Central poststroke pain is characterized by hemianesthesia associated with severe unrelenting chronic pain. To date, treatments have focused exclusively on modulation of sensory pathways with analgesia being the end point. Because the final pain experience stems from sensory as well as affective components, integrative approaches are needed to relieve patients from pain-related disability. Pain affect in this population is poorly understood, including anticipatory phenomena. We present neural correlates of pain anticipation studied using magnetoencephalography.
Keywords: pain anticipation, central poststroke pain, thalamic pain syndrome, magnetoencephalography, deep brain stimulation
Abstract
Central poststroke pain (CPSP) is characterized by hemianesthesia associated with unrelenting chronic pain. The final pain experience stems from interactions between sensory, affective, and cognitive components of chronic pain. Hence, managing CPSP will require integrated approaches aimed not only at the sensory but also the affective-cognitive spheres. A better understanding of the brain's processing of pain anticipation is critical for the development of novel therapeutic approaches that target affective-cognitive networks and alleviate pain-related disability. We used magnetoencephalography (MEG) to characterize the neural substrates of pain anticipation in patients suffering from intractable CPSP. Simple visual cues evoked anticipation while patients awaited impending painful (PS), nonpainful (NPS), or no stimulus (NOS) to their nonaffected and affected extremities. MEG responses were studied at gradiometer level using event-related fields analysis and time-frequency oscillatory analysis upon source localization. On the nonaffected side, significantly greater responses were recorded during PS. PS (vs. NPS and NOS) exhibited significant parietal and frontal cortical activations in the beta and gamma bands, respectively, whereas NPS (vs. NOS) displayed greater activation in the orbitofrontal cortex. On the affected extremity, PS (vs. NPS) did not show significantly greater responses. These data suggest that anticipatory phenomena can modulate neural activity when painful stimuli are applied to the nonaffected extremity but not the affected extremity in CPSP patients. This dichotomy may stem from the chronic effects of pain on neural networks leading to habituation or saturation. Future clinically effective therapies will likely be associated with partial normalization of the neurophysiological correlates of pain anticipation.
NEW & NOTEWORTHY
Central poststroke pain is characterized by hemianesthesia associated with severe unrelenting chronic pain. To date, treatments have focused exclusively on modulation of sensory pathways with analgesia being the end point. Because the final pain experience stems from sensory as well as affective components, integrative approaches are needed to relieve patients from pain-related disability. Pain affect in this population is poorly understood, including anticipatory phenomena. We present neural correlates of pain anticipation studied using magnetoencephalography.
central poststroke pain (CPSP), or poststroke thalamic pain, affects ∼8% of individuals 6 months to 1 year after a stroke without preference for sex or age group (Andersen et al. 1995). Almost all patients have a clinical presentation of spontaneous dysesthesias or allodynia as well as decreased sensation to pinprick, temperature, and touch. Unfortunately, the majority of affected individuals report the pain as moderate to severe (Andersen et al. 1995) and report high levels of pain-related disability (Vranken et al. 2011). Because of the overwhelming prevalence of stroke in the industrialized world, amounting to more than 5 million in the U.S. alone (Go et al. 2014), the prevalence of poststroke pain as a source of permanent disability deserves attention. Mechanisms associated with the onset of pain and pain-related disability need to be studied in hopes that new therapeutic approaches can be developed (Jensen and Lenz 1995).
Pharmacological treatment with multiple drugs including antidepressant, antiepileptic, and opioid medications can be effective, but treatment is often frustrating to both patients and physicians (Carrieri et al. 1998; Holtom 2000; Ray and Tai 1988). Motor cortex stimulation and deep brain stimulation (DBS) are sometimes attempted in select patients; however, outcomes are not as good in the CPSP population as in patients with peripheral neuropathies (Levy et al. 1987; Machado et al. 2007; Nguyen et al. 2000; Plow et al. 2012). The consistent lack of therapeutic success indicates the need for novel interventions. Poor clinical outcomes also reflect our poor understanding of the neural mechanisms underlying pain after stroke as well as the processes underlying chronification and pain-related disability.
Pain, in particular chronic pain, is not merely a sensory representation of intensity and location of injury. Rather, pain is represented by a complex neuromatrix, comprising various frontal, parietal, and limbic brain structures that are involved in the affective, cognitive, and discriminatory aspects to determine the final pain experience (Melzack 1999). We have proposed that rehabilitation from poststroke pain needs to target not only the sensory but also the affective and cognitive spheres of chronic pain (Machado et al. 2013; Plow et al. 2012). A key element of pain-related disability and chronification is the expectation or fear of pain (i.e., the anticipatory phenomena). Although our ability to anticipate pain cued by environmental stimuli is important for avoiding injury, anticipatory behavior becomes maladaptive in the chronic pain state and promotes avoidance of activities that cause pain (Vlaeyen and Linton 2000). These avoidant behaviors lead to sensitization, functional deficits, and disability (Vlaeyen et al. 1995; Waddell et al. 1993). For example, patients with CPSP commonly suffer from severe allodynia, elicited by touching of objects, which leads to a learned behavior of pain anticipation related to usage of the affected extremity. Such behavior typically limits the use of the extremity and social interaction, resulting in disability.
Anticipation of pain is a typical feature in chronic pain patients and involves continuous cognitive appraisal of sensory signals and heightened emotional processing to perceived imminent threat (Garland 2012; Palermo et al. 2015). We expect that electrical stimulation of the pathways related to the control of emotion will modulate the learned affective response to painful stimuli. A better understanding of the brain's processing of pain anticipation is hence critical for the development of novel therapeutic approaches, such as DBS, targeting affective-cognitive networks that modulate anticipatory phenomena and its impact on pain-related disability.
Pain anticipation in normal healthy subjects has been extensively studied using neuroimaging techniques (Brown and Jones 2008; Brown et al. 2008a, 2008b; Clark et al. 2008; Hauck et al. 2007). Prefrontal, cingulate, insular, and inferior parietal cortices are highly involved in pain anticipatory phenomena (Brown et al. 2014; Brown and Jones 2013). Recent studies also have shown that primary and secondary somatosensory cortices have involvement not only in pain perception but in pain anticipation, as well (Otsuru et al. 2011; Worthen et al. 2011). We have recently shown that the primary visual cortex (V1) can independently process and distinguish cues that signal upcoming pain from those that signal other stimuli or no stimuli (Machado et al. 2014). In this study, for the first time, we studied patients with refractory CPSP by using magnetoencephalography (MEG) to characterize the neural substrates of anticipatory phenomena preceding painful or nonpainful stimuli targeting the affected and nonaffected sides. We hypothesized that the neural correlates of pain anticipation would be amplified when cues indicative of nociceptive stimuli were presented to the affected side.
METHODS
Subjects
This study included 10 patients (6 male and 4 female; average age at time of enrollment, 51 ± 6 yr; range, 41–59 yr) diagnosed with CPSP and unilateral chronic pain. However, one male patient dropped out of the study after initial enrollment. In addition, we also studied 10 healthy controls (7 male and 3 female; age 45 ± 15 yr) whose data sets have been previously published (Gopalakrishnan et al. 2016; Machado et al. 2014). All research activities were approved by the Cleveland Clinic Institutional Review Board with signed informed consent. Patient's symptomology and clinical characteristics are described in Table 1.
Table 1.
Patients' symptomology and clinical characteristics
| Patient ID | Age at Enrollment, yr | Sex | Location of Lesion | Type of Stroke | Time Stroke to Baseline, yr | Pain Lateralization | Mean Pain at Enrollment (1–10) | Sensory Deficit in Painful Zone | Motor Deficit in Painful Zone |
|---|---|---|---|---|---|---|---|---|---|
| 01 | 49 | M | Left posterior cerebral artery dissection | Ischemic | 3 | Right | 7 | Severe | Moderate |
| 02 | 49 | M | Right medulla | Ischemic | 9 | Left | 6 | Severe | Moderate |
| 03 | 47 | F | Left basal ganglia | Hemorrhagic | 9 | Right | 9 | Severe | Moderate |
| 05 | 52 | M | Left thalamus; left parietal white matter | Hemorrhagic | 3 | Right | 7 | Severe | Moderate |
| 06 | 56 | M | Right thalamus | Ischemic | 1 | Left | 10 | Moderate | None |
| 07 | 49 | M | Left brain stem | Hemorrhagic | 1 | Right | 9 | Severe | Severe |
| 08 | 50 | M | Right corona radiata | Hemorrhagic | 2 | Right | 10 | Severe | Moderate |
| 09 | 41 | F | Left thalamus | Hemorrhagic | 4 | Left | 9 | Severe | Moderate |
| 10 | 59 | F | Right middle cerebral artery territory | Ischemic | 9 | Left | 10 | Severe | Severe |
| 11 | 59 | F | Right basal ganglia | Hemorrhagic | 6 | Left | 8 | Severe | Moderate |
Subject 01 dropped out of the study after enrollment, and subjects 03 and 10 were eliminated from data analysis due to their lack of attention to experimental procedures. Pain duration was the same as time stroke to baseline in all patients. M, male; F, female.
Experimental Procedure
Before beginning experimental procedures, all patients underwent T1-weighted magnetic resonance imaging (MRI) for anatomical coregistration of the neurophysiological data.
Preparatory steps.
After head position indicator coils were affixed, the patients' fiducials (nasion, left and right auricular) and head surface points were collected using a Fastrak digitizer (Polhemus, Colchester, VT) to allow for coregistration with MRI data. Before entering the MEG suite, patients were degaussed to decrease unwanted magnetic fields arising from metallic objects external and internal to their body.
Titration and familiarization for paradigm 1.
A contact heat-evoked potential stimulator (CHEPS) of the Medoc pathway system (Medoc, Ramat-Yoshai, Israel) was used to deliver the painful stimulus (PS). To determine each patient's individual pain threshold for the heat PS, the CHEPS thermode was attached to the volar surface of the forearms. The pain threshold for the nonaffected extremity (i.e., extremity not affected by a stroke) was titrated before the extremity affected by poststroke pain. A ramp-and-hold pattern of painful stimulus was used (rise rate, 70°C/s; hold, 2 s; fall rate, 40°C/s). Temperature was sequentially increased from 40°C to 50°C. This titration procedure was stopped when each subject reported a pain rating of 8 on the numerical rating scale from 0 to 10 (0 = no pain; 10 = worst pain imaginable). With these procedures, stimuli were not excruciatingly painful but were “intense and dreadful” enough to evoke an anticipatory neural response. The experimental paradigms (see Fig. 1) were explained in detail to each patient before they entered the MEG chamber. In this study, we used visual cues to evoke anticipation because of our prior experience showing the greater consistency of visual cues compared with other modalities in pain anticipatory studies (Gopalakrishnan et al. 2015). Patients were instructed to 1) stay alert and focused on the visual cues, 2) avoid blinking during the countdown as much as possible, and 3) remain as motionless as possible while data were being recorded.
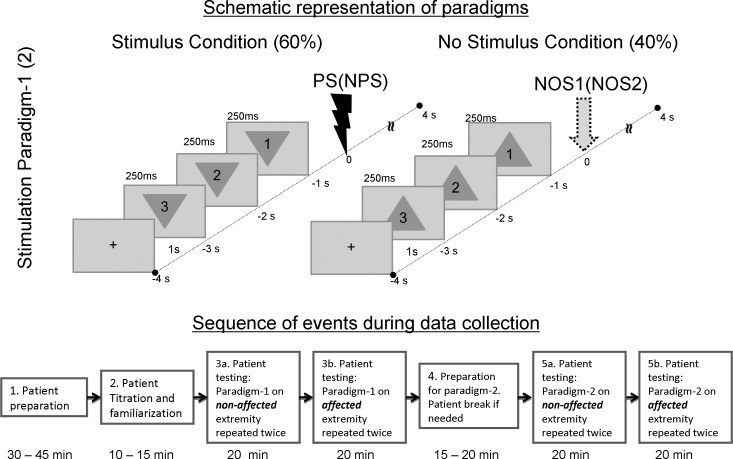
Fig. 1.
Illustration of paradigms 1 and 2. Top shows schematic representation of paradigms 1 and 2. In paradigm 1, visual countdown cues preceding a painful stimulus (PS) were presented as inverted triangles, whereas those preceding a no-stimulus (NOS) condition were presented as upright triangles. Paradigm 2 was the same as paradigm 1, except PS was replaced with a nonpainful stimulus (NPS). NOS1 refers to the no-stimulus condition in paradigm 1, whereas NOS2 refers to the no-stimulus condition in paradigm 2. Bottom shows the flow of events along with elapsed time during the data collection procedure.
Testing using paradigm 1.
Patients were seated upright in the MEG array (Elekta, Stockholm, Sweden) with their head fully inserted into the helmet. While seated, subjects viewed cues presented as a countdown on a screen for each experimental paradigm. Paradigm 1 consisted of 250-ms-long visual cues (with a 1-s start-to-start interval) presented as a countdown “3, 2, 1” embedded in a triangle. The type of stimulus succeeding the countdown was indicated by the orientation of the triangle. An inverted triangle indicated an imminent painful stimulus (PS), whereas an upright triangle signaled a no-stimulus (NOS) trial. Paradigm 1 consisted of 4 blocks of 60 pseudorandomized trials, with 60% signaling impending PS and the rest signaling NOS. The painful stimulus was applied to the nonaffected extremity for the first two blocks and then switched to the affected extremity for the last two blocks (steps 3a and 3b in Fig. 1). Each trial in a block was 8 s long and included 1 s of baseline, 3 s of prestimulus countdown or anticipatory period (Fig. 1), and 4 s of poststimulus (including stimulus and recovery) period before the next trial started. Patients were monitored continuously with a video camera to ensure alertness and continued attention to visual cues. Patients were asked to report an overall average pain rating (as a result of the PS) on a scale from 0 to 10 at the end of data collection for each extremity.
Titration and familiarization for paradigm 2.
A median nerve stimulator (Grass Instruments) was used to deliver nonpainful stimuli (NPS). The electrode was affixed to the median nerve at the wrist. The stimulus amplitude (voltage) was increased until a thumb twitch was evident. Based on feedback from patients, the voltage was either maintained or lowered until patients rated the associated pain level as 1 on a scale from 0 to 10 while maintaining their attention.
Testing using paradigm 2.
The procedure for paradigm 2 was exactly the same as for experimental paradigm 1 but utilized nonpainful median nerve stimulation (NPS) instead of the painful stimulus (PS).
Data Preprocessing
MEG data were collected either at 1,000 (recording bandwidth, 0–330 Hz) or 2,400 samples/s (recording bandwidth, 0–800 Hz). Magnetic interferences and external artifacts were filtered using the Neuromag MaxFilter algorithm (Taulu and Simola 2006). The raw data were resampled to 200 Hz. Initial signal preprocessing steps were performed using the fieldtrip toolbox (Oostenveld et al. 2011) and custom-built MATLAB algorithms (The MathWorks, Natick, MA). Out of all 306 sensor pairs, only data from 204 gradiometer pairs were studied because of their close proximity to cortical surface, and hence better signal-to-noise ratio (SNR). The gradiometer data were parsed to the onset of PS/NPS to segregate the 1-s baseline and the 3-s anticipatory period. Trials with SQUID quanta jump artifacts were removed from further analysis by means of thresholding the z-transformed value (Oostenveld et al. 2011). On average, 56 ± 3 (paradigm 1) and 53 ± 7 (paradigm 2) trials per block per subject underwent subsequent analysis. The trials were then subtracted for DC offset and bandpass filtered between 1 and 70 Hz using a 4th-order Butterworth zero-phase lag IIR filter in FieldTrip (Oostenveld et al. 2011). The preprocessed trials in each condition were averaged to compute evoked responses or event related fields (ERF) and then subjected to source analysis.
Our analysis focused on early evoked responses. Pain anticipation is a mechanism that protects the individual from external threats, and only early evoked responses (Gopalakrishnan et al. 2016) are meaningful for rapid behavioral responses. To facilitate rapid processing, the visual cues utilized in the experiment were simple and reliable to avoid complex cognitive requirements (Machado et al. 2014). The physical and spatial properties of the cues were kept consistent between conditions so that the difference seen can be unambiguously attributed to pain anticipation.
Data comparisons.
The experimental setup had four conditions: PS, NPS, NOS1 (no-stimulus condition associated with PS in paradigm 1), and NOS2 (no-stimulus condition associated with NPS in paradigm 2). Our primary interest was to study between-paradigm effects, i.e., the anticipatory effects during each countdown cue in paradigm 1 (PS) compared with paradigm 2 (NPS). In addition, we also studied the within-paradigm effects (PS vs. NOS1 and NPS vs. NOS2). The data are presented in two steps. First, the stimulus condition within each paradigm was compared with the no-stimulus condition to elicit both attention and anticipatory components, i.e., PS vs. NOS1 and NPS vs. NOS2. Second, stimulus conditions and the no-stimulus conditions in both paradigms were contrasted to elicit the pain anticipatory component, i.e., PS vs. NPS and NOS1 vs. NOS2.
Analysis of ERF Data
Data from orthogonal gradiometers were combined in each subject (using ft_combineplanar command in Fieldtrip; Oostenveld et al. 2011) for all conditions studied. To identify sensor clusters that showed significant effect, the ERFs were subjected to a nonparametric permutation statistical analysis (Maris and Oostenveld 2007). ERF activity between the two given conditions was initially clustered on the basis of adjacency in space and time using a dependent-samples two-sided t-test (thresholded at a t value corresponding to an α value of 0.05). Sample randomization was then performed 2,000 times. For each randomization, the cluster with the maximum sum was retained to compute the distribution histogram. Observed cluster sums that exceeded an α level of P < 0.01 based on a Monte-Carlo estimation were considered significant. The minimum cluster size was set to three sensors (with no maximum limit). We controlled for multiple comparisons by evaluating the cluster-level statistics under the permutation distribution of the maximum cluster-level statistic. Cluster topographies were plotted in intervals of 20 ms, highlighting sensors that showed significant effect for at least 10 ms. Depending on the latency, the effects were identified and labeled as P1, N1, P2, or N2.
Source Analysis of ERF Data
The source model was computed on 3T MPRAGE T1 magnetic resonance images that had been acquired from each patient. The pial surface and corresponding cortical parcellations (Desikan et al. 2006) were generated using FreeSurfer (Dale et al. 1999; Fischl et al. 1999). The individual heads/parcellations were then read into the open-source MATLAB toolbox Brainstorm (Tadel et al. 2011) to generate 15,000 dipolar sources on the cortical surface. Fiducials were manually chosen and used along with Isotrak head points to register MEG to MRI coordinates. Based on the segmentation of T1 images and the transformation matrix generated from above, the forward model was computed for each subject using a realistic single shell volume conductor model (Nolte 2003).
The averaged preprocessed trials were source localized using the minimum-norm estimate (MNE) technique (Baillet et al. 2001) with SNR for computation of the regularization parameter set to 3 (Tadel et al. 2011). The 1-s baseline period preceding each trial was used to evaluate the spatial noise covariance. MNE source estimates or time series were computed for each dipolar source for all three orientations (unconstrained) and then projected to its strongest orientation. The result of the source analysis was the transformation of 204-channel gradiometer data into 15,000 dipolar time series on the cortical surface. Our regions of interest (ROI) were restricted to networks associated with visual anticipation of pain (Machado et al. 2014; Senkowski et al. 2014). These included the frontoparietal networks (Lloyd et al. 2016; Palermo et al. 2015) including all of the prefrontal and parietal cortex, salience networks including operculoinsular (Lutz et al. 2013; Ploghaus et al. 1999) and cingulate cortices (Brown and Jones 2008; Brown et al. 2008a, 2008b; Vogt 2005), and medial temporal (Bauch et al. 2014) including entorhinal cortex (Ploghaus et al. 2001). The sensory cortices including somatosensory (Worthen et al. 2011) and visual cortex (Machado et al. 2014) were also included in the analysis. The mean time series corresponding to each anatomical ROI were computed.
Time-frequency (TF) spectral analysis was performed to convert the source time series into spectral components in the beta (13–30 Hz) and gamma bands (30–60 Hz) using complex Morlet wavelets (time-bandwidth parameter Fb = 7). Each frequency was then z-scored with respect to the baseline period at that frequency. Similar to the permutation analysis of ERF data described in Analysis of ERF data, TF spectrograms computed from the source time series were compared between conditions for each ROI. At the ERF level, the data had spatiotemporal dimensions, whereas spectrograms of source time series had spectrotemporal dimensions.
RESULTS
Patient Attention and Pain Experience
Two patients of nine were removed from analysis because of insufficient attention to visual cues. Despite unilateral chronic pain symptoms, the average temperature threshold to evoke 8/10 initial pain was similar for both the nonaffected (47.85 ± 1.34°C) and affected sides (47.57 ± 1.71°C). The average overall pain rating during each task was 8.35 ± 1.10 and 8.57 ± 0.78 for the nonaffected and affected extremities, respectively. Therefore, there was no significant change from the initial pain rating of 8/10.
ERF Data
Within-paradigm effects.
For paradigm 1, none of the three countdown cues were significantly different between PS and NOS1. Similarly, NPS vs. NOS2 comparison of countdown cues did not present any significant clusters. This was applicable to both affected and nonaffected extremities.
Cross-paradigm effects.
NONAFFECTED EXTREMITY.
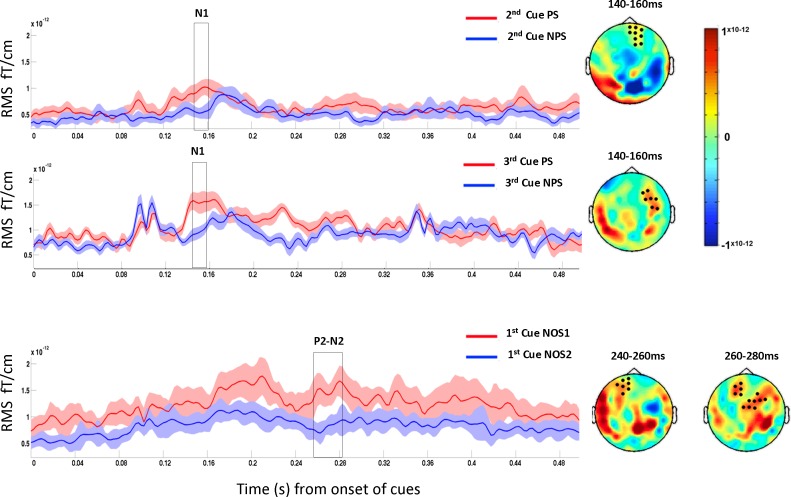
When comparing PS to NPS, we noted significant clusters from 140 to 160 ms in the second and third cues in PS, corresponding to N1 component of the visually evoked ERF. Interestingly, NOS1 showed significant clusters corresponding to P2-N2 component between 240 and 260 ms compared with NOS2. These results are shown in Fig. 2.
Fig. 2.
Cross-paradigm PS vs. NPS comparison (top and middle) and NOS1 vs. NOS2 comparison (bottom) from the nonaffected extremity. Cluster observed corresponding to the comparison of second cues (top) and third cues (middle) showed significant N1 component for PS. Significant P2 component was observed in NOS1 compared with NOS2 (bottom). Graphs (left) show the mean evoked responses from sensor cluster that showed significant responses (black dots on the topography displayed at right). Topographical maps (right) display difference between the conditions for the temporal window highlighted by the box on graph at left. Time axes shown are relative to the starting point of each cue.
AFFECTED EXTREMITY.
No significant differences were found between PS and NPS countdown cues.
Time-Frequency Data
The source-level results depicting spatio-spectrotemporal effects are presented in Figs. 3 and 4 for within-in paradigm and between-paradigm comparisons, respectively.
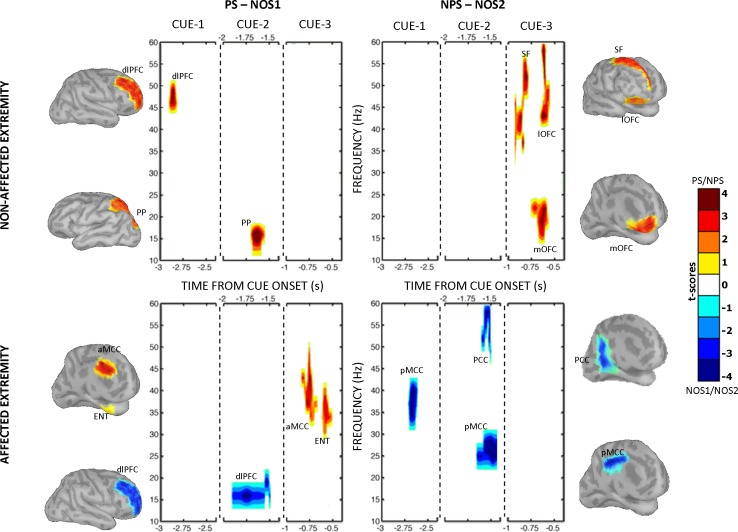
Fig. 3.
Within-paradigm time-frequency comparisons: PS (red) vs. NOS1 (blue) at left and NPS (red) vs. NOS2 (blue) at right from the nonaffected extremity (top) and affected extremity (bottom). Each panel has 3 subsections pertaining to 3 countdown cues. Only significant clusters (with sum of t values exceeding the permutation distribution at P < 0.01) are shown. An ROI label is displayed next to each time-frequency cluster. The cortical surface next to each panel displays the ROI with time series spread across the cortical dipoles and smoothed with a 10-mm full-width half-maximum Gaussian filter, purely for display purposes. Activations from pars triangularis, pars opercularis, and pars orbitalis are reported, labeled in Fig. 4 as inferior frontal gyrus (IF). The cingulate cortices were relabeled to better suit the nomenclature consistent with a 4-region neurobiological model (Vogt 2005; Vogt et al. 2005). Hence, areas noted in Desikan-Killiany atlas were retermed as follows: rostral anterior cingulate cortex as anterior cingulate cortex (ACC), caudal anterior cingulate as anterior mid-cingulate cortex (aMCC), posterior cingulate cortex as posterior mid-cingulate cortex (pMCC), and isthmus cingulate cortex as posterior cingulate cortex (PCC). Considering the close proximity of the midline regions and the limited spatial resolution of magnetoencephalography (MEG), midline regions were not lateralized. Because of potential contamination from movement-related artifacts, ROIs with motor functions (precentral and supplemental motor areas) were excluded from this analysis. dlPFC, dorsolateral prefrontal cortex; PP, posterior parietal cortex; SF, superior frontal cortex; lOFC, lateral orbitofrontal cortex; mOFC, medial orbitofrontal cortex; ENT, entorhinal cortex.
Fig. 4.
Cross-paradigm time-frequency comparisons: PS (red) vs. NPS (blue) at left and NOS1 (red) vs. NOS2 (blue) at right from the nonaffected extremity (top) and the affected extremity (bottom). Refer to the Fig. 3 legend for additional information. FP, frontal polar region; SMG, supramarginal gyrus.
Within-paradigm effects.
NONAFFECTED EXTREMITY.
When anticipating pain (PS vs. NOS1), patients evoked significant gamma-band activity in the right dorsolateral prefrontal cortex (dlPFC) during the first cue and beta-band activity in the superior parietal cortex during the second cue (Fig. 3, top left). When anticipating a nonpainful stimulus (NPS vs. NOS2), significant gamma-band activity was recorded during the third cue in the superior frontal and lateral orbitofrontal cortex (OFC) and beta-band activity in the medial OFC (Fig. 3, top right).
AFFECTED EXTREMITY.
Unlike on the nonaffected extremity, anticipating a nonpainful stimulus (NPS vs. NOS2) on the affected extremity did not evoke any significant oscillations (Fig. 3, bottom right). Pain anticipation (PS vs. NOS1) evoked significant gamma-band oscillations in the anterior mid-cingulate (MCC) and entorhinal cortex during the third cue, compared with the no-stimulus condition (Fig. 3, bottom left).
Cross-paradigm effects.
NONAFFECTED EXTREMITY.
Anticipating painful stimulus (PS vs. NPS) evoked significantly greater gamma-band oscillations in the frontal polar region during the second cue and beta-band oscillations in the supramarginal gyrus during the second and third cues (Fig. 4, top left). Compared with PS, NPS did not show any significant oscillations.
AFFECTED EXTREMITY.
Anticipating painful stimuli (PS vs. NPS) on the affected extremity did not evoke any significant increase in cortical oscillations (Fig. 4, bottom left). Interestingly, compared with PS, NPS evoked significantly larger gamma-band oscillations in the medial OFC during the second cue.
DISCUSSION
The purpose of the study was to characterize how patients unilaterally affected by CPSP respond to visual anticipatory cues that signal imminent painful, nonpainful, or no stimuli. This patient population is not only in great need of examination but also offers a unique model for this type of study because the nonaffected extremity can be tested as a control. To better account for possible differences underlying anticipatory phenomena, we report data from the nonaffected extremity separately from that for the affected extremity. We hypothesized that patients would exhibit an enhanced neural activation in the pain neuromatrix areas when anticipating painful stimuli on the affected extremity relative to the nonaffected hemibody. Contrarily, we found that pain anticipation related to the nonaffected extremity displayed correlates of enhanced anxiety and attention compared with the affected extremity. No differences were noted when cues that signaled painful or nonpainful stimuli were presented to the affected side. Although this observation may seem surprising, it needs to be interpreted within the context of chronic pain and its effect on the pain neuromatrix (Melzack 2005). In the neuromatrix model, Melzack proposes that our perception of self “normal body” is subserved by the integrity of the neuromatrix, which contains neural networks and predetermined patterns of signaling that determine our response to external stimuli. CPSP can be explained by deafferentation that results in a disruption of the neuromatrix's integrity, abnormally triggering preexisting patterns that result in the perception of relentless chronic pain. Constant exposure to pain may result in neural habituation to nociception or saturation, to the extent that the network will no longer be modulated by the threat of incoming nociceptive stimuli.
We have previously studied the anticipatory phenomena associated with incoming PS or NPS in normal healthy subjects (Machado et al. 2014). At the ERF level, we found that the first countdown cue evoked greater P2-N2 components, which were attributed to anticipatory alertness to incoming PS (Gopalakrishnan et al. 2016). Complementing these findings in the temporal domain, we found that the dlPFC, OFC, and MCC were dominated by gamma-band oscillations during early (naive) stages of PS (i.e., first half of the trials within the paradigm). Interestingly, once the contextual meaning of the visual cues was learned, the primary visual cortex (V1) presented robust modulation during the late phases of pain anticipation (i.e., second half of the trials within the paradigm). This modulation of V1 activity represents the ability to process pain anticipatory cues without the aid of frontal regions (Machado et al. 2014). In the present study, we found that patients with CPSP have significantly different responses to cues signaling incoming stimuli, particularly when these are applied to the affected hemibody.
We first evaluated the anticipatory phenomena associated with stimuli applied to the nonaffected extremity, followed by those applied to the affected extremity. Therefore, the data related to the nonaffected extremity represent the early (naive) phases of learning the contextual meaning of our visual cues, whereas data associated with the affected extremity represent the later (conditioned) phase. We chose to study event-related components because they represent electrophysiological biomarkers of affective, cognitive, and behavioral aspects of neural processing (Luck and Kappenman 2012). In addition, different frequency bands have been reported to have specific functional roles. Specifically, beta- and gamma-band activity has been reported to have a functional role in behavioral, affective, and cognitive processing in normal (Engel and Fries 2010; Jensen et al. 2007; Tiemann et al. 2010) and pathological states (Engel and Fries 2010; Herrmann and Demiralp 2005; Herrmann et al. 2010), including pain and pain anticipation in a healthy population (Schulz et al. 2012; Senkowski et al. 2014). The three-cue paradigm used in this study was intended to progressively evoke anticipation. The initial countdown cues were expected to evoke attention and alertness. Depending on the nature of the upcoming stimulus, we hypothesized that the last cue would evoke a heightened pain anticipatory neural activity.
Nonaffected Extremity
During cross-paradigm comparison, significant N1 and P2-N2 components localized to anterior sensors were recorded in PS and NOS1, respectively (Fig. 2). These findings imply that patients maintained a higher state of anticipatory alertness/attention throughout paradigm 1 (which was highly salient compared with paradigm 2 due to the involvement of a painful stimulus). These ERF findings were in close similarity to the ERF we observed in normal healthy controls (Gopalakrishnan et al. 2016).
For both within-paradigm and cross-paradigm comparisons (Figs. 3 and 4), PS showed consistent beta-band activity in the parietal cortex and gamma-band activity in the prefrontal cortex. This observation provides evidence for the involvement of the frontoparietal network during pain anticipation. The frontoparietal network has been highly implicated with attentional (Corbetta and Shulman 2002; Fries 2009) as well as cognitive control (Zanto and Gazzaley 2013). During anticipation of PS, our patients showed significant activity in the dlPFC and superior parietal regions. This early gamma activation of dlPFC was also evident in our normal healthy population (Machado et al. 2014). The lateral prefrontal cortex has been shown to be a key region in regulating behavior. Many mental disorders, including depression and obsessive compulsive disorder (OCD), have shown disruption in dlPFC functionality (Cole et al. 2014). Activation in dlPFC and parietal cortex (Behrmann et al. 2004; Moulton et al. 2012), especially during the initial cues, provides evidence of early increased attention. Several authors have also reported the activation of lateral prefrontal cortex in “pain catastrophizing,” a negative emotional response to upcoming painful stimuli (Quartana et al. 2009), and pain-related fear or anxiety in both healthy (Seminowicz and Davis 2006) and chronic pain patients (Gracely et al. 2004). We speculate that increased attention during the first cue could be directly related to regulation of affective dimension of pain, i.e., a counterbalancing preparatory strategy.
Interestingly, when NPS was anticipated, the last cue was associated with increased activity in OFC, areas of ventromedial and ventrolateral prefrontal cortex that have similar function to dlPFC in pain affect (Quartana et al. 2009). This area is also involved in the regulation of emotion and the fear response (Kringelbach and Rolls 2004). Therefore, the observed late OFC activity could represent the neural correlates of heightened attention/anxiety to stimuli that is not painful.
Affected Extremity
Contrary to our initial hypothesis, there were no significant differences in neural activity between the two main conditions (PS and NPS). When comparing ERFs, we found no significant differences in within-paradigm or cross-paradigm comparisons. This observation does not necessarily indicate that patients with CPSP do not fear or dread incoming stimuli to the affected extremity. Rather, we speculate a loss of stimulus salience consequent to the constant and unrelenting pain and the allodynia that patients with CPSP experience. This interpretation is corroborated by the lack of significant changes in beta- and gamma-band activity observed in PS relative to NPS on the affected side (Fig. 4). Unlike the nonaffected extremity, early increased frontoparietal attention to PS (relative to NOS1) was not evident on the affected side. Instead, we recorded a late (just prior to stimuli) increased activity in entorhinal cortex and aMCC, regions that have been associated with conditioned hyperalgesia (Ploghaus et al. 2001). Interestingly, MCC activity has been reported to interrupt attention network during pain anticipation (Brown and Jones 2008). When NPS was anticipated (relative to NOS2), we recorded diminished activity in posterior cingulate cortex and posterior mid-cingulate cortex (Fig. 3, bottom right), regions implicated in spatial orientation of attention to anticipated stimuli (Small et al. 2003). Increased activity in pain neuromatrix areas during NOS2 relative to NOS1 (Fig. 4, right column) further supports the highly salient nature of paradigm 2 in CPSP patients, possibly due to an allodynia-learned affective response.
Limitations
The study does present limitations to be considered. The initial sample consisted of 10 patients, but we removed 3, reducing our final number to 7 patients. Although this is a small n that could not be representative of the overall population, we note that severe and chronic CPSP is a rare disorder, and it is expected that any study with this population will have smaller samples. Furthermore, although the sample was not large, the clinical phenotype was quite consistent. All patients had the same diagnosis with a proven stroke and hemibody pain. All patients had severe and intractable pain despite aggressive medical management. Another potential limitation to consider is related to the type of stimuli. PS was induced with a temperature stimulus and NPS was provoked with an electrical stimulus. The difference in mechanism could cause a bias in the results. However, we argue that the impact would be greater on neural correlates of pain processing (i.e., processing after the stimulus) rather than pain anticipation. Lastly, we utilized a sequential rather than randomized paradigm to test the nonaffected and affected extremities. In all subjects, the nonaffected extremity was tested before the affected extremity, which might have had a conditioning effect. However, we preferred this nonrandomized framework in an attempt to reduce pain-related carryover effects that could bias data interpretation associated with stimulation of the nonaffected extremity.
Conclusions
To our knowledge, the present study is the first to examine neurophysiological correlates of pain anticipation in CPSP patients. Our data suggest that the pain neuromatrix areas were no longer modulated by cues signaling incoming stimuli to the affected extremity. This finding may be a neurophysiological correlate of anesthesia dolorosa or frequent allodynia in CPSP.
GRANTS
This work was supported by the Charles and Christine Carroll Family Endowed Chair in Functional Neurosurgery and National Institutes of Health New Innovator Award DO006469A.
DISCLOSURES
Andre Machado has the following conflicts to declare, none of which are pertinent to this research project or to this manuscript: consultant, Spinal Modulation and Functional Neuromodulation. Potential distribution from intellectual property: Enspire DBS, Cardionomics and ATI. Other authors have no disclosures.
AUTHOR CONTRIBUTIONS
R.G. performed experiments; R.G. analyzed data; R.G., R.C.B., S.F.L., J.T.G., D.P.F., and A.G.M. interpreted results of experiments; R.G. prepared figures; R.G. and A.G.M. drafted manuscript; R.G., R.C.B., S.F.L., J.T.G., D.P.F., and A.G.M. edited and revised manuscript; R.G., R.C.B., S.F.L., J.T.G., D.P.F., and A.G.M. approved final version of manuscript; A.G.M. conception and design of research.
REFERENCES
- Andersen G, Vestergaard K, Ingeman-Nielsen M, Jensen TS. Incidence of central post-stroke pain. Pain 61: 187–193, 1995. [DOI] [PubMed] [Google Scholar]
- Baillet S, Mosher JC, Leahy RM. Electromagnetic brain mapping. IEEE Signal Process Mag 18: 14–30, 2001. [Google Scholar]
- Bauch EM, Rausch VH, Bunzeck N. Pain anticipation recruits the mesolimbic system and differentially modulates subsequent recognition memory. Hum Brain Mapp 35: 4594–4606, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Curr Opin Neurobiol 14: 212–217, 2004. [DOI] [PubMed] [Google Scholar]
- Brown CA, El-Deredy W, Jones AK. When the brain expects pain: common neural responses to pain anticipation are related to clinical pain and distress in fibromyalgia and osteoarthritis. Eur J Neurosci 39: 663–672, 2014. [DOI] [PubMed] [Google Scholar]
- Brown CA, Jones AK. Psychobiological correlates of improved mental health in patients with musculoskeletal pain after a mindfulness-based pain management program. Clin J Pain 29: 233–244, 2013. [DOI] [PubMed] [Google Scholar]
- Brown CA, Jones AK. A role for midcingulate cortex in the interruptive effects of pain anticipation on attention. Clin Neurophysiol 119: 2370–2379, 2008. [DOI] [PubMed] [Google Scholar]
- Brown CA, Seymour B, Boyle Y, El-Deredy W, Jones AK. Modulation of pain ratings by expectation and uncertainty: behavioral characteristics and anticipatory neural correlates. Pain 135: 240–250, 2008a. [DOI] [PubMed] [Google Scholar]
- Brown CA, Seymour B, El-Deredy W, Jones AK. Confidence in beliefs about pain predicts expectancy effects on pain perception and anticipatory processing in right anterior insula. Pain 139: 324–332, 2008b. [DOI] [PubMed] [Google Scholar]
- Carrieri PB, Provitera VV, Lavorgna L, Bruno R. Response of thalamic pain syndrome to lamotrigine. Eur J Neurol 5: 625–626, 1998. [DOI] [PubMed] [Google Scholar]
- Clark JA, Brown CA, Jones AK, El-Deredy W. Dissociating nociceptive modulation by the duration of pain anticipation from unpredictability in the timing of pain. Clin Neurophysiol 119: 2870–2878, 2008. [DOI] [PubMed] [Google Scholar]
- Cole MW, Repovs G, Anticevic A. The frontoparietal control system: a central role in mental health. Neuroscientist 20: 652–664, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3: 201–215, 2002. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9: 179–194, 1999. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31: 968–980, 2006. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations–signalling the status quo? Curr Opin Neurobiol 20: 156–165, 2010. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 9: 195–207, 1999. [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci 32: 209–224, 2009. [DOI] [PubMed] [Google Scholar]
- Garland EL. Pain processing in the human nervous system: a selective review of nociceptive and biobehavioral pathways. Prim Care 39: 561–571, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 129: 399–410, 2014. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan R, Burgess RC, Plow EB, Floden D, Machado AG. A magnetoencephalography study of multi-modal processing of pain anticipation in primary sensory cortices. Neuroscience 304: 176–189, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan R, Burgess RC, Plow EB, Floden DP, Machado AG. Early event related fields during visually evoked pain anticipation. Clin Neurophysiol 127: 1855–1863, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, Williams DA, Clauw DJ. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 127: 835–843, 2004. [DOI] [PubMed] [Google Scholar]
- Hauck M, Lorenz J, Zimmermann R, Debener S, Scharein E, Engel AK. Duration of the cue-to-pain delay increases pain intensity: a combined EEG and MEG study. Exp Brain Res 180: 205–215, 2007. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol 116: 2719–2733, 2005. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Frund I, Lenz D. Human gamma-band activity: a review on cognitive and behavioral correlates and network models. Neurosci Biobehav Rev 34: 981–992, 2010. [DOI] [PubMed] [Google Scholar]
- Holtom N. Gabapentin for treatment of thalamic pain syndrome. Palliat Med 14: 167, 2000. [DOI] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci 30: 317–324, 2007. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Lenz FA. Central post-stroke pain: a challenge for the scientist and the clinician. Pain 61: 161–164, 1995. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol 72: 341–372, 2004. [DOI] [PubMed] [Google Scholar]
- Levy RM, Lamb S, Adams JE. Treatment of chronic pain by deep brain stimulation: long term follow-up and review of the literature. Neurosurgery 21: 885–893, 1987. [DOI] [PubMed] [Google Scholar]
- Lloyd DM, Helbig T, Findlay G, Roberts N, Nurmikko T. Brain areas involved in anticipation of clinically relevant pain in low back pain populations with high levels of pain behavior. J Pain 17: 577–587, 2016. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Kappenman ES. The Oxford Handbook of Event-Related Potential Components. New York: Oxford University Press, 2012, p. xxii. [Google Scholar]
- Lutz A, McFarlin DR, Perlman DM, Salomons TV, Davidson RJ. Altered anterior insula activation during anticipation and experience of painful stimuli in expert meditators. Neuroimage 64: 538–546, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A, Azmi H, Rezai AR. Motor cortex stimulation for refractory benign pain. Clin Neurosurg 54: 70–77, 2007. [PubMed] [Google Scholar]
- Machado AG, Baker KB, Plow E, Malone DA. Cerebral stimulation for the affective component of neuropathic pain. Neuromodulation 16: 514–518, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado AG, Gopalakrishnan R, Plow EB, Burgess RC, Mosher JC. A magnetoencephalography study of visual processing of pain anticipation. J Neurophysiol 112: 276–286, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164: 177–190, 2007. [DOI] [PubMed] [Google Scholar]
- Melzack R. Evolution of the neuromatrix theory of pain. The Prithvi Raj Lecture: presented at the third World Congress of World Institute of Pain, Barcelona 2004. Pain Pract 5: 85–94, 2005. [DOI] [PubMed] [Google Scholar]
- Melzack R. From the gate to the neuromatrix. Pain Suppl 6: 121–126, 1999. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Pendse G, Becerra LR, Borsook D. BOLD responses in somatosensory cortices better reflect heat sensation than pain. J Neurosci 32: 6024–6031, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JP, Lefaucher JP, Le Guerinel C, Eizenbaum JF, Nakano N, Carpentier A, Brugieres P, Pollin B, Rostaing S, Keravel Y. Motor cortex stimulation in the treatment of central and neuropathic pain. Arch Med Res 31: 263–265, 2000. [DOI] [PubMed] [Google Scholar]
- Nolte G. The magnetic lead field theorem in the quasi-static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Phys Med Biol 48: 3637–3652, 2003. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011: 156869, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuru N, Inui K, Yamashiro K, Urakawa T, Keceli S, Kakigi R. Effects of prior sustained tactile stimulation on the somatosensory response to the sudden change of intensity in humans: an magnetoencephalography study. Neuroscience 182: 115–124, 2011. [DOI] [PubMed] [Google Scholar]
- Palermo S, Benedetti F, Costa T, Amanzio M. Pain anticipation: an activation likelihood estimation meta-analysis of brain imaging studies. Hum Brain Mapp 36: 1648–1661, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci 21: 9896–9903, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Dissociating pain from its anticipation in the human brain. Science 284: 1979–1981, 1999. [DOI] [PubMed] [Google Scholar]
- Plow EB, Pascual-Leone A, Machado A. Brain stimulation in the treatment of chronic neuropathic and non-cancerous pain. J Pain 13: 411–424, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother 9: 745–758, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray DA, Tai YM. Increasing doses of naloxone hydrochloride by infusion to treat pain due to the thalamic syndrome. Br Med J (Clin Res Ed) 296: 969–970, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz E, Tiemann L, Witkovsky V, Schmidt P, Ploner M. γ Oscillations are involved in the sensorimotor transformation of pain. J Neurophysiol 108: 1025–1031, 2012. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain 120: 297–306, 2006. [DOI] [PubMed] [Google Scholar]
- Senkowski D, Hofle M, Engel AK. Crossmodal shaping of pain: a multisensory approach to nociception. Trends Cogn Sci 18: 319–327, 2014. [DOI] [PubMed] [Google Scholar]
- Small DM, Gitelman DR, Gregory MD, Nobre AC, Parrish TB, Mesulam MM. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. Neuroimage 18: 633–641, 2003. [DOI] [PubMed] [Google Scholar]
- Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput Intell Neurosci 2011: 879716, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51: 1759–1768, 2006. [DOI] [PubMed] [Google Scholar]
- Tiemann L, Schulz E, Gross J, Ploner M. Gamma oscillations as a neuronal correlate of the attentional effects of pain. Pain 150: 302–308, 2010. [DOI] [PubMed] [Google Scholar]
- Vlaeyen JW, Kole-Snijders AM, Rotteveel AM, Ruesink R, Heuts PH. The role of fear of movement/(re)injury in pain disability. J Occup Rehabil 5: 235–252, 1995. [DOI] [PubMed] [Google Scholar]
- Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain 85: 317–332, 2000. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6: 533–544, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Farber NB, Bush G. Architecture and neurocytology of monkey cingulate gyrus. J Comp Neurol 485: 218–239, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranken JH, Hollmann MW, van der Vegt MH, Kruis MR, Heesen M, Vos K, Pijl AJ, Dijkgraaf MG. Duloxetine in patients with central neuropathic pain caused by spinal cord injury or stroke: a randomized, double-blind, placebo-controlled trial. Pain 152: 267–273, 2011. [DOI] [PubMed] [Google Scholar]
- Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain 52: 157–168, 1993. [DOI] [PubMed] [Google Scholar]
- Worthen SF, Hobson AR, Hall SD, Aziz Q, Furlong PL. Primary and secondary somatosensory cortex responses to anticipation and pain: a magnetoencephalography study. Eur J Neurosci 33: 946–959, 2011. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A. Fronto-parietal network: flexible hub of cognitive control. Trends Cogn Sci 17: 602–603, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]