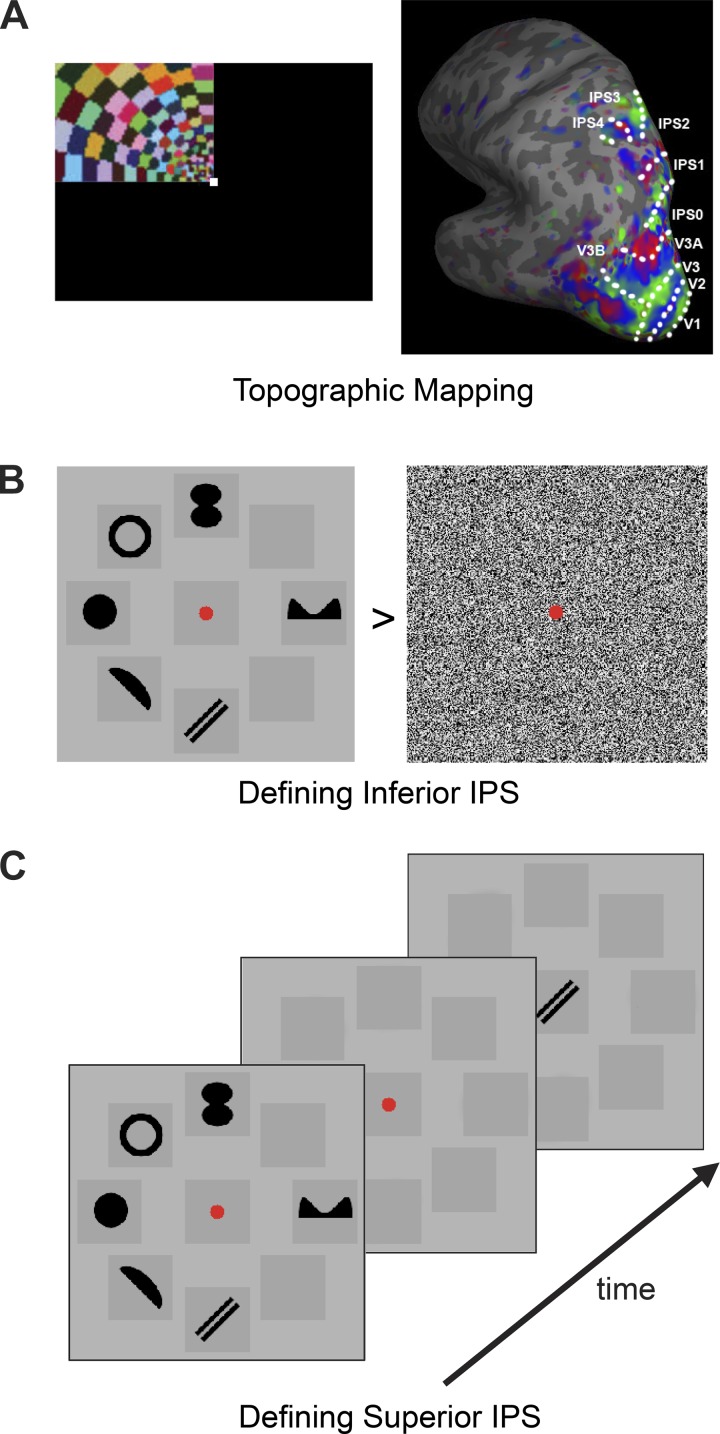
Topographic visual field representations of polar angle were mapped for each participant with flashing checker-board stimuli using standard techniques (DeYoe et al. 1996; Engel et al. 1994; Sereno et al. 1995; Swisher et al. 2007) with parameters optimized to reveal regions in the parietal cortex (Swisher et al. 2007). The polar angle wedge swept across the entire screen (23.4 × 17.5° of visual angle) and had an arc of 72°, flashed at 4 Hz, had a sweep period of 55.467s, and swept out 12 cycles per run (see Fig. 1A). For a more detailed description of the topographic mapping methods, see Swisher et al. (2007). The task varied slightly across participants. All participants were asked to detect a dimming in the visual display. For some participants the dimming occurred only at fixation, for others it occurred only within the polar angle wedge, and for some it could occur at both locations, commiserate with the various methodologies used in the literature (Swisher et al. 2007; Bressler and Silver 2010). No differences were seen in the regions obtained through each of these methods. Participants completed between four to six runs each, with each run lasting 11 min and 5.6 s. The total topographic mapping scanning time thus ranged from 44 to 66 min, similar to what others have used [e.g., Schluppeck et al. (2006) administered 8 to 16 runs with each lasting 4 min and 30 s, resulting in a total scanning time of 36 to 72 min]. The topographic mapping allowed for identification of cortical visual areas including V1, V2, V3, V3A, V3B, V4, as well as IPS0, IPS1, IPS2, IPS3, and IPS4. Topographic areas beyond IPS4 were not consistently seen in all participants and so no analysis was done on higher IPS regions.
Fig. 1.
Defining topographic intraparietal sulcus (IPS) regions (A), inferior IPS (B), and superior IPS (C). To define topographic IPS regions, rotating polar angel wedge stimuli were used. The resulting representative IPS topographic regions obtained were shown. To define inferior IPS, observers viewed blocks of shape and noise stimuli and performed a jitter detection task on the stimuli. Inferior IPS was defined as a region in the inferior part of IPS showing strong responses to the shape than to the noise stimuli. To define superior IPS, observers viewed either 1, 2, 3, 4, or 6 black target shapes presented around fixation and, after a brief delay, judged whether the probe shown at fixation matched one of the target shapes. Superior IPS was defined as a region in the superior part of IPS that tracked observers' behavioral performance across the different set sizes.
Compared with the covert attention task used by Silver et al. (2005) and the delayed saccade task (i.e., a working memory task) used by Schluppeck et al. (2005, 2006), the mapping method that we used here produced similar if not more prominent maps than the other two methods (see Fig. 7 of Swisher et al. 2007 in which the maps produced by the method used here and by the saccade working memory task were compared side-by-side within the same participant). If anything, the method we used here was able to localize IPS3 and IPS4, two higher topographic regions that were not reported in Silver et al. (2005) and Schluppeck et al. (2005 and 2006). Thus the mapping method that we used here has been properly validated and, if anything, tends to produce stronger maps than those obtained with a saccade or a working memory task.
Inferior and superior IPS regions were localized following the procedures used by Xu and Chun (2006). To localize inferior IPS, participants viewed blocks of either noise images or images containing six black shapes (see Fig. 1B). A total of seven distinctive shapes were used. All displays subtended 13.78 × 13.78° of visual angle and were presented on a light gray background. A given shape subtended maximally 3.18 × 3.18° of visual angle. Each image was presented for 500 ms followed by a 300-ms blank interval. To ensure equal attention across noise and shape images, participants detected a slight spatial jitter, occurring randomly in one out of every 10 images. Each participant completed two runs, each lasting 4 min and 40 s and containing 160 shape images and 160 noise images.
To localize superior IPS, participants completed a VSTM shape experiment, similar to the simple shape experiments in Xu and Chun (2006). In each trial, participants saw one, two, three, four, or six black shapes presented briefly around a central fixation, and after a short delay, judged whether a probe shape presented at fixation was present in the original display (see Fig. 1C). The shapes and locations used here were identical to those used in the inferior IPS localizer. Each trial lasted 6 s and consisted of fixation (1,000 ms), sample display (200 ms), blank delay (1,000 ms), test display/response period (2,500 ms), and response feedback (1,300 ms) at fixation. Each run also contained blank fixation trials in which only a fixation dot was present throughout the 6-s trial duration. Trial presentation order was pseudorandom and balanced in a run (Xu and Chun 2006; Todd and Marois 2004). Each participant completed two runs, with each run lasting 7 min and 42 s and containing 76 trials. They included 12 trials per set size, 2 practice trials at the beginning, and 1 filler trial each at the beginning and end of the trial sequence for counterbalancing purposes. The practice and filler trials were removed from further analysis. Behavioral VSTM capacity for each set size was calculated using Cowan's K (Cowan 2001).
Main VSTM experimental paradigm.
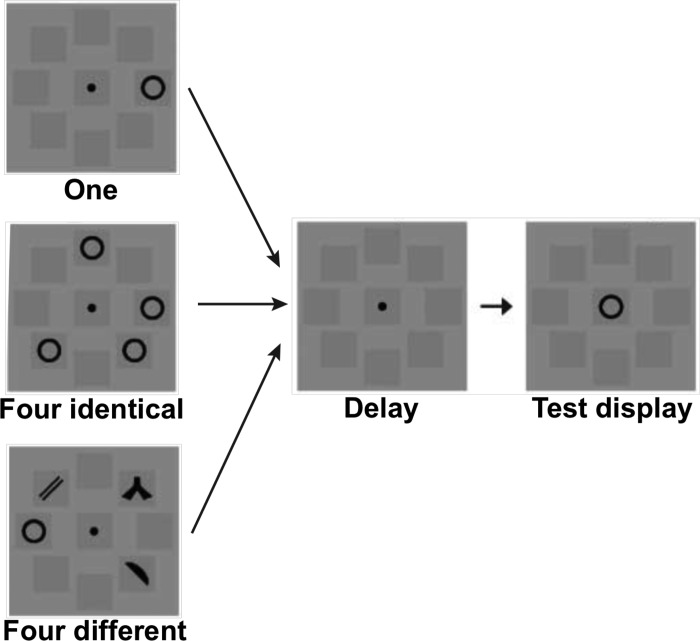
Previously, Xu (2009) has shown strong location- and feature-based encoding differences in inferior and superior IPS using a VSTM design in which participants had to remember either one object, four identical objects, or four different objects. This experimental design allows for the examination of the effects of increasing the amount of location information without a concurrent increase in featural information (by comparing one vs. four identical objects), as well as the converse, in which the amount of featural information increases but the amount of location information remains constant (by comparing four identical vs four different objects). To look for similar effects across topographic regions of IPS, we replicated the design of that experiment here. Participants were shown a display with either one, four identical, or four different black objects presented around a central fixation, and were asked to remember their shapes only (see Fig. 2). After a delay, a single object was presented at fixation and participants responded as to whether or not the object was present in the original display. During the sample phase, each item was presented in one of eight locations defined by dark gray squares (which were always present) to aid individuation and decrease any effects of grouping. As in the superior IPS localizer, seven distinct shapes were used and all displays subtended 13.78 × 13.78° of visual angle, with any given object subtending maximally 3.18 × 3.18° of visual angle. Each trial lasted for 6-s and consisted of fixation (1,000 ms), sample display (200 ms), blank delay (1,000 ms), test display/response period (2,500 ms), and response feedback, presented at fixation (1,300 ms). Blank fixation trials were also included in which only a fixation dot was present throughout the 6-s trial duration. Trial order was pseudorandom and counterbalanced within a run. Participants completed two runs, with each run lasting 6 min and 54 s and containing 68 trials. They included 16 experimental trials for each of the 4 conditions, 2 practice trials at the beginning, and 1 filler trial each at the beginning and end of the trial sequence for counterbalancing purposes. The practice and filler trials were removed from further analysis. Behavioral VSTM capacity for each set size was calculated using Cowan's K (Cowan 2001).
Fig. 2.
Stimuli and task used in the main visual short-term memory (VSTM) experimental paradigm. Observers viewed either 1, 4 identical, or 4 different black target shapes shown around the fixation and, after a brief delay, judged whether the probe shape shown at fixation matched one of the target shapes. The 1 and 4 identical conditions were matched in the amount of feature information shown but different in the number of spatial locations shown, whereas the 4 identical and 4 different conditions were matched in the number of spatial locations shown but different in the amount of feature information shown.
fMRI Methods
The data were acquired on a Siemens Tim Trio 3T scanner with a 32-channel head coil at the Center for Brain Science at Harvard University (Cambridge, MA). Participants took part in one to three scanning sessions. In one session a high-resolution (1.0 × 1.0 × 1.3 mm) anatomical image was collected for surface reconstruction. In a subset of the participants, the functional tasks were completed in this same session. Others had previously completed this anatomical scan and were brought back for one or two functional imaging sessions, depending on the available time, to collect localizer and VSTM data. Before functional images were collected in each session, T1-weighted gradient-echo images were collected in the same slice prescription as the functional scans to allow each session to be registered to the participant's high-resolution anatomical scan. Functional data were acquired using T2*-weighted gradient-echo, echo-planar sequences. Topographic data were collected using 42, 3-mm thick (3.125 × 3.125 mm in plane, no skip) slices oriented just off parallel from the AC-PC line to cover the full brain (TR = 2.6 s, TE = 30 ms, flip angle = 90°). These parameters were chosen to closely match those used by Swisher et al. (2007). Data for the inferior and superior IPS localizers as well as the main VSTM experiment were collected using 24, 5-mm thick slices (3.75 × 3.75 mm in plane, no skip) parallel to the AC-PC line (TR = 2s, TE = 30 ms, flip angle = 85° for inferior IPS and TR = 1.5 s, TE = 29 ms, flip angle = 90° for superior IPS and the main VSTM experiment).
Data Analysis
fMRI data were analyzed using the Freesurfer software package (Dale et al. 1999; Fischl et al. 1999, 2001). Data preprocessing included motion correction and intensity normalization. Computer representations of each cortical hemispheric surface were unfolded and inflated.
Topographic visual regions were defined by looking for phase reversals using standard techniques (Engel et al. 1997; Sereno et al. 1995; DeYoe et al. 1996; Swisher et al. 2007). We followed the procedure developed by Swisher et al. (2007) to obtain our IPS topographic regions. An example of the mapping result is reported in Fig. 1A.
Following Xu and Chun (2006), inferior IPS was defined as a region near the intersection of IPS and the transverse occipital sulcus that showed higher activation for multiple objects compared with noise (P < 0.01). Following Todd and Marois (2004) and Xu and Chun (2006), superior IPS was analyzed using a multiple regression analysis performed on the data from the VSTM task. The regression coefficient for each set size was weighted by each participant's corresponding behavioral K score estimate for that set size. Superior IPS was defined as a region that showed significant activation in the regression analysis (P < 0.05) overlapping or near the region previously reported in Talairach coordinates.
The amount of overlap between regions was calculated as the percentage of inferior or superior IPS that overlapped with each topographic region. In other words, it was the area (in square mm) of overlap between inferior or superior IPS and each topographic region divided by the total area of inferior or superior IPS, and then multiplied by 100. The percentage of overlap with each topographic region was then averaged across participants to obtain the average percentage of overlap between inferior or superior IPS and each topographic region. To obtain the total percentage of overlap between inferior or superior IPS and all of topographic IPS, the average percentage of overlaps was summed over all the IPS topographic regions.
The data from the main VSTM experiment were analyzed in a method similar to that of Xu (2009). Specifically, time course for each participant in each stimulus condition was extracted for each of the regions of interest (ROIs) and then converted into percent signal change relative to fixation. Peak responses for each ROI were derived by collapsing the time courses for all the conditions and determining the time point with the greatest signal amplitude in the averaged response for each participant. The resulting peak responses were then averaged across participants.
RESULTS
Anatomical Overlap
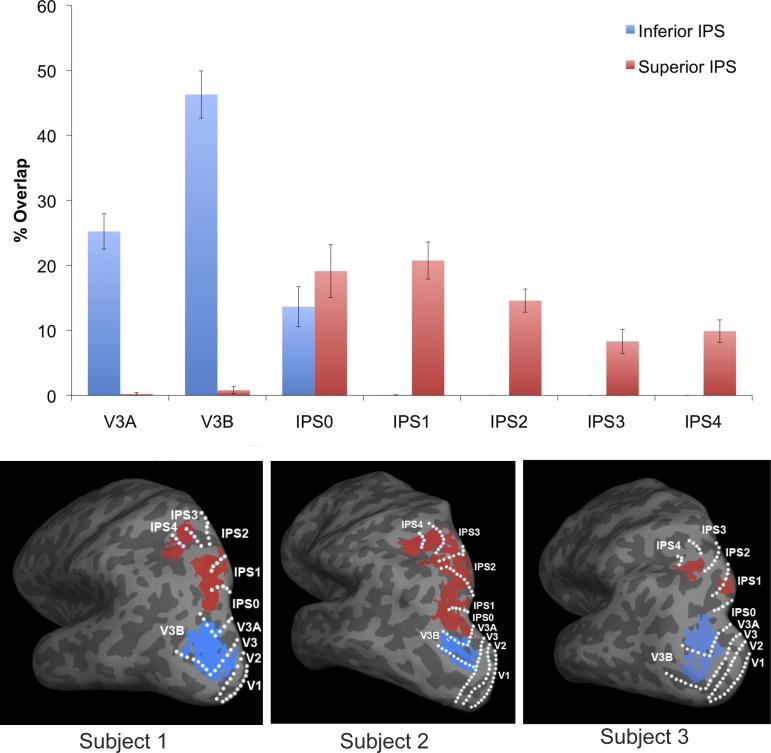
We examined the anatomical overlap between independently localized inferior IPS, superior IPS, and the IPS topographic regions in each participant. Overall, there was considerable overlap of inferior IPS and superior IPS with the topographic regions of IPS (inferior IPS: 85.2%, superior IPS: 73.6%). Although both showed a fairly high degree of overlap with topographic regions, superior IPS did show significantly less overlap that inferior IPS (all t values > 2.6, all P < 0.05). Additionally, inferior IPS showed more consistency in the degree of overlap across individuals (ranging from 71 to 92%) than superior IPS (46 to 97%). The same overall pattern of results was seen even when the two participants who did not participate in the functional task were removed.
Overall, there was a main effect of hemisphere [F(1,11) = 5.5, P < 0.05], with the right hemisphere showing a slightly higher degree of overlap than the left (83.2 vs. 75.6%, respectively). There was also a significant interaction among hemisphere, topographic ROI, and inferior and superior IPS regions [F(6,11) = 57.9, P < 0.001]. Post hoc t-tests showed that the hemispheric difference was mainly due to superior IPS overlapping to a higher degree with topographic IPS2 in the right hemisphere than in the left [t(11) = 3.4, P < 0.01].
There was a main effect of topographic ROI [F(6,11) = 18.0, P < 0.001], such that the amount of overlap with inferior IPS and superior IPS, as a whole differed, in different IPS topographic regions, as well as a main effect of inferior/superior IPS ROIs [F(1,11) = 6.8, P < 0.05]. Importantly, there was an interaction between topographic ROI and inferior/superior IPS ROIs [F(6,11) = 57.9.9, P < 0.001], indicating that the overlap with the IPS topographic regions differed between inferior IPS and superior IPS, which is expected. Post hoc t-tests showed that inferior IPS significantly overlapped with V3A, V3B, and IPS0 (all t values > 4.8, P values < 0.001). The percentage of overlap was higher with V3B than with either V3A or IPS0 (t values > 3.6, P values < 0.01, see Fig. 3), and with V3A than IPS0 [t(11) = 2.8, P < 0.05]. Superior IPS showed significant overlap with IPS0-4 (t values > 4.9, P values < 0.001), with less overlap with IPS3-4 than IPS0-2 in general. Specifically, superior IPS showed less overlap with IPS3-4 than with IPS1 (t values > 3.1, P values < 0.05) or IPS0 (trending for IPS4, t > 1.9, P < 0.08), and there was a greater percentage of overlap with IPS2 than with IPS3 [t(11) = 2.3, P < 0.05], with the difference in the overlap between IPS2 and IPS4 in the right direction but not significant (P = 0.13). Overall, the amount of overlap between superior IPS and IPS0, IPS1 and IPS2 were comparable (t values < 1.0, P values > 0.09). The amount of overlap between superior IPS and IPS3 and IPS4 were also comparable (P = 0.6).
Fig. 3.
The amount of anatomical overlap between inferior/superior IPS regions and the IPS topographic regions, in the average of all participants (top) and in 3 representative participants (bottom). While inferior IPS had the greatest overlap with V3B, superior IPS had the greatest overlap with IPS0, IPS1, and IPS2. Error bars indicate means ± SE.
Main VSTM Experiment
To determine the role of topographic IPS regions in visual object selection and encoding, we examined how activity differed in these regions when participants were asked to remember one object, four identical objects, or four different objects in a VSTM task. This combination of conditions allowed us to distinguish between the selection of objects by their location and the encoding and storage of VSTM object featural information. If a region is primarily involved in location-based processing and is not sensitive to object featural information, it should show higher levels of activation when more objects are on the screen, regardless of whether they are identical or different, resulting in equally high activation for four identical objects and four different objects and low activation for one object. After all, all four locations will have to be selected and their features encoded before one knows whether these locations contain identical or different feature information. A location-based process is thus essential in extracting feature information from a visual display. If a region is involved in encoding detailed object features, then its activation would only increase when the number of distinctive features to be encoded increases, resulting in no difference between four identical and one object but higher activation for four different objects. Previously, this task has been shown to elicit location-based selection in inferior IPS and feature-based encoding and storage in superior IPS (Xu 2009).
Behavioral results.
VSTM capacities, calculated using Cowan's K (Cowan 2001) were as follows: one object: 0.97 ± 0.01, four identical objects (treated as one single object): 0.96 ± 0.02, and four different objects: 3.08 ± 0.20. Capacities for one and four identical objects were both at ceiling and did not differ from each other [t(9) = 0.5, P > 0.6], but both were lower than for four different objects (t values > 10.3, P values < 0.001). Reaction times for the correct trials were: one object: 558 ± 54 ms, four identical objects: 544 ± 55 ms, and four different objects: 768 ± 64 ms. Participants were significantly slower for four different objects than for either one or four identical objects (t values > 6.9, P values < 0.001), with four identical objects being slightly faster than one object [t(9) = 2.4, P < 0.05].
fMRI results from inferior IPS and superior IPS.
In a repeated-measures ANOVA (ROI × condition × hemisphere), we found a main effect of ROI [F(1,9) = 5.1, P < 0.05] with inferior IPS showing less activity than superior IPS. We also found a main effect of stimulus condition [F(2,9) = 39.9, P < 0.0001], in which activity was higher for four different objects than for either one or four identical objects (t values > 4.2, P values < 0.01) and for four identical objects than for one object [t(9) = 4.1 P < 0.01]. There was a main effect of hemisphere [F(1,9) = 5.2, P < 0.05], in which the left hemisphere showed slightly higher activity than the right hemisphere, and an interaction between ROI and hemisphere, showing that only superior IPS showed a hemispheric effect, with higher activity in the left hemisphere than the right [t(9) = 3.0, P < 0.05]. Inferior IPS showed no hemispheric differences (P > 0.8). There were no other hemispheric interactions (P values > 0.3).
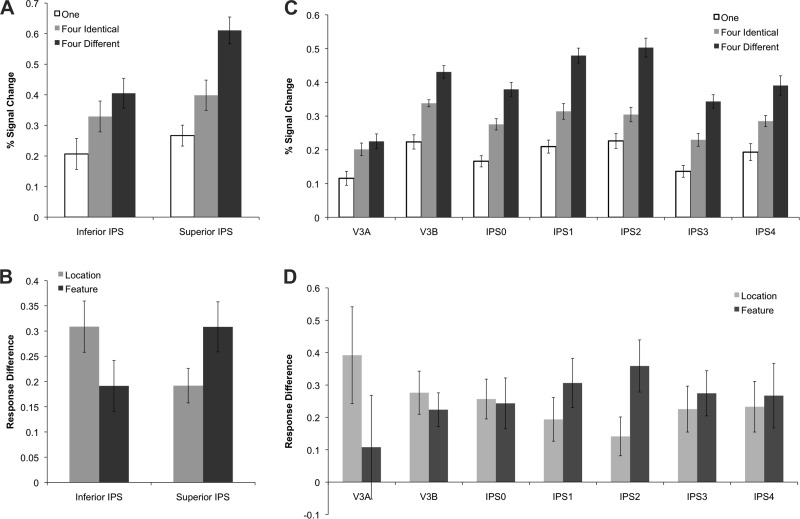
Although both areas showed significantly higher activation for four objects (regardless of whether they were identical or different) than for one object (t values > 3.9, P values < 0.01), as well as higher activation for four different relative to four identical objects (t values > 2.3, P values < 0.05), inferior and superior IPS differed in the degree of location- and feature-based processing as reflected in the significant interaction between ROI and stimulus condition [F(2,9) = 21.9, P < 0.001] (see Fig. 4A).
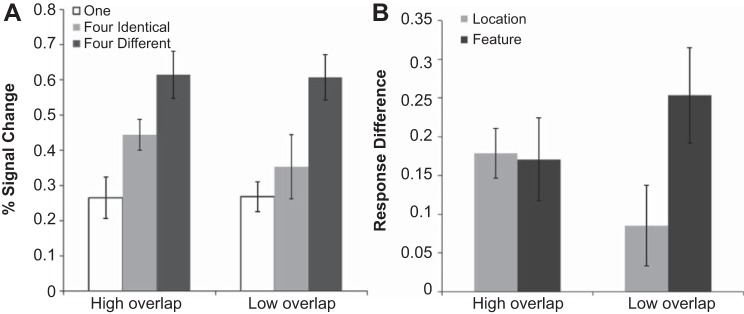
Fig. 4.
Activity for each condition in the main VSTM experiment in superior and inferior IPS (A), along with the normalized response difference (see materials and methods) showing location-based processing (4 identical objects minus 1 object) and featural-based processing (4 different objects minus 4 identical objects) (B). The same is also shown for the IPS topographic regions (C and D). The strength of location-based processing was stronger in inferior than superior IPS, and the reverse was true for feature-based processing. Similarly, along the IPS topographic regions, there seemed to be a shift in the balance of location vs feature-based processing from a location bias in the lower IPS regions V3A, V3B, and IPS0 to a feature bias in higher IPS regions IPS1 and IPS2. Error bars indicate means ± SE.
To better measure the relative strength of these two types of processing and to account for overall response amplitude differences between brain regions, following Xu (2009), we first normalized the responses by anchoring, in each brain region, the averaged responses for the one and the four different conditions to be 0.5 and 1, respectively. We then examined activity related to location-based processing by calculating response difference between the four identical and the one object conditions, as the amount of featural information was identical in both of these conditions. Likewise, we examined activity related to feature-based processing by calculating response difference between the four different and the four identical objects conditions, as the amount of location information was equated between these two conditions (see Fig. 4B). With these measures, as with the un-normalized data, we found a significant interaction between location- and feature-based processing and brain regions [F(1,9) = 8.5, P < 0.05], with a stronger location-based processing in inferior IPS [t(9) = 2.9, P < 0.05] and a stronger feature-based processing in superior IPS [t(9) = 2.3, P < 0.05], consistent with findings from Xu (2009).
fMRI results from parietal topographic regions.
When activity was examined in topographic IPS (V3A-IPS4) across the three object conditions, we found a main effect of stimulus condition [F(2,9) = 26.5, P < 0.001], such that, as in the inferior/superior IPS ROIs analyses, four different objects elicited more activity than both four identical objects and one object, and four identical objects produced more activity than one object (all t values > 3.4, P values < 0.01). These results were further confirmed in detailed pair-wise comparisons in each region. Specifically, all topographic ROIs showed less activity for one object than for either four identical or four different objects (all t values > 2.3, P values < 0.05), and all ROIs, except V3A (P > 0.5), showed higher activity for four different than for four identical objects (all t values > 2.6, P values < 0.05). We also found a main effect of ROI [F(6,9) = 6.1, P < 0.001] and a significant main effect of hemisphere [F(1,9) = 6.5, P < 0.05], with the left hemisphere showing higher activity than the right hemisphere. However, an interaction between ROI and hemisphere [F(6,9) = 4.2, P < 0.01] and subsequent post hoc t-tests showed that this hemisphere effect is localized to IPS4 only [t(9) = 4.8, P < 0.001], with no other ROI showing a significant hemisphere effect (P > 0.1). No other hemispheric interactions reached significance (P > 0.7).
Despite the similarity in response pattern across the different IPS regions, as in our inferior and superior IPS analyses, we observed a significant interaction between ROI and condition [F(12,9) = 4.2, P < 0.001] (see Fig. 4B), indicating that these regions differed in their relative strength of location- and feature-based processing.
To directly compare location- and feature-based processing while accounting for overall response difference across the different IPS regions, as with the data from the inferior and superior IPS ROIs, we first normalized the responses by anchoring in each brain region the averaged responses for the one and the four-different conditions to be 0.5 and 1, respectively. We then directly compared the processing of location information (four identical vs. one object conditions) and feature information (four different vs four identical objects conditions). With these measures, as with the un-normalized data, we found a significant interaction between location- and feature-based processing and brain regions [F(6,9) = 3.2, P < 0.01]. Consistent with what was seen in inferior and superior IPS, there seemed to be a shift in the balance of location- vs. feature-based processing from a location bias in the lower IPS regions V3A, V3B, and IPS0 to a feature bias in higher IPS regions IPS1 and IPS2. These findings are expected given the amount of anatomical overlap between regions and the response profiles of inferior and superior IPS in location- and feature-based processing.
The variability of location- and featured-based processing in superior IPS based on the degree of overlap with parietal topographic regions.
To examine how the amount of overlap between superior IPS regions and parietal topographic regions would affect location- and featured-based responses in superior IPS, we divided our participants evenly into two groups, those who showed a higher level of overlap (>78%) with topographic IPS regions and those who had a relatively lower level of overlap (see Fig. 5; the data shown here were not normalized, as was done in earlier analyses, because the overall response amplitudes were comparable between the two group of participants). For participants with a higher degree of overlap (n = 5, mean overlap = 87%), we found significant differences between all three conditions (t values > 2.9, P values < 0.05). For participants with a lower degree of overlap (n = 5, mean overlap = 68%), while we still found a significant difference between the four different and the four identical conditions [t(4) > 3.7, P < 0.05], and between the four different and one object conditions [t(9) > 5.2, P < 0.01], we found no difference between the four identical and one object conditions (P > 0.17). Thus in individuals with a higher degree of overlap we see both location- and feature-based processing in superior IPS, but in individuals with a lower degree of overlap we see only feature-based processing. These results are somewhat expected given the location-based organization of the IPS topographic regions. Nevertheless, this may explain why in Xu (2009) feature-based encoding was much stronger in superior IPS than in the present study, as participants in that study may have had lower levels of overlap between superior IPS and parietal topographic areas, similar to the low overlap group in the current study.
Fig. 5.
Superior IPS responses for the group of participants showing a higher degree of overlap with the IPS topographic regions and those showing a lower degree of overlap with the IPS topographic regions (A), along with the amount of location- and feature-based processing (see materials and methods) in both groups (B). Individuals with a lower degree of overlap showed little location-based processing, but a significant amount of feature-based processing, whereas those with a higher degree of overlap showed an equal amount of location- and feature-based processing. Error bars indicate means ± SE.
DISCUSSION
The goal of the present research was to provide a direct link between two disparate literatures related to the role of IPS in visual cognition. By localizing superior and inferior IPS regions in relation to the topographic IPS regions in individual participants, we were able to provide a common ground with which to explore the role of object selection and encoding, as well as location- and feature-based processing within IPS sub regions.
We were able to localize inferior IPS to the V3A/B region, at the bottom of IPS around the intersection of IPS and the transverse occipital sulcus, in line with previous reports. Superior IPS, on the other hand, overlapped with higher IPS topographic regions, showing a greater overlap with IPS0-2 than V3A/B or IPS3-4. Overall, inferior IPS showed an 85.2% overlap with topographic IPS regions, greater than what was seen in superior IPS, who showed a 73.6% overlap (see Fig. 3). Additionally, inferior IPS more consistently overlapped with topographic regions across participants than superior IPS did.
Functionally, both inferior and superior IPS showed location- and feature-based processing. Given the high degree of overlap with topographic regions, it is perhaps unsurprising that we found location-based encoding in these regions. While these results seem to fit with those of Harrison et al. (2010) and suggest a primarily location-based role for processing in IPS, we did find a dissociation between inferior and superior IPS, such that processing in inferior IPS was biased towards location based processing, while processing in superior IPS was biased towards feature based processing, similar to that seen in Xu (2009). All topographic IPS regions showed both location- and feature-based processing, as well as an increase in feature-based encoding going from inferior to superior topographic regions that peaked in IPS1-2. Thus IPS1-2 seemed to be the focal point for the shift from more location to more feature based processing within the topographic IPS regions.
The amount of overlap between superior IPS and the IPS topographic regions varied greatly across participants. To understand the functional significance of these results, we divided our participants into two groups based the amount of this overlap. Interestingly, in the high-overlap group, we found the presence both location- and feature-based processing in superior IPS, but in the low-overlap group, we only found significant feature-based processing in superior IPS. The latter result is consistent with what was reported in Xu (2009). This nontopographic superior IPS region may be related to the area found by Harrison et al. (2010) within the right inferior parietal lobule that appears to track the amount of featural information, but not location information, in a display. This also indicates that the incomplete overlap between superior IPS and IPS topographic regions could not be driven by the imprecision of fMRI data acquisition. However, they reflected functional differences between these regions. Further studies are needed to understand how variation in the amount of overlap between superior IPS and the IPS topographic regions across individual would impact performance. For example, it is possible that individuals with a higher degree of overlap would be more sensitive to location information during feature encoding and storage.
Overall, the combined findings from both inferior and superior IPS as well as the topographic IPS regions indicate that as one proceeds up the sulcus from inferior to superior IPS, and up the hierarchy of the topographic IPS regions, the amount of feature-based processing increases. Along the lateral medial axis there is another shift, such that more medial areas, those that overlap with topographic regions, show stronger location based processing than more lateral areas, which do not.
Given the large overlap between inferior IPS and the V3A/B region it would be expected that these regions would show similar functional profiles. Indeed, the role of inferior IPS in object selection and individuation as proposed by Xu and Chun (2009) is consistent with the role of the V3A/B region reported by others. Specifically, previous work has linked V3A/B with the kinetic occipital (KO) region and with processes related to the binding of contours in space and time (Zeki et al 2003; Georgieva et al 2009). Other work has associated V3A/B with IPS regions in terms of the processes involved in focusing and suppression attention (Donner et al 2008). If we consider the binding of contours found within V3A/B to be a component process of object individuation, then the role of V3A/B in the attentional selection and individuation of objects is precisely the role proposed for inferior IPS by Xu and Chun (2009).
The incomplete overlap between the IPS topographic regions and superior IPS shows that the parietal region involved in VSTM storage is not completely defined by topographic representation. This makes sense, as VSTM storage is not always location dependent. More importantly, this incomplete overlap indicates that while these topographic regions capture the representation of location information along the IPS, they are insufficient in characterizing the representation of VSTM information in parietal cortex. This is consistent with the results from a recent study in which we found that fMRI MVPA decoding from none of the IPS topographic regions consistently correlated with behavioral VSTM performance, but VSTM response amplitude-defined superior IPS did (Bettencourt and Xu 2016). Thus there seems to exist distinct but partially overlapping functional regions within IPS, involved in location processing and visual information encoding and storage, respectively. A similar partial overlap with the IPS topographic maps has also been found for number and size processing (Harvey et al. 2013; 2015).
In monkey neurophysiology studies, it has been shown that IPS neurons selective for shape features are at the same time strongly influenced by spatial position (Romero et al. 2014; Janssen et al. 2008; see also Toth and Assad 2002). Rishel et al. (2013) further showed that there are distinct and independent saccade-related spatial signals and nonspatial category signals in LIP at both the single-neuron and population levels. Because neural recording studies can only sample neurons within an area rather than delineating borders between functional areas, it is unknown whether LIP neurons exhibiting spatial and category selectivity show a complete or partial overlap. If these two types of visual information are relayed by nonoverlapping neurons upstream, there is likely a partial overlap in LIP. In the human IPS, the present study shows a partial overlap between location- and feature-based processing. This suggests that a similar partial overlap may exist in macaque IPS as well. Further studies are needed to verify this prediction. Overall, the present study shows that understanding the multiplex role of IPS in visual cognition may not be reduced to examining the functions of the different IPS topographic regions only, but rather, it could only be achieved by understanding how regions identified by different tasks and methods may colocalize with each other.
Harrison et al. (2010) questioned the dichotomy of inferior and superior IPS in object individuation and identification by demonstrating a predominance of location-based encoding in IPS with minimal featural encoding. However, the IPS localizer used in that study did not show a plateau function in behavioral VSTM capacity measure, making it possible that the single IPS region localized within this study may correspond only to the inferior IPS region or parietal regions involved in spatial attention-related processing. Magen et al. (2009) has argued that attentional demand, rather than visual information processing and storage, may best explain the parietal fMRI responses associated with VSTM tasks. However, Magen et al. (2009), in their experiment 1, used a group based IPS ROI instead of an individual observer defined ROI. This likely reduced the precision of their results. Although individual observer-defined IPS ROIs were used in experiment 2, Magen et al. (2009) examined a single IPS ROI and did not separately investigate the unique role of inferior and superior IPS. Indeed, the response pattern from that experiment seemed to suggest a mixture of results from both the inferior and superior IPS. Lastly, the IPS ROIs examined in Magen et al. (2009) appeared to be much more medial, in the vicinity of the parietal lobule, compared with those reported by Todd and Marois (2004) and Xu and Chun (2006). Taking all of these into account, the results from Magen et al. (2009) do not seem to provide strong evidence arguing against the dissociation seen between the inferior and the superior IPS as proposed in Xu and Chun (2006 and 2009) and reported here.
In our study, we measured feature-based encoding as a difference between the encoding of four different and four identical objects. Although task difficulty also increased when more feature information was encoded, we do not think task difficulty contributed to the observed feature-based encoding effect. This is because previous studies have shown that activities within inferior and superior IPS reflect the number of objects individuated and encoded and not task difficulty, such that increases in difficulty without concurrent increases in the number of individuated and encoded objects do not affect activity levels (Todd and Marois 2004; Xu and Chun 2006). Similarly, it has been shown that VSTM related activity in IPS0-4 plateaus with capacity limits, regardless of whether task difficulty increases beyond this limit (Sheremata et al 2010). For these reasons, we believe any differences between the encoding of four different and four identical objects reflect the difference in feature encoding and not task difficulty. In our more recent study using fMRI multivoxel pattern analysis, we found strong decoding of VSTM content in superior IPS that closely tracked VSTM behavioral performance (Bettencourt and Xu 2016). This provides further support that visual information can be directly represented in superior IPS.
The superior and inferior IPS regions we describe here are activations partially overlapping with topographically defined areas. They are defined as regions in the inferior and superior parts of the IPS, respectively, and, as stated in the Introduction, referred to as inferior and superior IPS only for simplicity. Although these two regions could be given more specific functional names, given the involvement of the human parietal cortex across a number of different cognitive tasks, naming parietal regions by the specific functions they play in a specific task context could be rather limited and potentially misleading. Perhaps realizing this, except for a few action-defined parietal regions, the general literature on the human parietal cortex has labeled functional regions by their anatomical locations, rather than by the specific cognitive functions identified. One advantage of this naming tradition is that it could facilitate comparisons across studies and tasks.
Overall, this study provides an important first step in bridging disparate literatures related to the role of IPS in cognitive processing. Because location- and feature-based encoding are building blocks of visual cognition, determining the exact manner in which these two types of processing interact across IPS regions may serve as a basis for understanding the underlying mechanisms and role of the subregions within IPS across cognitive domains. Our results suggest that instead of a strict processing distinction within IPS, there appears to be two partially overlapping functional regions within IPS, with one involved in location processing and the other involved in visual information encoding and storage. This resulted in more inferior and medial regions showing stronger location-based processing and more superior and lateral regions showing stronger feature-based processing. Thus understanding the multiplex role of IPS in visual cognition may not be reduced to only examining the functions of the different IPS topographic regions, which best capture the representation of location information along the IPS, but rather, it could only be accomplished by understanding how regions identified by different tasks and methods may colocalize with each other.
GRANTS
This research was supported by National Eye Institute Grants 1R01-EY-022355 (to Y. Xu) and F32-EY-022874 (to K. C. Bettencourt).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.C.B. and Y.X. conception and design of research; K.C.B. performed experiments; K.C.B. analyzed data; K.C.B. and Y.X. interpreted results of experiments; K.C.B. prepared figures; K.C.B. and Y.X. drafted manuscript; K.C.B. and Y.X. edited and revised manuscript; K.C.B. and Y.X. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jascha Swisher for topographic mapping script, Sarah Cohan for proofreading the final draft of the manuscript, and members of the Harvard Vision Lab for valuable comments on this study.
REFERENCES
- Arcaro MJ, Pinsk MA, Li X, Kastner S. Visuotopic organization of macaque posterior parietal cortex: an fMRI study. J Neurosci 31: 2064–2078, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt KC, Xu Y. Decoding under distraction reveals distinct occipital and parietal contributions to visual short-term memory representation. Nat Neurosci 19: 150–157, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis 10: 433–436, 1997. [PubMed] [Google Scholar]
- Bressler DW, Silver MA. Spatial attention improves reliability of fMRI retinotopic mapping signals in occipital and parietal cortex. Neuroimage 53: 526–533, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3: 201–215, 2002. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci 24: 87–114, 2001. [DOI] [PubMed] [Google Scholar]
- Culham JC, Valyear KF. Human parietal cortex in action. Curr Opin Neurobiol 16: 205–212, 2006. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9: 179–194, 1999. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Piazza M, Pinel P, Cohen L. Three parietal circuits for number processing. Cogn Neuropsychol 20: 487–506, 2003. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Carman GJ, Bandettini P, Glickman S, Wieser J, Cox R, Miller D, Neitz J. Mapping striate and extrastriate visual areas in human cerebral cortex. Proc Natl Acad Sci USA 93: 2382–2386, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner TH, Sagi D, Bonneh YS, Heeger DJ. Opposite neural signatures of motion-induced blindness in human dorsal and ventral visual cortex. J Neurosci 28: 10298–10310, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex 7: 181–192, 1997. [DOI] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 20: 70–80, 2011. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 9: 195–207, 1999. [DOI] [PubMed] [Google Scholar]
- Georgieva S, Peeters R, Kolster H, Todd JT, Orban GA. The processing of three-dimensional shape from disparity in the human brain. J Neurosci 29: 727–742, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb J, Balan P. Attention as a decision in information space. Trends Cogn Sci 14: 240–248, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A, Jolicoeur P, Marois R. “What” and “where” in the intraparietal sulcus: an fMRI study of object identity and location in visual short-term memory. Cereb Cortex 20: 2478–2485, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BM, Fracasso A, Petridou N, Dumoulin SO. Topographic representations of object size and relationships with numerosity reveal generalized quantity processing in human parietal cortex. Proc Natl Acad Sci USA 112: 13525–13530, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BM, Klein BP, Petridou N, Dumoulin SO. Topographic representation of numerosity in the human parietal cortex. Science 341: 1123–1126, 2013. [DOI] [PubMed] [Google Scholar]
- Janssen P, Srivastava S, Ombelet S, Orban G. Coding of shape and position in macaque lateral intraparietal area. J Neurosci 28: 6679–6690, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konen CS, Kastner S. Representation of eye movements and stimulus motion in topographically organized areas of human posterior parietal cortex. J Neurosci 28: 8361–8375, 2008a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konen CS, Kastner S. Two hierarchically organized neural systems for object information in human visual cortex. Nat Neurosci 11: 224–231, 2008b. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Mapping of architectonic subdivisions in the macaque monkey, with emphasis on parieto-occipital cortex. J Comp Neurol 428: 79–111, 2000a. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sen-sorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol 428: 112–137, 2000b. [DOI] [PubMed] [Google Scholar]
- Magen H, Emmanouil TA, McMains SA, Kastner S, Treisman A. Attentional demands predict short-term memory load response in posterior parietal cortex. Neuropsychologia 47: 1790–1798, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: two cortical pathways. Trends Neurosci 6: 414–417, 1983. [Google Scholar]
- Pelli DG. The videotoolbox software for visual psychophysics: Transforming numbers into movies. Spat Vis 10: 437–442, 1997. [PubMed] [Google Scholar]
- Rishel CA, Huang G, Freedman DJ. Independent category and spatial encoding in parietal cortex. Neuron 77: 969–979, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MC, Pani P, Janssen P. Coding of shape features in the macaque anterior intraparietal area. J Neurosci 34: 4006–4021, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D, Glimcher P, Heeger DJ. Topographic organization for delayed saccades in human posterior parietal cortex. J Neurophysiol 94: 1372–1384, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D, Curtis CE, Glimcher PW, Heeger DJ. Sustained activity in topographic areas of human posterior parietal cortex during memory-guided saccades. J Neurosci 26: 5098–5108, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268: 889–893, 1995. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science 294: 1350–1354, 2001. [DOI] [PubMed] [Google Scholar]
- Sheremata SL, Bettencourt KC, Somers DC. Hemispheric asymmetry in visuotopic posterior parietal cortex emerges with visual short-term memory load. J Neurosci 30: 12581–12588, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Kastner S. Topographic maps in human frontal and parietal cortex. Trends Cogn Sci 13: 488–495, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol 94: 1358–1371, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straw AD. Vision Egg: an open-source library for realtime visual stimulus generation. Front Neuroinformatics 2: 4, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swisher JD, Halko MA, Merabet LB, McMains SA, Somers DC. Visual topography of human intraparietal sulcus. J Neurosci 27: 5326–5337, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature 428: 751–754, 2004. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cogn Affect Behav Neurosci 5: 144–155, 2005. [DOI] [PubMed] [Google Scholar]
- Toth LJ, Assad JA. Dynamic coding of behaviourally relevant stimuli in parietal cortex. Nature 415: 165–168, 2002. [DOI] [PubMed] [Google Scholar]
- Vesia M, Prime SL, Yan X, Sergio LE, Crawford JD. Specificity of human parietal saccade and reach regions during transcranial magnetic stimulation. J Neurosci 30: 13053–13065, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 9: 445–453, 2005. [DOI] [PubMed] [Google Scholar]
- Xu Y. Encoding color and shape from different parts of an object in visual short-term memory. Percept Psychophys 64: 1260–1280, 2002. [DOI] [PubMed] [Google Scholar]
- Xu Y. Representing connected and disconnected shapes in human inferior intraparietal sulcus. Neuroimage 40: 1849–1856, 2008. [DOI] [PubMed] [Google Scholar]
- Xu Y. Distinctive neural mechanisms supporting visual object individuation and identification. J Cogn Neurosci 21: 511–518, 2009. [DOI] [PubMed] [Google Scholar]
- Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature 440: 91–95, 2006. [DOI] [PubMed] [Google Scholar]
- Xu Y, Chun MM. Visual grouping in human parietal cortex. Proc Natl Acad Sci USA 104: 18766–18771, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Chun MM. Selecting and perceiving multiple visual objects. Trends Cogn Sci 13: 167–174, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S, Perry RJ, Bartels A. The processing of kinetic contours in the brain. Cereb Cortex 3: 189–202, 2003. [DOI] [PubMed] [Google Scholar]