In this paper, we investigated an increase on the dose of exercise used to promote the enhancement of axon regeneration and functional recovery following peripheral nerve injury. The more strenuous exercise of walking an upwardly sloped treadmill resulted in enhanced regeneration of motor axons and muscle reinnervation, but functional recovery was poorer than found using exercise on a level treadmill or no exercise at all.
Keywords: nerve regeneration, exercise, kinematics, EMG activity
Abstract
Following peripheral nerve injury, moderate daily exercise conducted on a level treadmill results in enhanced axon regeneration and modest improvements in functional recovery. If the exercise is conducted on an upwardly inclined treadmill, even more motor axons regenerate successfully and reinnervate muscle targets. Whether this increased motor axon regeneration also results in greater improvement in functional recovery from sciatic nerve injury was studied. Axon regeneration and muscle reinnervation were studied in Lewis rats over an 11 wk postinjury period using stimulus evoked electromyographic (EMG) responses in the soleus muscle of awake animals. Motor axon regeneration and muscle reinnervation were enhanced in slope-trained rats. Direct muscle (M) responses reappeared faster in slope-trained animals than in other groups and ultimately were larger than untreated animals. The amplitude of monosynaptic H reflexes recorded from slope-trained rats remained significantly smaller than all other groups of animals for the duration of the study. The restoration of the amplitude and pattern of locomotor EMG activity in soleus and tibialis anterior and of hindblimb kinematics was studied during treadmill walking on different slopes. Slope-trained rats did not recover the ability to modulate the intensity of locomotor EMG activity with slope. Patterned EMG activity in flexor and extensor muscles was not noted in slope-trained rats. Neither hindblimb length nor limb orientation during level, upslope, or downslope walking was restored in slope-trained rats. Slope training enhanced motor axon regeneration but did not improve functional recovery following sciatic nerve transection and repair.
NEW & NOTEWORTHY
In this paper, we investigated an increase on the dose of exercise used to promote the enhancement of axon regeneration and functional recovery following peripheral nerve injury. The more strenuous exercise of walking an upwardly sloped treadmill resulted in enhanced regeneration of motor axons and muscle reinnervation, but functional recovery was poorer than found using exercise on a level treadmill or no exercise at all.
traumatic peripheral nerve injuries are common, and even though axons in injured peripheral nerves have a significant capacity for regeneration, functional recovery following peripheral nerve injury is poor (Brushart, 2011; Frostick et al. 1998). Slow axon regeneration is the most cited reason for poor functional recovery and has emerged as a target for therapies designed to treat peripheral nerve injuries (Hoke and Brushart 2010). Brief electrical stimulation and exercise are two such experimental therapies. A single hour of 20-Hz continuous electrical stimulation at the time of injury results in enhancement of both sensory and motor axon regeneration (Gordon and English 2015). After 2 wk of moderate daily treadmill walking, regenerating axons in cut peripheral nerves of mice grow more than twice as far as untreated controls (Sabatier et al. 2008) and axons of significantly more motoneurons regenerate successfully (English et al. 2009). Similar findings have been reported by other laboratories using a wide variety of different exercise regimens (reviewed in Sabatier and English 2015; Udina et al. 2011). Because brief electrical stimulation and exercise are experimental therapies that are assumed to activate neurons, they have been termed activity dependent (Udina et al. 2011).
In rats that were exercised following sciatic nerve transection and repair, modest improvements in hindblimb kinematics and patterns of extensor muscle EMG activity during locomotion were reported (Boeltz et al. 2013). However, no restoration of the timing of locomotor activity in the reinnervated tibialis anterior was noted and the ability of animals to conserve limb length during upslope walking remained compromised. This observed limitation in functional recovery prompted us to consider whether the dose of exercise that was applied was adequate and that more intense exercise might lead to even greater functional recovery (Field-Fote 2009). In prior studies of the effects of treadmill exercise on axon regeneration following peripheral nerve injury, whether conducted in our laboratory or by others, the exercise was performed on a level treadmill. One way of increasing the dose of exercise might be to perform the exercise with the treadmill inclined upward. The mechanical requirements of walking upslope are more strenuous than level walking and in intact animals, the activity of both flexor and extensor muscles is increased during upslope walking (Sabatier et al. 2011b). Whether upslope exercise increases the activity of axotomized motoneurons is not known, but when we exercised mice on an upwardly inclined treadmill after sciatic nerve transection and repair, axons of significantly more motoneurons regenerated more successfully than those found in mice after level training (Sabatier and English 2015). Irrespective of the precise mechanism, increasing the dose of exercise by conducting it on an upwardly sloped treadmill had a more potent effect on enhancing axon regeneration than level exercise.
The overall goal of this study was to evaluate whether this greater enhancement of motor axon regeneration is accompanied by greater improvement in functional outcomes. We focused on three distinct outcomes: the timing of reinnervation of muscles; the timing and extent of restoration of the monosynaptic H reflex; and kinematics and EMG activity during slope walking. A preliminary report of some of our findings has been made (Sabatier et al. 2011a).
METHODS
Animals and surgical procedures.
Experiments were conducted using 11 female Lewis rats and included experimental rats exercised on a level treadmill (LT, n = 3); and animals exercised on a treadmill with the belt inclined 20° (ST, n = 8). For statistical comparisons, these new data were compared with data from untrained rats (UT, n = 5) and from LT rats that were reported previously (Boeltz et al. 2013). Data were acquired from all rats before sciatic nerve transection and repair and were used as intact controls for comparisons within groups. Female rats and the Lewis strain were chosen because they have a reduced probability of responding to reinnervation with autotomy (Carr et al. 1992). The number of animals used was based on an a priori power sample size analysis (α = 0.05, Power = 0.8), based on the variability in the outcome measures used in this study that was observed in prior studies from our laboratory. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Emory University and conformed to the Guidelines for the Use of Animals in Research of the Society for Neuroscience.
While the animals were under isoflurane anesthesia, a bipolar stimulating cuff electrode was placed around the right tibial nerve just distal to its branching from the sciatic nerve and secured in place with sutures. This cuff electrode was used to stimulate tibial axons to evoke EMG activity to monitor the time course of muscle reinnervation. Fine wire EMG electrodes were inserted into the right tibialis anterior (TA) and soleus (SOL) muscles and secured in place by sutures. All wires were led subcutaneously to a connector mounted on the animal's skull. This electrode configuration is identical to one that has been described in more detail elsewhere (Hamilton et al. 2011). All rats were acclimated to walking on a treadmill before this implantation surgery.
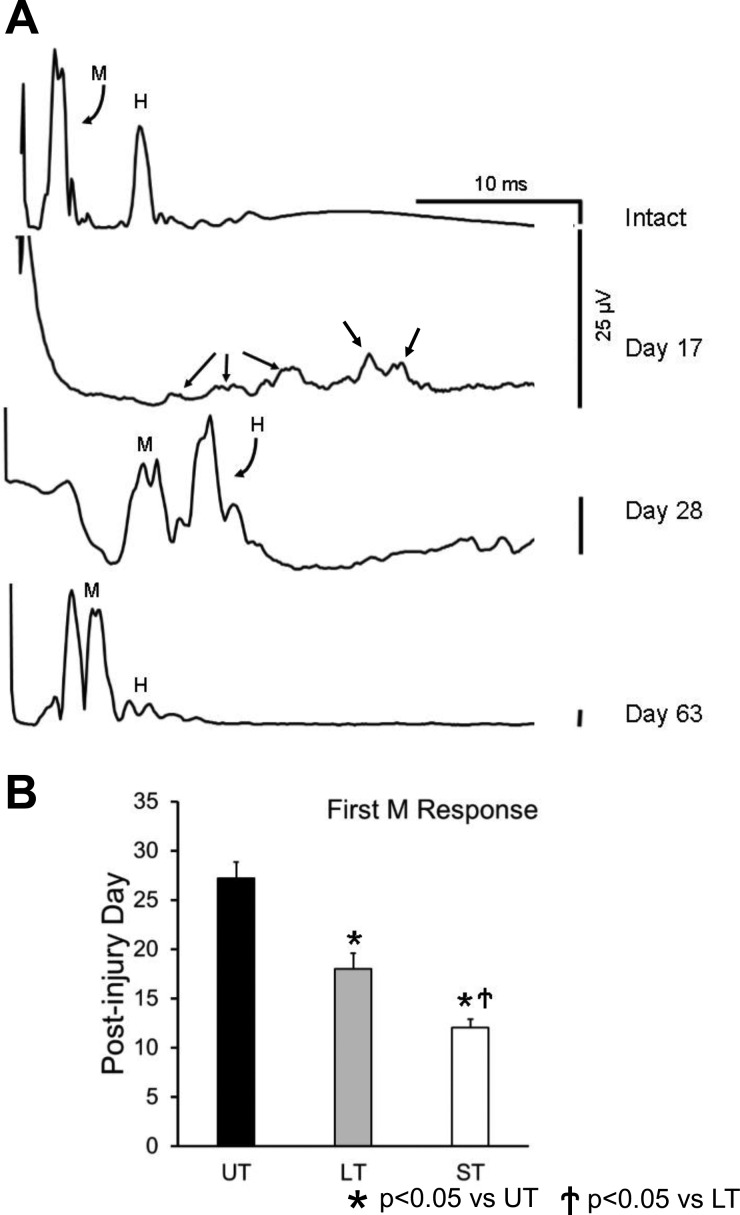
Baseline recordings of stimulus-evoked EMG activity were made in awake rats, while in their cages, 1 and 2 wk following electrode implantation. Ongoing EMG activity in SOL was monitored using a laboratory computer system, and when the average rectified activity over a 20-ms period was within a user-defined voltage window, a short (0.3 ms) constant voltage stimulus was delivered to the right tibial nerve via the implanted cuff. The evoked EMG activity in SOL was sampled at 10 kHz, beginning 20 ms before stimulation (considered background EMG activity) and ending 50 ms after the pulse, and stored on disc. The intensity of stimulation was increased very gradually to study the entire range of evoked muscle responses. To prevent fatigue, stimuli were applied no more frequently than once every 3 s. Two evoked responses were found: a short latency (typically 0.5–4.0 ms in intact rats) M response produced by direct activation, via motor axon stimulation, of any innervated muscle fibers; and a longer latency (typically 4.0–10.0 ms) H reflex, produced by monosynaptic afferent activation of motoneurons (see Fig. 2A).
Fig. 2.
A: examples of evoked EMG responses recorded from soleus muscle of a rat in response to stimulation of the tibial nerve before (intact) and at different times after transection and surgical repair of the sciatic nerve proximal to the stimulation site are shown. Potentials designated as direct muscle (M) responses and monosynaptic H reflexes are indicated. Except for the earliest posttransection time shown (17 days), all traces are from recordings made at a stimulus intensity at which the largest H reflex was evoked, so that the posttransection timing of restoration of this reflex can be observed. At all of these postinjury times, larger M responses than shown here can be evoked at larger stimulus intensities. Each trace represents the average of at least 100 stimulus presentations. This animal was treated with two wk of daily exercise on an upslope inclined treadmill. Before day 17 after sciatic nerve transection and repair, no significant evoked soleus EMG activity could be recorded from this rat. At this earliest posttransection time that an EMG response could be evoked in this rat, multiple small potentials are observed (day 17, small arrows), making differentiation of these responses as M or H impossible. Scale bars for each of the traces are the same: 25 μV. B: mean posttransection days (±SE) when a direct muscle (M) response first could be evoked are shown for untreated (UT) rats and rats treated with exercise on a level (LT) or upslope inclined (ST) treadmill (n = 5 for UT, n = 7 for LT, n = 8 for ST).
Slope walking was used as a measure of functional recovery. While rats walked on a motor-driven treadmill, either level or inclined up or down 20°, at a modest speed (16 m/min at each incline), locomotor EMG activity was recorded from SOL and TA via the implanted electrodes. Data were amplified (×1,000), band pass filtered (100-1,000 Hz), sampled at 10 kHz, and recorded to disc. Video records of the rats were obtained using a Dragonfly Express (Point Grey, Richmond, British Columbia, Canada) high-speed video camera (120 fps) and streamed to AVI files using the IEEE1394 (FireWire) interface. These video records were synchronized to the recorded EMG activity.
Once these baseline data were collected, the rats then were re-anesthetized and the right sciatic nerve was cut, with sharp scissors, above the level of the cuff electrode and then repaired by end-to-end anastomosis of the cut stumps using two 10-0 nylon sutures. Beginning on the third postoperative day, rats were treated with treadmill exercise, walking either on a level treadmill (n = 3, in LT group) or up a 20° slope (n = 8, in ST group), 1 h per day, 5 days per wk for 2 wk, at a treadmill speed of 10 m/min.
Evoked EMG activity.
The timing and extent of muscle reinnervation were studied using stimulus-evoked EMG activity. Recording sessions were initiated 1 wk after nerve repair surgery and continued for the duration of the study. Initially, evoked SOL muscle activity in ST rats was tested daily to determine the earliest evidence for muscle reinnervation. Thereafter, recordings were made weekly. The amplitude of the M response was measured as the average rectified voltage, corrected for any background EMG activity, in a defined short latency time window. In intact rats, this time window is 0.5–4.0 ms, but during reinnervation, this window changes slightly. All of the evoked potentials occur at a longer latency, probably depending on the extent of myelination of reinnervating axons (English et al. 2007). An average M response amplitude that was significantly greater (paired t-test, P < 0.05) than the prestimulus background EMG intensity was considered evidence that some muscle reinnervation had occurred. The mean posttransection day that reinnervation was first noted was compared between ST rats and data we had reported previously from UT and LT groups using a one-way ANOVA. For this and other measures used in this study, if the omnibus test resulting from the ANOVA was significant, the significance of differences between pairs of groups was evaluated using post hoc [Fisher's least significant differences (LSD)] paired testing. Significance was set at P < 0.05.
In untrained rats, we have reported a linear increase in the maximum M response amplitude during the weeks following initial muscle reinnervation (Boeltz et al. 2013). To compare the time course of muscle reinnervation in LT and ST groups to that of this UT group, changes in maximal M response amplitudes recorded over 11 wk following muscle sciatic nerve transection and repair were fitted with a linear regression model and the significance of differences in regression coefficients was compared between LT and ST rats and our published findings for UT rats using ANOVA.
The timing and extent of restoration of the monosynaptic H reflex were studied using the same approach in the same three groups. At the earliest postinjury times that EMG potentials could be evoked from stimulating regenerated axons in the tibial nerve, a clear distinction between direct muscle (M) responses and monosynaptic H reflexes was not possible. However, by 5 wk after sciatic nerve transection and repair, the two responses were clearly separated in time and could be studied. From that time point forward, evaluation of the time course of recovery of the H reflex was studied as described above for the M response.
EMG activity during treadmill locomotion.
At 10 wk after nerve repair surgery, activity in reinnervated muscles during treadmill locomotion was recorded, as described above. Video records were used to select step cycles for analysis of steady-state locomotion: when the rat was moving at constant speed with the treadmill belt, not riding it backwards or accelerating forward. Multiple 15-s locomotion trials were collected for each slope to ensure at least 10 step cycles were recorded where the rat was walking at a constant speed. A step cycle was defined as beginning with the first video frame where the foot was removed from the treadmill belt and ended at the last video frame of the stance phase.
The extracted EMG activity from individual selected step cycles was rectified and low pass filtered at 10 Hz and then time normalized to 100 time bins using a cubic spline interpolation routine written in Labview. Data from 10 step cycles on each slope at each recording session were then averaged to yield a single activation profile for each muscle for each slope studied. The value of the bin containing the largest voltage in these averages, among the three slopes, was used to scale the EMG intensity in these profiles for all three slopes. Intensity of activity was then represented as percent of maximum EMG intensity. To evaluate slope-related changes in scaled locomotor EMG intensity, different sets of measurements were made for the two muscles studied. For TA, the average percentage of maximum EMG intensity during the first 10% of the step cycle (first 10 bins following toe off) was determined during walking on the three slopes. For SOL, the average percentage of maximum EMG intensity at the mid-point of the step cycle (50th percentile) was determined. These measures of EMG intensity during locomotion on different slopes were then compared between the four different groups studied (intact, UT, LT, ST) using ANOVA. This method is similar to that reported by others (Gregor et al. 2006). Using this approach, we have shown previously that these measures of EMG intensity are modulated with slope in intact rats (Sabatier et al. 2011b).
Factor analysis using principal components was used to evaluate differences in locomotor EMG activity profiles in the three different treatment groups. This approach is a dimensionality reduction method that uses structure detection to express two or more correlated variables with one or more uncorrelated variables that are termed principal components (Bishop 1995). The proportion of overall variance explained by each principal component is termed its eigenvalue. The first principal component necessarily accounts for the largest proportion of the variance in the data (i.e., it has the largest eigenvalue). Each succeeding principal component explains the maximum amount of the remaining variance and is uncorrelated to previous principle component. Analysis was conducted from locomotor EMG data from intact (n = 7), UT (n = 4, Boeltz et al 2013), LT (n = 3), and ST (n = 6) groups. Each of 120 EMG profiles (3 slopes × 20 rats × 2 muscles), each representing the average of 10 selected step cycles, was incorporated into the data set. Maximum eigenvalues were obtained using Varimax normalization to obtain the maximum variance explained by each principal component (Widmer et al. 2003). Principle component (factor) loadings were determined for SOL and TA EMG activity during level, downslope, and upslope walking. Each factor loading represents the correlation coefficient between the EMG activity profile and the regression line representing each principal component. The factor loadings for each principal component were averaged for each slope and each of the four rat groups (intact, UT, LT, and ST). Significance of differences in factor loadings were evaluated using ANOVA and post hoc paired testing where appropriate.
Hindblimb kinematics during treadmill locomotion.
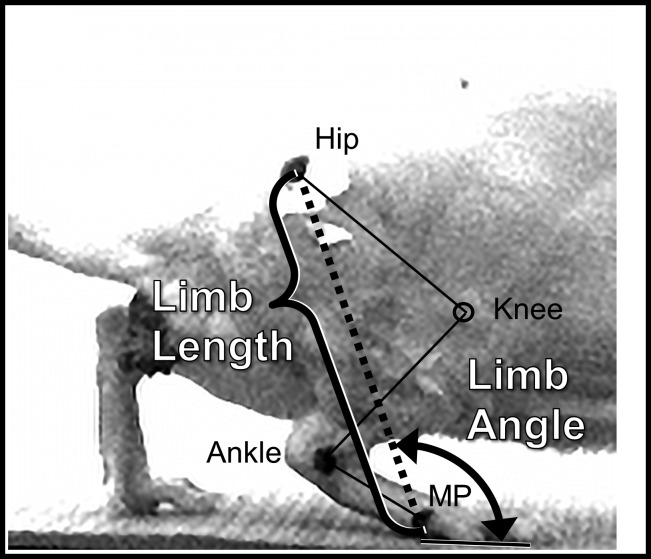
Before each locomotor trial, each rat was anesthetized using isoflurane and the skin over the greater trochanter, lateral malleolus, and lateral-most metatarsophalangeal (MP) joint were marked with dark spots (Fig. 1). The positions of these markers were digitized from video records of the step cycles chosen for EMG analysis (see above). These markers have been shown to be reliable indicators of the positions of the underlying hip and MP joints (Bauman and Chang 2010). The positions of the markers over the trochanter and MP joints were used to calculate a global kinematic vector that is a measure of the length and orientation of the hindblimb. The magnitude of this vector is the distance between the two markers, limb length. The direction of the vector is the angle formed by this line and the treadmill belt and was termed limb angle (Fig. 1). For our analysis, limb length was scaled to the length of the femur, as measured directly in anesthetized rats. This adjusted limb length was used to correct for differences in sizes between animals and for any changes in the sizes of the animals during the course of the study. These two components of the vector were tracked over step cycles from the digitized points. As a measure of central tendency of the components, we determined the median adjusted limb length and median limb angle from at least 10 step cycles on each of 3 slopes for each rat studied. Significance of differences in the average medians between intact rats and rats 10 wk after sciatic nerve transection and repair that had been left untreated, had been treated for 2 wk with level treadmill walking and had been treated for 2 wk with upslope treadmill walking was evaluated using ANOVA and post hoc paired testing, as described above. Significance was set at P < 0.05.
Fig. 1.
Illustration of the components of the global kinematic variable studied. In this view of the right hindblimb of an intact rat walking on a treadmill, taken from videos captured in our laboratory as part of this study, the positions of the hip, ankle, and lateral most metatarsophalangeal (MP) joints are indicated by solid marked spots on the animal. The approximate position of the knee joint is indicated by an open circle placed over the image. The magnitude of the global kinematic variable is limb length and is defined as the distance between the markers for the hip and MP joints (dashed line). The direction of this vector is Limb Angle, defined as the angle formed by this line and the treadmill belt.
RESULTS
Effects of upslope exercise on muscle reinnervation.
Beginning 1 wk after sciatic nerve transection and repair, ST animals were tested daily until significant evoked EMG responses were observed. Examples of direct muscle (M) responses and H reflexes, monosynaptic reflexes driven by peripheral nerve stimulation, which were evoked in SOL by tibial nerve stimulation are shown from the same rat before and at different times after sciatic nerve transection and repair and 2 wk of daily upslope treadmill training in Fig. 2A. Much as we have described previously (English et al. 2007), the M response and H reflex occurred at characteristic latencies in intact animals but at the earliest signs of muscle reinnervation following sciatic nerve transection and repair (Fig. 2A, day 17), the responses were at considerably longer latency and represented as multiple, small potentials. The precise temporal separation of M responses and H reflexes at this stage of reinnervation is entirely arbitrary. Over time, both of the evoked EMG responses occurred at shorter latencies and became much more easily recognized (Fig. 2A).
Mean values (±SE) of the first posttransection day on which EMG responses could be evoked in UT, LT, and ST rats are shown in Fig. 2B. To evaluate the differences between the three groups, these data were subjected to an ANOVA and a significant (P < 0.01) difference was found. With the use of post hoc paired testing (LSD), significant differences were found between both trained groups vs. UT (P < 0.01) and between LT and ST groups (P < 0.02). Thus the time to the earliest indication of muscle reinnervation is significantly shorter in ST rats than in LT or UT animals.
Effects of upslope exercise on recovery of evoked EMG potentials.
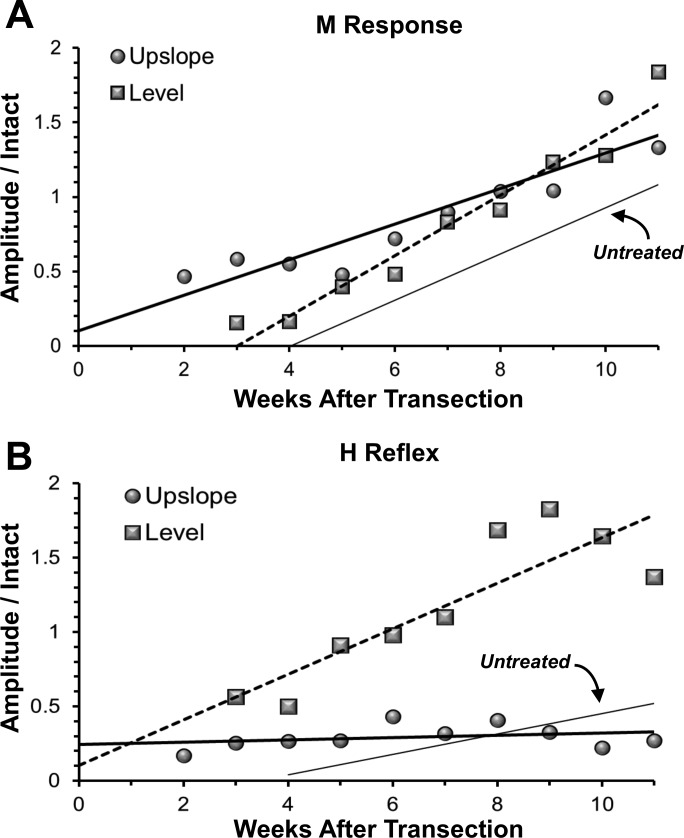
The maximum amplitude of any returned M responses and H reflexes was studied at weekly intervals over an 11 wk posttransection survival time. At each time studied, the amplitude of the largest responses observed from each rat was scaled to the amplitude of the corresponding maximal response recorded from that rat before sciatic nerve transection and repair. These data are summarized in Fig. 3, A and B. Data from LT and ST rats are shown as square and circular symbols, respectively. Each symbol represents the mean of animals in the group at the time. In each graph, the data were fit with a least squares linear regression model. Also included in each graph is a thin regression line for UT rats (n = 5), which is based on Boeltz et al. (2013).
Fig. 3.
Changes in maximum amplitudes of the M response (A) and H reflex (B) as a function of posttransection time are shown for untreated rats (solid thin line), rats exercised on a level treadmill (squares and dashed line), and rats exercised on an upslope treadmill (circles and solid thick line). In all cases, the maximum amplitude of the evoked responses recorded at posttransection times were scaled to the amplitude of the same potential in the same rat recorded before sciatic nerve transection and repair. Data from each group were fit with a linear regression line. Data recorded from animals at posttransection times earlier than 4 wk, when temporal separation of M responses and H reflexes is problematic, are not included in this analysis. The regression line for the UT rats is based on data from Boeltz et al. (2013).
In all rats following sciatic nerve transection and repair, the amplitude of the scaled M response increased linearly over the 11-wk study period (Fig. 3A). The correlation coefficients were quite high and all regression coefficients were significantly different from zero. The slope of the line fit to the data for LT rats was significantly (P < 0.01) greater than that found in UT rats. The slope of the line fit to the data for ST rats was not significantly different from that observed in UT rats. A significant difference (P < 0.01) in the intercept was found between these two groups (ST vs UT), likely resulting from the earlier initial muscle reinnervation found in ST rats (see above). The net result of these differences is that by the end of the study period, the scaled M-response amplitudes of LT and ST rats, while both significantly greater than found in UT rats, were not significantly different from each other. Thus slope training results in a significant enhancement of motor axon regeneration and muscle reinnervation but in a slightly different manner than found after level training.
It is notable in Fig. 3A, that the amplitudes of the evoked M responses in both the LT and ST rats are greater than the amplitudes of the M responses recorded from the same animals before sciatic nerve transection and repair (scaled responses >1.0). Numerous factors could contribute to this observation, as we have discussed previously (Boeltz et al. 2013). We believe that the most likely explanation is that the recording environment of the chronically implanted electrodes is different after muscle reinnervation than during pretransection recordings. Although the electrical properties of the electrodes are not different, their relationship to the muscle fibers that are the source of the EMG activity may be different. Even 11 wk after transection and repair of the sciatic nerve and robust muscle reinnervation, muscle atrophy remains significant and the different anatomical relationship between the electrode tips and these smaller muscle fibers may contribute to an altered recording environment.
There is a pronounced enhancement of H-reflex amplitude over time in LT rats, relative to UT controls (Fig. 3B). Both the slope and intercept of the regression line were significantly (P < 0.01) greater than those found in UT rats. In ST rats, there was virtually no increase in the scaled H response amplitude during the recovery period studied. Neither the correlation coefficient nor the slope of the line fit to the data was significantly different from zero. The amplitude of the H reflex did not change in ST rats as muscle fiber reinnervation progressed.
Efficacy of the H reflex.
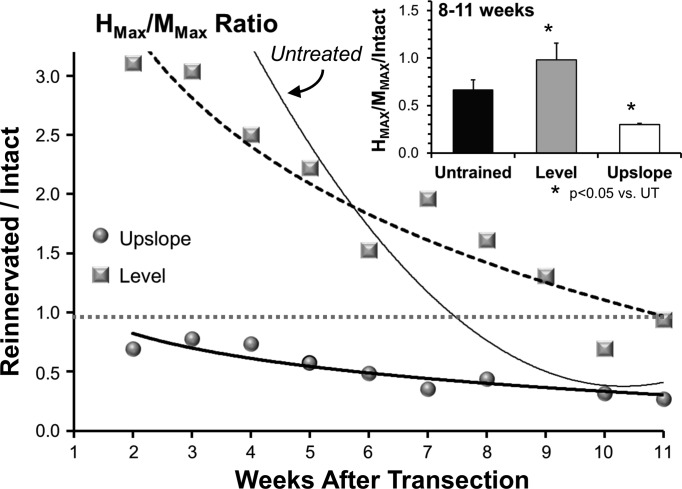
An additional measure made from the evoked EMG activity is the Hmax/Mmax ratio, a rough measure of the proportion of the available motoneuron pool that is recruited into the H reflex. We scaled the Hmax/Mmax ratio recorded at weekly intervals after sciatic nerve transection and repair to the same ratio recorded before nerve transection (Fig. 4). In both UT and LT rats, this measure of the efficacy of the H reflex recorded from reinnervated muscles was significantly exaggerated until at least 8 wk after nerve transection. In UT rats, the Hmax/Mmax ratio continued to decline and stabilized at approximately half the amount found in intact animals (Fig. 4, inset). In LT animals, the decline in the ratio was more gradual and stabilized at a level not significantly different from that found in intact animals (1.0). In ST rats, no period of exaggerated Hmax/Mmax ratio was observed. The efficacy of the H reflex in these animals declined gradually, and by 8–11 wk after nerve transection and repair, it was significantly smaller than found in all other groups of rats (Fig. 4, inset).
Fig. 4.
Changes in the efficacy of the monosynaptic H reflex recorded from the soleus muscle at different times after sciatic nerve transection and repair are shown for rats treated with two wk of daily exercise conducted either on a level treadmill (squares and dashed line) or one inclined upslope (circles and thick line). In each case the ratio of the maximum evoked H reflex was scaled to the amplitude of the maximal evoked M response, and this Hmax/Mmax ratio was then scaled to the same ratio recorded in the same rat before sciatic nerve transection and repair. Lines were approximated to the data using a second order polynomial fit, for illustrative purposes only. The thin solid line is based on similar data from untreated rats (Boeltz et al. 2013). The horizontal dotted line at 1.0 represents a ratio of Hmax/Mmax identical to that found before nerve injury. Data recorded from animals at posttransection times earlier than 4 wk, when temporal separation of M responses and H reflexes is problematic, are not included in this analysis. Inset: the mean scaled Hmax/Mmax ratios measured during the last 3 wk of the study (8–11 wk after nerve transection and repair) ± SE are shown for the 3 groups.
Effects of upslope exercise on EMG activity of reinnervated muscles during slope walking.
Two types of analysis were performed to evaluate the effect of slope training on locomotor EMG activity recorded from reinnervated SOL and TA muscles: slope-dependent changes in locomotor EMG intensity and the pattern of activity of reinnervated muscles.
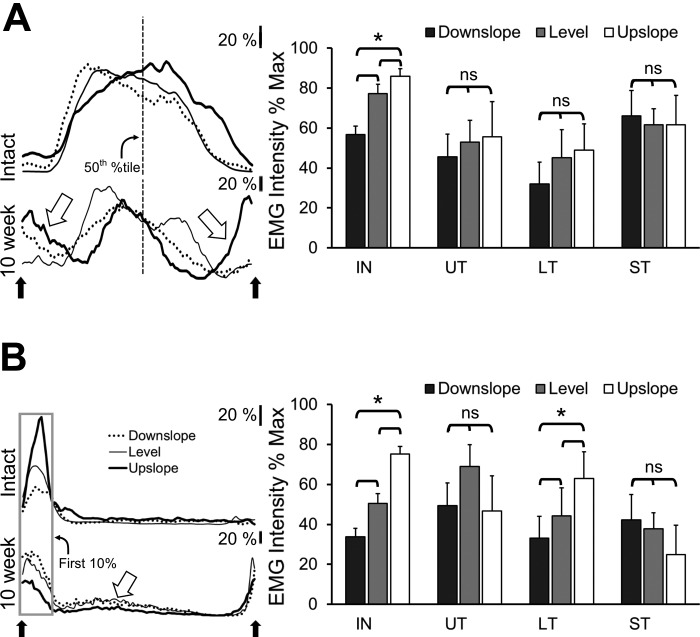
Typical locomotor activity profiles for TA and SOL during downslope, level, and upslope walking are shown for a single ST rat before and 10 wk after sciatic nerve transection and repair in Fig. 5, left. These traces have been scaled (see above) to show the relative EMG intensity changes with slope. Mean scaled locomotor EMG intensity data during slope walking are summarized for intact, UT, LT, and ST rats in Fig. 5, right, for SOL (A) and TA (B). For each group, significance of differences in these mean EMG intensities with slope was evaluated using ANOVA. For seven intact rats the amplitude of EMG activity increased with slope for both muscles (P < 0.01). This significant amplitude modulation in intact rats was lost in four UT rats, as we have shown previously (Sabatier et al. 2011b) and was not restored in five ST rats. In the seven LT rats studied, significant amplitude modulation of TA but not SOL EMG intensity with slope was found.
Fig. 5.
Changes in the intensity of locomotor EMG activity in soleus (SOL; A) and tibialis anterior (TA; B) with treadmill slope are shown. Left: traces of rectified and integrated EMG activity recorded during walking on a level, upslope, and downslope treadmill, are shown. These traces were recorded from a single ST rat before (top traces in A and B) and 10 wk after (bottom traces in A and B) transection and repair of the ipsilateral sciatic nerve. Each trace represents the activity of the muscle found in a step cycle and is an average of activity recorded in 10 step cycles, each beginning and ending when the paw was removed from the treadmill belt (up arrows). All traces were scaled to the maximum EMG intensity found during steps on the 3 different slopes. Scale bars = 20% of this maximum voltage. For analysis of SOL intensity, the activity level at the mid-point of the step cycle was measured (vertical dashed line). For TA, the average activity during the first 10% of the step cycle was used as a measure of intensity (boxed area). In the traces from recordings made posttransection and repair, large open arrows point to activity that differs notably from that observed before nerve injury. On the right side of each panel, the mean (+SE) scaled locomotor EMG intensity found during level, upslope, and downslope walking is shown for intact (IN), UT, LT, and ST groups of rats.
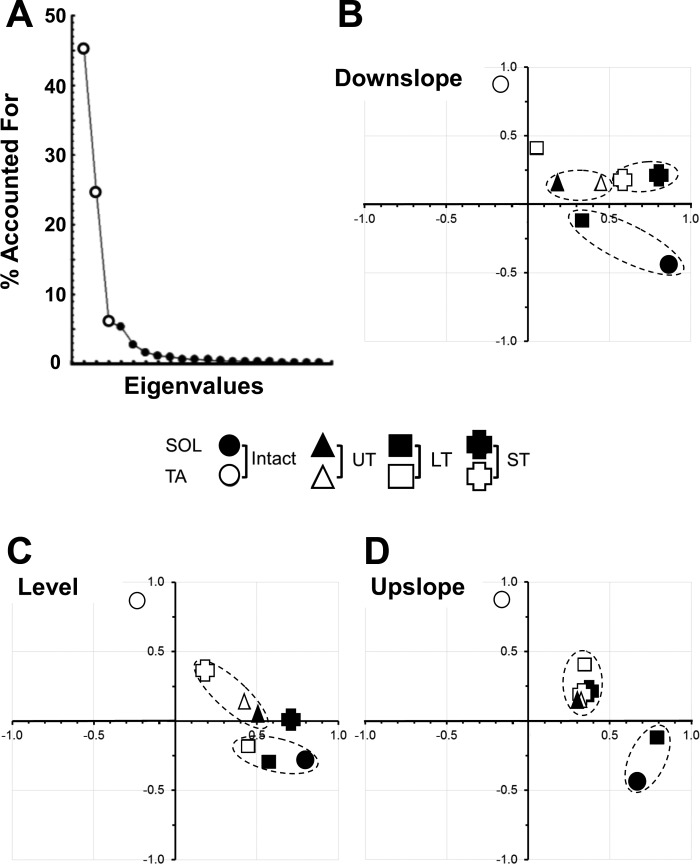
To compare the patterns of activation of TA and SOL during slope walking in the different groups, we used the dimensionality reduction approach afforded by factor analysis using principal components. For our analysis, we chose to study the first three factors, as they accounted for >84% of the variance within this data set (Fig. 6A). The omnibus test from the ANOVA was significant for all three factors studied (P < 0.01). Differences between groups and muscles were evaluated with paired, post hoc testing and were considered significant if any one of the three factors differed significantly (LSD, P < 0.05).
Fig. 6.
EMG activity in SOL and TA was synchronized to selected step cycles from video analyses during level, downslope, and upslope walking, and the extracted step cycle patterns for intact rats and rats in UT, LT, and ST groups were combined into one data set and subjected to factor analysis using principal components. A: screen plot showing the proportion of the overall variance in the data set studied that is accounted for by the different eigenvalues. Note that nearly 94% of this variance can be accounted for by the first 3 eigenvalues (larger symbols). B–D: the means of the first 2 factors derived from locomotor activity in SOL and TA during downslope (B), level (C) and upslope (D) walking are plotted against each other. The values for the different groups are shown as different symbols. Values for which none of the first 3 factors differed significantly from each other are enclosed in dashed ellipses.
Mean factor loadings are shown for the first two factors for downslope, level, and upslope walking in Fig. 6, B–D. Dashed ellipses surround symbols for groups where no significant differences were found for any of the three factors. All other symbols should be considered to be represent values that differed significantly from all others for at least one factor. Data are shown from recordings made from intact rats and from rats 10 wk after transection and repair of the sciatic nerve in the UT, LT, and ST groups. Open symbols represent data from TA and filled symbols represent data from SOL. Because factor loadings are correlation coefficients, they can range between ± 1.0. In intact rats, the first two factors for SOL and TA were found in very different regions of this factor loading space (Fig. 6, round symbols) and reflected the strict reciprocal activation pattern of these muscles during locomotion on all three slopes (Sabatier et al. 2011b); they differed significantly from one another for all of the three factors considered. Mean factor loading values for both TA and SOL in UT rats (Fig. 6, triangular symbols) were significantly different (P < 0.05) from the corresponding values in intact rats and also not significantly different from one another, on all three walking slopes. We have argued that this finding is a reflection of a degree of coactivation of these muscles in these animals (Boeltz et al. 2013; Sabatier et al. 2011b). In LT rats (Fig. 6, square symbols), factor loadings for SOL, but not TA are not significantly different from those of intact rats, on all three slopes. In ST rats (Fig. 6, plus symbols), factor loadings for both SOL and TA were significantly different from those of both SOL and TA in intact rats at all three slopes. During level walking, the factor loadings for SOL and TA in ST rats were significantly different from each other (Fig. 6C, open symbols), suggesting that the patterns of activation of the two functional antagonists were different. However, factor loadings for TA and SOL in ST rats also were significantly different from those on intact animals. The significant difference in factor loadings for SOL and TA was lost when the ST rats walked either up or down slopes (Fig. 6, B and D), most notably during upslope walking. The pattern of EMG activity in these reinnervated muscles of ST rats during slope walking is not restored to that found in intact animals.
Effects of upslope exercise on hindblimb kinematics during slope walking.
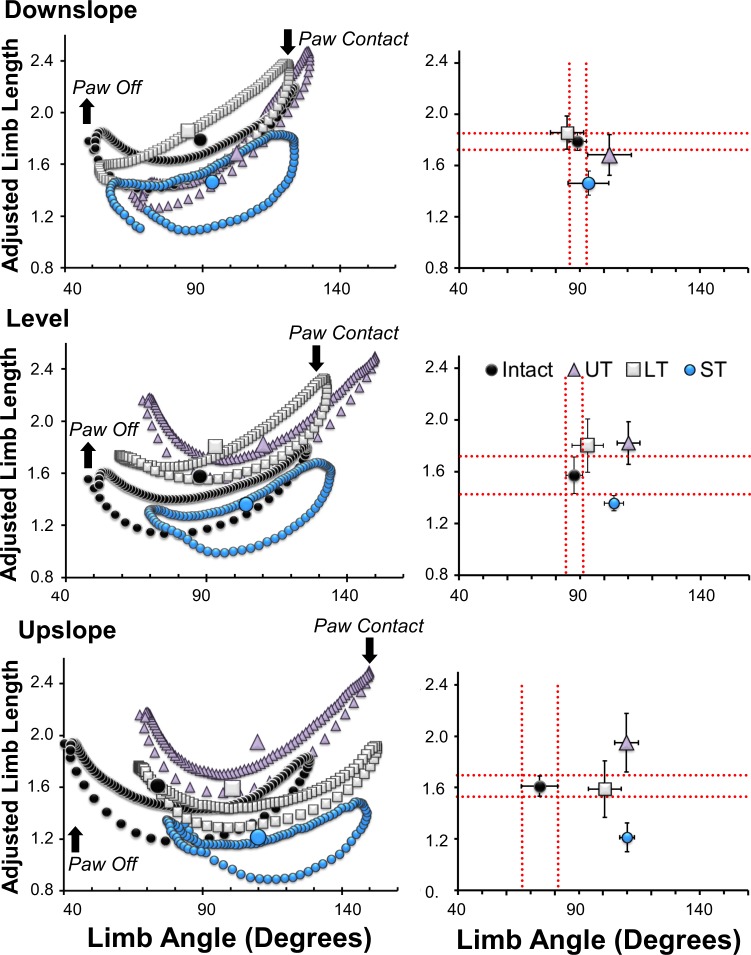
A global limb length vector was studied during level, upslope, and downslope walking in intact rats and in UT, LT, and ST rats 10 wk after sciatic nerve transection and repair (Fig. 1). Data from the current study were for intact and ST rats. Data from earlier published studies for UT and LT rats (Boeltz et al. 2013; Sabatier et al. 2011b) are included here for comparison. Coordinated changes in the magnitude (adjusted limb length) and direction (limb angle) of this vector during step cycles on level, upslope, and downslope inclined treadmill walking are shown for intact, UT, LT, and ST groups in Fig. 7, left. In each of these graphs, the medians of the two components, chosen as a measure of central tendency, are shown as larger symbols. In Fig. 7, right, the means ± 95% confidence intervals for intact, UT, LT, and ST rats are shown. Dashed lines mark the extent of these limits observed in intact rats (ST rats before nerve transection, n = 8).
Fig. 7.
Changes in the components of the global hindblimb kinematic vector during downslope, level, and upslope locomotion are shown. Left: the trajectories of the magnitude (limb length as a proportion of femur length) and direction (limb angle, see Fig. 1) of this vector during single step cycles are shown for rats before (black symbols) and ten wk after transection and repair of the sciatic nerve in untrained rats (UT, triangles), rats exposed to 2 wk of training on a level treadmill (LT, square symbols), and rats exposed to 2 wk of exercise on an upslope inclined treadmill (ST, blue symbols). In each animal, trajectories from at least ten step cycles on each slope were averaged. Upward and downward pointing arrows indicate the times of paw off and paw contact with the treadmill belt. The larger symbols at left indicate the average median adjusted limb length and limb angle for these animals. At right, the means (±95% confidence intervals) of these medians are shown for the intact, UT, LT, and ST animals. Data from UT rats are based on Boeltz et al. (2013) and Sabatier et al. (2011b). Dashed lines delineate the mean ± 95% confidence intervals for adjusted limb length and limb angle for intact rats.
During walking on different slopes, the trajectories of the two vector components during a step cycle change in predictable ways in intact rats (Fig. 7) (Sabatier et al. 2011b). During downslope walking there is a prolonged limb lengthening during late swing and a shortened lengthening during late stance. Conversely, during upslope walking limb lengthening near the end of the swing phase is very rapid but limb lengthening just before the end of stance is prolonged and limb angle is smaller. On all slopes, the average median adjusted limb lengths are in a similar, limited range and the variability in this kinematic measure is the smallest of several observed, i.e., limb length is conserved (Boeltz et al. 2013; Chang et al. 2009; Sabatier et al. 2011b).
Ten weeks after sciatic nerve transection and repair and muscle reinnervation, limb movements were changed. As we have shown previously (Sabatier et al. 2011b), adjusted limb lengths are significantly longer than intact rats in UT rats during upslope walking (Fig. 7, right, triangle symbols). During level walking, this restoration of limb length is only achieved by a significant increase in limb angle. In LT rats, both limb length and limb angle are found in the same range as intact rats during both level and downslope walking. During upslope walking, limb length is within the limits found in intact animals but this is achieved only by a significant increase in limb angle (Fig. 7, right, square symbols) (Sabatier et al. 2011b). In this study, we observed that in rats trained on an upward sloping treadmill limb length could be conserved when walking under any of the three slope conditions; the range of lengths observed was small. However, average median adjusted limb lengths were significantly shorter than in other groups on all slopes, suggesting that the ST rats adopted a crouched posture when walking. Limb angle was significantly larger during level and upslope walking in ST rats (Fig. 7, right, open circles). Thus ST rats were actually less able to restore their hindblimb movements in response to changes in slope than UT rats.
DISCUSSION
Following peripheral nerve injury, axons can regenerate and reinnervate peripheral targets, but functional recovery is poor (Brushart 2011; Frostick et al. 1998). The slow and inefficient process of axon regeneration is often cited as a cause for the poor functional outcomes found in patients, so that enhancing the process of axon regeneration is now a goal for the development of experimental therapies aimed at treating peripheral nerve injuries (Hoke and Brushart 2010). Both brief electrical stimulation and different forms of exercise are examples of such therapies that promote both the elongation of regenerating axons and the participation of more neurons in the process of regeneration (Gordon and English 2015). All of these experimental therapies are thought to increase activity in the neurons whose axons are regenerating and are often termed activity dependent (Udina et al. 2011). In studies from our laboratory, we found that moderate daily walking on a level treadmill enhances motor axon regeneration and leads to modest improvements in locomotor function (Boeltz et al. 2013; Sabatier et al. 2008). We increased the dose of exercise by inclining the treadmill belt upward and this resulted in axons of even more motoneurons regenerating successfully (Sabatier and English 2015). The present study was undertaken to evaluate whether this more strenuous form of exercise also would result in greater improvements in functional recovery than level exercise.
The most important finding of this study is that upslope treadmill training does not lead to improvements in functional recovery. We assayed functional recovery using slope walking. Slope walking is a novel behavioral task that exploits the ability of rats to modulate muscle activity and limb movements in response to the very different biomechanical demands of walking up and down slopes (Carlson-Kuhta et al. 1998; Maas et al. 2009). When intact rats walk up and down slopes, they change the movements of their hindblimbs and alter the magnitude and pattern of EMG activity in the SOL and TA muscles to meet the different mechanical demands imposed by the slopes (Sabatier et al. 2011b). In UT rats, this ability to adapt is lost. No modulation of locomotor EMG activity in reinnervated muscles with slope is found, coactivation of functional antagonists persists, and the ability to conserve limb length when walking on inclines is poor (Sabatier et al. 2011b). In LT rats, modulation of the intensity of locomotor EMG activity in the reinnervated TA but not SOL muscles with slope is restored, and the pattern of activity in SOL, although not TA, found during slope walking is reinstated. The ability to conserve limb length when walking on slopes is returned modestly (Boeltz et al. 2013). We had hypothesized that in ST rats, where more axons of motoneurons would be expected to have regenerated successfully, an even greater improvement in functional recovery might be found. However, by all measures of functional recovery used in this study, upslope training resulted in less improvement in ST animals than found in LT rats.
However, consistent with our earlier observations on upslope-trained mice, the upslope treadmill exercise applied following sciatic nerve transection and repair in rats did result in enhancement of motor axon regeneration and muscle fiber reinnervation. Stimulus-evoked M responses in the soleus muscles were noted significantly earlier after nerve injury in ST animals than in either UT or LT rats. The subsequent maturation of this response in reinnervated muscles of ST rats was at the same rate as found in UT rats, so that by 11 wk after nerve transection and repair, even though the amplitudes of the M responses in ST and LT animals were not significantly different from each other, they both were significantly greater than found in UT rats.
The mechanism behind this greater enhancement of motor axon regeneration with increased dose of exercise is not clear at this time, at least in part because the cellular mechanism by which exercise promotes axon regeneration following peripheral nerve injury is not well established. The simplest explanation might be that more motoneurons are recruited into activity by the central pattern generators for locomotion during upslope than level walking. In intact rats, the amplitude of EMG activity in both TA and SOL during locomotion increases with slope (Sabatier et al. 2011b). Although we have shown that increased motoneuron activity is sufficient to stimulate motor axon regeneration (Ward et al. 2016), the activity of axotomized motoneurons during locomotion is not well known and may actually be less than observed in intact animals (Gordon et al. 1980). In the present study, we show that the slope-related amplitude modulation of locomotor EMG activity found in the SOL and TA muscles of intact rats is lost in the reinnervated muscles of UT and ST rats. If this loss of amplitude modulation is in any way a reflection of the activity of axotomized motoneurons during the treadmill exercise, then greater activity or/and recruitment of more motoneurons into activity during upslope walking might not be an explanation for the greater enhancement of motor axon regeneration noted here. Other explanations, including changes in motoneuron excitability (Jaiswal and English 2015) need to be considered. Exercise may be widely thought of as an activity-dependent therapy (Udina et al. 2011), but details of the nature of the activity remain poorly understood.
How then might the poorer functional recovery in ST rats, relative to LT animals be explained? In both groups of animals motor axon regeneration has been enhanced, but functional recovery is improved only in LT rats. Several factors could contribute to poorer recovery in ST than LT rats, but we feel that the most striking difference observed between ST and LT rats is the relatively poor effect of upslope treadmill training on proprioceptive sensory feedback from the reinnervated muscles. Upslope training did not promote the restoration of a monosynaptic H reflex, whereas nearly full restoration of the amplitude of the H reflex was observed in LT rats (Boeltz et al. 2013). In fact, by 11 wk after nerve injury, the magnitude of the H reflex recorded from ST rats was significantly smaller than that found in UT rats. It is possible that the significant reduction in the H reflex observed in ST rats could be explained by a negative effect of slope training on sensory axon regeneration in the periphery, a reduction in the excitability of the motoneurons that have reinnervated muscles relative to that found in LT rats, or/and changes in the synaptic inputs to those motoneurons.
One consequence of the more marked enhancement of motor axon regeneration observed in ST animals (Sabatier and English 2015) might be a reduction in the initial access of regenerating sensory axons to available endoneurial tubes in the distal segments of cut nerves, resulting in successful regeneration of fewer sensory axons than found in LT animals. Whether this proposed effect on sensory axon regeneration would result in the observed reduction of the amplitude of the H reflex evoked from reinnervated muscles is not known at this time. The excitability of the axotomized motoneurons in both ST and LT rats during the exercise period is not known. The significant differences in slope-related amplitude modulation of locomotor EMG activity recorded from reinnervated muscles of LT, but not ST, rats in this study might feed speculation that upslope training results in motoneurons whose intrinsic excitability is decreased, relative to LT rats, but this is also not known. Many of the synaptic inputs responsible for the H reflex found on the somata and proximal-most dendrites of motoneurons are withdrawn following peripheral nerve transection (Alvarez et al. 2011). In UT rats, this loss is permanent (Alvarez et al. 2011; Rotterman et al. 2014) and is thought to underlie the permanent loss of the stretch reflex in self-reinnervated muscles (Cope and Clark 1993; Cope et al. 1994). In LT rats and mice, this synaptic loss is not detectable (Brandt et al. 2015; Krakowiak et al. 2015). Whether an effect on synaptic withdrawal is found in ST rats is not known. Irrespective of the source of reduction in the H reflex found in ST animals, it is clear is that the effectiveness of sensory feedback from the large, presumably proprioceptive afferent neurons in reinnervated muscles is significantly reduced in the ST rats.
Additionally, we feel that a role for proprioceptive feedback to the injured motoneurons from afferent neurons from intact muscle synergists also should be considered in explaining the diminished functional recovery in ST rats, relative to LT animals. Using simple hindblimb stretching, Caudle et al. (2015) found a significant loss of locomotor function, including a reduction in the amplitude of spinal reflexes induced by stimulation of tail afferent axons, in different spinal cord injury models. They concluded that stretching muscles reduces the excitability of hindblimb motoneurons. The changes observed are not restricted to muscle stretch but include “any and all daily afferent input” (p. 276). During exercise following sciatic nerve transection and repair, we assume that the sources of such afferent input are only from innervated structures, such as synergist muscles.
Compensatory activity in knee extensor (quadriceps) muscles during walking might be expected following sciatic nerve transection and the need for the increase in muscle force these muscles would provide would be even greater when walking upslope. Despite the likelihood that most of this increased force during upslope walking would be produced during shortening, rather than stretch of these muscles, as has been noted in cats (Gregor et al. 2006), one might anticipate significant differences in short latency length-dependent excitatory (Eccles et al. 1957; Wilmink and Nichols 2003) and oligosynaptic force-dependent inhibitory (Ross and Nichols 2009; Wilmink and Nichols 2003) feedback from intact synergists onto injured SOL motoneurons when animals are trained on an upslope inclined treadmill rather than a level treadmill. Whether this difference in afferent feedback results in changes in spinal cord function/excitability of the same magnitude as that observed by Caudle et al. (2015) is not known, and how the differences might contribute to the poor functional outcomes we observed in ST rats is not known at this time, but could be a source of future investigations.
GRANTS
This work was supported by Grant HD-032571 from the National Center for Medical Regeneration Research of the Eunice S. Kennedy National Institute of Child Health and Development.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.C., S.C., A.J., K.L.W., K.O., and S.P. performed experiments; J.C., S.C., A.J., K.L.W., K.O., S.P., and A.W.E. analyzed data; J.C., S.C., A.J., K.L.W., K.O., S.P., and A.W.E. interpreted results of experiments; J.C., S.C., A.J., K.L.W., K.O., S.P., and A.W.E. prepared figures; J.C., S.C., A.J., K.L.W., K.O., and A.W.E. drafted manuscript; J.C., S.C., A.J., K.L.W., K.O., S.P., and A.W.E. edited and revised manuscript; J.C., S.C., A.J., K.L.W., K.O., S.P., and A.W.E. approved final version of manuscript; A.W.E. conception and design of research.
ACKNOWLEDGMENTS
We thank Jennifer Nicolini and Amanda Mulligan for valuable technical assistance. We have benefitted from several conversations with our colleagues Manning Sabatier, T. Richard Nichols, and Mark Lyle on the role of proprioceptive feedback in movement control. We also thank Drs. Nichols, Patricia J. Ward, and Poonam Jaiswal for careful reading of earlier versions of the manuscript.
REFERENCES
- Alvarez FJ, Titus-Mitchell HE, Bullinger KL, Kraszpulski M, Nardelli P, Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. I. Loss of VGLUT1/IA synapses on motoneurons. J Neurophysiol 106: 2450–2470. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman JM, Chang YH. High-speed X-ray video demonstrates significant skin movement errors with standard optical kinematics during rat locomotion. J Neurosci Meth 186: 18–24. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C. Neural Networks for Pattern Recognition. Oxford, UK: Oxford Univ. Press; 1995. [Google Scholar]
- Boeltz T, Ireland M, Mathis K, Nicolini J, Poplavski K, Rose SJ, Wilson E, English AW. Effects of treadmill training on functional recovery following peripheral nerve injury in rats. J Neurophysiol 109: 2645–2657. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Evans JT, Mildenhall T, Mulligan A, Konieczny A, Rose SJ, English AW. Delaying the onset of treadmill exercise following peripheral nerve injury has different effects on axon regeneration and motoneuron synaptic plasticity. J Neurophysiol 113: 2390–2399. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM. Nerve Repair. New York: Oxford Univ. Press, 2011, p. 463. [Google Scholar]
- Carlson-Kuhta P, Trank TV, Smith JL. Forms of forward quadrupedal locomotion. II. A comparison of posture, hindlimb kinematics, and motor patterns for upslope and level walking. J Neurophysiol 79: 1687–1701, 1998 [DOI] [PubMed] [Google Scholar]
- Carr MM, Best TJ, Mackinnon SE, Evans PJ. Strain differences in autotomy in rats undergoing sciatic nerve transection or repair. Ann Plast Surg 28: 538–544. 1992. [PubMed] [Google Scholar]
- Caudle KL, Atkinson DA, Brown EH, Donaldson K, Seibt E, Chea T, Smith E, Chung K, Shum-Siu A, Cron CC, Magnuson DS. Hindlimb stretching alters locomotor function after spinal cord injury in the adult rat. Neurorehabil Neural Repair 29: 268–277. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YH, Auyang AG, Scholz JP, Nichols TR. Whole limb kinematics are preferentially conserved over individual joint kinematics after peripheral nerve injury. J Exp Biol 212: 3511–3521. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope TC, Clark BD. Motor-unit recruitment in self-reinnervated muscle. J Neurophysiol 70: 1787–1796. 1993. [DOI] [PubMed] [Google Scholar]
- Cope TC, Bonasera SJ, Nichols TR. Reinnervated muscles fail to produce stretch reflexes. J Neurophysiol 71: 817–820. 1994. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol 137: 22–50. 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Chen Y, Carp JS, Wolpaw JR, Chen XY. Recovery of electromyographic activity after transection and surgical repair of the rat sciatic nerve. J Neurophysiol 97: 1127–1134. 2007. [DOI] [PubMed] [Google Scholar]
- English AW, Cucoranu D, Mulligan A, Sabatier M. Treadmill training enhances axon regeneration in injured mouse peripheral nerves without increased loss of topographic specificity. J Comp Neurol 517: 245–255. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field-Fote EC. Does the dose do it? J Neurol Phys Ther 33: 177–178. 2009. [DOI] [PubMed] [Google Scholar]
- Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery 18: 397–405. 1998. [DOI] [PubMed] [Google Scholar]
- Gordon T, Hoffer JA, Jhamandas J, Stein RB. Long-term effects of axotomy on neural activity during cat locomotion. J Physiol 303: 243–263. 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, English AW. Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur J Neurosci 43: 336–350, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor RJ, Smith DW, Prilutsky BI. Mechanics of slope walking in the cat: quantification of muscle load, length change, and ankle extensor EMG patterns. J Neurophysiol 95: 1397–1409. 2006. [DOI] [PubMed] [Google Scholar]
- Hamilton SK, Hinkle ML, Nicolini J, Rambo LN, Rexwinkle AM, Rose SJ, Sabatier MJ, Backus D, English AW. Misdirection of regenerating axons and functional recovery following sciatic nerve injury in rats. J Comp Neurol 519: 21–33. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke A, Brushart T. Introduction to special issue: Challenges and opportunities for regeneration in the peripheral nervous system. Exp Neurol 223: 1–4. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal PB, English AW. Pharmacogenetic enhancement of functional recovery from sciatic nerve transection. Neuroscience Meeting Planner (Online). Chicago, IL: Soc. Neurosci, 2015, 517.04/R19.2015. [Google Scholar]
- Krakowiak J, Liu C, Papudesu C, Ward PJ, Wilhelm JC, English AW. Neuronal BDNF signaling is necessary for the effects of treadmill exercise on synaptic stripping of axotomized motoneurons. Neural Plast 2015: 11, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas H, Gregor RJ, Hodson-Tole EF, Farrell BJ, Prilutsky BI. Distinct muscle fascicle length changes in feline medial gastrocnemius and soleus muscles during slope walking. J Appl Physiol 106: 1169–1180. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross KT, Nichols TR. Heterogenic feedback between hindlimb extensors in the spontaneously locomoting premammillary cat. J Neurophysiol 101: 184–197. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotterman TM, Nardelli P, Cope TC, Alvarez FJ. Normal distribution of VGLUT1 synapses on spinal motoneuron dendrites and their reorganization after nerve injury. J Neurosci 34: 3475–3492. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier M, Redmon N, Schwartz G, English A. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp Neurol 211: 489–493. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier M, Nicolini J, Kaufman M, English AW. Upslope treadmill training is a potent promoter of axon regeneration in cut peripheral nerves. Neuroscience Meeting Planner (Online). Washington, DC: Soc. Neurosci, 2011a, Program No. 784.09. [Google Scholar]
- Sabatier MJ, To BN, Nicolini J, English AW. Effect of slope and sciatic nerve injury on ankle muscle recruitment and hindlimb kinematics during walking in the rat. J Exp Biol 214: 1007–1016. 2011b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier MJ, English AW. Pathways mediating activity-induced enhancement of recovery from peripheral nerve injury. Exerc Sport Sci Rev 43: 163–171. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udina E, Cobianchi S, Allodi I, Navarro X. Effects of activity-dependent strategies on regeneration and plasticity after peripheral nerve injuries. Ann Anat 193: 347–353. 2011. [DOI] [PubMed] [Google Scholar]
- Ward PJ, Jones LN, Mulligan A, Goolsby W, Wilhelm JC, English AW. Optically-induced neuronal activity is sufficient to promote functional motor axon regeneration in vivo. PLoS One 11: e0154243 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer CG, Carrasco DI, English AW. Differential participation by rabbit masseter compartments in different oral behaviors. Exp Brain Res 150: 297–307. 2003. [DOI] [PubMed] [Google Scholar]
- Wilmink RJ, Nichols TR. Distribution of heterogenic reflexes among the quadriceps and triceps surae muscles of the cat hindblimb. J Neurophysiol 90: 2310–2324. 2003. [DOI] [PubMed] [Google Scholar]