The relative use of vestibular vs. somatosensory information for human balance control is unclear. When standing with eyes closed, subjects with vestibular loss were most unstable within a critical surface tilt velocity range of 2 to 8 deg/s. We propose that the vestibular system is critical for controlling balance at tilt velocities around normal postural sway, whereas graviceptive control is used at lower velocities, and proprioceptively triggered automatic postural responses are used at higher velocities.
Keywords: vestibular, postural stability, human, surface tilt, somatosensory, proprioception
Abstract
Vestibular information is known to be important for postural stability on tilting surfaces, but the relative importance of vestibular information across a wide range of surface tilt velocities is less clear. We compared how tilt velocity influences postural orientation and stability in nine subjects with bilateral vestibular loss and nine age-matched, control subjects. Subjects stood on a force platform that tilted 6 deg, toes-up at eight velocities (0.25 to 32 deg/s), with and without vision. Results showed that visual information effectively compensated for lack of vestibular information at all tilt velocities. However, with eyes closed, subjects with vestibular loss were most unstable within a critical tilt velocity range of 2 to 8 deg/s. Subjects with vestibular deficiency lost their balance in more than 90% of trials during the 4 deg/s condition, but never fell during slower tilts (0.25–1 deg/s) and fell only very rarely during faster tilts (16–32 deg/s). At the critical velocity range in which falls occurred, the body center of mass stayed aligned with respect to the surface, onset of ankle dorsiflexion was delayed, and there was delayed or absent gastrocnemius inhibition, suggesting that subjects were attempting to actively align their upper bodies with respect to the moving surface instead of to gravity. Vestibular information may be critical for stability at velocities of 2 to 8 deg/s because postural sway above 2 deg/s may be too fast to elicit stabilizing responses through the graviceptive somatosensory system, and postural sway below 8 deg/s may be too slow for somatosensory-triggered responses or passive stabilization from trunk inertia.
NEW & NOTEWORTHY
The relative use of vestibular vs. somatosensory information for human balance control is unclear. When standing with eyes closed, subjects with vestibular loss were most unstable within a critical surface tilt velocity range of 2 to 8 deg/s. We propose that the vestibular system is critical for controlling balance at tilt velocities around normal postural sway, whereas graviceptive control is used at lower velocities, and proprioceptively triggered automatic postural responses are used at higher velocities.
the relative importance of vestibular information for postural control depends on task and environmental conditions (Fitzpatrick et al. 2002; Hlavacka et al. 1995; Horak et al. 2002; Inglis et al. 1995; Mergner et al. 2002, 2003). For example, vestibular information is not critical for maintaining stance posture on a stationary, horizontal surface, a condition in which the support surface can provide a stable frame of reference for posture control (Inglis and Macpherson 1995; Macpherson and Inglis 1993; Mergner et al. 2002, 2003; Nashner et al. 1982). Vestibular information is also unnecessary for eliciting appropriately timed postural responses in the legs to rapid translations of a horizontal surface, which are triggered by somatosensory feedback (Horak et al. 1990; Inglis and Macpherson 1995; Inglis et al. 1995; Macpherson and Inglis 1993). However, vestibular information has long been known to be important for balance when the surface is unstable from tilting, oscillating or sway-referencing, especially when vision is not available (Allum and Pfaltz 1985; Creath et al. 2002; Maurer et al. 2000; Nashner et al. 1982; Peterka and Loughlin 2004).
Vestibular information may be most critical when subjects attempt to balance on a surface that is tilting at particular velocities that are difficult to detect by the somatosensory system, normally the first line of defense in signal postural disequilibrium (Peterka 2002). When the support surface tilts at about the same rate as natural body sway (2–8 deg/s), the somatosensory system alone may not be able to differentiate between body sway on a stable surface and surface tilt under a stable body (Mergner et al. 2003). However, if the surface tilts faster than natural body sway, the stretch of muscle spindles signal the surface perturbation, and if the surface tilts more slowly than natural body sway, integrative pressure changes (graviception) can signal the surface perturbation. In contrast, when the surface is tilted at the rate at which a body naturally falls (around 4 deg/s), we predict that vestibular information is most important because somatosensory signals are less available (assuming vision is also unavailable).
Although the effects of continuous surface oscillations on steady-state postural control have been reported (Creath et al. 2008; Maurer et al. 2000; Peterka 2002), no previous publication has systematically examined the effects of a wide range of discrete, surface-tilt velocities on postural responses in people with vestibular loss. During oscillating surface tilts, subjects with vestibular loss and eyes closed fall within only one or two cycles if the tilting amplitude is 8 deg or more (Creath et al. 2008; Maurer et al. 2000; Peterka and Loughlin 2004). In contrast, discrete, ramp-and-hold surface tilts have the advantage of testing subjects with vestibular loss across a wider velocity range without falling, because balance needs to be maintained for only one-half of a tilt cycle.
We hypothesize that vestibular information will be particularly important at tilt velocities too fast (>8 deg/s) for passive or graviceptive control mechanisms and too slow (<2 deg/s) for rapid postural responses (Maurer et al. 2000).
Although surface tilts are known to cause postural instability in patients with vestibular loss, the underlying cause remains unclear. Vestibular information may be particularly important for maintaining postural equilibrium during surface tilts because it provides gravity-specific information that is necessary for the nervous system to distinguish whether the body is swaying over a stationary surface, or the surface is tilting under the stationary body (Mergner and Rosemeier 1998). Without vestibular information, people may misinterpret changes in somatosensory information that accompany surface tilt to mean that their body, not the surface, is tilting (Mergner 2007; Mergner and Rosemeier 1998; Nashner et al. 1982). People with vestibular loss could produce a destabilizing postural response that causes falling if they actively aligned their bodies to the tilting surface instead of to gravitational vertical (Macpherson et al. 2007; Nashner et al. 1982). Alternatively, instability could occur because vestibular signals are necessary to trigger a stabilizing postural response (Allum and Pfaltz 1985; Carpenter et al. 2001; Diener et al. 1988). In the present study, the strategy of body kinematic responses in response to surface rotations was used to understand how the vestibular system contributes to stabilization of body segments. Ground reaction forces and electromyographic (EMG) postural responses were used to determine whether instability was a result of active destabilizing responses or delayed or absent equilibrium response.
In this study, we characterized postural responses to cosine function ramp-and-hold surface tilts across a wide range of tilt velocities between 0.05 and 32 deg/s in subjects with bilateral vestibular loss and compared the responses with those of control subjects to determine the velocities at which vestibular information is most important. Subjects were tested with both their eyes open and eyes closed to determine how surface-tilt velocity affects the degree to which vision can stabilize posture, even when vestibular information is not available.
METHODS
Subjects
Nine subjects with profound bilateral vestibular loss (age, 54 ± 7 yr) and nine age-matched, control subjects (age, 54 ± 7 yr) participated in the study. Table 1 summarizes the diagnosis, onset, and severity of vestibular loss based on gain of the horizontal vestibule-ocular reflex from rotatory chair rotation at 0.05 Hz. Six subjects had ototoxity from gentamycin, one had a neurectomy on one side in addition to ototoxity, and the rest had idiopathic vestibular loss. All subjects with vestibular loss were healthy, active individuals, with symptom onset at least 2 yr before the experiment. Subjects were screened via a health history and medical examination before experimental testing to ensure that they were free of musculoskeletal and neurological impairments that could affect postural control. Control subjects were also screened to ensure they did not have vestibular, musculoskeletal, or neurological impairments. All subjects gave their informed consent before participating in the study. The Institutional Review Board at Oregon Health & Science University approved the experimental protocol.
Table 1.
Characteristics of subjects with vestibular loss
| Subject | Sex | Age | Etiology | Onset, yr after | H-VOR Gain* |
|---|---|---|---|---|---|
| 1 | F | 52 | Ototoxicity | 5 | 0.070 |
| 2 | F | 53 | Idiopathic | 7 | 0.005 |
| 3 | F | 46 | Idiopathic | 9 | 0.006 |
| 4 | M | 58 | Ototoxicity | 4 | 0.047 |
| 5 | M | 53 | Right neurectomy | 5 | 0.025 |
| Left ototoxicity | |||||
| 6 | F | 53 | Idiopathic | 3 | 0.032 |
| 7 | M | 69 | Ototoxicity | 5 | 0.022 |
| 8 | M | 49 | Ototoxicity | 2 | 0.150 |
| 9 | M | 49 | Ototoxicity | 2 | 0.210 |
H-VOR, horizontal vestibule-ocular reflex from rotatory chair rotation at 0.05 Hz; normal range = 0.4–0.8.
Experimental Protocol
Subjects stood on a force platform that tilted 6 deg, toes-up at eight different ramp velocities (0.25, 0.5, 1, 2, 4, 8, 16, and 32 deg/s) with eyes open and eyes closed. The eight velocity conditions were randomized with a catch condition of no surface movement to compose a block of nine trials. Each experimental session consisted of 6–8 blocks of trials (a total of 54–72 trials per subject), alternating visual conditions by block. The first block was always an eyes-open condition, with the first trial of each block starting with a different velocity with eyes open to minimize first trial, startle effects (Allum et al. 2011). To avoid fatigue, subjects were given a 10- to 15-min rest break after every two blocks of trials. The length of the session was 2–3 h.
Surface tilts were driven by a computer-controlled hydraulic system, with the axis of surface rotation at approximately ankle height (2 cm) (Horak et al. 2002). Figure 1 shows the surface tilt displacement, velocity, and acceleration characteristics for the 4 and 32 deg/s velocity conditions. The forcing function that was used to drive the surface tilt consisted of a constant velocity profile with a cosine function at the start and end of the ramp. The forcing function was designed to limit acceleration and create gradual, rather than abrupt, onset and offset of surface tilt so that we could study how equilibrium is maintained without activating short- or medium-latency, somatosensory-triggered destabilizing bursts of the gastrocnemius muscles, particularly during the slower surface tilts of ≤4 deg/s (Allum and Pfaltz 1985; Diener et al. 1984, 1988; Horak et al. 1990). The forcing-function controlling-surface position also created a period (1 to 22 s) of constant velocity (zero acceleration) for all but the two fastest velocity conditions that showed a peak velocity of 16 and 32 deg/s (see Fig. 1). The peak accelerations ranged from 0.74 to 50 deg·s−1·s−1. Onset and offset of surface tilt was defined by the first detectable change in surface acceleration. Durations of surface tilts ranged from 0.187 to 24 s for the fastest to slowest rotations.
Fig. 1.
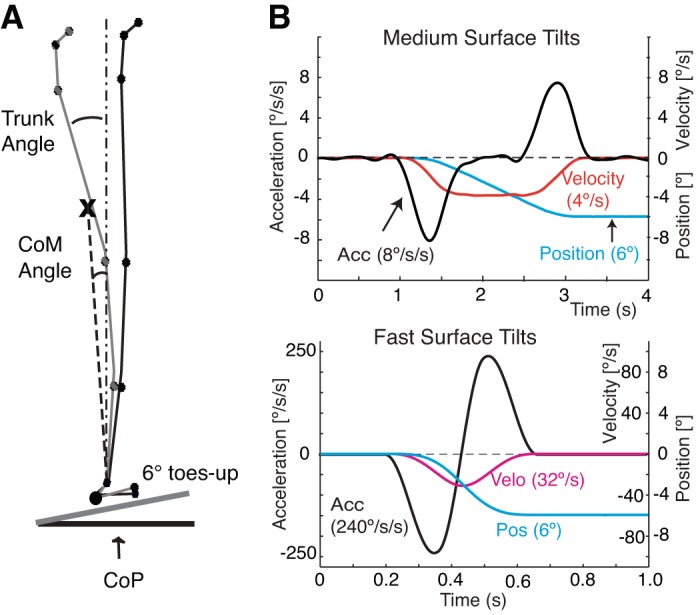
A: definitions of trunk angle and CoM angle and position of eight kinematic reflective markers from a representative control subject standing on the support surface when flat and after tilting toes-up by 6 deg. Trunk angle was measured as the difference from gravitational vertical with the axis of angle on the greater trochanter. CoM angle was measured as the difference from gravitational vertical with the axis of angle on the lateral malleolus. B: characteristics of platform position displacement, velocity, and acceleration for the 4 deg/s and 32 deg/s velocity conditions. CoM, center of mass.
For each trial, subjects stood on the force platform with their head facing forward and arms crossed. Their feet were spaced a comfortable distance apart, approximately hip width. The base of the support width was held constant across trials. Subjects were instructed to look at an art poster located 2 m straight ahead of them to maintain a constant initial gaze direction across trials. Subjects were instructed to imagine they were looking at the poster when their eyes were closed. Center of pressure (CoP) was monitored online throughout the experiment to keep initial CoP alignment constant (within 1 cm) before each trial. Subjects wore a safety harness that was tethered to an overhead beam to ensure safety during trials. The safety harness was adjusted so that it gave no light touch, or directional or spatial cues for postural alignment. In addition, a research assistant stood next to and closely guarded subjects for each trial. The assistant touched or supported the backs of subjects falling backward, but did not help return them to vertical posture. The time of fall onset was detected by a sudden reduction in weight on the force plates under the feet.
Each trial consisted of a 4-s baseline period of quiet stance; a variable period of surface tilt; followed by 10 s of quiet stance on the stationary, inclined surface. The duration of each trial depended on the velocity of tilt and ranged from 20 to 38 s. For each trial, subjects were cued that the trial was about to start. Data collection began a short, but variable, time after the verbal cue (0.5–5 s). This delay was brief to minimize the amount of time that subjects with vestibular loss stood with eyes closed and was variable so that subjects could not predict the precise timing of rotation onset.
Kinematics
Body kinematics were recorded at 60 Hz using a four-camera, motion analysis system (Motion Analysis, Santa Rosa, CA), and eight reflective markers placed on the left side of the body. Marker data were low-pass filtered with a 6-Hz, dual-pass Butterworth filter. Marker data were used to calculate the orientation of body segment angles (head, trunk, thigh, and shank), the position of joint angles (ankle, knee, and hip), and the location of the body's CoM. CoM displacement was calculated by identifying the summed position of the CoM of five body segments. The CoM for each segment was calculated using segment kinematics and anthropometric data (Chandler et al. 1975; Vaughan et al. 1992). The orientation of body segments in the sagittal plane was defined with reference to gravitational-vertical, with positive-going angular change representing forward inclination (Fig. 1A). The head segment was defined as from the eye to the external auditory meatus, the trunk segment was defined as from the greater trochanter to the acromion, the thigh segment was defined as from the lateral condyle to the greater trochanter, and the shank segment was defined as from the lateral malleolus to the lateral condyle. Change in joint angle was referenced to the initial, baseline posture. For the ankle angle, positive values indicate increasing dorsiflexion.
Forces
Subjects stood on a custom-made dual-plate force platform with four vertical force sensors under each foot. Forces were sampled at 120 Hz, and the CoP was calculated from the combined vertical forces of each force plate. CoP displacements were low-pass filtered at 10 Hz using a dual-pass, second-order, Butterworth filter.
Electromyography
EMG activity in four ankle muscles was differentially recorded using surface electrodes to determine whether backward leans and falls during surface tilts were a result of a passive failure to respond to the surface perturbation or whether they were due to active postural responses. The four muscles consisted of the left and right medial gastrocnemius (GAS) and the left and right anterior tibialis (TIB). EMG signals were amplified (× 2–7K) band-pass filtered (70–2,000 Hz), full-wave rectified, and smoothed by integrating at a cutoff frequency of 100 Hz before sampling at 480 Hz. Latencies of muscle (and CoP) onsets were measured from the onset of surface tilt, defined as the first detectible change in acceleration of surface rotation. Amplitude of integrated EMG was measured as the area under the rectified EMG from 200 to 900 ms during the initial postural response. To minimize the effect of individual differences in EMG amplitudes across subjects, the EMG data were normalized by the mean value from the interval 1 s before surface tilt onset to 1.5 s after tilt onset.
Data Analyses
We analyzed how the velocity of surface tilt and availability of vestibular and visual information affected 1) postural stability, 2) relative dependence on the surface vs. gravity as an orientation reference, and 3) postural responses to the surface tilt.
To quantify postural stability, each trial was coded as a fall or a no-fall trial. Trials were identified as a fall trial if the subject needed to be caught or touched by the research assistant and/or the safety harness to prevent falling, if the subject took one or more steps, or if the subject touched or held onto the research assistant. We pooled all trials within each condition (velocity, subject group, and vision) and calculated the percentage of total trials in which a fall occurred. We also quantified postural stability by measuring the peak trunk angular displacement in the sagittal plane that occurred in response to the surface tilts. In the case of a fall that required a catch by the assistant, maximum trunk displacement was determined at the time of the catch. Because subjects with vestibular loss lost their balance in more than 50% of the trials with their eyes closed during the 2, 4, and 8 deg/s surface tilts, measured peak backward trunk tilt at these tilt velocities underestimate how far the trunk might have displaced without intervention by the assistant.
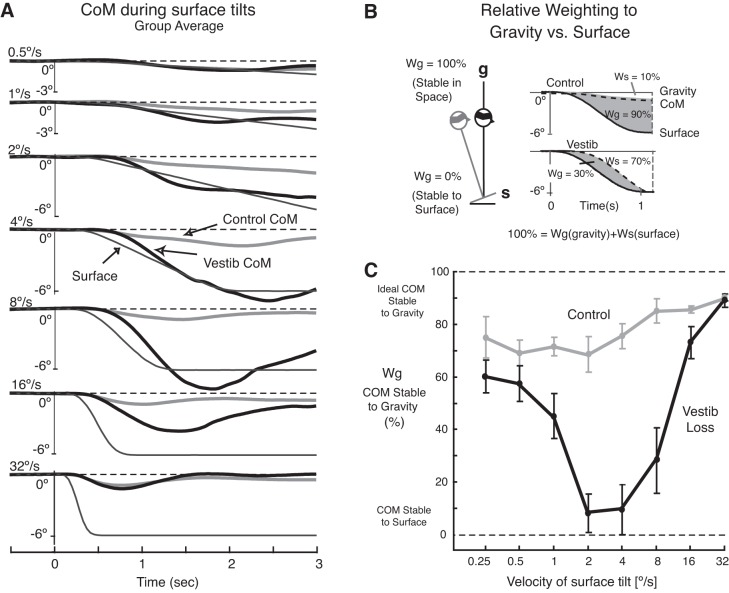
To determine how strongly subjects depended on the surface vs. gravity as a reference for postural orientation when the surface tilted and subjects' eyes were closed, we measured the CoM angle anchored at the lateral malleolus (Fig. 1A), and quantified the extent to which subjects maintained their CoM aligned with respect to the tilting surface vs. to gravitational vertical during the surface tilts. Figure 5B summarizes how the “relative sensory weightings” of the CoM orientation to the surface and to gravity were calculated. Sensory weighting was defined as differences between the integrals of change in surface orientation and change in CoM angle orientation during the periods of surface tilt. A weighting to gravity (Wg) of 100% indicates that subjects maintained alignment of the CoM angle stable with respect to gravitational vertical (space) during surface rotations. In contrast, a Wg of 0% indicates that subjects maintained alignment of the CoM stable with respect to the surface during surface rotations. Thus, the percent weighting to gravity vs. to the surface was normalized across the different surface tilt velocity conditions and excluded falls, which occurred toward the end, or after the end, of the surface tilt.
Fig. 5.
Relative weighting of gravity vs. to the surface as a reference for postural control during surface tilts with eyes closed. A: displacement of the body CoM during the period of surface tilts for subjects with vestibular loss (thick black lines) and control subjects (thick gray lines) is compared across tilt velocity conditions starting with the slowest, 0.5 deg/s condition (top) and progressing to the fastest, 32 deg/s condition (bottom). The CoM is shown as pitch angular rotation, anchored at the ankle joint, with backward tilts downward. B: the method used to quantify the proportion that a subject depended on gravity (Wg) vs. the support surface (Ws) as a reference for keeping the body's CoM stable during surface tilts. C: effect of surface tilt velocity on the mean (± SE) relative weighting to gravity (Wg) for subjects with vestibular loss (black line) and control subjects (gray line) in the eyes-closed condition.
Postural responses were measured to determine whether the backward body CoM, displacements as a result of surface tilt, reflected active orientation of the body to maintain a constant body-to-surface relationship or whether it reflected passive body motion because of a failure to respond quickly enough to the surface perturbation. Postural response magnitude was quantified as the maximum, initial forward CoP displacement, which was destabilizing. We also measured the latency to change from forward, to a backward, stabilizing CoP displacement direction. Postural response latency was measured as onset of postural muscle electromyography with respect to the onset of surface tilt and to change in CoP direction. We calculated the percent of trials with an active GAS EMG burst because GAS activity in response to toes-up rotations moves the CoP forward, which pushes the CoM backward, resulting in falls (Nashner 1976). We also determined the onset latencies of GAS inhibition, TIB burst onset, and ankle dorsiflexion, all which would return the body to an equilibrium position during toes-up surface tilts. Onset latencies were determined on a trial-by-trial basis as the time when EMG activity or change in ankle angle exceeded 3 SD of the mean baseline for at least 200 ms. The baseline period was defined as the 2 s immediately preceding the onset of surface tilt.
Differences between subjects with vestibular loss and control subjects across tilt velocity conditions were analyzed using a mixed model (between group and repeated) ANOVA (2 groups × 8 velocity conditions × 2 visual conditions) for each variable.
RESULTS
Effect of Tilt Velocity on Postural Stability and Postural Alignment
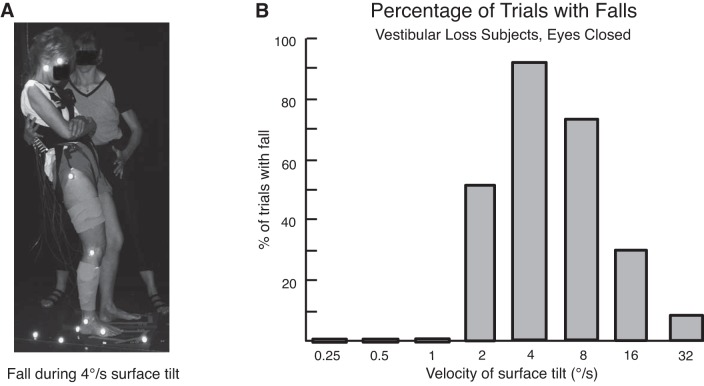
Subjects with vestibular loss were less stable than control subjects when standing on a tilting surface with their eyes closed, and their degree of postural instability depended on surface tilt velocity. In subjects with vestibular loss, surface tilts resulted in loss of balance that required a catch or a step (falls) in the majority of trials when the surface tilted at mid-range velocities of 2 to 8 deg/s (Fig. 2A). In fact, Fig. 2B shows that in the 4 deg/s condition, falls occurred in 94% (34/37) of trials. In contrast, falls did not occur when the surface rotated at the slowest velocities (0.25 to 1 deg/s), and falls occurred in only 8% (3/37) of trials at the fastest (32 deg/s) condition. Falls were always in the backward direction during 2 to 16 deg/s velocity tilts (Fig. 2A). At the fastest (32 deg/s) tilt condition, falls occurred only three times, and one of these falls was in the forward direction. Subjects with vestibular loss never fell when their eyes were open. Control subjects never fell when their eyes were open or closed.
Fig. 2.
A: example of a subject with vestibular loss falling backward during a surface tilt of 4 deg/s. Note the larger trunk, than shank, backward tilt. B: percentage of trials that resulted in a fall is compared across tilt velocities for subjects with vestibular loss in the eyes-closed condition. A fall was defined as either being caught by the research assistant or by taking a step to maintain balance. Subjects with vestibular loss did not fall when their eyes were open. Control subjects did not fall.
The velocity of surface tilt influenced not only the incidence of falls in subjects with vestibular loss, but it also influenced the initial direction and peak amplitude of trunk displacement (Fig. 3). In control subjects, regardless of whether their eyes were open or closed, initial trunk sway was in the backward direction at slow-tilt velocities (≤4 deg/s) and in the forward direction at fast-tilt velocities (≥8 deg/s). In contrast to control subjects, subjects with vestibular loss with their eyes closed showed backward trunk displacement at all tilt velocities, except during the fastest (32 deg/s) tilt condition.
Fig. 3.
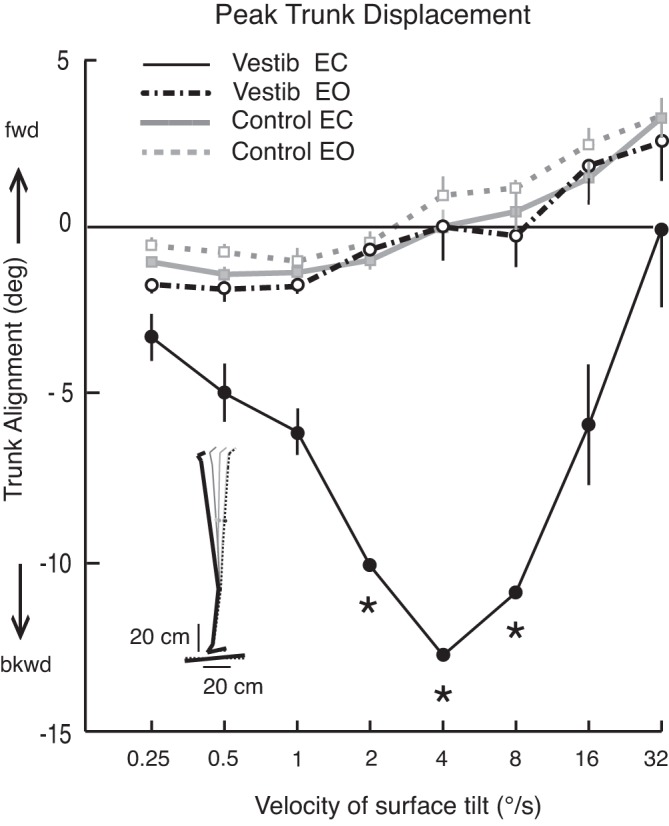
Effect of surface tilt velocity on peak trunk displacement (means ± SE) is compared between subject groups (subjects with vestibular loss are depicted with black lines and symbols, control subjects are depicted with gray lines and symbols) and visual conditions (open circles, eyes-open; filled circles, eyes-closed). Note that the greatest difference between subjects with vestibular loss and control subjects took place at mid-range velocities of 2 to 8 deg/s when subjects' eyes were closed. In fact, more than 50% of trials at 2 to 8 deg/s resulted in loss of balance, and peak backward lean at these velocities could only be estimated. The estimated values are indicated by asterisks and represent the peak trunk lean at the time that subjects were caught by the research assistant.
The amplitude of backward trunk displacement in subjects with vestibular loss was greatest at the mid-range velocities of 2 to 8 deg/s, with gradually less-backward trunk displacement as tilt velocity increased or decreased (Fig. 3). Peak trunk displacement was statistically significantly different between the control and vestibular loss groups for all velocities of tilt with eyes closed (but not eyes open), between 1 and 16 deg/s (P < 0.01). Trunk displacement was not significantly different between subjects with vestibular loss with their eyes open and control subjects with their eyes open or closed (Fig. 3).
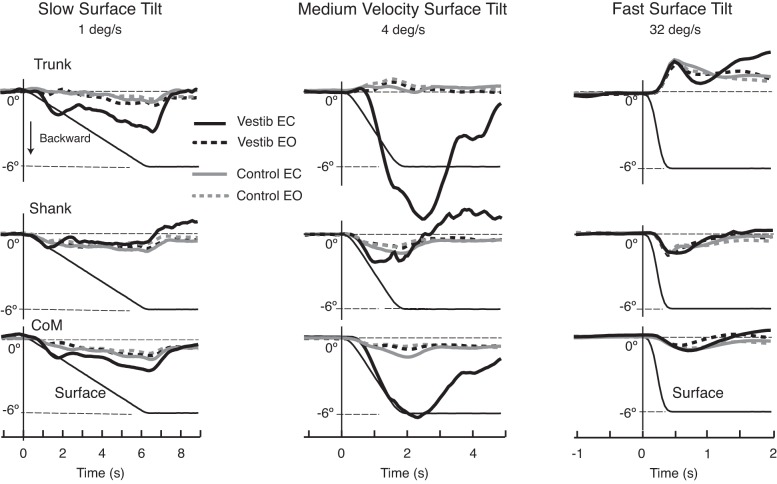
Representative examples of trunk, shank, and CoM kinematics in response to a slow (1 deg/s), medium (4 deg/s), and fast (32 deg/s) tilt velocity in Fig. 4 show that body kinematics were similar among groups at all velocities when subjects' eyes were open. With eyes closed, however, the kinematics of control subjects and those with vestibular loss were similar only at the slow- and fast-tilt velocities, but very different at the medium-tilt velocities. In response to the slow, 1 deg/s surface tilt, both groups leaned their trunk, shank, and CoM backward, although the subjects with vestibular loss tracked the backward tilt of the surface longer than control subjects did (P < 0.01; Fig. 4, left). In contrast, at the fastest, 32 deg/s surface tilts, both groups flexed their hips, resulting in forward trunk with backward shank and CoM tilt (Fig. 4, right). At medium velocities (2–8 deg/s), subjects with vestibular loss with their eyes closed showed very large amplitude trunk and CoM, but not shank, backward displacement, and falls, whereas control subjects kept their trunks relatively upright with a similar amount of backward shank and CoM displacements (Fig. 4, middle).
Fig. 4.
Changes in postural responses during slow (1 deg/s), medium (4 deg/s), and fast (32 deg/s) surface tilts. The plots are aligned with onset of surface tilt. For each velocity, the group average trunk, shank, and CoM displacement during surface tilts is compared between groups (subjects with vestibular loss in black, control subjects in gray) and between visual conditions (dashes, eyes-open; solid lines, eyes-closed). Notice that the largest between-group differences occurred when the surface tilted at 4 deg/s and subjects' eyes were closed (solid lines). The thin black line in all figures is the platform 6 deg rotation displacement. The onset and offset of surface displacement was defined based on first detectable change in surface acceleration.
The CoM rate of rotation in subjects with vestibular loss was approximately the same as the rate of rotation of the support surface at 4 deg/s (P > 0.05, Fig. 4). In contrast, backward trunk tilt was larger and faster than the rate of surface tilt (P < 0.01). Unlike the backward trunk and CoM tilts, the lower legs of subjects with vestibular loss did not tilt backward throughout the entire period of surface rotation, but instead, the ankle and knee flexed (Fig. 2A and Fig. 4 middle).
Postural Alignment with Respect to the Support Surface Vs. to Gravity
Backward CoM tilts with eyes closed in the group of subjects with vestibular loss tracked the velocity and amplitude of surface tilt during tilts of 0.5 to 8 deg/s, maintaining an approximately constant CoM orientation with respect to the support surface (Fig. 5A). Backward CoM tilts were largest at mid-range velocities of 4 and 8 deg/s, and very small at the fastest (32 deg/s) and slowest (0.5 deg/s) tilt velocities (Fig. 5A). In contrast to subjects with vestibular loss, control subjects tilted their CoM backward approximately the same 0.5 deg, regardless of the surface tilt velocity (P > 0.05).
A gravitational weighting factor (Wg) was calculated for the eyes-closed conditions to quantify the degree to which each subject aligned their CoM with respect to gravity vs. to the support surface. Wg was calculated separately for each trial and is shown as the shaded area in the two examples (Fig. 5B). Wg is the difference between the area of support surface rotation (solid line) and area of CoM rotation (dotted line), as a percentage of the area of support surface rotation: Wg = 100∗(∫ αsurf − ∫ αCoM)/∫ αsurf in Fig. 5B. A relative Wg near 0% indicates that the amplitude of angular CoM displacement matched that of surface tilt so the CoM was stabilized with respect to the support surface. A relative Wg near 100% indicates that the CoM was stable with respect to gravity as the surface tilted.
Figure 5C shows that control subjects heavily favored a gravity-based reference in controlling their CoM for all velocity conditions, with Wg ranging from 75 to 90%. The upper plot in Fig. 5B from a representative, control subject shows what happens when the body's CoM stays nearly aligned with respect to gravity during surface tilt; in this example, CoM angular displacement is very small, so Wg equals 90%.
In contrast to the control subjects, subjects with vestibular loss have a large relative weighting to the surface for the 2, 4, and 8 deg/s conditions (Fig. 5C). The plot from a representative subject with vestibular loss (Fig. 5B) shows what happens when the CoM rotates in the same direction and amplitude as the support surface; in this example, Wg equals 30% and the support surface (Ws) equal equals 70%. Subjects with vestibular loss showed a similar, high weighting to gravity similar to that of control subjects at the very slow (Wg = 60%) and very fast (Wg = 90%) tilt velocities, respectively (P > 0.05). However, at the mid-range velocities of 1–8 deg/s, subjects with vestibular loss showed predominant postural weighting to a surface-reference (P < 0.001). In fact, during 2 and 4 deg/s surface tilts, subjects with vestibular loss weighted their postural orientation to gravity (Wg) less than 10%.
Effect of Surface Tilt Velocities on Postural Responses
To determine whether subjects with vestibular loss were failing to respond to the surface tilt and passively falling backward or, instead, were actively orientating to the rotating support surface, we examined changes in the activity of postural muscles and the resulting CoP and ankle dorsiflexion motion during the surface rotations. Postural responses suggest that instability and falls in response to the 4 deg/s surface tilts were the result of abnormal, active postural responses and not passive tilts due to lack of postural responses for several reasons.
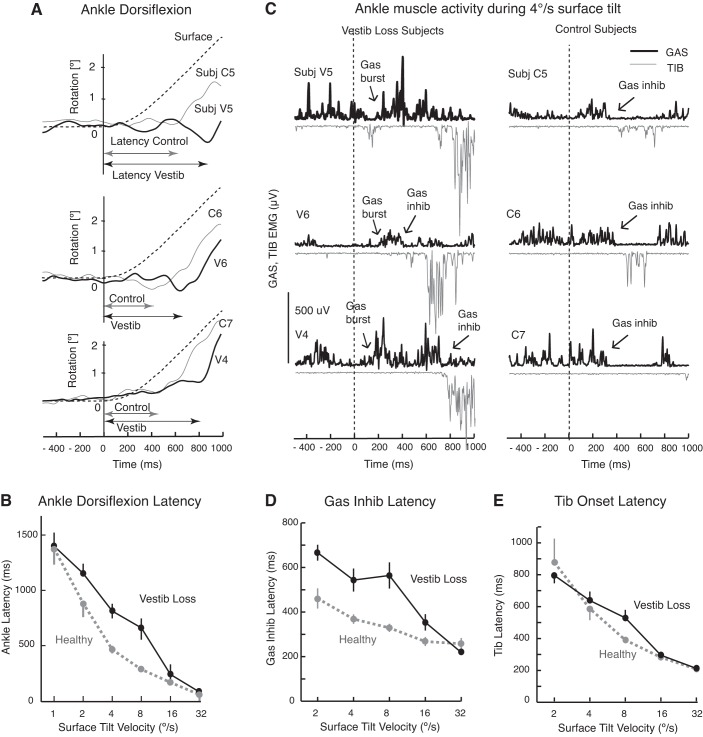
First, the subjects with vestibular loss had later onsets of corrective ankle dorsiflexion than the control subjects. Close inspection of the onset of ankle dorsiflexion latencies in three representative control subjects and three subjects with vestibular loss in Fig. 6A and group means in Fig. 6B shows that the ankle joint began its stabilizing dorsiflexion significantly later in subjects with vestibular loss than in control subjects during the medium-velocity surface tilts of 4 and 8 deg/s (P < 0.001 and P < 0.01, respectively). The difference in ankle joint onset latencies for the 2 deg/s condition approached significance (P = 0.096). In other words, subjects with vestibular loss tended to keep a constant ankle angle longer while the surface was tilting at the medium-tilt velocities than control subjects did. Allowing the ankle to dorsiflex when the surface tilts underneath the body allows the body to stay upright against gravity rather than lean backward.
Fig. 6.
Evidence of active postural destabilization in subjects with vestibular loss with their eyes closed. A: examples of delays to initiation of ankle dorsiflexion in response to 4 deg/s surface tilts from three representative subjects with vestibular loss (V4, V5, and V6) compared with three control subjects (C5, C6, and C7). B: comparison of latencies to ankle dorsiflexion after the surface began to tilt between vestibular loss (black tracings) and control groups (gray tracings) across surface tilt velocities (group means ± SE). C: gastrocnemius (GAS) and anterior tibialis (TIB) muscle bursts and inhibition in response to 4 deg/s surface tilts from the same representative subjects as in A. D and E: average latency of GAS inhibition and TIB activation between the groups, respectively, but we could not reliability measure these for the slowest surface tilts (0.5 deg/s). Note the large GAS burst and late or incomplete GAS inhibition in the subjects with vestibular loss. Standard errors were sometimes too small to be observed around the means in B, D, and E.
Second, the delayed onset of ankle dorsiflexion during medium-velocity tilts could be explained by a difference in the active postural response in subjects with vestibular loss compared with control subjects. Subjects with vestibular loss showed larger and prolonged activation of GAS ankle muscle activity in the initial response to surface tilt as observed in three representative subjects (Fig. 6C). Although control subjects occasionally showed a small GAS activation, they consistently inhibited background GAS activity during the toes-up rotation, unlike subjects with vestibular loss, as can be observed in data from three representative control subjects in Fig. 6C. Inhibition of background GAS activity was significantly delayed in subjects with vestibular loss at the medium-tilt velocities of 2, 4, 8, and 16 deg/s (all post hoc tests P < 0.01, Fig. 6D). In addition, the percent of trials with a GAS burst was consistently larger in subjects with vestibular loss (range, 23–60%) compared with that in control group (range, 0–22%). In contrast to the abnormal postural responses during medium-velocity surface tilts, the timing of GAS activity was similar between groups during fast, 32 deg/s surface tilts (Fig. 6, D and E). Unlike GAS, onset of stabilizing TIB muscle activity in response to surface tilts were not significantly different between groups, except at the 8 deg/s velocity condition, in which subjects with vestibular loss showed later TIB onset latencies (Fig. 6E, P < 0.01).
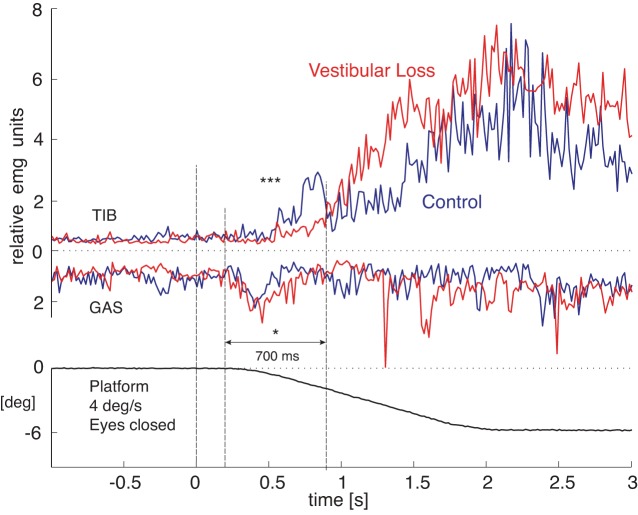
Third, integrated GAS EMG activity that destabilized posture was larger in subjects with vestibular loss than in control subjects (916 ± 229 vs. 698 ± 136 relative units × ms, P = 0.03). In addition, the integrated TIB EMG activity to stabilize posture was much smaller in subjects with vestibular loss than control subjects (324 ± 154 vs. 882 ± 277 relative units × ms, P < 0.0001). Figure 7 compares the group-averaged EMG activity for the 4 deg/s surface tilt that resulted in falls in every subjects with vestibular loss.
Fig. 7.
Group-averaged TIB and GAS electromyographic (EMG) activity in response to the 4 deg/s surface tilt resulting in a fall in subjects with vestibular loss and their eyes closed. The group of subjects with vestibular loss (red lines) showed significantly less TIB integrated EMG (IEMG) than the control group (black lines, ***P < 0.001) and significantly more GAS IEMG activity (*P < 0.05) during the initial postural response (200–900 ms after onset of surface tilt). Each subjects' contribution to the group average was from their mean of three responses. EMG integrals were normalized to the mean value 1 s prior to platform rotation onset to 1.5 s after platform onset to control for individual differences in EMG amplitudes.
Fourth, as a consequence of their larger GAS and smaller TIB activity, subjects with vestibular loss showed a significantly larger and longer lasting initial forward CoP displacement in response to the surface tilt than control subjects did (peak CoP displacements of 2.19 ± 0.19 cm for subjects with vestibular loss and 0.67 ± 0.17 cm for control subjects, P < 0.001). In addition, the destabilizing forward CoP displacement reversed direction significantly later in subjects with vestibular loss (latencies of 510.1 ± 28.3 ms for subjects with vestibular loss and 394.0 ± 20.8 ms for control subjects, P < 0.01) during 4 deg/s rotations. The larger and longer-lasting forward-directed CoP displacements reflects destabilizing ankle plantar flexor torque that actively pushed the body backward.
DISCUSSION
This study presents the novel finding that vestibular information is critical for human postural control only within a specific, mid-range of tilt velocities (2–8 deg/s). Our results also show that subjects with vestibular loss fall when they stand on a tilting surface because they actively produce destabilizing postural responses, not because of a passive fall due to a lack of a postural response.
Velocity Dependence
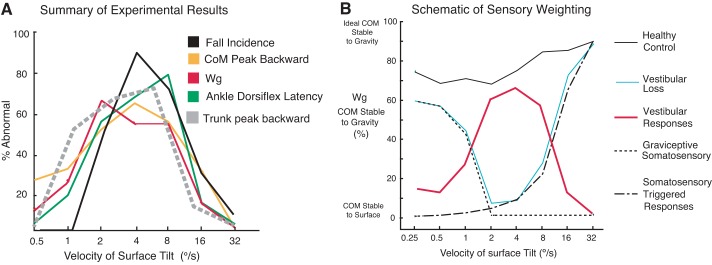
This study showed a U-shaped relationship in postural stability across surface tilt velocities in subjects with vestibular loss with their eyes closed. Figure 8A summarizes our primary results by superimposing differences in group average performance measures between subjects with vestibular loss and control subjects across tilt velocities. Measures of postural stability, postural responses, and relative sensory weighting to a gravity reference frame in subjects with vestibular loss were near normal only at the slowest and fastest tilt velocities. Postural behavior was most abnormal in subjects with vestibular loss when the surface tilted at 2–8 deg/s. Subjects with vestibular loss fell even though the direction of the surface tilt was predictable, allowing them to prepare for a toes-up surface tilt. They fell only during 2–16 deg/s tilts even though the velocity conditions were randomized. Unlike subjects with vestibular loss, control subjects with their eyes closed never fell during surface tilts, and had similar postural stability across all tilt velocities.
Fig. 8.
A: summary of experimental results as % differences in group average performance measures between subjects with vestibular loss and control subjects across tilt velocities in the eyes-closed conditions. B: a schematic of approximate sensory weightings of vestibular and somatosensory inputs for postural orientation across surface tilt velocities based on our results. Our Wg data from groups of control subjects (black solid line) and subjects with vestibular loss (blue solid line) are summarized. The graviceptive somatosensory influence (horizontal dotted lines) and somatosensory-triggered postural responses (vertical dashed lines) follow the ability of our group of subjects with vestibular loss to maintain stability at low and high velocities, respectively. We propose that vestibular responses (red line = control data − vestibular loss Wg data) are most critical at tilt velocities too fast for control by graviceptive somatosensory inputs and too slow for control by somatosensory-triggered postural responses.
At these critical tilt velocities resulting in postural instability, the trunk and CoM, but not the lower leg, tilted backward as the surface began to tilt and subjects subsequently fell backward. Trunk tilt, but not leg tilt, during surface tilts is consistent with the notion that vestibular information may be especially important for upper body orientation to gravity and that proprioception is used to orient the trunk, not the legs, with respect to the surface (Buchanan and Horak 1999, 2001; Creath et al. 2008; Mergner et al. 2002).
When the surface tilted slowly, the trunks and CoM of all subjects tilted backward, but the backward displacement was more pronounced in subjects with vestibular loss when their eyes were closed. Our results are consistent with previous studies showing that control subjects partially orient their bodies to the surface during very slow transient surface tilts (Ivanenko et al. 1997; Maurer et al. 2006; Peterka and Loughlin 2004) or low-frequency continuous surface rotations (Peterka and Loughlin 2004), and that subjects with vestibular loss show even larger body tilt in the direction of tilt (Creath et al. 2002). When the surface tilted most quickly (32 deg/s), the postural responses of subjects with vestibular loss were more like the responses of control subjects than at any other tilt velocity. At this fast tilt velocity, the timing and direction of body displacements and the onsets of muscle activity were similar for the two groups. In our study, fast surface tilts resulted in hip flexion and forward trunk tilt similar to those reported in previous studies (Carpenter, et al. 2001).
The unique characteristics of our surface tilt perturbations may have helped identify a specific vestibular dependence at mid-range velocities. Our surface tilts had smoothed onsets and offsets to avoid activating short-latency, somatosensory-triggered postural responses to rapid accelerations, particularly for velocities ≤4 deg/s (Allum and Pfaltz 1985; Diener et al. 1984; Inglis et al. 1994; Macpherson et al. 2007). In addition, the slower surface tilts of ≤4 deg/s had periods of constant velocity, allowing us to analyze how much the body aligned to the tilting surface. The range of tilt velocity conditions in our study is also much wider than any other studies that used either faster (Carpenter et al. 2001) or slower (Hlavacka et al. 1996) sets of velocities to study postural control on tilting surfaces in subjects with vestibular loss.
Consistent with previous studies, vision effectively compensated for the lack of vestibular information across all transient tilt velocities (Allum and Pfaltz 1985; Creath et al. 2008; Maurer et al. 2000; Mergner et al. 2009; Peterka and Benolken 1995). In contrast to transient tilts, subjects with vestibular loss and their eyes closed cannot maintain their balance when the surface translates sinusoidally at a wide range of velocities, and only half of the subjects maintained balance with their eyes open (Buchanan and Horak 2001). On a continuously oscillating surface, subjects with vestibular loss fell because of increasing trunk drift, even though their legs showed a normal stabilizing postural response (Buchanan and Horak 2001). Stabilizing the legs while the trunk fell backward is similar to how our subjects fell during medium-velocity surface tilts.
Possible Reasons for Velocity-Specific Instability
Figure 8B presents a schematic that summarizes how different types of sensory information may contribute to postural control across a range of surface tilt velocities. Our results show that vestibular information is critical at medium velocities (2–8 deg/s), suggesting that somatosensory (proprioceptive, joint, and cutaneous) information is insufficient within this velocity range. During very-slow-tilt velocities, subjects with profound bilateral vestibular loss with their eyes closed can rely upon very-low-threshold somatosensory inputs. In fact, a somatosensory “graviceptor system” that operates at very low frequencies (or velocities) has been proposed (Cnyrim et al. 2009; Maurer et al. 2000; Mergner et al. 2009). Graviceptors consist of gravitational information from alimentary pressure sensors (Vaitl et al. 2002) and Golgi tendon organs, which provide torque-related information; deep-pressure sensors under the feet; and otoliths, when available (Maurer et al. 2006). In contrast, during very-fast-tilt velocities, both control subjects and subjects with profound bilateral vestibular loss with their eyes closed can rely upon high-threshold muscle spindle inputs that trigger medium-latency, automatic postural responses (Inglis et al. 1994; Nardone and Schieppati 2004; Stapley et al. 2002) or they do not have enough time to become unstable because of inertia.
Why are subjects with vestibular loss particularly unstable when the surface tilts at medium velocities but not at slower or faster velocities? Possible explanations include velocity-specific differences in 1) sensory system dynamics, 2) biomechanical constraints, and 3) central interpretation of multisensory inputs during postural sway.
Sensory dynamics.
The vestibular and somatosensory systems preferentially operate at different velocities of postural sway because of differences in sensory thresholds and sensitivities (Mergner et al. 2003). For example, vestibular semicircular canal activation has higher thresholds than somatosensory afferents, consistent with increasing importance of vestibular inputs as the rate of surface tilt increases (Wardman et al. 2003). Vestibular canal thresholds have been reported at 1 deg/s (Maurer et al. 2006), and our subjects with vestibular loss began to fall at tilt velocities faster than this. In contrast, at and below tilts of 1 deg/s, the subjects with vestibular loss who had their eyes closed may have stabilized their posture using the graviceptive system. Foot proprioception and/or pressure sensation may be important at low frequencies as suggested by the finding that ischemic block at the ankle results in increased body sway at slow, but not fast, tilts (Diener and Dichgans 1988).
Gradually increasing use of vestibular information with increasing postural sway amplitude has also been described in previous studies of subjects standing on continuously rotating surfaces (Creath et al. 2008; Peterka and Benolken 1995). What is surprising in our study of discrete surface tilts is that vestibular information became less important, again, during fast-tilt velocities. Although the fast tilts were above threshold for somatosensory-triggered automatic postural responses, we did not observe short-latency (50 ms) postural responses at any velocities because we designed our perturbation to have very slow initial accelerations and decelerations (Diener et al. 1984; Nashner 1976). However, both our control subjects and those with vestibular loss showed destabilizing, medium-latency (100 and 105 ms) GAS postural responses at the fastest-tilt velocities (16 and 32 deg/s), as well as long-latency stabilizing TIB responses as reported previously (Allum et al. 2011; Nashner 1976).
Biomechanical constraints.
In addition to velocity-dependent differences in sensory sensitivity, a second reason for velocity dependence of postural stability in subjects with vestibular loss could be related to how biomechanical constraints affect the response of the body to the rotating surface. For example, the background stiffness of the ankle joint affects how much it will be passively dorsiflexed by the force applied by the rotating surface. At medium velocities, we observed that ankle dorsiflexion began later in subjects with vestibular loss than it did in control subjects, and this delay could be related to increased stiffness. In addition, during the fast, 16 deg/s or 32 deg/s surface tilts, trunk inertia may have resisted backward movement, as suggested by the forward, rather than backward, rotation of the trunk. The rapid, passive forward trunk rotation may have reduced backward displacement of the body's CoM and may have provided additional somatosensory cues about the direction of postural perturbation.
Central multisensory interpretation.
A third, most likely reason why subjects with vestibular loss were particularly unstable at medium velocities of tilt may be related to misinterpretation of multisensory inputs during postural sway. Tilt velocities of 2–4 deg/s for 6-deg surface tilts are similar to the body's natural frequency when standing quietly (Chiari et al. 2002). Because the surface was tilting at similar velocities as a body sway, the nervous system may have difficulty distinguishing between surface and body tilt without a gravitational reference. Somatosensory feedback alone may be inadequate to distinguish surface tilt from body sway, so the vestibular system helps determine whether the surface is stable with respect to space and can, therefore, be used as a postural reference (Mergner et al. 2002). In fact, as the trunk leaned backward during medium-velocity surface tilts, subjects with vestibular loss maintained a consistent orientation of their body CoM to the surface instead of stabilizing their postural orientation with respect to gravity, even at the expense of falling (Fig. 4A and Fig. 5).
If vestibular information is critical for distinguishing surface tilt from body tilt, why didn't subjects fall during the very slow tilt velocities of ≤1 deg/s? In fact, body CoM did closely track the rotating surface during the initial 1–2 s of surface tilt (Fig. 4). Although the CoM continued to tilt backward slightly for the remaining duration of the 6-deg surface tilt, after the surface tilted about 1 deg, the CoM slowed its tilt dramatically (Fig. 4A). In other words, even subjects without vestibular function or vision available could brake their backward CoM tilt using somatosensory information. Another study shows a similar saturation of leaning in control subjects with a longer latency brake in subjects with vestibular loss during sinusoidal rotations on an inclined surface (Schweigart and Mergner 2008). Mergner proposes a “sensory reweighting switch” from a surface reference to a gravitational reference to account for this switch from a “body on support” to “body in space” orientation. Based on a multisegmental postural model, they propose that subjects with vestibular loss use a local proprioceptive loop to stabilize their bodies with respect to the tilting surface and later, a second somatosensory graviceptive central signal indicating “foot in space,” probably from foot pressure receptors, to prevent the body from continuing to tilt backward (Mergner et al. 2009). Control subjects brake their tilt to switch to a body-in-space reference more quickly because they have use of both somatosensory and vestibular graviception mechanisms (Mergner et al. 2009).
Destabilizing Postural Responses at Medium Velocities
Subjects with vestibular loss may be falling during medium-velocity surface tilts for two reasons. First, without vestibular information, subjects may fail to detect the postural perturbation that results from the surface tilt, and therefore fail to produce a postural stabilizing response in time to prevent falling. Alternatively, subjects may actively respond to the surface tilt, but produce a destabilizing postural response that contributes to the fall because of their misinterpretation of somatosensory information.
Results of our study agree with those of previous studies suggesting that subjects with vestibular loss are not simply failing to respond, but are actively contributing to their backward falls (Macpherson et al. 2007). The abnormal ankle muscle activity, abnormal direction and magnitude of surface reactive forces, and delay of stabilizing ankle dorsiflexion during medium-velocity surface tilts suggests that subjects with vestibular loss are actively contributing to their own falls. Subjects with vestibular loss showed a burst of GAS activity when control subjects showed a period of complete GAS inhibition during the initial response to the surface rotation. Unlike control subjects, the GAS burst in subjects with vestibular loss resulted in a plantar flexor torque that caused forward displacement of the CoP. This plantar flexion significantly delayed the ankle dorsiflexion observed in control subjects when their GAS was inhibited. This lack of dorisflexion thereby resulted in an unchanging orientation of their lower legs to the surface for several hundred milliseconds longer than control subjects.
The tilt of the lower legs with the surface started the backward lean of the trunk. Backward trunk leaning was accelerated both by the effects of gravity and by the addition of large TIB activation that dorsiflexed the ankles, returning the lower legs, but not the upper body, toward upright. Thus, although it appears that subjects with vestibular loss attempted to align their lower legs to vertical, their trunks continued to tilt backward (Carpenter et al. 2001). Platform deceleration could also potentially contribute to backward falls as observed in cats with bilateral vestibular loss (Macpherson et al. 2007). This explanation for falling is unlikely in our study because subjects tended to fall at similar times regardless of the differently timed surface decelerations in the 2, 4, 8, and 16 deg/s velocity conditions.
Our finding that subjects with vestibular loss produce destabilizing postural responses that exacerbate falling during discrete surface tilts is consistent with an early study of the effects of 15 deg/s toes-up surface tilts in a few subjects with vestibular loss (Nashner 1976). In Nashner's study, control subjects gradually reduced destabilizing GAS activity with repeated rotations, but the subjects with vestibular loss did not show this adaptation. By extending the range of tilt velocities, we identified an important inhibition of GAS in control subjects and show that even slower surface tilt velocities resulted in more instability in subjects with vestibular loss, even when they had 64 trials to adapt to the toes-up rotations (Carpenter et al. 2001).
An active destabilizing response to surface tilts has also been reported in cats standing on a tilting surface (Macpherson et al. 2007). Like our human subjects, the cats showed lack of inhibition of GAS activity to surface tilts, contributing to falling in the direction of surface tilt. Unlike our study in which vision was able to effectively compensate for lack of vestibular information, the cats fell even when vision was available. However, the cats were studied in the acute state, 1–3 wk after labyrinthectomy, and showed postural instability even in quiet stance, whereas our subjects were studied at least 2 yr after loss of vestibular function, were very stable in quiet stance (even with their eyes closed), and thus were able to compensate with vision as a postural reference.
A limitation of the study is that we did not record data from a large number of muscles that may be activated differently in people with vestibular loss than age-matched controls, and that they may be contributing to destabilization in addition to abnormal activation of the GAS (Carpenter et al. 2001).
Summary
We propose that the most important role of the vestibular system in stance posture is to orient the upper body with respect to gravity, especially when the surface is unreliable as a postural reference. Our study identifies critical velocities for vestibular control of human posture. These critical velocities (2–8 deg/s) may be too fast for slow, graviceptive control and too slow for triggering fast, automatic postural responses from proprioceptive inputs. When patients with vestibular loss fall, they contribute to their falls by producing abnormal postural responses that attempt to maintain upper body alignment with respect to the changing surface reference. Patients with vestibular loss are unstable when they stand on a moving surface not because they fail to trigger fast, automatic postural responses, but because they fail to quickly switch from a surface-reference frame to a gravity reference frame for postural orientation.
GRANTS
Supported by a National Institute on Deafness and Other Communication Disorders grant and National Institutes on Aging Grant AG0006457 to F. Horak, and by a Foundation of American Physical Therapy Association Scholarship to J. Kluzik.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.B.H. and F.H. conception and design of research; F.B.H. and F.H. performed experiments; J.K. and F.H. analyzed data; F.B.H. and F.H. interpreted results of experiments; J.K. and F.H. prepared figures; F.B.H. drafted manuscript; F.B.H. edited and revised manuscript; F.B.H. and F.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Charles Russell for excellent technical assistance, Dr. Robert Peterka for providing results of vestibular function tests and for helpful discussions, and Sophie Stapley and Sharna Clark-Donovan for assistance with data collection.
REFERENCES
- Allum JH, Pfaltz CR. Visual and vestibular contributions to pitch sway stabilization in the ankle muscles of normals and patients with bilateral peripheral vestibular deficits. Exp Brain Res 58: 82–94, 1985. [DOI] [PubMed] [Google Scholar]
- Allum JH, Tang KS, Carpenter MG, Oude Nijhuis LB, Bloem BR. Review of first trial responses in balance control: influence of vestibular loss and Parkinson's disease. Hum Mov Sci 30: 279–295, 2011. [DOI] [PubMed] [Google Scholar]
- Buchanan JJ, Horak FB. Emergence of postural patterns as a function of vision and translation frequency. J Neurophysiol 81: 2325–2339, 1999. [DOI] [PubMed] [Google Scholar]
- Buchanan JJ, Horak FB. Vestibular loss disrupts control of head and trunk on a sinusoidally moving platform. J Vestib Res 11: 371–389, 2001. [PubMed] [Google Scholar]
- Carpenter MG, Allum JH, Honegger F. Vestibular influences on human postural control in combinations of pitch and roll planes reveal differences in spatiotemporal processing. Exp Brain Res 140: 95–111, 2001. [DOI] [PubMed] [Google Scholar]
- Chandler R, Clauser C, McConville J, Reynolds H, Young J. Investigation of the Inertial Properties of the Human Body. Springfield, VA: National Technical Information Service, 1975. [Google Scholar]
- Chiari L, Rocchi L, Cappello A. Stabilometric parameters are affected by anthropometry and foot placement. Clin Biomech 17: 666–677, 2002. [DOI] [PubMed] [Google Scholar]
- Cnyrim C, Mergner T, Maurer C. Potential roles of force cues in human stance control. Exp Brain Res 194: 419–433, 2009. [DOI] [PubMed] [Google Scholar]
- Creath R, Kiemel T, Horak F, Jeka JJ. Limited control strategies with the loss of vestibular function. Exp Brain Res 145: 323–333, 2002. [DOI] [PubMed] [Google Scholar]
- Creath R, Kiemel T, Horak F, Jeka JJ. The role of vestibular and somatosensory systems in intersegmental control of upright stance. J Vestib Res 18: 39–49, 2008. [PMC free article] [PubMed] [Google Scholar]
- Diener HC, Dichgans J. On the role of vestibular, visual and somatosensory information for dynamic postural control in humans. Prog Brain Res 76: 253–262, 1988. [DOI] [PubMed] [Google Scholar]
- Diener HC, Dichgans J, Bootz F, Bacher M. Early stabilization of human posture after a sudden disturbance: influence of rate and amplitude of displacement. Exp Brain Res 56: 126–134, 1984. [DOI] [PubMed] [Google Scholar]
- Diener HC, Horak FB, Nashner LM. Influence of stimulus parameters on human postural responses. J Neurophysiol 59: 1888–1905, 1988. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RC, Marsden J, Lord SR, Day BL. Galvanic vestibular stimulation evokes sensations of body rotation. Neuroreport 13: 2379–2383, 2002. [DOI] [PubMed] [Google Scholar]
- Hlavacka F, Krizkova M, Horak FB. Modification of human postural response to leg muscle vibration by electrical vestibular stimulation. Neurosci Lett 189: 9–12, 1995. [DOI] [PubMed] [Google Scholar]
- Hlavacka F, Mergner T, Krizkova M. Control of the body vertical by vestibular and proprioceptive inputs. Brain Res Bull 40: 431–435, 1996. [DOI] [PubMed] [Google Scholar]
- Horak FB, Buchanan J, Creath R, Jeka J. Vestibulospinal control of posture. Adv Exp Med Biol 508: 139–145, 2002. [DOI] [PubMed] [Google Scholar]
- Horak FB, Nashner LM, Diener HC. Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res 82: 167–177, 1990. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Horak FB, Shupert CL, Jones-Rycewicz C. The importance of somatosensory information in triggering and scaling automatic postural responses in humans. Exp Brain Res 101: 159–164, 1994. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Macpherson JM. Bilateral labyrinthectomy in the cat: effects on the postural response to translation. J Neurophysiol 73: 1181–1191, 1995. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Shupert CL, Hlavacka F, Horak FB. Effect of galvanic vestibular stimulation on human postural responses during support surface translations. J Neurophysiol 73: 896–901, 1995. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Levik YS, Talis VL, Gurfinkel VS. Human equilibrium on unstable support: the importance of feet-support interaction. Neurosci Lett 235: 109–112, 1997. [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Everaert DG, Stapley PJ, Ting LH. Bilateral vestibular loss in cats leads to active destabilization of balance during pitch and roll rotations of the support surface. J Neurophysiol 97: 4357–4367, 2007. [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Inglis JT. Stance and balance following bilateral labyrinthectomy. Prog Brain Res 97: 219–228, 1993. [DOI] [PubMed] [Google Scholar]
- Maurer C, Mergner T, Bolha B, Hlavacka F. Vestibular, visual, and somatosensory contributions to human control of upright stance. Neurosci Lett 281: 99–102, 2000. [DOI] [PubMed] [Google Scholar]
- Maurer C, Mergner T, Peterka RJ. Multisensory control of human upright stance. Exp Brain Res 171: 231–250, 2006. [DOI] [PubMed] [Google Scholar]
- Mergner T. Modeling sensorimotor control of human upright stance. Prog Brain Res 165: 283–297, 2007. [DOI] [PubMed] [Google Scholar]
- Mergner T, Maurer C, Peterka RJ. A multisensory posture control model of human upright stance. Prog Brain Res 142: 189–201, 2003. [DOI] [PubMed] [Google Scholar]
- Mergner T, Maurer C, Peterka RJ. Sensory contributions to the control of stance: a posture control model. Adv Exp Med Biol 508: 147–152, 2002. [DOI] [PubMed] [Google Scholar]
- Mergner T, Rosemeier T. Interaction of vestibular, somatosensory and visual signals for postural control and motion perception under terrestrial and microgravity conditions–a conceptual model. Brain Res Brain Res Rev 28: 118–135, 1998. [DOI] [PubMed] [Google Scholar]
- Mergner T, Schweigart G, Fennell L, Maurer C. Posture control in vestibular-loss patients. Ann NY Acad Sci 1164: 206–215, 2009. [DOI] [PubMed] [Google Scholar]
- Nardone A, Schieppati M. Group II spindle fibres and afferent control of stance. Clues from diabetic neuropathy. Clin Neurophysiol 115: 779–789, 2004. [DOI] [PubMed] [Google Scholar]
- Nashner LM. Adapting reflexes controlling the human posture. Exp Brain Res 26: 59–72, 1976. [DOI] [PubMed] [Google Scholar]
- Nashner LM, Black FO, Wall C 3rd. Adaptation to altered support and visual conditions during stance: patients with vestibular deficits. J Neurosci 2: 536–544, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol 88: 1097–1118, 2002. [DOI] [PubMed] [Google Scholar]
- Peterka RJ, Benolken MS. Role of somatosensory and vestibular cues in attenuating visually induced human postural sway. Exp Brain Res 105: 101–110, 1995. [DOI] [PubMed] [Google Scholar]
- Peterka RJ, Loughlin PJ. Dynamic regulation of sensorimotor integration in human postural control. J Neurophysiol 91: 410–423, 2004. [DOI] [PubMed] [Google Scholar]
- Schweigart G, Mergner T. Human stance control beyond steady state response and inverted pendulum simplification. Exp Brain Res 185: 635–653, 2008. [DOI] [PubMed] [Google Scholar]
- Stapley PJ, Ting LH, Hulliger M, Macpherson JM. Automatic postural responses are delayed by pyridoxine-induced somatosensory loss. J Neurosci 22: 5803–5807, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitl D, Mittelstaedt H, Saborowski R, Stark R, Baisch F. Shifts in blood volume alter the perception of posture: further evidence for somatic graviception. Int J Psychophysiol 44: 1–11, 2002. [DOI] [PubMed] [Google Scholar]
- Vaughan C, Davis B, O'Connor J. Dynamics of Human Gait. Cape Town, South Africa: Kiboho Publishers, 1992. [Google Scholar]
- Wardman DL, Taylor JL, Fitzpatrick RC. Effects of galvanic vestibular stimulation on human posture and perception while standing. J Physiol 551: 1033–1042, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]