Abstract
Purpose
This study was conducted to determine whether alpha lipoic acid (ALA) promotes the survival of retinal ganglion cells (RGCs) in a rat model of optic nerve crush (ONC) injury and to investigate the neuroprotective mechanisms of ALA in the retina in this ONC injury model.
Methods
Adult male Sprague-Dawley rats (180–220 g) were subjected to ONC injury surgery. ALA (63 mg/kg) was injected intravenously 1 day before or after the ONC injury. Animals were euthanized after 10 days, and the number of ganglion cells positive for RNA-binding protein with multiple splicing (Rbpms), which is an RGC marker, were counted on the whole mount retinas. In addition, immunofluorescence and immunoblotting were performed to examine the localization and levels of erythropoietin receptor (EPOR) and neurotrophin-4/5 (NT4/5) in the retinas in all experimental groups. To determine whether the EPO/EPOR signaling pathway was involved in the ALA antioxidant pathway, the rats were subjected to ruxolitinib (INCB018424, 0.25 mg/kg, bid, intraperitoneal, i.p.) treatment after the animals were injected intravenously with ALA 1 day before ONC injury.
Results
The average number of Rbpms-positive cells/mm2 in the control group (sham-operated group), the ONC group, the ALA-ONC group, and the ONC-ALA group retinas was 2219±28, 418±8, 848±22, and 613±18/mm2, respectively. The ALA-ONC and ONC-ALA groups showed a statistically significantly increased RGC survival rate compared to the ONC group. There were statistical differences in the RGC survival rates between the ALA-ONC (39%) and ONC-ALA groups (28%; p<0.05). Immunofluorescent labeling showed that EPOR and NT4/5 expression was significant in the retinal ganglion cell layer (GCL). At the same time, western blot analysis revealed that ALA induced upregulation of EPOR protein and NT4/5 protein expression in the retina after ONC injury. However, INCB018424 reversed the protective effects of ALA on the ONC retinas.
Conclusions
ALA has neuroprotective effects on RGCs after ONC injury. Moreover, prophylactic administration of ALA may have a stronger neuroprotective effect against ONC-induced damage. Based on these data, we also conclude that the endogenous EPO/EPOR signaling pathway may contribute to the protective effects of ALA in the retina after ONC injury.
Introduction
Mechanical axonal injury and a lack of neurotrophic support for retinal ganglion cell (RGC) bodies induces apoptosis and necrosis of retinal ganglion cells [1], which results in visual dysfunction and can lead to blindness by giving rise to diseases such as glaucoma [2,3]. Blunt trauma, including optic canal fractures and expansile intracranial lesions, may result in partial axonal injury instead of complete nerve transection. The optic nerve is a white-matter tract composed principally of RGC axons, and injury of the optic nerve is similar to brain axonal injury [4-6]. Currently, there is no effective therapy for diseases involving optic nerve injury, such as glaucoma and ischemic optic neuropathy [7,8]. To investigate the mechanisms and neuroprotective treatment of disease associated with optic nerve injury, we chose a partial axonal injury model. Optic nerve crush (ONC) injury is a model of acute RGC injury that produces rapid degeneration of axons and significant changes in RGC morphology that are readily standardized [9]. We thus decided to use this model as previously described [10-12] with a slight modification, to understand the process of axonal degeneration and RGC death involved in traumatic optic neuropathy and glaucoma [13,14].
The mechanism of RGC death after ONC in adult animals is not fully understood. Many studies have demonstrated that neurotrophic factor deprivation [15,16] and oxidative stress [17] contribute to RGC loss. A substantial body of evidence suggests that reactive oxygen species (ROS) are part of the signaling pathway in RGC death after ONC [18,19]. RGC axons within the globe are functionally specialized and are richly endowed with many mitochondria. Mitochondria produce the energy required for nerve conduction in the unmyelinated part of ganglion cell axons. Thus, optic nerve crush injury–induced RGC apoptosis may at least partially be due to mitochondrial malfunction [20,21]. Alpha lipoic acid (ALA) and its reduced form, dihydrolipoic acid (DHLA), have powerful antioxidant effects. ALA is a disulfide compound found naturally in mitochondria that serves as the coenzyme involved in the carbohydrate utilization necessary for the production of ATP in mitochondria. Evidence shows that ALA is a superb antioxidant that enhances mitochondrial function [20,22,23]. ALA inhibits mitochondrial calcium transport that may be associated with its beneficial effects which are observed in neurodegenerative disorders [10]. ALA provides protection to the retina as a whole, and to ganglion cells in particular from ischemia–reperfusion injury [24] and optic nerve crush [20]. Recent studies have revealed that ALA exerts a neuroprotective effect against oxidative stress in retinal neurons [25,26].
However, whether treatment with ALA has protective effects on RGCs in the ONC retina, and whether there are differences in outcome with administration of ALA before versus after ONC is unclear. Erythropoietin (EPO) and erythropoietin receptor (EPOR) are expressed in the retina [27,28], and an endogenous EPO/EPOR signaling system may participate in intrinsic recovery mechanisms and play an endogenous neuroprotective role in the survival of RGCs after retinal injury [29]. EpoR depends on Janus kinase 2 (JAK2), which is a non-receptor tyrosine kinase, and its active form binds the Box1/Box2 region of EpoR [30]. EPO binding to the EpoR homodimer triggers a conformational change in the receptor cytoplasmic domain, bringing the JAK2 proteins in close proximity to each other and resulting in transphosphorylation and activation of JAK2 and EpoR [31]. Neurotrophin-4/5 (NT4/5), which is a neurotrophin that shares many homologies with brain-derived neurotrophic factor (BDNF), and its receptor, tropomyosin related kinase receptor B (TrkB), are produced locally in the retina [32-35]. Multiple studies have demonstrated that administration of NT4/5 increases RGC survival in ONC retinas [36-39]. The NT4/5-TrkB survival signaling pathway has been implicated in the protection of RGCs against retinal injury, such as ONC, vitreoretinopathy, and glaucoma [35,37,40-42]. Although research in this area is substantial, the effectiveness of ALA as a neuroprotectant acting via the endogenous EPO/EPOR signaling pathway or the NT4/5 molecule in the ONC retina has not been investigated. The objective of this study was to determine whether ALA has protective effects on retina neuronal cells against retinal ONC injury and to identify the mechanisms involved in this process.
Methods
Animals
Seventy-four 6-week-old male Sprague-Dawley (SD) rats with bodyweight of 180–220 g were housed in a 12 h:12 h light-dark cycle environment. Rats had free access to food and water during the experiments. All experiments were performed in accordance with the Peking University guidelines for animal research and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The experimental animal protocol used throughout this study was approved by the Peking University Institutional Animal Care and Use Committee (IACUC).
Animal model of retinal ONC
ONC surgery was conducted as previously described [10,11] with a slight modification. Briefly, rats were deeply anesthetized with an intraperitoneal injection using a cocktail of ketamine (80 mg/kg) and xylazine (8 mg/kg). An incision was made on the temporal conjunctiva, the lateral rectus muscle was dissected, and the optic nerve was exposed with sharp forceps tips, followed by blunt dissection. The left optic nerve was then crushed 1 mm distal to the globe for 10 s with a 40 g power vascular clamp (TKF-5–40, AROSurgical Corp, Newport Beach, CA), carefully avoiding vessels. The right eye was treated in the same manner, but crushing of the optic nerve was omitted to establish a sham-operated control group.
Experimental design
ALA (300 mg:12 ml, c = 121 mmol/l, C8H14O2S2) was purchased from STADA (Dresden, Germany). About 0.5 ml ALA was administered in one intravenous (i.v.) bolus (63 mg/kg bodyweight) 1 day before or after ONC surgery. Control animals received vehicle alone. Ruxolitinib (INCB018424, 0.25 mg/kg, bid at a fixed time; Selleck Chemicals, Shanghai, China), which is a Jak2 inhibitor, was administered via intraperitoneal (i.p.) injection (for 10 days until the animals were killed) after retinal ONC injury following 1 day of pretreatment with ALA. INCB018424 (10 mg) was prepared by dissolving the compound in 1 ml of dimethyl sulfoxide (DMSO) as stock solution (10 mg/ml). The pH was adjusted to 7.4, and the solution was diluted to a final volume with 0.9% NaCl. Rats were randomly divided into five groups: The sham-operated group (the optic nerve was exposed with sharp forceps tips and blunt dissection without crushing the optic nerve, Control, n = 17); the ONC group (animals were subjected to retinal optic nerve crush only, ONC, n = 17); the ALA-ONC group (animals were injected intravenously with ALA 1 day before ONC, ALA-ONC, n = 17); the ONC-ALA group (animals were injected intravenously with ALA 1 day after ONC, ONC-ALA, n = 17); and the ALA-ONC+I group (animals were injected intravenously with ALA 1 day before ONC, followed by INCB018424, bid, i.p., ALA-ONC+I, n = 6). All experimental animals were euthanized by administration of an overdose of sodium pentobarbital 10 days after ONC injury.
Tissue preparation and whole mount retinal immunohistochemistry
Rats were euthanized with an overdose of sodium pentobarbital, the temporal pole of each retina was burn marked for orientation, and the eyes were removed and dissected (n = 30). After the cornea, lens, and vitreous body were removed, the retina was removed from the eyecup after 2 min in 4% paraformaldehyde (Cat. No. 15710, Electron Microscopy Sciences, Ft. Washington, PA), dissected from the RPE, separated by four radial cuts in the retinal periphery, and mounted on a nitrocellulose membrane filter (GSWP02500, Millipore, Bedford, MA), followed by fixation for an additional 60 min in a 24-well tissue culture cluster (Cat. No. 3524, Corning Inc., Corning, MA). Retinas were then washed four times with 0.01 M PBS (1X; 85 mM Na2HPO4, 15 mM NaH2PO4, 150 mM NaCl, pH 7.4) for 6 min per wash on a shaker (unless otherwise stated, all steps in the following protocol were performed on a shaker). The retinas were then incubated in 2% Triton X-100 (Sigma-Aldrich, St. Louis, MO) and 0.5% DMSO (Sigma-Aldrich) in 0.01 M PBS for 2–3 days at 4 °C and then blocked (CAS-Block, Invitrogen, Frederick, MD) for 3–4 h at room temperature. The blocking solution was removed, and a rabbit polyclonal antibody against RNA-binding protein with multiple splicing (Rbpms; 1:1,000, ProSci, Poway, CA), diluted in blocking solution, was immediately added to the retinas, which were then incubated for 3–5 days at 4 °C. After rinsing four times to remove excess primary antibody, the retinas were incubated with secondary antibody DyLight 488 anti-rabbit immunoglobulin G (IgG; 1:2,000, Vector Labs, Inc., Burlingame, CA) and diluted in blocking solution for 3–4 h at room temperature. The cell nuclei were then counterstained with 4’-6-diamidino-2-phenylindole (DAPI, Cat. No. 10236276001, Roche, Basel, Switzerland). Following the final washing, the retinas were mounted on glass slides with the ganglion cell layer up, air-dried, and coverslipped using antifade mounting medium (Cat. No. 17985–10, Electron Microscopy Sciences, Hatfield, PA).
Image processing and counting of surviving RGCs
The entire whole mount retina was photographed with a 2.5× objective using fluorescent microscopy (BX51, Olympus, Tokyo, Japan). The actual image area of a single shot was 3207 × 2415 μm2 (n = 6 per group). Photographic images were montaged with graphic software Adobe Photoshop CS5 (Adobe Systems, Inc., San Jose, CA). Mean densities of Rbpms-positive cells in the GCL of the retina were estimated as previously described [16,43,44]. Briefly, Rbpms-positive cells were counted by the same observer from photographs of 12 rectangular (436 × 327 μm2/microscope fields) areas of each retina, three in each quadrant (superior, inferior, nasal, and temporal) at distances of 1, 2, and 3 mm from the optic disc (OD) using a 20× objective lens. Cell counts were performed in an area approximately the same distance from the optic disc (1, 2, and 3 mm from the OD). Twelve microscopic fields were counted per retina, corresponding to approximately 3.5% of the retinal area. The average number of Rbpms-positive cells/mm2 was calculated. Quantification was performed in a blinded manner.
Immunohistochemistry using radial sections
Immunofluorescence was used to examine the localization of EPOR-positive cells and NT4/5-positive cells in the retina (n = 24). In addition, radial sections were also used to double label EPOR or NT4/5 with Rbpms, which is a specific marker for RGCs [45,46]. Eyes were enucleated, postfixed in 4% freshly prepared paraformaldehyde for 40 min to 1 h, and then immersed in 30% sucrose solution (in 0.01 M PBS) at 4 °C overnight. The following day, retinas were embedded in optimum cutting temperature (OCT). Sections were cut transversely along the temporal-nasal axis of the eyeball. To ensure comparability, only sections containing the optic nerve stump were used for this study (n = 6 per group). Cryosections (12 µm) were thawed, air-dried, and washed three times with 0.01 M PBS (pH 7.4). Tissue specimens were treated with 3% bovine serum albumin (BSA; Sigma-Aldrich) in 0.3% Triton X-100 for 60 min at room temperature and then incubated with either rabbit polyclonal antibody against EPOR (1:100, sc-5624, Santa Cruz Biotech Inc., Dallas, TX) or goat polyclonal antibody against NT4/5 (1:100, SAB2500696, Sigma-Aldrich), and with guinea pig polyclonal antibody against Rbpms (1:1,000, ProSci) as double primary antibodies. Immunoreactivity was evaluated using two fluorescein isothiocyanate (FITC)-labeled secondary antibodies (Abcam Inc., Cambridge, MA), and cell nuclei were counterstained with 4’-6-diamidino-2-phenylindole (DAPI; Cat. No. 10236276001, Roche). As a control for secondary antibody specificity, some retinas were processed in parallel for immunofluorescence staining, with the omission of the primary antibody. These specimens showed no detectable signal. Preparations were evaluated using fluorescence microscopy (BX51, Olympus, Tokyo, Japan).
Western blotting
Rats were euthanized using an overdose of sodium pentobarbital 10 days after ONC, the eyes were enucleated immediately, and the retinal tissues were extracted (n = 20). Tissues were washed with precooled 0.01 M PBS (pH 7.4), and ultrasonically homogenized at 4 °C in 100 ml of RIPA Lysis buffer (pH 7.4, 50 mM Tris-Hcl, 150 mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate (SDS), Cat. No. C1053, Applygen, Beijing, China), 1X All-in-One (100X, P1260, Applygen), and 1X Proteinase inhibitor cocktail (50X, P1265, Applygen). Using a bicinchoninic acid protein assay reagent kit (P1511, Applygen), the protein concentration was measured (n = 5 per group). Equal amounts of protein (20 µg/lane) were separated with 10% or 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). Next, the proteins were transferred to Immun-Blot Polyvinylidene Difluoride (PVDF) Membrane (162–0177, Bio-Rad Laboratories, Hercules, CA), which was then blocked and probed with one of the following primary antibodies: rabbit polyclonal antibody against EPOR (1:1,000, sc-5624, Santa Cruz Biotech), goat polyclonal antibody against NT4/5 (1:1,000, SAB2500696, Sigma-Aldrich), or mouse monoclonal anti–β-actin (1:2,000, A1978, Sigma-Aldrich). Peroxidase-conjugated donkey anti-rabbit IgG (H+L; 1:2,000, Jackson ImmunoResearch Laboratories, West Grove, PA), donkey anti-goat IgG-horseradish peroxidase (HRP; 1:2,000, sc-2020, Santa Cruz Biotech), and goat anti-mouse IgG-peroxidase (1:4,000, Cat. No. HRPGTXMS.05, KPL Inc., Gaithersburg, MD) were used. Protein bands were visualized using Amersham Biosciences ECL western blotting detection reagent (GE Healthcare Life Science, Uppsala, Sweden) according to the manufacturer’s instructions. For quantification, blots from at least five independent experiments (five animals per group) were used and quantified using Image J software.
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Analysis among multiple groups for single variable data was performed using one-way ANOVA analysis, followed by Bonferroni post hoc tests using SPSS 17.0 for Windows Software (SPSS Inc., Armonk, NY). A p value of less than 0.05 was considered statistically significant.
Results
ALA protects RGCs from ONC injury
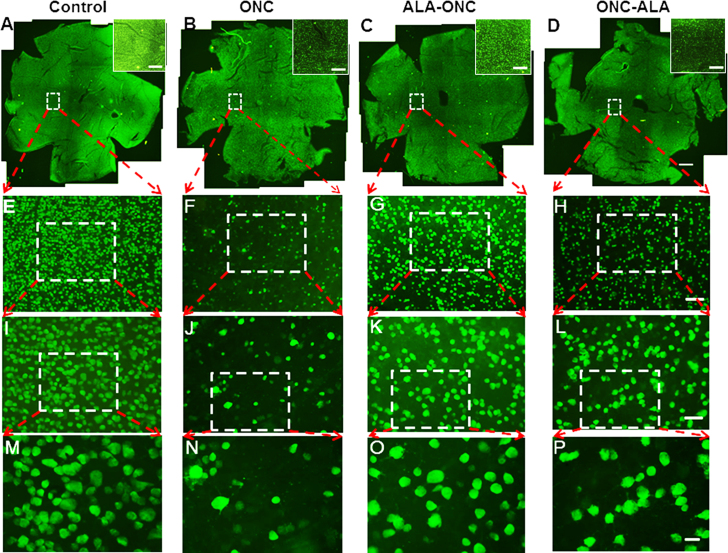
To determine whether treatment with ALA has protective effects on RGCs in the ONC retina, Rbpms, which is a specific marker for RGCs, was used to quantify the loss of RGCs due to ONC-induced damage. RGCs of various soma size were immunoreactive to Rbpms, and Rbpms-positive cells were distributed across the entire retina. The retinal distribution patterns of Rbpms-positive cells under different experimental conditions are shown in Figure 1. A significant decrease in the number of RGCs was observed 10 days after ONC injury (Figure 1N) compared to the control group (Figure 1M). The extent of cell death was reduced in the ALA-ONC group compared to the ONC group (Figure 1O). In the ONC-ALA group, cell loss induced by ONC injury was also prevented (Figure 1P). Graphic quantification of RGCs induced by ONC in all experimental groups is shown in Figure 2. RGC densities in four experimental groups at distances of 1 mm (Figure 2A), 2 mm (Figure 2B), and 3 mm (Figure 2C) from the OD are shown in the partial content of Table 1. As shown in Figure 2D, the average number of Rbpms-positive cells/mm2 was consistent with the mean density at identical distances from the OD. The average RGC density in the control group retinas was 2219±28/mm2. The RGC density in the ONC group, ALA-ONC group, and ONC-ALA group decreased to 418±8, 848±22, and 613±18/mm2, respectively. Rbpms-positive cell loss was 81% of the RGC population in the ONC group compared with the control group (n = 6 per group). This is somewhat comparable to previously reported results obtained after ONC in adult SD rats. A recent study showed 51% RGC survival at 7 days, 10% RGC survival at 1 month, and 4% RGC survival at 6 months after ONC [47]. In previous research, ONC-induced RGC loss first appeared at 7 days, and after 12 days, only 32% of the RGC population remained in the retina [37]. In the ALA-ONC and ONC-ALA groups, RGC loss was limited to approximately 61% and 72%, respectively. These results demonstrate the RGC survival rate was increased in the retinas of the ALA-ONC and ONC-ALA groups compared to the ONC group, and the RGC survival rate was higher in the ALA-ONC group (39%) compared with the ONC-ALA group (28%; p<0.05). The neuroprotective effect of precrush treatment with ALA (the ALA-ONC group) on RGCs was more effective than that seen in post-crush treatment with ALA (the ONC-ALA group). These results suggest that prophylactic administration of ALA may yield better protective effects in neurodegenerative diseases, such as glaucoma.
Figure 1.
Density distribution of retinal ganglion cells (RGCs) in whole mount retinas under different conditions. Panels A to D (25× magnification, amplified images in white boxes) reveal the retinal distribution patterns of RNA-binding protein with multiple splicing (Rbpms)-positive cells under different experimental conditions. The retinal orientation (clockwise) is temporal, inferior, nasal, and superior poles. Sampling location: 1.5 mm temporal to the optic disc. Scale bar = 800 μm. Panels E to H (100× magnification) show magnified micrographs of Rbpms-positive cells (white dashed lines encircle areas from panels A–D). Sampling field size: 877 × 660 μm2. Scale bar = 100 μm. Panels I through L (200× magnification) exhibit retinal micrographs from panels E to H (white dashed lines encircle the areas). Sampling field size: 439 × 330 μm2. Scale bar = 50 μm. Panels M to P (400× magnification) are magnified micrographs of Rbpms-positive cells (white dashed lines encircle areas from panels I–L). Sampling field size: 219×165 μm2. Scale bar = 20 um. A–D: 2.5× objective lens; E–H: 10× objective lens; I–L: 20× objective lens; M–P: 40× objective lens. AEIM = Control (sham-operated animal); BFJN = ONC (optic nerve crush animal); CGKO = ALA-ONC (ALA-pretreated animal 1 day before ONC); DHLP = ONC-ALA (ALA-treated animal 1 day after ONC).
Figure 2.
ALA protects retinal ganglion cells (RGCs) from ONC-induced damage. A, B, C, D: Quantitative analysis of RNA-binding protein with multiple splicing (Rbpms)-positive cells (mean ± standard error of the mean [SEM], n = 6 per group). A, B, C: Mean density was estimated in an area approximately the same distance from the optic disc (OD): 1 mm (A), 2 mm (B), and 3 mm (C) from the OD. D: The average number of Rbpms-positive cells/mm2 was calculated. *** p<0.001 compared to control, ## p<0.01, ### p<0.001 compared to optic nerve crush (ONC) injury, + p<0.05, +++ p<0.001 compared to the alpha lipoic acid (ALA)-ONC group at the same time point.
Table 1. Quantification of Rbpms-positive retinal ganglion cells (RGCs; cells/mm2) in whole mount retinas in different experimental groups (n=6 per group, mean ± SEM).
| Group | Distance from Center of Optic Nerve Head (mm) |
Average | ||
|---|---|---|---|---|
| 1 mm | 2 mm | 3 mm | ||
| Control | 2435±35 | 2328±49 | 1893±35 | 2219±28 |
| ONC | 422±13 | 464±5 | 369±19 | 418±8 |
| ALA-ONC | 933±45 | 896±25 | 714±22 | 848±22 |
| ONC-ALA | 672±28 | 628±24 | 540±11 | 613±18 |
| ALA-ONC+I | 546±38 | 579±32 | 550±39 | 558±33 |
| F1 | 767.28 | 801.95 | 862.77 | 1588.12 |
| P1-value (<) | 0.000 | 0.000 | 0.000 | 0.000 |
| F2 | 58.63 | 91.26 | 38.09 | 87.41 |
| P2-value (<) | 0.000 | 0.000 | 0.000 | 0.000 |
Note: F1 and P1-value: one-way ANOVA analysis among the four groups Control, ONC, ALA-ONC and ONC-ALA; F2 and P2-value: one-way ANOVA analysis among the three groups ONC, ALA-ONC and ALA-ONC+I; Control: sham-operated animal; ONC: optic nerve crush animal; ALA-ONC: ALA animal pretreated 1d before ONC; ONC-ALA: ALA-treated animal 1d after ONC; ALA-ONC+I: ALA animal pretreated 1d before ONC, followed by INCB018424.
ALA induces the upregulation of EPOR expression in the retina after ONC injury
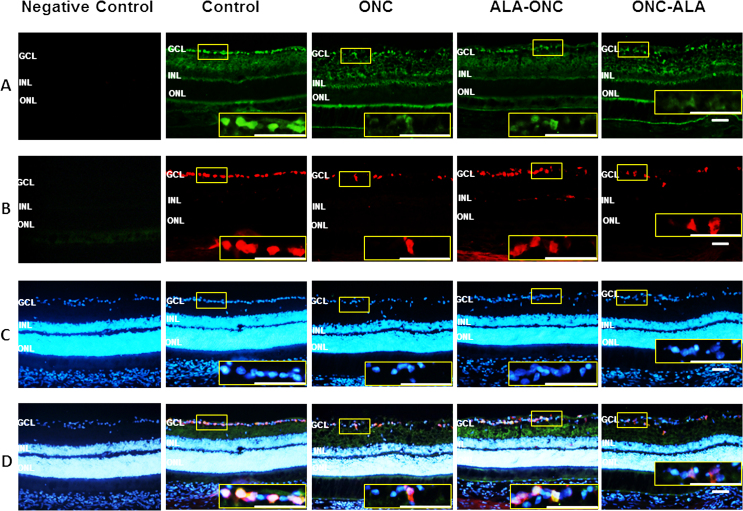
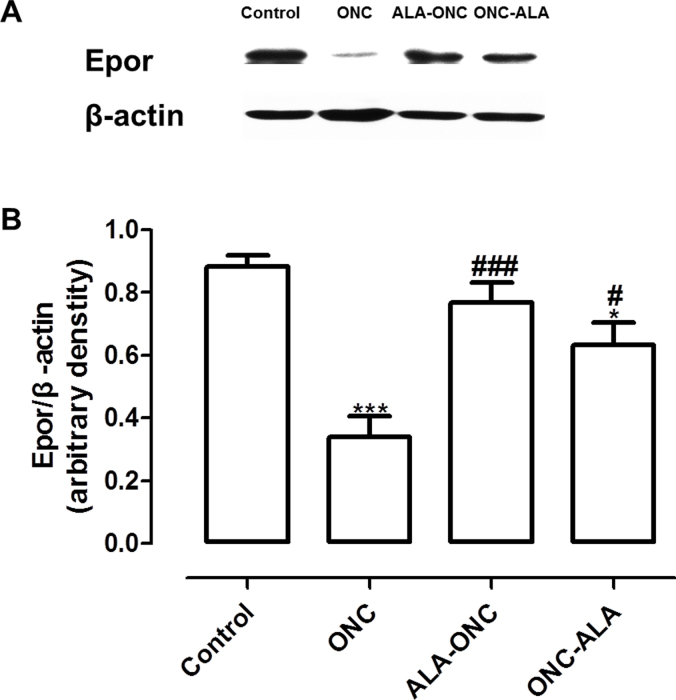
To determine whether the EPO/EPOR signaling pathway contributes to the retinal protective effects of ALA after ONC injury, we immunostained for EPOR and Rbpms in retinal cross sections from different experimental groups. With three-label immunohistochemical staining using antibodies against EPOR, Rbpms, and DAPI (Figure 3), we observed intense immunoreactivity in the GCL of the control group rats and weak immunoreactivity in the ONC groups. There was no immunoreactivity in the controls without a primary antibody (negative control). In the ALA-ONC and ONC-ALA groups, EPOR immunoreactivity was statistically significant enhanced compared with the ONC group. Double-label staining of the control retinas for EPOR and Rbpms showed that EPOR was highly expressed in the GCL but not in the RGCs. In the other groups, the alteration in EPOR expression was consistent with Rpbms but was slightly greater than that of Rpbms. Semiquantitative analysis of the EPOR protein was confirmed with immunoblotting. As shown in Figure 4A, the level of EPOR in the ONC retina was low compared with that in the control groups. ALA induced an increase in EPOR expression in the retinal GCL. Statistically significantly enhanced EPOR expression was observed in the ALA-ONC group compared with the ONC group, but there was no statistically significant difference compared with the control group. There was no statistically significant difference in EPOR levels in the ALA-ONC group and the ONC-ALA group. These results show that the EPOR protein contributes to the protective effect of ALA on the retina after ONC injury.
Figure 3.
Evaluation of EPOR expression in the retina with immunohistochemistry. A: Representative micrographs of retinal sections obtained from each group stained with antierythropoietin receptor (EPOR) antibody. B: EPOR expression (green, amplified images in yellow boxes) was observed in the GCL and colocalized with RNA-binding protein with multiple splicing (Rbpms; red, in the GCL, amplified images in yellow boxes). C: Rbpms and 4’-6-diamidino-2-phenylindole (DAPI; blue, in the GCL, amplified images in yellow boxes). D: Merged images (in the GCL, amplified images in yellow boxes) of the EPOR. Control = sham-operated animal; ONC = optic nerve crush animal; ALA-ONC = alpha lipoic acid (ALA) animal pretreated 1 day before ONC; ONC-ALA = ALA-treated animal 1 day after ONC. Scale bar = 50 µm. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer.
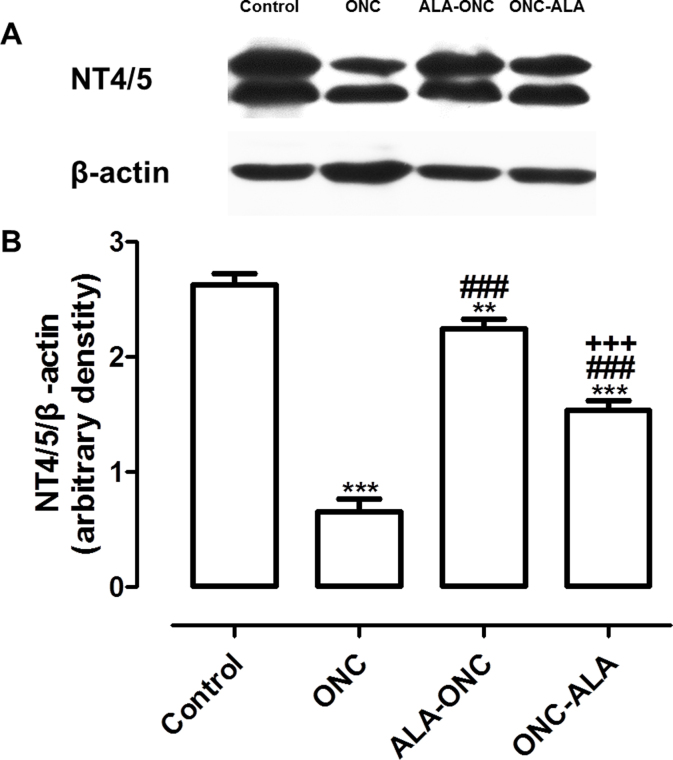
Figure 4.
ALA upregulates the expression of EPOR in the retina after ONC injury. A: Representative immunoblotting showing erythropoietin receptor (EPOR) protein levels in the whole retina. B: Densitometric analysis of EPOR expression relative to the loading control (mean ± standard error of the mean [SEM], n = 5 per group). Control: sham-operated animal; ONC = optic nerve crush animal; ALA-ONC = alpha lipoic acid (ALA) animal pretreated 1 day before ONC; ONC-ALA = ALA-treated animal 1 day after ONC. * p<0.05, *** p<0.001 compared to the control group, # p<0.05, ### p<0.001 compared to the ONC groups.
ALA upregulates the level of NT4/5 in retinal tissue after ONC injury
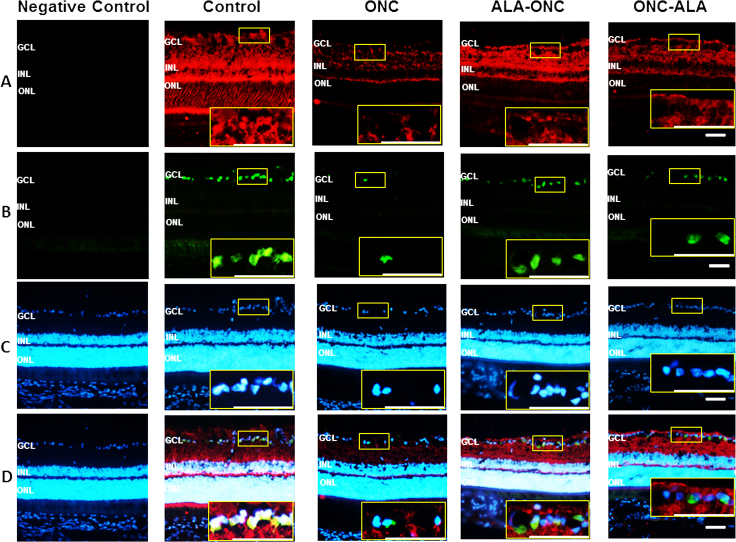
To determine whether ALA increases NT4/5 expression, we performed immunohistochemistry and immunoblotting studies for NT4/5 in different experimental groups. Using immunohistochemistry, no staining was detected in the controls without a primary antibody (negative control, Figure 5A). We observed a change in NT4/5 expression in the RGCs after ONC (ONC, Figure 5A), compared with the retinas that had no lesions (control, Figure 5A). In the ALA-ONC and ONC-ALA groups, we discovered intense immunoreactivity in the surviving RGCs in the GCL (Figure 5A) compared with the ONC group. As shown in Figure 5D, NT4/5 immunoreactivity colocalized with Rbpms (Figure 5B) and DAPI (Figure 5C). These findings show NT4/5 expression is located in the cells of the retinal GCL, and especially in the RGCs. Western blot experiments confirmed changes in NT4/5 expression in protein lysates prepared from the retinas of the four experimental groups that were consistent with the immunohistochemistry results (Figure 6A). The level of NT4/5 in the ONC retina was low compared with that in the control group (Figure 6B, p<0.001). Treatment with ALA (the ALA-ONC and ONC-ALA groups) statistically significantly enhanced NT4/5 expression compared with the ONC group (Figure 6B, p<0.001), and the control group showed higher expression compared with these two groups (Figure 6B, the ALA-ONC group, p<0.05; the ONC-ALA group, p<0.001). In the comparison of the ALA-ONC group with the ONC-ALA group, the relative optical density of NT4/5 in the ALA-ONC animals was statistically significantly higher (Figure 6B, p<0.001). These results suggest that the NT4/5 signaling pathway also contributes to the protective effects of ALA on the retina after ONC injury.
Figure 5.
Evaluation of NT4/5 expression in the retina with immunohistochemistry. A: Representative micrographs of retinal sections obtained from each group stained with anti-neurotrophin-4/5 (NT4/5) antibody (red, in the GCL, amplified images in yellow boxes). D: Merged images (in the GCL, amplified images in yellow boxes) of NT4/5 expression (A). B: RNA-binding protein with multiple splicing (Rbpms; green, in the GCL, amplified images in yellow boxes). C: 4’-6-diamidino-2-phenylindole (DAPI; blue, in the GCL, amplified images in yellow boxes). Control = sham-operated animal; ONC = optic nerve crush animal; ALA-ONC = alpha lipoic acid (ALA) animal pretreated 1 day before ONC; ONC-ALA = ALA-treated animal 1 day after ONC. Scale bar = 50 µm. GCL = ganglion cell layer; INL = inner nuclear layer; ONL = outer nuclear layer.
Figure 6.
ALA upregulates expression of NT4/5 in the retina after ONC injury. A: Representative immunoblotting showing neurotrophin-4/5 (NT4/5) protein levels in the whole retina. B: Densitometric analysis of NT4/5 expression relative to the loading control (mean ± standard error of the mean [SEM], n = 5 per group). Control = sham-operated animal; ONC = optic nerve crush animal; ALA-ONC = ALA animal pretreated 1 day before ONC; ONC-ALA = alpha lipoic acid (ALA)-treated animal 1 day after ONC. ** p<0.01, *** p<0.001 compared to the control group, ### p<0.001 compared to the ONC group, +++ p<0.001 compared to the ALA-ONC group at the same time point.
INCB018424 administration reverses the protective effects of ALA on ONC retinas
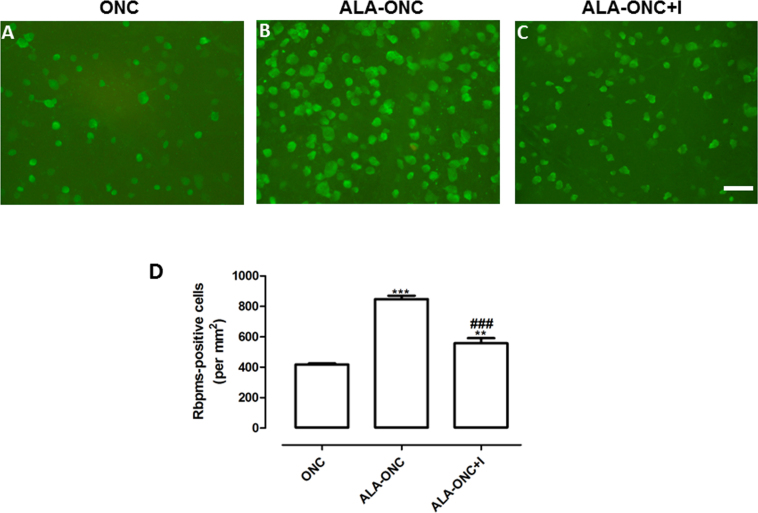
To determine whether EPOR protein expression is involved in the protective effect of ALA on the RGCs in ONC retinas, a specific Jak2 inhibitor, INCB018424 ,was used in this study. JAK2 is the most critical molecule of the EPO/EPOR signaling system. As shown in Figure 7, INCB018424 treatment statistically significantly reduced the number of Rbpms-expressing cells in the ALA-ONC+I group compared with the ALA-ONC group (Figure 7D, p<0.001). In the comparison of the ONC group with the ALA-ONC+I group, the number of Rbpms-expressing cells in the ALA-ONC+I animals was still higher (Figure 7D, p<0.01). Together, these results provide evidence that the protective effects of ALA on ONC retinas are at least partially mediated by EPOR.
Figure 7.
INCB018424 administration reverses the protective effects of ALA on ONC retinas. A-C: Representative micrographs (200× magnification) of retinal whole mounts obtained from three groups stained with anti-RNA-binding protein with multiple splicing (Rbpms) antibody (green). Sampling location: 2 mm temporal to the optic disc. Sampling field size: 439 × 330 μm2 (20× objective lens). Scale bar: 50 μm. D: Quantitative analysis of Rbpms-positive cells under different experimental conditions (mean ± standard error of the mean [SEM], n = 6 per group). The average number of Rbpms-positive cells/mm2 was calculated. ONC = optic nerve crush animal; ALA-ONC = alpha lipoic acid (ALA) animal pretreated 1 day before ONC; ALA-ONC+I = ALA animal pretreated 1 day before ONC, followed by INCB018424. *** p<0.001, ** p<0.01 compared to the ONC group, ### p<0.001 compared to the ALA-ONC group at the same time point.
Discussion
This study showed that ALA protects against oxidative stress and neurotrophic factor deprivation in ONC retinas. Treatment with ALA not only alleviated or retarded ONC-induced retinal damage but also simultaneously increased the expression of the EPOR protein and increased NT4/5 protein expression in the ONC retina. There was a significant difference in our data in the average number of Rbpms-positive cells/mm2 in the control group and the ONC group, in agreement with previous studies [4,9,25,45,47,48]. R-a-lipoic acid has a dramatic neuroprotective effect against oxidative stress–induced death of the retinal neuronal RGC-5 cell line [25]. Dietary therapy containing ALA results in oxidative stress reduction and an increase in the RGC survival rate in the DBA/2J model of glaucoma [49]. In our study, the average RGC density in the control group retinas at different concentric distances (1, 2, and 3 mm from the OD) was 2435±35, 2328±49, and 1893±35/mm2, respectively. The central and medial regions of the rat retina were more densely populated than the periphery. This trend of RGC density (Rbpms-positive cells/mm2) at different concentric distances reflects the normal distribution of RGCs throughout the retina of the adult SD rat, in agreement with previous reports [50-52]. In the present study, the average RGC density in the control group, ONC group, ALA-ONC group, and ONC-ALA group retinas was 2219±28, 418±8, 848±22, and 613±18/mm2, respectively. The trend of RGC density (Rbpms-positive cells/mm2) at different concentric distances (1, 2, and 3 mm from the OD) parallels the total average RGC density in the retina of the four experimental groups. For example, the number of Rbpms-positive cells/mm2 at a distance of 2 mm from the OD in the four groups was 2328±49, 464±5, 896±25, and 628±24/mm2, respectively (in the order designated above). These results indicate that pretreatment with ALA can improve the RGC survival rate in rats after retinal ONC, which is consistent with a previous report that showed R-a-lipoic acid (R-LA) induced protection in the mouse [25]. Together with previous studies [25,49], these results provide evidence that ONC-induced oxidative stress is attenuated by ALA. In addition, pre- and post-treatment with ALA provides protection to the retina as a whole, and in particular to RGCs from ischemia–reperfusion injuries [24]. There were statistical differences in the RGC survival rates between the ALA-ONC group (39%) and the ONC-ALA group (28%; p<0.05). These data suggest that prophylactic administration of ALA produces a better neuroprotective effect in ONC-induced damage. This result is most likely due to the fact precrush treatment with ALA initiates the body’s immune defense mechanism and produces protective factors before the injury, which ultimately reduces damage to RGCs in ONC retinas. Once injury has occurred, there is a greater protective effect in the ALA-ONC group than in the ONC-ALA group. However, the average density of the Rbpms-positive cells did not return to the level of the control group (p<0.001). Repeated injections of ALA over 7 days offered no better protection for the injured RGCs than a single injection (data not shown). However, in the current study we did not examine RGC survival later than 10 days after ONC and administration of ALA, and thus do not know whether single injection ALA can have long-lasting neuroprotective effects on the survival of injured RGCs. Previous studies have shown neuroprotection of axotomized rat RGCs is a transient, rather than a persistent, effect [36,53]. The RGC density for animals that received growth factor injections was <200 cells per mm2 (9% of normal) at 6 and 8 weeks [53]. Fourteen days after axotomy, RGC density in the groups treated with growth factor was 839±39/mm2 [36]. Further study will be needed to confirm whether the protection afforded by ALA in ONC retinas is temporary or prolonged.
EPOR expression was found mainly in the GCL and the INL in the retinas consistent with findings in previous studies [54-57]. There was intense immunoreactivity in the GCL in the control group rats consistent with previous reports [56-58]. Fu et al. demonstrated that endogenous EPO/EPOR signaling may participate in intrinsic recovery mechanisms and play an endogenous neuroprotective role in the survival of RGCs after retina injury [29]. EPO binding to the EpoR homodimer triggers a conformational change in the receptor cytoplasmic domain, bringing the JAK2 proteins in close proximity to each other and resulting in transphosphorylation and activation of JAK2 and EpoR [31]. EpoR activation initiates downstream cascades via different signaling pathways including signal transducer and activator of transcription (STAT), phosphoinositide 3-kinase (PI3K)/AKT, and mitogen-activated protein kinase (MAPK) via adaptor proteins such as Src homology containing protein (SHC) [59,60]. JAK2 is the most critical molecule of the EPO/EPOR signaling system. In the present study, the ALA-ONC and ONC-ALA groups showed statistically significantly increased EPOR protein expression in the GCL of the retinas compared with the ONC group (p<0.001). The number of Rbpms-expressing cells in the ALA-ONC+I group was statistically significantly higher than that in the ALA-ONC group (p<0.001). INCB018424, a specific Jak2 inhibitor, reversed the protective effects of ALA on the ONC retinas. The protective effects of ALA were diminished on the ONC retinas by the administration of inhibitors of the EPO/EPOR signaling system. ALA may protect RGCs against ONC injury by activating the endogenous EPO/EPOR survival signaling pathway. Recent evidence suggests that ALA may induce endogenous antioxidant pathways that sustain antioxidant effects for a period of time longer than expected if ALA acts only as an ROS scavenger [61]. In addition, Weishaupt et al. demonstrated that there was no change in the EPOR protein after optic nerve transection [57], but we discovered the level of EPOR in the ONC retina was low compared with that of the control group in the present study. Previous studies have shown the RGC loss after axotomy in adult rats varies depending on the severity of the insult or the type of injury (optic nerve cut versus crush) [62-65] and the relative distance of the lesion site from the cell somata [65]. This difference in the RGC death rate could be due to the difference in response to crush versus incision and/or that ONC was performed using watchmaker’s forceps or 40 g power vascular clamps. Thus, this difference in EPOR expression in the present study and this previous study may be related to the severity of injury (crush versus incision), the duration of the insult, or the relative distance of the lesion site from the corresponding cell bodies. In future studies, it will be important to determine how change comes about upstream or downstream of the EPO/EPOR signaling pathway.
Under conditions of neurotrophin deprivation, the survival of immunopurified RGCs is enhanced statistically significantly by treatment with EPO [57]. Parrilla-Reverter et al. demonstrated that intravitreal administration of NT4 increases RGC survival in intraorbital nerve crush (IONC) retinas for up to 12 days post-injury [37]. In addition, in accordance with our results, the EPO/EPOR signaling pathway may contribute to the protective effects of ALA on the retina after ONC injury. We thus examined whether the improved survival rate of RGCs after ALA treatment in ONC retinas is also caused by upregulation of NT4/5. The trend of change in NT4/5 protein expression in all experimental groups in the present study is consistent with the change in average RGC density. For example, the NT4/5 level in the ALA-ONC animals was statistically significantly higher than that in the ONC-ALA animals for NT4/5 expression (p<0.001) and average RGC density (p<0.05). In addition, treatment with ALA (the ALA-ONC and ONC-ALA groups) statistically significantly enhanced NT4/5 expression compared with the ONC group (p<0.001).
From the experimental data, we found that NT4/5 protein expression in the control group was higher compared with that in the ALA-ONC and ONC-ALA groups. The level of the NT4/5 protein in the ALA-ONC group failed to return to the control level, but the EPOR protein in the ALA-ONC group returned to a level near the control level. There were statistically significant differences in levels of NT4/5 protein in the ALA-ONC and ONC-ALA animals, but there were no significant differences in the EPOR protein levels in the ALA-ONC and ONC-ALA animals. These subtle differences in the NT4/5 and EPOR protein levels may explain the differences in effects on RGCs before and after the administration of ALA in the ALA-ONC and ONC-ALA groups. These differences may also result from the fact that increases in EPOR are the cause of alpha lipoic acid neuroprotection, but the increase in NT4/5 may represent the outcome and not the cause of alpha lipoic acid neuroprotection. Effects before and after the application of ALA in this ONC model may result from different mechanisms, and this merits further investigation. Unfortunately, previous studies regarding prior and post-application of medication have not focused on different mechanisms [24,38]. Moreover, none of these studies attempted to identify different mechanisms of action for the neuroprotective effects of ALA. We report that there may be different mechanisms involved in the RGC neuroprotective effect in precrush treatment with ALA and post-crush treatment with ALA in ONC injury. The present study offers novel data on the different mechanisms of neuroprotection in the ALA-ONC and ONC-ALA groups. This is the first time there has been an investigation of this aspect of this problem.
In conclusion, the present study demonstrates that ALA has protective effects on the RGCs in ONC retinas, particularly in the ALA-ONC group. In addition, the research suggests that the EPO/EPOR signaling pathway and the molecule NT4/5 contribute to the protective effects of ALA in the retina after ONC injury and suggests they play differing roles in ALA neuroprotective effects after ONC injury. These data raise the possibility of a new approach to therapy for and ways of thinking about optic nerve injury diseases. However, further studies are required to resolve important issues such as the downstream effects of the EPO/EPOR signaling pathways and to evaluate the functional state of rescued RGCs.
Acknowledgments
This work was supported by National Basic Research Program of China (973 Program, 2012CB825503, 2011CB707502 to JGao).
References
- 1.Bien a Seidenbecher CI, Böckers TM, Sabel B a, Kreutz MR. Apoptotic versus necrotic characteristics of retinal ganglion cell death after partial optic nerve injury. J Neurotrauma. 1999;16:153–63. doi: 10.1089/neu.1999.16.153. [DOI] [PubMed] [Google Scholar]
- 2.Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31:152–81. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Yoles E, Schwartz M. Elevation of intraocular glutamate levels in rats with partial lesion of the optic nerve. Arch Ophthalmol. 1998;116:906–10. doi: 10.1001/archopht.116.7.906. [DOI] [PubMed] [Google Scholar]
- 4.Zhang ZZ, Gong YY, Shi YH, Zhang W, Qin XH, Wu XW. Valproate promotes survival of retinal ganglion cells in a rat model of optic nerve crush. Neuroscience. 2012;224:282–93. doi: 10.1016/j.neuroscience.2012.07.056. [DOI] [PubMed] [Google Scholar]
- 5.Klöcker N, Zerfowski M, Gellrich NC, Bähr M. Morphological and functional analysis of an incomplete CNS fiber tract lesion: Graded crush of the rat optic nerve. J Neurosci Methods. 2001;110:147–53. doi: 10.1016/s0165-0270(01)00435-6. [DOI] [PubMed] [Google Scholar]
- 6.Dieterich DC, Trivedi N, Engelmann R, Gundelfinger ED, Gordon-Weeks PR, Kreutz MR. Partial regeneration and long-term survival of rat retinal ganglion cells after optic nerve crush is accompanied by altered expression, phosphorylation and distribution of cytoskeletal proteins. Eur J Neurosci. 2002;15:1433–43. doi: 10.1046/j.1460-9568.2002.01977.x. [DOI] [PubMed] [Google Scholar]
- 7.Hayreh SS. Ischemic optic neuropathies - where are we now? Graefes Arch Clin Exp Ophthalmol. 2013;251:1873–84. doi: 10.1007/s00417-013-2399-z. [DOI] [PubMed] [Google Scholar]
- 8.Diekmann H, Fischer D. Glaucoma and optic nerve repair. Cell Tissue Res. 2013;353:327–37. doi: 10.1007/s00441-013-1596-8. [DOI] [PubMed] [Google Scholar]
- 9.Kalesnykas G, Oglesby EN, Zack DJ, Cone FE, Steinhart MR, Tian J, Pease ME, Quigley HA. Retinal ganglion cell morphology after optic nerve crush and experimental glaucoma. Invest Ophthalmol Vis Sci. 2012;53:3847–57. doi: 10.1167/iovs.12-9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Homma K, Koriyama Y, Mawatari K, Higuchi Y, Kosaka J, Kato S. Early downregulation of IGF-I decides the fate of rat retinal ganglion cells after optic nerve injury. Neurochem Int. 2007;50:741–8. doi: 10.1016/j.neuint.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Tan H-B, Shen X, Cheng Y, Jiao Q, Yang Z-J, Zhong Y-S. Evaluation of a partial optic nerve crush model in rats. Exp Ther Med. 2012;4:401–4. doi: 10.3892/etm.2012.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng D-F, Chen E-T, Li X-Y, Liu Y, Wang Y. Standardizing optic nerve crushes with an aneurysm clip. Neurol Res. 2010;32:476–81. doi: 10.1179/016164110X12556180206158. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz M. Optic nerve crush: protection and regeneration. Brain Res Bull. 2004;62:467–71. doi: 10.1016/S0361-9230(03)00076-5. [DOI] [PubMed] [Google Scholar]
- 14.Wang R, Xu J, Xie J, Kang Z, Sun X, Chen N, Liu L, Xu J. Hyperbaric oxygen preconditioning promotes survival of retinal ganglion cells in a rat model of optic nerve crush. J Neurotrauma. 2010;27:763–70. doi: 10.1089/neu.2009.1005. [DOI] [PubMed] [Google Scholar]
- 15.Quigley H a McKinnon SJ, Zack DJ, Pease ME, Kerrigan-Baumrind LA, Kerrigan DF, Mitchell RS. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000;41:3460–6. [PubMed] [Google Scholar]
- 16.Weber AJ, Viswanáthan S, Ramanathan C, Harman CD. Combined application of BDNF to the eye and brain enhances ganglion cell survival and function in the cat after optic nerve injury. Invest Ophthalmol Vis Sci. 2010;51:327–34. doi: 10.1167/iovs.09-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta VK, You Y, Li JC, Klistorner A, Graham SL. Protective effects of 7,8-dihydroxyflavone on retinal ganglion and RGC-5 cells against excitotoxic and oxidative stress. J Mol Neurosci. 2013;49:96–104. doi: 10.1007/s12031-012-9899-x. [DOI] [PubMed] [Google Scholar]
- 18.Clutton S. The importance of oxidative stress in apoptosis. Br Med Bull. 1997;53:662–8. doi: 10.1093/oxfordjournals.bmb.a011637. [DOI] [PubMed] [Google Scholar]
- 19.Levkovitch-Verbin H, Harris-Cerruti C, Groner Y, Wheeler LA, Schwartz M, Yoles E. RGC death in mice after optic nerve crush injury: oxidative stress and neuroprotection. Invest Ophthalmol Vis Sci. 2000;41:4169–74. [PubMed] [Google Scholar]
- 20.Osborne NN. Pathogenesis of ganglion “cell death” in glaucoma and neuroprotection: focus on ganglion cell axonal mitochondria. Prog Brain Res. 2008;173:339–52. doi: 10.1016/S0079-6123(08)01124-2. [DOI] [PubMed] [Google Scholar]
- 21.Osborne NN, del Olmo-Aguado S. Maintenance of retinal ganglion cell mitochondrial functions as a neuroprotective strategy in glaucoma. Curr Opin Pharmacol. 2013;13:16–22. doi: 10.1016/j.coph.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Santos JM. Kowluru R a. Role of mitochondria biogenesis in the metabolic memory associated with the continued progression of diabetic retinopathy and its regulation by lipoic acid. Invest Ophthalmol Vis Sci. 2011;52:8791–8. doi: 10.1167/iovs.11-8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moini H, Packer L, Saris N-EL. Antioxidant and Prooxidant Activities of α-Lipoic Acid and Dihydrolipoic Acid. Toxicol Appl Pharmacol. 2002;182:84–90. doi: 10.1006/taap.2002.9437. [DOI] [PubMed] [Google Scholar]
- 24.Chidlow G, Schmidt K-G, Wood JPM, Melena J, Osborne NN. Alpha-lipoic acid protects the retina against ischemia-reperfusion. Neuropharmacology. 2002;43:1015–25. doi: 10.1016/s0028-3908(02)00129-6. [DOI] [PubMed] [Google Scholar]
- 25.Koriyama Y, Nakayama Y, Matsugo S, Kato S. Protective effect of lipoic acid against oxidative stress is mediated by Keap1/Nrf2-dependent heme oxygenase-1 induction in the RGC-5 cellline. Brain Res. 2013;1499:145–57. doi: 10.1016/j.brainres.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 26.Ji D, Majid ASA, Yin ZQ. α-Lipoic acid attenuates light insults to neurones. Biol Pharm Bull. 2013;36:1060–7. doi: 10.1248/bpb.b12-00941. [DOI] [PubMed] [Google Scholar]
- 27.Xie Z, Wu X, Qiu Q, Gong Y, Song Y, Gu Q, Li C. Expression pattern of erythropoietin and erythropoietin receptor in experimental model of retinal detachment. Curr Eye Res. 2007;32:757–64. doi: 10.1080/02713680701531074. [DOI] [PubMed] [Google Scholar]
- 28.Juul SE. Yachnis a T, Christensen RD. Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum Dev. 1998;52:235–49. doi: 10.1016/s0378-3782(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 29.Fu Q-L, Wu W, Wang H, Li X, Lee VWH, So K-F. Up-regulated endogenous erythropoietin/erythropoietin receptor system and exogenous erythropoietin rescue retinal ganglion cells after chronic ocular hypertension. Cell Mol Neurobiol. 2008;28:317–29. doi: 10.1007/s10571-007-9155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Funakoshi-Tago M, Pelletier S, Moritake H, Parganas E, Ihle JN. Jak2 FERM domain interaction with the erythropoietin receptor regulates Jak2 kinase activity. Mol Cell Biol. 2008;28:1792–801. doi: 10.1128/MCB.01447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livnah O, Stura EA, Middleton SA, Johnson DL, Jolliffe LK, Wilson IA. Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science. 1999;283:987–90. doi: 10.1126/science.283.5404.987. [DOI] [PubMed] [Google Scholar]
- 32.Caminos E, Becker E, Martín-Zanca D, Vecino E. Neurotrophins and their receptors in the tench retina during optic nerve regeneration. J Comp Neurol. 1999;404:321–31. doi: 10.1002/(sici)1096-9861(19990215)404:3<321::aid-cne4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 33.Lamballe F, Klein R, Barbacid M. The trk family of oncogenes and neurotrophin receptors. Princess Takamatsu Symp. 1991;22:153–70. [PubMed] [Google Scholar]
- 34.Jelsma TN, Friedman HH, Berkelaar M, Bray GM, Aguayo AJ. Different forms of the neurotrophin receptor trkB mRNA predominate in rat retina and optic nerve. J Neurobiol. 1993;24:1207–14. doi: 10.1002/neu.480240907. [DOI] [PubMed] [Google Scholar]
- 35.Johnson EC, Guo Y, Cepurna WO, Morrison JC. Neurotrophin roles in retinal ganglion cell survival: lessons from rat glaucoma models. Exp Eye Res. 2009;88:808–15. doi: 10.1016/j.exer.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peinado-Ramón P, Salvador M, Villegas-Pérez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37:489–500. [PubMed] [Google Scholar]
- 37.Parrilla-Reverter G, Agudo M, Sobrado-Calvo P, Salinas-Navarro M, Villegas-Pérez MP, Vidal-Sanz M. Effects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: a quantitative in vivo study. Exp Eye Res. 2009;89:32–41. doi: 10.1016/j.exer.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 38.Clarke DB, Bray GM, Aguayo AJ. Prolonged administration of NT-4 / 5 fails to rescue most axotomized retinal ganglion cells in adult rats. Vision Res. 1998;38:1517–24. doi: 10.1016/s0042-6989(97)00341-6. [DOI] [PubMed] [Google Scholar]
- 39.Cui Q, Lu Q, So KF, Yip HK. CNTF, not other trophic factors, promotes axonal regeneration of axotomized retinal ganglion cells in adult hamsters. Invest Ophthalmol Vis Sci. 1999;40:760–6. [PubMed] [Google Scholar]
- 40.Cheng L, Sapieha P, Kittlerova P, Hauswirth WW, Di Polo A. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J Neurosci. 2002;22:3977–86. doi: 10.1523/JNEUROSCI.22-10-03977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghazi-Nouri SMS, Ellis JS, Moss S, Limb GA, Charteris DG. Expression and localisation of BDNF, NT4 and TrkB in proliferative vitreoretinopathy. Exp Eye Res. 2008;86:819–27. doi: 10.1016/j.exer.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Machalińska A, Kawa M, Pius-Sadowska E, Nowak W, Rogińska D, Kaczyńska K, Baumert B, Wiszniewska B, Józkowicz A, Dulak J, Machaliński B. Long-term neuroprotective effects of NT-4-engineered mesenchymal stem cells injected intravitreally in a mouse model of acute retinal injury. Invest Ophthalmol Vis Sci. 2013;54:8292–305. doi: 10.1167/iovs.13-12221. [DOI] [PubMed] [Google Scholar]
- 43.Dvoriantchikova G, Barakat D, Brambilla R, Agudelo C, Hernandez E, Bethea JR, Shestopalov VI, Ivanov D. Inactivation of astroglial NF-kappa B promotes survival of retinal neurons following ischemic injury. Eur J Neurosci. 2009;30:175–85. doi: 10.1111/j.1460-9568.2009.06814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vecino E, García-Crespo D, García-Grespo D, García M, Martinez-Millán L, Sharma SC, Carrascal E. Rat retinal ganglion cells co-express brain derived neurotrophic factor (BDNF) and its receptor TrkB. Vision Res. 2002;42:151–7. doi: 10.1016/s0042-6989(01)00251-6. [DOI] [PubMed] [Google Scholar]
- 45.Kwong JMK, Quan A, Kyung H, Piri N, Caprioli J. Quantitative analysis of retinal ganglion cell survival with Rbpms immunolabeling in animal models of optic neuropathies. Invest Ophthalmol Vis Sci. 2011;52:9694–702. doi: 10.1167/iovs.11-7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hörnberg H, Wollerton-van Horck F, Maurus D, Zwart M, Svoboda H, Harris WA, Holt CE. RNA-binding protein Hermes/RBPMS inversely affects synapse density and axon arbor formation in retinal ganglion cells in vivo. J Neurosci Off J Soc Neurosci. 2013;33:10384–95. doi: 10.1523/JNEUROSCI.5858-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nadal-Nicolás FM, Sobrado-Calvo P, Jiménez-López M, Vidal-Sanz M, Agudo-Barriuso M. Long-Term Effect of Optic Nerve Axotomy on the Retinal Ganglion Cell Layer. Invest Ophthalmol Vis Sci. 2015;56:6095–112. doi: 10.1167/iovs.15-17195. [DOI] [PubMed] [Google Scholar]
- 48.Vigneswara V, Berry M, Logan A, Ahmed Z. Pharmacological inhibition of caspase-2 protects axotomised retinal ganglion cells from apoptosis in adult rats. PLoS ONE. 2012;7:e53473. doi: 10.1371/journal.pone.0053473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inman DM, Lambert WS, Calkins DJ, Horner PJ. α-Lipoic acid antioxidant treatment limits glaucoma-related retinal ganglion cell death and dysfunction. PLoS ONE. 2013;8:e65389. doi: 10.1371/journal.pone.0065389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nadal-Nicolás FM, Jiménez-López M, Salinas-Navarro M, Sobrado-Calvo P, Alburquerque-Béjar JJ, Vidal-Sanz M, Agudo-Barriuso M. Whole number, distribution and co-expression of brn3 transcription factors in retinal ganglion cells of adult albino and pigmented rats. PLoS ONE. 2012;7:e49830. doi: 10.1371/journal.pone.0049830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nadal-Nicolás FM, Salinas-Navarro M, Jiménez-López M, Sobrado-Calvo P, Villegas-Pérez MP, Vidal-Sanz M, Agudo-Barriuso M. Displaced retinal ganglion cells in albino and pigmented rats. Front Neuroanat. 2014;8:99. doi: 10.3389/fnana.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salinas-Navarro M, Mayor-Torroglosa S, Jiménez-López M, Avilés-Trigueros M, Holmes TM, Lund RD, Villegas-Pérez MP, Vidal-Sanz M. A computerized analysis of the entire retinal ganglion cell population and its spatial distribution in adult rats. Vision Res. 2009;49:115–26. doi: 10.1016/j.visres.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 53.Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci USA. 1994;91:1632–6. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Colella P, Iodice C, Di Vicino U, Annunziata I, Surace EM, Auricchio A. Non-erythropoietic erythropoietin derivatives protect from light-induced and genetic photoreceptor degeneration. Hum Mol Genet. 2011;20:2251–62. doi: 10.1093/hmg/ddr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caprara C, Britschgi C, Samardzija M, Grimm C. The erythropoietin receptor is not required for the development, function, and aging of rods and cells in the retinal periphery. Mol Vis. 2013;2014:307–24. [PMC free article] [PubMed] [Google Scholar]
- 56.Böcker-Meffert S, Rosenstiel P, Röhl C, Warneke N, Held-Feindt J, Sievers J, Lucius R. Erythropoietin and VEGF promote neural outgrowth from retinal explants in postnatal rats. Invest Ophthalmol Vis Sci. 2002;43:2021–6. [PubMed] [Google Scholar]
- 57.Weishaupt JH. Effect of Erythropoietin Axotomy-Induced Apoptosis in Rat Retinal Ganglion Cells. Invest Ophthalmol Vis Sci. 2004;45:1514–22. doi: 10.1167/iovs.03-1039. [DOI] [PubMed] [Google Scholar]
- 58.Zhong L, Bradley J, Schubert W, Ahmed E, Adamis AP, Shima DT, Robinson GS, Ng YS. Erythropoietin promotes survival of retinal ganglion cells in DBA/2J glaucoma mice. Invest Ophthalmol Vis Sci. 2007;48:1212–8. doi: 10.1167/iovs.06-0757. [DOI] [PubMed] [Google Scholar]
- 59.Quelle FW, Wang D, Nosaka T, Thierfelder WE, Stravopodis D, Weinstein Y, Ihle JN. Erythropoietin induces activation of Stat5 through association with specific tyrosines on the receptor that are not required for a mitogenic response. Mol Cell Biol. 1996;16:1622–31. doi: 10.1128/mcb.16.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao W, Kitidis C, Fleming MD, Lodish HF, Ghaffari S. Erythropoietin stimulates phosphorylation and activation of GATA-1 via the PI3-kinase/AKT signaling pathway. Blood. 2006;107:907–15. doi: 10.1182/blood-2005-06-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petersen Shay K, Moreau RF, Smith EJ, Hagen TM. Is alpha-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity. IUBMB Life. 2008;60:362–7. doi: 10.1002/iub.40. [DOI] [PubMed] [Google Scholar]
- 62.Parrilla-Reverter G, Agudo M, Nadal-Nicolás F, Alarcón-Martínez L, Jiménez-López M, Salinas-Navarro M, Sobrado-Calvo P, Bernal-Garro JM, Villegas-Pérez MP, Vidal-Sanz M. Time-course of the retinal nerve fibre layer degeneration after complete intra-orbital optic nerve transection or crush: a comparative study. Vision Res. 2009;49:2808–25. doi: 10.1016/j.visres.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 63.Agudo M, Pérez-Marín MC, Lönngren U, Sobrado P, Conesa A, Cánovas I, Salinas-Navarro M, Miralles-Imperial J, Hallböök F, Vidal-Sanz M. Time course profiling of the retinal transcriptome after optic nerve transection and optic nerve crush. Mol Vis. 2008;14:1050–63. [PMC free article] [PubMed] [Google Scholar]
- 64.Agudo M, Pérez-Marín MC, Sobrado-Calvo P, Lönngren U, Salinas-Navarro M, Cánovas I, Nadal-Nicolás FM, Miralles-Imperial J, Hallböök F, Vidal-Sanz M. Immediate upregulation of proteins belonging to different branches of the apoptotic cascade in the retina after optic nerve transection and optic nerve crush. Invest Ophthalmol Vis Sci. 2009;50:424–31. doi: 10.1167/iovs.08-2404. [DOI] [PubMed] [Google Scholar]
- 65.Villegas-Pérez MP, Vidal-Sanz M, Rasminsky M, Bray GM, Aguayo AJ. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J Neurobiol. 1993;24:23–36. doi: 10.1002/neu.480240103. [DOI] [PubMed] [Google Scholar]