SUMMARY
Zika virus (ZIKV) is an emerging flavivirus that causes congenital abnormalities and Guillain-Barré syndrome. ZIKV infection also results in severe eye disease characterized by optic neuritis, chorioretinal atrophy, and blindness in newborns and conjunctivitis and uveitis in adults. We evaluated ZIKV infection of the eye using recently developed mouse models of pathogenesis. ZIKV-inoculated mice developed conjunctivitis, pan-uveitis and infection of the cornea, iris, optic nerve, and the ganglion and bipolar cells in the retina. This phenotype was independent of the entry receptors Axl or Mertk, as Axl−/−, Mertk−/− or Axl−/− Mertk−/− DKO mice sustained similar levels of infection as control animals. We also detected abundant viral RNA in tears, suggesting that virus may be secreted from lacrimal glands or shed from the cornea. This model provides a foundation for studying ZIKV-induced ocular disease, defining mechanisms of viral persistence, and developing therapeutic approaches for viral infections of the eye.
Graphical abstract
INTRODUCTION
ZIKV is a mosquito-transmitted flavivirus that is closely related to Dengue (DENV), West Nile (WNV), and yellow fever (YFV) viruses. Beyond the clinical syndromes of microcephaly, intrauterine growth restriction, and fetal demise, several clinical studies have observed eye malformations and pathology in neonates born to mothers infected with ZIKV during pregnancy (de Paula Freitas et al., 2016; McCarthy, 2016; Miranda et al., 2016; Sarno et al., 2016; Ventura et al., 2016a; Ventura et al., 2016b). Manifestations of eye disease in newborns with ZIKV include chorioretinal atrophy, optic neuritis, bilateral iris colobomas, intraretinal hemorrhages, lens subluxation, and blindness (de Paula Freitas et al., 2016; Miranda et al., 2016; Ventura et al., 2016a; Ventura et al., 2016b).
Viral infection in the eye can cause inflammation of uveal tissues (retina, choroid, iris, and ciliary body), also termed uveitis, which can lead to permanent vision loss if untreated (Niederkorn, 2006). In 2014, an Ebola virus (EBOV) infected patient in the convalescent phase presented with uveitis. The aqueous humor of this patient’s eye contained persistent EBOV RNA well after clearance of virus in non-immune privileged sites (Varkey et al., 2015). A subsequent study identified 57 EBOV survivors with uveitis, suggesting that infectious virus or viral RNA in the eye may have triggered this complication (Tiffany et al., 2016). Other animal models have suggested that some DNA viruses (e.g., ectromelia virus) may persist in the eye and recrudesce after immunosuppression (Sakala et al., 2015). ZIKV causes conjunctivitis in 10 to 15% of infected adults, and uveitis occurred in a patient several weeks after initial infection. Fluid sampled from the anterior chamber of this patient’s eye contained viral RNA (Furtado et al., 2016), suggesting that ZIKV can replicate within the eye at some stage of its clinical syndrome.
ZIKV does not replicate efficiently in adult wild-type (WT) mice; this phenotype may be explained by the ability of ZIKV to antagonize human but not mouse STAT2, which is activated by signaling through the type I and type III interferon (IFN) receptors (Ashour et al., 2009; Grant et al., 2016). Accordingly, we and others recently described models of ZIKV pathogenesis after congenital and adult infection in mice deficient in signaling through the type I IFN receptor (Aliota et al., 2016; Lazear et al., 2016; Miner et al., 2016; Rossi et al., 2016). Mice lacking the type I IFN receptor (Ifnar1−/− mice) developed neuroinvasive disease after ZIKV infection, which caused death in younger animals although a fraction of older adult mice survived infection. Infection of Ifnar1−/− females with a French Polynesian strain of ZIKV resulted in fetal demise and intrauterine growth restriction in Ifnar1+/− fetuses (Miner et al., 2016), but ocular pathology was not assessed.
Some flaviviruses are thought to attach to or enter target cells by interacting with TAM receptors (Tyro3, Axl, or Mertk) via their ligands, Gas6 and Protein S, which bind to phosphatidylserine displayed on the surface of the virion. In vitro studies have suggested that TAM receptors can facilitate infection with WNV, DENV, and ZIKV either by promoting binding (Hamel et al., 2015; Meertens et al., 2012) or by activating TAM receptors (Bhattacharyya et al., 2013), which negatively regulates signaling through Ifnar1 and results in a more permissive environment for replication. Recent focus has been placed on ZIKV infection and Axl, because it is expressed highly on neuroprogenitor cells (Nowakowski et al., 2016) and subtypes of trophoblasts (Tabata et al., 2016), which are key cellular targets of ZIKV.
Here, we evaluated whether ZIKV infects and injures the eyes of mice. Although we did not detect eye pathology in congenitally infected mice, ZIKV infected the iris, retina, and optic nerve of adult mice, and caused conjunctivitis, pan-uveitis, and neuroretinitis without global photoreceptor abnormalities. Acute uveitis was associated with high levels of ZIKV RNA and infectious virus in the eye and detectable viral RNA in the tears and lacrimal glands. ZIKV RNA persisted within the eye through the convalescent stage, while infectious virus was cleared within 28 days. Although Axl and Mertk are expressed in eye tissues (Prasad et al., 2006; Valverde et al., 2004), infection studies in Axl−/− and Mertk−/− mice revealed no difference in ocular or brain infection, suggesting that these TAM receptors lack an essential entry or signaling role in these tissues. Our experiments establish that ZIKV infects specific target cells in different regions of the eye and provide a model for the development and testing of treatments for acute and persistent viral infections in the eye.
RESULTS
ZIKV infection of the eye with viral RNA shedding into tears
ZIKV does not replicate efficiently in WT C57BL/6 mice, in part because ZIKV NS5 antagonizes human but not mouse STAT2 (Grant et al., 2016), which transmits signals downstream of Ifnar1, a component of the type I IFN receptor. To overcome this limitation, we treated mice with an anti-Ifnar1 blocking antibody (Lazear et al., 2016; Miner et al., 2016), and inoculated subcutaneously low-passage ZIKV contemporary clinical isolates, including strains from French Polynesia (H/PF/2013) or Brazil (Paraiba 2015). WT adult mice treated with anti-Ifnar1 mAb and inoculated with these ZIKV isolates do not develop clinically apparent disease, although viremia and infection of multiple organs occurs including in immune privileged sites such as the testes (Lazear et al., 2016). In comparison, Ifnar1−/− mice develop neuroinvasive infection, causing some of these animals to succumb to infection (Aliota et al., 2016; Lazear et al., 2016; Rossi et al., 2016). In anti-Ifnar1 mAb-treated animals, we detected RNA of ZIKV H/PF/2013 or Paraiba 2015 in the eye at day 2 after infection that increased at day 6 (Fig 1A). Similar results were obtained after infection of Ifnar1−/− mice with Paraiba 2015, with high intraocular levels of ZIKV RNA accumulating by day 7 (Fig 1B).
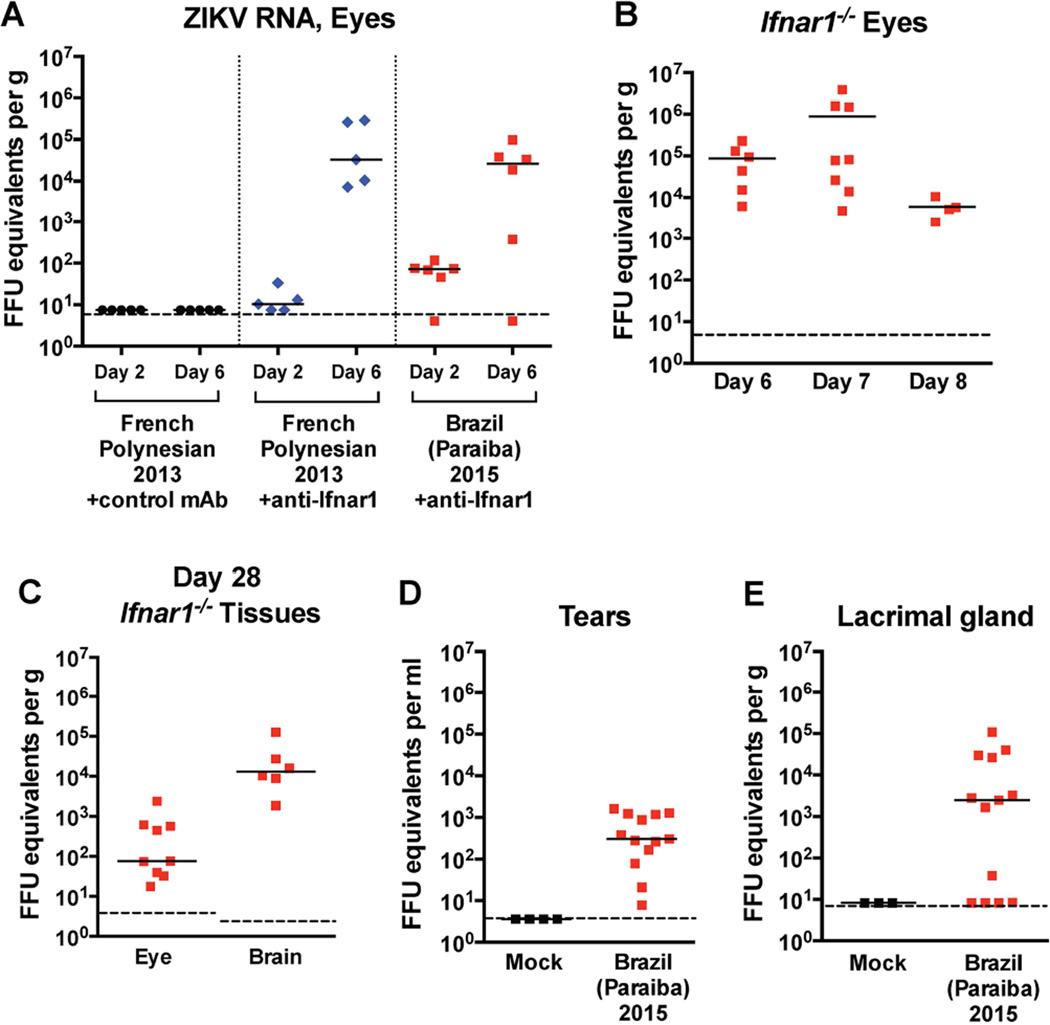
Figure 1. Viral burden in the eyes, tears, and lacrimal gland of ZIKV-infected animals.
Four-week-old mice were infected with 103 FFU of ZIKV Brazil (Paraiba 2015). Viral burden was measured by qRT-PCR assay. (A) Viral burden in the eyes of WT mice on days 2 and 6 after infection with ZIKV Brazil (Paraiba 2015) or French (H/PF/2013). Mice were treated with 2 mg of an anti-Ifnar1 or control mAb one day prior to infection.(B) Viral burden in the eyes of Ifnar1−/− mice on days 6, 7, and 8 after infection with ZIKV Brazil (Paraiba 2015). (C) Viral burden in the eyes and brain of Ifnar1−/− mice on day 28 after infection with ZIKV Brazil (Paraiba 2015). (D) Viral RNA in tears of mock- and ZIKV-infected Ifnar1−/− mice on day 7 after infection with ZIKV Brazil (Paraiba 2015). (E) Viral RNA in the lacrimal gland of mock- and ZIKV-infected Ifnar1−/− mice on day 7 after infection with ZIKV Brazil (Paraiba 2015). Symbols are derived from individual animals and pooled from 2 or 3 independent experiments. Bars indicate the mean of 5 to 13 mice per group. Dotted lines represent the limit of sensitivity of the assay.
Given the data on persistence of eye infection in humans after infection with EBOV and ZIKV (Furtado et al., 2016; Varkey et al., 2015), we assessed infection in mice 28 days after inoculation. Notably, ZIKV RNA persisted in several tissues including the eyes, brain, spleen, and other organs long after the virus was cleared from serum (Fig 1C and data not shown). During the 2015 EBOV epidemic, there was concern for person-to-person spread through ocular secretions including tears (Varkey et al., 2015). As such, we next tested whether ZIKV RNA was present in tear fluid after eye lavage of infected animals with 10 µl of PBS. We detected ZIKV RNA in tear fluid (~ 3 × 102 focus-forming units (FFU) equivalents per ml) and in the lacrimal gland (~2.4 × 103 FFU equivalents per ml) on day 7 after infection (Fig 1D and E), suggesting that infectious virus, viral RNA, or ZIKV-infected cellular debris were present in ocular secretions.
To determine whether ZIKV RNA in eyes (day 7 and day 28) and tears (day 7) was infectious, we inoculated AG129 mice via an intraperitoneal route with ocular homogenates or tear fluid; these mice were utilized because they lack receptors for both type I and II IFN signaling and are highly vulnerable to ZIKV infection even after inoculation with 1 plaque forming unit (PFU) (Aliota et al., 2016), which we confirmed (data not shown). Inoculation with eye homogenates obtained from Ifnar1−/− mice infected with Paraiba 2015 at day 7 resulted in death of AG129 mice, which occurred uniformly by day 10 (Fig 2A). In contrast, mice inoculated with eye homogenates from day 28 or tears from day 7 did not develop signs of ZIKV infection (Fig 2A, and data not shown). These data suggest that infectious virus was not produced in the eye after the acute phase of infection. In Ifnar1−/− mice infected with ZIKV Paraiba 2015, we observed conjunctivitis, although this occurred in some but not all animals (Fig 2B and data not shown). In the AG129 mice inoculated with day 7 eye homogenates, we observed greater ocular pathology including severe conjunctivitis with extraocular exudate in all animals, compared to milder disease in mice receiving a similar dose of the parental ZIKV Paraiba 2015 (Fig 2B and C). Nonetheless, we observed similar titers in the spleen, brain, and eyes of AG129 mice inoculated with either parental or eye-derived virus (Fig 2D). We considered whether the severe pathology seen with the eye-derived virus might be due to a virus adaptation that enhances ocular tropism or injury. To evaluate this hypothesis, we performed next generation sequencing of eye-, spleen-, and brain-derived virus from ZIKV-infected Ifnar1−/− animals (Fig S1 and Table S1). Although we did not identify any substitutions that were absent in the inoculating virus, we observed a large increase in frequency of a single NS2A nucleotide mutation (C→T at genome position 3895 from ~10 to ~80% in all biological replicates and in all tissues tested), which resulted in an alanine to valine change.
Figure 2. Survival and viral burden in AG129 mice inoculated with tears and eye homogenate derived infected Ifnar1−/− mice.
Four-week-old AG129 mice were inoculated via an intraperitoneal route with 104 FFU of parental ZIKV Brazil (Paraiba 2015) or eye-derived virus obtained from Ifnar1−/− mice. (A) Kaplan-Meier survival curve in mice inoculated with day 7 or 28 eye homogenates from Ifnar1−/− mice, or 10 µl of tears obtained 7 days after ZIKV infection of Ifnar1−/− mice. (B) A representative photograph demonstrating gross ocular pathology and exudate in mice inoculated with 104 FFU of parental ZIKV Brazil (Paraiba 2015) or eye-derived virus obtained from Ifnar1−/− mice. (C) Representative hematoxylin and eosin stained eye sections from AG129 mice infected with parental and eye-derived ZIKV. Black arrowheads indicate inflammatory cell infiltrates in the posterior region of the eye. Scale bars = 100 µm for upper panels and 75 µm for lower panels. (D) Viral burden assessed by qRT-PCR assay in the spleen, brain, and eyes of AG129 mice inoculated with 104 FFU of parental ZIKV Brazil (Paraiba 2015) or eye-derived virus obtained from Ifnar1−/− mice. Symbols are derived from individual animals and pooled from 2 or 3 independent experiments. Bars indicate the mean of 2 to 5 to mice per group. Dotted lines represent the limit of sensitivity of the assay. See also Figure S1.
Axl and Mertk are not required for ZIKV infection of the eye and brain in vivo
The TAM receptors (Tyro3, Axl, and Mertk) are a family of receptor tyrosine kinases whose ligands, Gas6 and Protein S, bind to phosphatidylserine on the surface of apoptotic cells and enveloped viruses (Meertens et al., 2012). As prior studies have suggested that Axl may act as an attachment or entry receptor for ZIKV (Hamel et al., 2015; Nowakowski et al., 2016; Savidis et al., 2016) and TAM receptors are expressed in multiple cell types in the eye (Prasad et al., 2006; Valverde et al., 2004), we hypothesized Axl or its related TAM receptor, Mertk might be required for efficient ZIKV replication in ocular tissues. Unexpectedly, Axl−/−, Mertk−/−, and Axl/Mertk−/− DKO mice that were treated with anti-Ifnar1 mAb exhibited similar levels of ZIKV RNA in the serum, spleen, brain, and eyes on day 6 after infection compared to similarly treated WT control animals (Fig 3A–D). Thus, in mice deficient in IFN signaling, Axl and Mertk are not required for ZIKV infection in several tissues including the eyes.
Figure 3. Viral burden in WT, Axl−/−, Mertk−/− or Axl−/− Mertk−/− DKO mice.
Four-to-six-week-old WT, Axl−/−, Mertk−/− or Axl−/− Mertk−/− DKO mice were treated with 2 mg of anti-Ifnar1 mAb one day prior to subcutaneous infection with 103 FFU of ZIKV Brazil (Paraiba 2015). On day 6 after infection, viral burden was measured by qRT-PCR in the eye (A), brain (B), serum (C) and spleen (D). Symbols are derived from individual animals and pooled from 2 independent experiments. Bars indicate the mean of 8 to 14 mice per group. Dotted lines represent the limit of sensitivity of the assay. Data were analyzed by Kruskal-Wallis one-way ANOVA with a Dunn’s multiple comparison test (P > 0.1 for comparison of all genotypes to the WT in all tissues).
ZIKV infects the eyes and brain of young WT mice
Congenital ZIKV infection in humans causes ocular pathology including optic neuritis, retinal mottling, and chorioretinal atrophy (de Paula Freitas et al., 2016; Miranda et al., 2016; Ventura et al., 2016a; Ventura et al., 2016b). This could be a consequence of direct eye infection, or it may be due to secondary brain defects that disrupt eye development. To test whether ocular pathology occurs in mice infected with ZIKV in utero, we modified a previously described congenital infection model with Ifnar1−/− dams and Ifnar1+/+ sires such that Ifnar1+/− fetuses develop intrauterine growth restriction and brain injury but not isolated microcephaly (Miner et al., 2016). After infection with ZIKV Paraiba 2015 at E6.5 or E12.5 (equivalent to late first and second trimester, respectively), we confirmed the presence of ZIKV RNA in the heads of infected Ifnar1+/− fetuses 6 to 7 days later (E13.5 and E18.5) by qRT-PCR (Fig 4A). As reported previously (Miner et al., 2016), substantive demise of Ifnar1+/− fetuses was observed by E13.5 after ZIKV infection of Ifnar1−/− dams at E6.5, which precluded analysis of fetal ocular tissues. However, fetuses treated with an anti-Ifnar1 mAb survived ZIKV infection on E6.5 but did not show ocular abnormalities by histological analysis (Fig 4B). When Ifnar1−/− dams were inoculated later in gestation (E12.5), intrauterine growth restriction occurred without fetal demise (Fig 4C). Again, no histologically apparent pathology or developmental abnormality was observed at E18.5 in the eyes of Ifnar1+/− fetuses (Fig 4D).
Figure 4. Histology and viral burden in the eye after congenital and early postnatal infection with ZIKV.
In panels (A–E), pregnant WT mice treated with 2 mg of an anti-Ifnar1 mAb or Ifnar1−/− mice were infected subcutaneously with 103 FFU of ZIKV Brazil (Paraiba 2015 WT), except where the French Polynesian strain is indicated in panel (E). (A) Viral burden was assessed by qRT-PCR on E13.5 in WT fetuses or E18.5 in Ifnar1+/− fetuses following E6.5 and 12.5 infection, respectively. (B)Representative hematoxylin and eosin stained eye sections from mock- and ZIKV-infected WT fetuses on E13.5. (C) A representative photograph demonstrating the average size of mock- and ZIKV-infected Ifnar1+/− fetuses on E18.5. (D) Representative hematoxylin and eosin stained eye sections from mock- and ZIKV-infected Ifnar1+/− fetuses on E18.5. The retinal detachment in the mock-infected sample is a commonly seen artifact of sectioning. (E) Viral burden in the eyes of WT pups on postnatal day 8 after in utero ZIKV infection of anti-Ifnar1 mAb-treated WT pregnant dams at the indicated gestational date with the indicated strain. (F–H) WT pups were inoculated subcutaneously on postnatal day 8 with 103 FFU of ZIKV Brazil (Paraiba 2015 WT) and harvested 6 days later. (F) Viral burden was assessed by qRT-PCR in the spleen, brain, and eye. (G) Activated caspase-3 staining of mock- (left panel) and ZIKV-infected (right panel) WT pup brains including the visual pathway (optic chiasm, optic tract, and lateral geniculate nucleus (LGN)). The image was generated by combining images of several coronal sections in the same animal into a single merged figure. (H) Activated caspase-3 staining of a ZIKV-infected WT pup brain highlighting apoptosis in the visual cortex (red box) at higher magnification (right panel). Results in A–H are from at least 2–3 independent experiments with 7 to 16 animals for viral burden analysis and 2 to 4 mice for histological analysis. Scale bars = 25 µm for (B), 60 µm for (D), 500 µm for (G) and 1 mm (H). Dotted lines represent the limit of sensitivity of the assay.
Since we observed delayed clearance of ZIKV from the eyes of adult mice (see Fig 1C), we tested for viral persistence in the eyes of congenitally infected pups on postnatal day 8. After testing five different experimental conditions including infection of anti-Ifnar1 mAb-treated pregnant WT mice at different gestational dates with ZIKV H/PF/2013 or Paraiba 2015, we detected viral RNA in eyes of only 2 of 41 congenitally infected animals (Fig 4E). These results suggest that ZIKV may not infect the eyes of fetuses efficiently in this model of in utero infection, even though it crosses the placenta (Miner et al., 2016). In contrast, postnatal infection of WT mice with ZIKV on day 8 after birth caused a subset of animals to become moribund, and ZIKV RNA was detected readily in the spleen, brain, and eyes 8 days later (Fig 4F). These data establish that ZIKV infection of the eye occurs in young mice even with intact type I IFN signaling. Examination of the brains of these postnatally infected animals revealed apoptosis (as detected by activated caspase-3 staining) in several central nervous system (CNS) regions including the optic tract, lateral geniculate nucleus, and the visual cortex, all components of the visual processing pathway (Fig 4G and H).
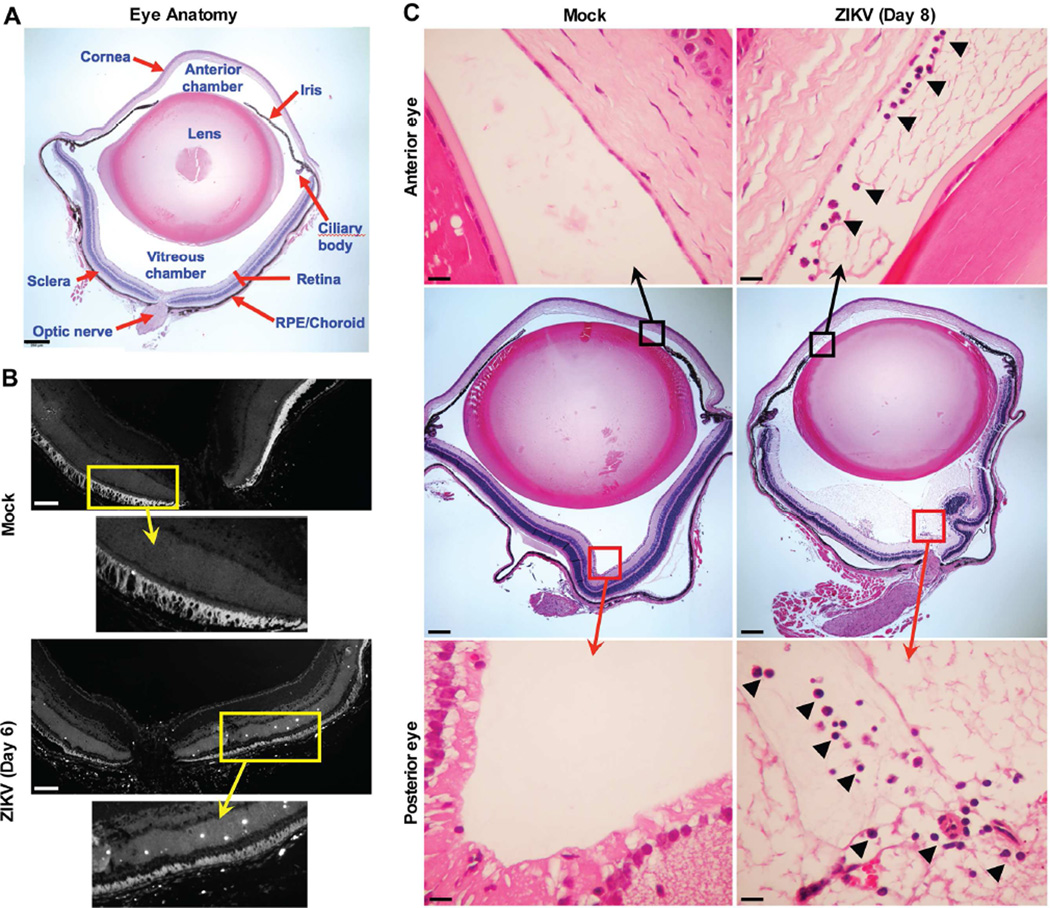
Anatomically, the eye is divided into the anterior (cornea, iris, ciliary body, lens) and posterior (vitreous, retina, retinal pigment epithelium, choroid, and optic nerve) compartments, each with specialized cells and functions (Fig 5A). We evaluated the extent of eye injury in Ifnar1−/− or anti-Ifnar1 mAb-treated adult animals infected with ZIKV. Histopathological analysis revealed TUNEL-positive cells in the neurosensory retina (Fig 5B, white punctate staining). Hematoxylin and eosin staining showed anterior uveitis with infiltration of inflammatory cells in the anterior chamber (Fig 5C, upper right panel). The posterior eye also exhibited evidence of uveitis with inflammatory cell infiltration, exudate, and debris in the vitreous humor(Fig 5C, lower right panel). We next assessed the fundus of the eye for gross structural damage using biomicroscopic and fundoscopic examination; no differences were observed between mock- and ZIKV-infected Ifnar1−/− or anti-Ifnar1 mAb-treated mice on day 7 (Fig S2 and data not shown). These experiments suggest that ZIKV infection does not induce significant pan-retinal abnormalities. Fluorescein angiography in ZIKV-infected Ifnar1−/− mice also revealed no evidence of vascular leakage or intra-retinal hemorrhages (Fig S2). Thus, ZIKV infection in adult mice causes pan-uveitis without major structural damage or effects on vascular permeability.
Figure 5. Histology of the eye in adult Ifnar1−/− mice after infection with ZIKV.
Four-to-eight-week-old Ifnar1−/− mice were inoculated subcutaneously with 103 FFU of ZIKV Brazil (Paraiba 2015). On days 6 or 8 after infection, eyes were harvested. (A) Anatomy of an uninfected eye, demonstrating anterior (cornea, lens, iris, and ciliary body) and posterior (sclera, retina, retinal pigment epithelium/choroid, and optic nerve) structures. (B) TUNEL staining of the neurosensory retina of a mock (upper panel) or ZIKV-infected animal on day 6 (lower panel). Regions shown in higher magnification are indicated by a yellow box and arrow. Scale bars = 100 µm. (C) Hematoxylin and eosin stained eye sections from mock (left panels) and ZIKV-infected animals on day 8 (right panels). Regions shown in higher magnification are indicated by a box and displayed in the upper and lower panels. Black arrowheads indicate inflammatory cell infiltrates in the anterior (upper panels) and posterior (lower panels) chambers of the eye. Scale bars = 250 µm (middle panels) and 25 µm (upper and lower panels). The data and images in this Figure are representative of two independent experiments with 2 to 4 animals per group. See also Figure S2 and S3.
To determine whether ZIKV infection of the eye caused functional deficits, we performed ex vivo electroretinography (ERG), which measures the transmission of light by photoreceptor cells. ERG testing of dissected eyes from Ifnar1−/− mice on day 7 after ZIKV infection revealed no apparent defects in photoreceptor function (Fig S3A–D). Similar results were obtained on days 7, 14, and 28 in ZIKV-infected WT mice treated with anti-Ifnar1 mAb (Fig S3E–H and data not shown). Thus, ZIKV infection and persistence in the eye does not cause global photoreceptor dysfunction. This ERG evaluation, however, does not rule out mild or focal defects in photoreceptor function or whether neuronal dysfunction of the inner retina and damage to the optic nerve, optic tract, or visual cortex occurs, any of which could result in blindness or selective visual field defects.
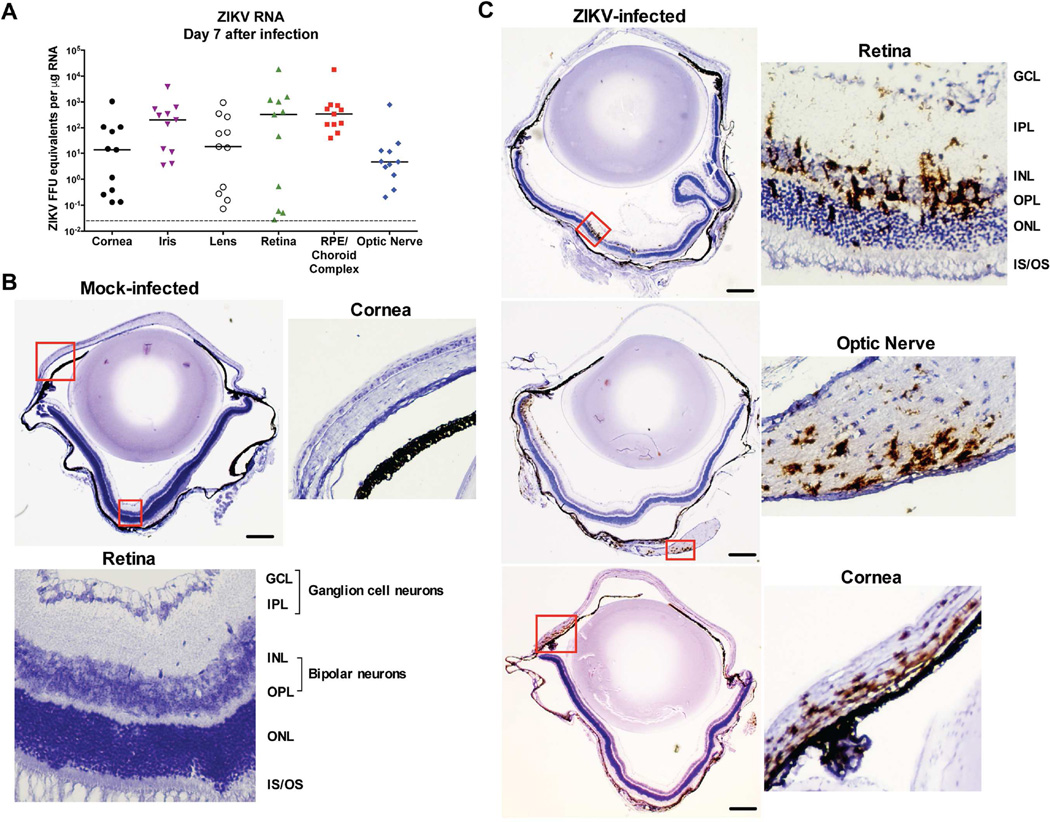
We evaluated the cellular tropism of ZIKV in the eye. Initially, we performed microdissection and measured ZIKV RNA levels in different compartments of the eye on day 7 after infection (Fig 6A). We included analysis of the anterior (cornea, iris, and lens) and posterior (neurosensory retina, retinal pigment epithelium/choroid complex, and optic nerve) chambers (see Fig 3B). ZIKV RNA was detected in all eye regions with ~100-fold higher levels in the retinal pigment epithelium/choroid complex compared to the optic nerve (P < 0.05, Fig 6A). To confirm these findings, eyes were collected at 6 to 8 days after inoculation and assessed for viral RNA by in situ hybridization (ISH). Mock-infected animals stained for ZIKV RNA and infected animals stained with a negative control probe against a bacterial gene had no staining in the cornea, optic nerve, or retina (Fig 6B and data not shown). In comparison, abundant ZIKV RNA was apparent in the bipolar and ganglion cell neurons of the neurosensory retina (Fig 6C, upper panel), the optic nerve (Fig 6C, middle panel), and the cornea of infected animals (Fig 6C, lower panel).
Figure 6. Viral burden and localization in the anterior and posterior regions of the eye.
Four-to-eight-week-old Ifnar1−/− mice were inoculated subcutaneously with 103 FFU of ZIKV Brazil (Paraiba 2015). Eyes were harvested for microdissection and quantitation of ZIKV RNA by qRT-PCR (day 7) or for ZIKV RNA detection by ISH (day 6 or 8). (A) Viral burden in the microdissected cornea, iris, lens, retina, retinal pigment epithelium/choroid, and optic nerve on day 7 after infection. Symbols are derived from individual animals and pooled from two independent experiments. Bars indicate the mean of 8 to 14 mice per group. Dotted lines represent the limit of sensitivity of the assay. Data were analyzed by Kruskal-Wallis one-way ANOVA with a Dunn’s multiple comparison test (P > 0.05 only for samples from optic nerve compared to RPE/choroid complex). (B and C) RNA ISH using a ZIKV-specific probe to stain eye sections of mock- (B) or ZIKV-infected (C) Ifnar1−/− mice. Red boxes indicate regions shown at higher magnification in adjacent panels. High magnification views in (B) indicate regions of the cornea (right panel) and retina (bottom panel). High magnification views in (C) show regions of the retina (right panel), optic nerve (middle panel), and cornea (lower panel). ISH data are representative of two independent experiments with at least two animals per group. Scale bar equals 200 µm.
DISCUSSION
Understanding the consequences of ocular infection by viruses has become of greater concern because of the potential implications related to viral persistence and spread. Case reports of human patients infected with herpes simplex virus-1 (HSV-1), EBOV, ZIKV, and WNV suggest some viruses have the capacity to cause significant acute ocular pathology including uveitis and keratitis (Chong et al., 2004; Furtado et al., 2016; Kuchtey et al., 2003; Varkey et al., 2015). Since ZIKV does not efficiently infect WT mice, we tested whether it targets the eyes of mice using a published model of ZIKV pathogenesis in adult animals deficient in type I IFN immunity as well as immunocompetent neonatal mice and fetuses. Although we did not observe evidence of congenital ocular disease, which has been described in humans (de Paula Freitas et al., 2016; McCarthy, 2016; Ventura et al., 2016a; Ventura et al., 2016b), ZIKV infected the eye and caused conjunctivitis and severe uveitis in immunodeficient adult mice. RNA ISH and virological analysis revealed that ZIKV infects the cornea, vascularized choroid, the bipolar and ganglion layers of the retina, and the optic nerve. Although the mechanism by which ZIKV causes eye disease remains undefined, our studies provide an animal model that can be used to define host factors that restrict pathogenesis and test candidate antiviral therapies directly within the eye.
The most common form of ZIKV-induced ocular disease is conjunctivitis, which occurs in 10 to 15% of patients, but whether conjunctivitis is a direct consequence of ZIKV infection of the eye is not known. In contrast, ZIKV-induced uveitis is less common, although it has been described in humans (Furtado et al., 2016). How ZIKV triggers uveitis remains uncertain, although inflammatory cell recruitment may contribute to this based on our histological findings. Direct infection of the eye may cause inflammation as a consequence of virus-induced cell death, cytokine production, and/or leukocyte recruitment. Alternatively, intracellular or extracellular viral RNA can act as a pathogen-associated molecular pattern (PAMP) and activate inflammatory responses via stimulating pattern recognition receptors (e.g., Toll-like or Rig-I-like receptors) even in the absence of infectious virus. In humans with EBOV- and ZIKV-induced uveitis, viral RNA was detectable within the intraocular fluid. We found that mice infected with ZIKV had no infectious virus in the eye by day 28 even though high levels of ZIKV RNA were present. Consistent with this observation, there is an extensive literature describing WNV-induced ocular disease in humans including uveitis, chorioretinitis, and retinal artery occlusion (Hershberger et al., 2003; Kaiser et al., 2003; Kuchtey et al., 2003). Neurotropic flaviviruses may first invade the brain, then infect the optic tract, and later transit in a retrograde direction into the eye along the optic nerve. Alternatively, ocular infection may result from hematogenous spread of virus across the blood-retinal barrier. Additional studies are needed to define the precise mechanisms of ocular invasion by ZIKV.
ZIKV infects both the eyes and testes in humans and in animal models (Lazear et al., 2016; Musso et al., 2015), suggesting that immune privileged organs may support replication even weeks after resolution of viremia and clinical symptoms. Hepatitis C virus, a related Flaviviridae family member, can infect the human cornea (Lee et al., 2001) and is transmitted by corneal transplantation (Tugwell et al., 2005). As ZIKV has capacity to infect the cornea of mice, human studies may be needed to confirm whether ZIKV analogously infects the human cornea. If corneal infection by ZIKV were established in humans, then widespread ocular infection during epidemics could necessitate testing by eye banks to ensure that ZIKV is not present in corneas of infected donors.
Congenital ocular disease caused by ZIKV may be due to direct targeting of cells in the fetal eye. Alternatively, it could occur as part of a neurodevelopmental defect, which is seen in microcephaly cases even in the absence of an infectious cause (Derbent et al., 2004). Although a recent study suggested ocular pathology was present in SJL mouse pups that were infected with ZIKV in utero, histological characterization of their eyes was not demonstrated (Cugola et al., 2016). We did not observe histological abnormalities of the eyes of congenitally infected Ifnar1+/− fetuses from C57BL/6 Ifnar1−/− dams. Studies with other mouse and non-human primate models are needed to clarify the requirements and basis for ZIKV-induced congenital ocular disease.
TAM receptors may enhance attachment and entry of flaviviruses, including ZIKV (Hamel et al., 2015; Meertens et al., 2012; Savidis et al., 2016). This hypothesis has been strengthened by a correlation between levels of Axl expression and vulnerability of certain neuronal subtypes to ZIKV infection (Nowakowski et al., 2016). However, our studies in Axl−/−, Mertk−/−, and Axl−/−Mertk−/− mice revealed no effect of a loss of expression of these TAM receptors on ZIKV replication, suggesting that Axl and Mertk are not required for CNS or ocular infection in mice. These results are analogous to prior studies with WNV where an absence of Axl and/or Mertk paradoxically resulted in enhanced infection in the brain that was due in part to alterations in the permeability of endothelial cells lining the blood-brain barrier (Miner et al., 2015). Our data do not exclude the possibility that Axl may still act as an entry factor for ZIKV in specific cells in other tissue compartments (e.g., trophoblasts in the placenta or subsets of neurons in the CNS).
In summary, we have described a mouse model of ocular disease that demonstrates ZIKV tropism in specific regions of the eye, pan-uveitis, shedding of viral RNA into tears, and persistence in immunodeficient adult mice. We also confirmed that ZIKV infects the eye of immunocompetent neonatal WT mice. Studies are planned to define the cellular mechanisms by which ZIKV invades and infects the eye and results in inflammation. Further analysis of the host and virus factors that facilitate ocular infection and mechanisms of immune-mediated clearance may lead to interventions that enhance elimination of viruses from immune privileged sites including the eye.
EXPERIMENTAL PROCEDURES
Ethics statement
This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols were approved by the Institutional Animal Care and Use Committee at the Washington University School of Medicine (Assurance number A3381-01). Inoculations were performed under anesthesia induced and maintained with ketamine hydrochloride and xylazine, and all efforts were made to minimize animal suffering.
Viruses and titration
The ZIKV strain H/PF/2013 (French Polynesia, 2013) was provided by the Arbovirus Branch of the Centers for Disease Control and Prevention with permission (X. de Lamballerie, Aix Marseille Université) (Baronti et al., 2014). The ZIKV strain Paraiba (Brazil, 2015) was provided by Steve Whitehead (NIH). ZIKV stocks were propagated in Vero cells and titrated by focus-forming assay (FFA) as previously described (Lazear et al., 2016; Miner et al., 2016).
Mouse experiments
Ifnar1−/− mice (Hwang et al., 1995) were backcrossed onto a C57BL/6 background. Axl−/−, Mertk−/−, and Axl−/−Mertk−/− mice have been published (Lu and Lemke, 2001) and were backcrossed for ten generations. Mice were bred in a specific-pathogen-free facility at Washington University, or purchased (WT animals) from Jackson Laboratories. In some experiments, WT mice were treated with 2 mg of an antiIfnar1 blocking mouse MAb (MAR1-5A3) or isotype control mouse MAb (GIR-208) (produced by Leinco Technologies) (Sheehan et al., 2006; Sheehan et al., 2015) by intraperitoneal injection prior to infection with ZIKV. Four to eight-week-old mice were inoculated with ZIKV by subcutaneous (footpad) route with 103 FFU in 50 µl of PBS. Mice were sacrificed at day 2, 6, 7, 8, or 28 depending on the experimental design, and organs were harvested. To study congenital infection, anti-Ifnar1 mAb-treated WT pregnant mice were infected with the indicated strains of ZIKV at E6.5, E9.5, E12.5, or E15.5 and tissues were harvested at postnatal day 8. For some experiments pregnant Ifnar1−/− females carrying Ifnar1+/− fetuses were infected on E12.5. Ifnar1+/− fetal tissues were harvested on E18.5 as previously described (Miner et al., 2016). For studies in neonatal WT animals, mice were inoculated subcutaneously on postnatal day 7 or 8 and then tissues harvested 6 days later and analyzed for the presence of viral RNA. For comparison of parental (Paraiba 2015) and mouse-passaged ZIKV, AG129 mice (van den Broek et al., 1995) were inoculated via an intraperitoneal route with 104 FFU of parental ZIKV or equivalent dose (based on viral RNA) from eye homogenates collected from Ifnar1−/− mice on day 7 or day 28 after ZIKV infection. Alternatively, AG129 mice were inoculated via an intraperitoneal route with 10 µl of tears (diluted in 150 µl of PBS) collected from Ifnar1−/− mice on day 7 after infection with ZIKV (Paraiba 2015).
Measurement of viral burden
Tissues were weighed and homogenized with zirconia beads in a MagNA Lyser instrument (Roche Life Science) in 250 µl or 500 µl of PBS. Tissue samples and serum from ZIKV-infected mice were extracted with the RNeasy Mini Kit (tissues) or Viral RNA Mini Kit (serum) (Qiagen). ZIKV RNA levels were determined by one-step quantitative reverse transcriptase PCR (qRT-PCR) on an ABI 7500 Fast Instrument using standard cycling conditions. Viral burden was expressed on a log10 scale as viral RNA equivalents per gram or per ml after comparison with a standard curve produced using serial 10-fold dilutions of ZIKV RNA. A published primer set was used to detect ZIKV RNA (Lanciotti et al., 2008; Miner et al., 2016).
Tears and lacrimal gland collection
ZIKV-infected mice were euthanized on day 7 after infection. Tear fluid from both eyes was collected after gentle lavage with 10 µl of PBS using FP plus multiflex tips (Fisher Scientific). Intact lacrimal gland lobules were isolated by dissection.
TUNEL staining
Cell death in the neurosensory retina of Ifnar1−/− mice was assessed 7 days after ZIKV infection by TUNEL staining. Briefly, paraffin sections of eyes from ZIKV-infected mice were pretreated with proteinase K and stained with ApopTag fluorescein in situ apoptosis detection kit (Millipore) according to the manufacturer’s instructions. Images of the retina were collected and merged into a composite image.
Brain sectioning and immunostaining for activated caspase-3
Following ZIKV infection, animals were anesthetized and perfused via intracardiac injection with 4% paraformaldehyde in Tris-HCl buffer. Brains were then sectioned at 70 µm with a vibratome and stained for activated caspase-3 as described previously (Miner et al., 2016).
RNA in situ hybridization (ISH)
RNA ISH was performed using RNAscope 2.5 (Advanced Cell Diagnostics) according to the manufacturer’s instructions. Briefly, formalin-fixed paraffin-embedded tissue sections were deparaffinized by incubating for 60 min at 60°C. Endogenous peroxidases were quenche d with hydrogen peroxide for 10 min at room temperature. Slides were then boiled for 15 min in RNAscope Target Retrieval Reagents and incubated for 30 min in RNAscope Protease Plus before probe hybridization. The probe targeting ZIKV RNA was designed and synthesized by Advanced Cell Diagnostics (Catalog #467771). Positive (targeting plr2a gene) and negative (targeting bacterial gene dapB) control probes also were obtained from Advanced Cell Diagnostics (Catalog #312471 and #310043, respectively). Tissues were counterstained with Gill’s hematoxylin and visualized using standard bright-field microscopy.
Data analysis
All data were analyzed with GraphPad Prism software. For viral burden analysis, the log10 transformed titers and levels of viral RNA were analyzed by the Mann-Whitney test or Kruskal-Wallis one-way ANOVA. A P value of < 0.05 indicated statistically significant differences.
Supplementary Material
Acknowledgments
This study was supported by grants from the NIH (R01 AI073755, R01 AI101400, and R01 AI104972 (to M.S.D.), R01 EY019287 (to R.S.A.), P30 EY02687 (Vision Core Grant), and R01 AI067380 (to G.D.E.). R.S.A. also was supported by a Physician-Scientist Award from Research to Prevent Blindness, the Schulak Family Gift Fund for Retinal Research, and the Jeffrey Fort Innovation Fund. J.J.M. was supported by a Rheumatology Research Foundation Scientist Development Award. K.N. was supported by the Intellectual and Developmental Disabilities Research Center at Washington University (NIH/NICHD U54 HD087011). Additional funding comes from an unrestricted grant to the Department of Ophthalmology and Visual Sciences of Washington University School of Medicine from Research to Prevent Blindness. M.S.D. is a consultant for Inbios, Visterra, Sanofi, and Takeda Pharmaceuticals, is on the Scientific Advisory Boards of Moderna and OraGene, and is a recipient of research grants from Moderna, Sanofi, and Visterra.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
J.J.M. and A.M.S. performed infections, tissue harvests, RNA isolation, qRT-PCR analysis, and some of the histological analysis. A. Sene performed ERG, fundoscopy, fluorescein angiography, TUNEL staining, and some of the histological analysis. A. Sene and N.B. dissected lacrimal glands, eye regions, and collected tears. J.M.R. and A.M.S. performed all RNA ISH experiments. J.G. performed the ZIKV French Polynesian strain infection and harvest. A. Santeford performed tissue sectioning and histological analysis. F.M. and K.N. performed histological analysis of the brain. J.W., C.R., and G.D.E. performed the next generation sequencing and analysis. A. Sene wrote parts of the initial draft. J.J.M. wrote the initial complete draft of the manuscript. J.J.M., M.S.D., and R.S.A. edited the manuscript. J.J.M, M.S.D, and R.S.A are corresponding authors. As a group, the corresponding authors shared responsibility for all data, figures, and text, determination of authorship, approval of content by authors, adherence to editorial policies, declaration of conflict of interests, and ensuring figures are representative of the original data. M.S.D. was the Lead Contact for journal communication and is the primary contact for reagent and resource sharing.
REFERENCES
- Aliota MT, Caine EA, Walker EC, Larkin KE, Camacho E, Osorio JE. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Negl Trop Dis. 2016;10:e0004682. doi: 10.1371/journal.pntd.0004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour J, Laurent-Rolle M, Shi P-Y, García-Sastre A. NS5 of Dengue Virus Mediates STAT2 Binding and Degradation. Journal of Virology. 2009;83:5408–5418. doi: 10.1128/JVI.02188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baronti C, Piorkowski G, Charrel RN, Boubis L, Leparc-Goffart I, de Lamballerie X. Complete coding sequence of zika virus from a French polynesia outbreak in 2013. Genome announcements. 2014;2:e00500–e00514. doi: 10.1128/genomeA.00500-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Zagórska A, Lew Erin D, Shrestha B, Rothlin Carla V, Naughton J, Diamond Michael S, Lemke G, Young John AT. Enveloped Viruses Disable Innate Immune Responses in Dendritic Cells by Direct Activation of TAM Receptors. Cell Host & Microbe. 2013;14:136–147. doi: 10.1016/j.chom.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong E-M, Wilhelmus KR, Matoba AY, Jones DB, Coats DK, Paysse EA. Herpes simplex virus keratitis in children. American Journal of Ophthalmology. 2004;138:474–475. doi: 10.1016/j.ajo.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JLM, Guimarães KP, Benazzato C, Almeida N, Pignatari GC, Romero S, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–271. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula Freitas B, de Oliveira Dias J, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed zika virus congenital infection in salvador, brazil. JAMA Ophthalmology. 2016;134:529–535. doi: 10.1001/jamaophthalmol.2016.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbent M, Agras PI, Gedik Ş, Oto S, Alehan F, Saatçi Ü. Congenital cataract, microphthalmia, hypoplasia of corpus callosum and hypogenitalism: Report and review of Micro syndrome. American Journal of Medical Genetics Part A. 2004;128A:232–234. doi: 10.1002/ajmg.a.30109. [DOI] [PubMed] [Google Scholar]
- Furtado JM, Espósito DL, Klein TM, Teixeira-Pinto T, da Fonseca BA. Uveitis Associated with Zika Virus Infection. New England Journal of Medicine. 2016;375:394–396. doi: 10.1056/NEJMc1603618. [DOI] [PubMed] [Google Scholar]
- Grant A, Ponia Sanket S, Tripathi S, Balasubramaniam V, Miorin L, Sourisseau M, Schwarz Megan C, Sánchez-Seco Mari P, Evans Matthew J, Best Sonja M, et al. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host & Microbe. 2016;19:882–890. doi: 10.1016/j.chom.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, et al. Biology of Zika Virus Infection in Human Skin Cells. Journal of Virology. 2015;89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershberger VS, Augsburger JJ, Hutchins RK, Miller SA, Horwitz JA, Bergmann M. Chorioretinal lesions in nonfatal cases of West Nile virus infection. Ophthalmology. 2003;110:1732–1736. doi: 10.1016/S0161-6420(03)00720-6. [DOI] [PubMed] [Google Scholar]
- Hwang SY, Hertzog PJ, Holland KA, Sumarsono SH, Tymms MJ, Hamilton JA, Whitty G, Bertoncello I, Kola I. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:11284–11288. doi: 10.1073/pnas.92.24.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser PK, Lee MS, Martin DA. Occlusive vasculitis in a patient with concomitant west nile virus infection. American Journal of Ophthalmology. 2003;136:928–930. doi: 10.1016/s0002-9394(03)00866-3. [DOI] [PubMed] [Google Scholar]
- Kuchtey RW, Kosmorsky GS, Martin D, Lee MS. Uveitis associated with West Nile virus infection. Archives of Ophthalmology. 2003;121:1648–1649. doi: 10.1001/archopht.121.11.1648. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerging infectious diseases. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. A Mouse Model of Zika Virus Pathogenesis. Cell Host & Microbe. 2016;19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Naor J, Alhindi R, Chinfook T, Krajden M, Mazzulli T, Rootman DS. Detection of Hepatitis C Virus in the Corneas of Seropositive Donors. Cornea. 2001;20:37–40. doi: 10.1097/00003226-200101000-00007. [DOI] [PubMed] [Google Scholar]
- Lu Q, Lemke G. Homeostatic Regulation of the Immune System by Receptor Tyrosine Kinases of the Tyro 3 Family. Science. 2001;293:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- McCarthy M. Severe eye damage in infants with microcephaly is presumed to be due to Zika virus. BMJ. 2016;352:i855. doi: 10.1136/bmj.i855. [DOI] [PubMed] [Google Scholar]
- Meertens L, Carnec X, Lecoin Manuel P, Ramdasi R, Guivel-Benhassine F, Lew E, Lemke G, Schwartz O, Amara A. The TIM and TAM Families of Phosphatidylserine Receptors Mediate Dengue Virus Entry. Cell Host & Microbe. 2012;12:544–557. doi: 10.1016/j.chom.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016 doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, Daniels BP, Shrestha B, Proenca-Modena JL, Lew ED, Lazear HM, Gorman MJ, Lemke G, Klein RS, Diamond MS. The TAM receptor Mertk protects against neuroinvasive viral infection by maintaining blood-brain barrier integrity. Nat Med. 2015;21:1464–1472. doi: 10.1038/nm.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda HAd, II, Costa MC, Frazão MAM, Simão N, Franchischini S, Moshfeghi DM. Expanded Spectrum of Congenital Ocular Findings in Microcephaly with Presumed Zika Infection. Ophthalmology. 2016;123:1788–1794. doi: 10.1016/j.ophtha.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau V. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21:552. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. 2006;7:354–359. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- Nowakowski Tomasz J, Pollen Alex A, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein Arnold R. Expression Analysis Highlights AXL as a Candidate Zika Virus Entry Receptor in Neural Stem Cells. Cell Stem Cell. 2016;18:591–596. doi: 10.1016/j.stem.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad D, Rothlin CV, Burrola P, Burstyn-Cohen T, Lu Q, Garcia de Frutos P, Lemke G. TAM receptor function in the retinal pigment epithelium. Molecular and Cellular Neuroscience. 2006;33:96–108. doi: 10.1016/j.mcn.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, Langsjoen RM, Paessler S, Vasilakis N, Weaver SC. Characterization of a Novel Murine Model to Study Zika Virus. The American Journal of Tropical Medicine and Hygiene. 2016;94:1362–1369. doi: 10.4269/ajtmh.16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakala IG, Chaudhri G, Scalzo AA, Eldi P, Newsome TP, Buller RM, Karupiah G. Evidence for Persistence of Ectromelia Virus in Inbred Mice, Recrudescence Following Immunosuppression and Transmission to NaÔve Mice. PLoS Pathog. 2015;11:e1005342. doi: 10.1371/journal.ppat.1005342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarno M, Sacramento GA, Khouri R, do Ros·rio MS, Costa F, Archanjo G, Santos LA, Nery N, Jr, Vasilakis N, Ko AI, et al. Zika Virus Infection and Stillbirths: A Case of Hydrops Fetalis, Hydranencephaly and Fetal Demise. PLoS Negl Trop Dis. 2016;10:e0004517. doi: 10.1371/journal.pntd.0004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidis G, McDougall William M, Meraner P, Perreira Jill M, Portmann Jocelyn M, Trincucci G, John Sinu P, Aker Aaron M, Renzette N, Robbins Douglas R, et al. Identification of Zika Virus and Dengue Virus Dependency Factors using Functional Genomics. Cell Reports. 2016;16:232–246. doi: 10.1016/j.celrep.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Sheehan KC, Lai KS, Dunn GP, Bruce AT, Diamond MS, Heutel JD, Dungo-Arthur C, Carrero JA, White JM, Hertzog PJ, et al. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2006;26:804–819. doi: 10.1089/jir.2006.26.804. [DOI] [PubMed] [Google Scholar]
- Sheehan KC, Lazear HM, Diamond MS, Schreiber RD. Selective Blockade of Interferon-alpha and -beta Reveals Their Non-Redundant Functions in a Mouse Model of West Nile Virus Infection. PLoS One. 2015;10:e0128636. doi: 10.1371/journal.pone.0128636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, Harris E, Pereira L. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host & Microbe. 2016;20:155–166. doi: 10.1016/j.chom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany A, Vetter P, Mattia J, Dayer J-A, Bartsch M, Kasztura M, Sterk E, Tijerino AM, Kaiser L, Ciglenecki I. Ebola Virus Disease Complications as Experienced by Survivors in Sierra Leone. Clinical Infectious Diseases. 2016;62:1360–1366. doi: 10.1093/cid/ciw158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugwell BD, Patel PR, Williams IT, Hedberg K, Chai F, Nainan OV, Thomas AR, Woll JE, Bell BP, Cieslak PR. Transmission of Hepatitis C Virus to Several Organ and Tissue Recipients from an Antibody-Negative Donor. Annals of Internal Medicine. 2005;143:648–654. doi: 10.7326/0003-4819-143-9-200511010-00008. [DOI] [PubMed] [Google Scholar]
- Valverde P, Obin MS, Taylor A. Role of Gas6/Axl signaling in lens epithelial cell proliferation and survival. Experimental Eye Research. 2004;78:27–37. doi: 10.1016/j.exer.2003.10.002. [DOI] [PubMed] [Google Scholar]
- van den Broek MF, Müller U, Huang S, Aguet M, Zinkernagel RM. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. Journal of Virology. 1995;69:4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varkey JB, Shantha JG, Crozier I, Kraft CS, Lyon GM, Mehta AK, Kumar G, Smith JR, Kainulainen MH, Whitmer S, et al. Persistence of Ebola Virus in Ocular Fluid during Convalescence. New England Journal of Medicine. 2015;372:2423–2427. doi: 10.1056/NEJMoa1500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura CV, Maia M, Bravo-Filho V, Góis AL, Belfort R., Jr Zika virus in Brazil and macular atrophy in a child with microcephaly. The Lancet. 2016a;387:228. doi: 10.1016/S0140-6736(16)00006-4. [DOI] [PubMed] [Google Scholar]
- Ventura CV, Maia M, Travassos SB, et al. RIsk factors associated with the ophthalmoscopic findings identified in infants with presumed zika virus congenital infection. JAMA Ophthalmology. 2016b;134:912–918. doi: 10.1001/jamaophthalmol.2016.1784. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.