Abstract
A main obstacle arising when using ex-situ hyperpolarization to increase the sensitivity of biomolecular NMR, is the fast relaxation that macromolecular spins undergo upon being transferred from the polarizer to the spectrometer where their observation takes place. To cope with this limitation the present study explores the use of hyperpolarized water, as a means to enhance the sensitivity of nuclei in biomolecules. Methods to achieve proton polarizations in excess of 5% in water transferred into the NMR spectrometer were devised, as were methods enabling this polarization to last for up to 30 sec. Upon dissolving aminoacids and polypeptides sited at the spectrometer into such hyperpolarized water, a substantial enhancement of certain biomolecular amide and amine proton resonances was observed. This exchange driven 1H enhancement was further passed on to sidechain and to backbone nitrogens, owing to spontaneous one-bond Overhauser processes. 15N signal enhancements >500 over 11.7 T thermal counterparts could thus be imparted, in a kinetic process that enabled multi-scan signal averaging. Besides potential bioanalytical uses, this approach opens interesting possibilities in the monitoring of dynamic biomolecular processes -including solvent accessibility and exchange process.
Keywords: nuclear magnetic resonance, dynamic nuclear polarization, hyperpolarized water, biomolecular spectroscopy, sensitivity enhancement
1. Introduction
Recent developments in high-field dynamic nuclear polarization (DNP), can greatly enhance the sensitivity of nuclear magnetic resonance (NMR) in solids and liquids.1–8 Most promising among these methods, particularly within the context of solution-phase NMR spectroscopy and imaging (MRI), is the dissolution DNP approach. Dissolution DNP improves NMR’s sensitivity by executing the nuclear hyperpolarization ex situ, on a custom polarizer where the targeted sample is co-mixed with a stable (often organic) radical, and cooled into an amorphous frozen glass.9 After exposing such cryogenic system to suitable microwave radiation, the very high polarization of the electron spins (≥90%) is efficiently transferred to the surrounding nuclei in bulk. This microwave-driven polarization transfer happens over minutes or hours at T ≤ 1.5 K; the sample is subsequently returned to the liquid state by exposing it to hot vapors, and the resulting liquid is then flushed from the polarizer into the NMR/MRI probe/coil for a rapid inductive-based detection10. This ex situ method can create nuclear polarizations in excess of 30%,11–17 and for the case of small molecules its sudden-dissolution nature can preserve much of these earnings for subsequent liquid-phase NMR observations. Such sensitivity gains can be truly outstanding, akin to years of non-stop conventional signal averaging18–20. Still, when considering the use of this setup for biomolecular applications, a serious limitation arises. This derives from the short relaxation times that characterize biomolecules, particularly in the very low (<0.1 T) magnetic fields that the dissolved sample has to negotiate between the polarizer and the spectrometer. Indeed relaxation rates in excess of a kHz are typical of medium-sized biomolecules tumbling with ns correlation times,21–23 implying that in the 1-3 sec timescales that the dissolution DNP method requires for the sample to traverse through a low-field region, most of the hard-earned polarization gains will be lost. The sample hyperpolarization will be further depleted by the additional relaxation induced by the paramagnetic polarizing agent, which gets dissolved and transferred together with the targeted sample into the NMR spectrometer.
A number of alternatives have emerged over recent years to deal with this limitation. Most general among these solutions is arguably the proposal by Kockenberger et al,24 which employs a dual-magnet approach whereby the solid sample is transported from an upper DNP magnet into a lower NMR magnet where samples are melted and observed. While also in this setup the sample transverses a low-field region in-between the magnets it does so as a cryogenic pellet, opening an opportunity for preserving the hyperpolarization of even large biomolecules thanks to their cryogenic state. In a scheme that follows more closely the original ex situ DNP setup, Hilty and coworkers have recently described a dissolution device that maximizes sample transport speed while minimizing turbulence through a system of back-pressure regulation.25,26 Using this system and a modified Hypersense polarizer a total sample dissolution-to-NMR delay of 1.2 s was achieved; short enough to endow the original ex situ approach with 300-3000× sensitivity gains for certain 13C sites in perdeuterated, unfolded polypeptides.20 Yet another interesting option recently demonstrated within the context of DNP-enhanced biomolecular NMR, focuses the hyperpolarization on perdeuterated 15N-labeled systems, which were allowed to slowly exchange their deuterons with protons of water acting as dissolution solvent.27 As this H/D exchange process takes place once the sample has reached the high-field NMR magnet and probe, the 15N sensitivity enhancement is preserved and can be passed onwards to protons.
The present study examines an alternative way to cope with these limitations, that uses hyperpolarized water as a mean to enhance the sensitivity of biomolecular nuclei. We find that water protons could be spin-aligned rapidly in a cryogenic DNP setup, delivering polarizations of ≈5% after their dissolution and transfer to the NMR scanner. This enhancement could be made relatively long-lived, thanks to extended relaxation times T1 realized by gentle heating and by adding a co-solvent that executed a post-melting radical extraction. Even factoring all dilution and relaxation losses, the ensuing method led to magnetizations that where over 100× larger than thermal counterparts involving pure water placed in a high field magnet. Upon using this hyperpolarized water to dilute a biomolecule waiting in the NMR spectrometer, a number of amine and amide groups underwent rapid exchange of their protons with H2O, leading to a clear enhancement of their 1H resonances. This incorporation of hyperpolarized protons also led to an Overhauser-driven heteronuclear effect, whereby 15N sites that were chemically bound to solvent-exchanging protons underwent a spontaneous magnetization enhancement. 15N signal enhancements equating to hundreds of times the thermal equilibrium 15N polarization could thus be recorded for both backbone and sideband amide and amine sites; these effects could last over significant times, opening the possibility of exploiting them in mutli-scan acquisitions. Besides enabling new bioanalytical capabilities via their sensitivity enhancements, this kind of experiment opens new opportunities to monitor dynamic biomolecular processes involving water H-exchange as reporter –including studies of protein folding and solvent accessibility.
2. Materials and Methods
Dynamic Nuclear Polarization
Water hyperpolarization was achieved by dissolving 25 mM of TEMPO radical (Sigma Aldrich, St. Louis, MO) in a 1.5:1 H2O:glycerol (v/v) solution. Samples –usually of 150 μL or less– were hyperpolarized in an Oxford Instruments (Tubney Woods, Abingdon, UK) Hypersense® 3.35 T polarizer operating at 1.5 K, by irradiating a co-frozen TEMPO radical with ≤180 mW at ~94.1 GHz. Following DNP samples were dissolved in 99.9% D2O (Sigma Aldrich) and heptane (Sigma Aldrich) as specified in the text, and transferred to the NMR by a 10 bar pulse of pressurized helium gas applied over 1.5 seconds. 1H dilution factors in these dissolution DNP experiments were determined by measuring the absorbance of equivalent samples containing known quantities of dissolved red food coloring; these are reported for various conditions in Table 1 of the Supplementary Information. The absorbance values in the latter samples were measured on an Ultrospec 2100pro UV/Visible spectrophotometer (Amersham Biosciences, Piscataway, NJ) at a 492 nm wavelength, using a 17.8 MΩ·cm H2O sample as blank.
Sample preparation
For the exchangeable 1H NMR experiments (Figure 4), a concentrated sample of partially deuterated arginine was prepared by dissolving this amino acid at natural abundance and in powdered form (≥98% pure, Sigma Aldrich, St. Louis, MO) in 99.9% D2O (Sigma Aldrich, St. Louis, MO), adjusting the pH to ~3 with concentrated HCl, and drying it by rotary evaporation. The procedure was repeated and the remaining powder was dissolved in 3 mL 99% D2O to a final concentration of 1 M. This arginine sample was inserted into the 10 mm NMR tube subsequently used in the hyperpolarized water injection experiments. For the water-derived 15N enhancement experiments of small molecules (Figures 5 and 6), sample volumes and concentrations included: 500 μL of 200 mM 15N-urea (Cambridge Isotopes, Cambridge, MA), 350 μL of 500 mM 15N-alanine (Cambridge Isotopes), and 0.7 mL of 2.1 M natural abundance arginine at pH~3 (Sigma Aldrich). All samples were prepared in 99.9% D2O, and analyzed in 10 mm NMR tubes. Finally, for the water-derived 15N enhancement experiments of biomolecules (Figure 7), modified aldehyde reductase (40 kDa) was cloned into pET28_TEVH and expressed in BL21 (DE3) bacteria using 4 L of M9 minimal media supplemented with 15N labeled ammonium chloride. The bacterial lysate was applied to a Ni column (HisPrep FF 16/10, GE Healthcare Biosciences, Uppsala, Sweden) and eluted with imidazole to yield a partially purified protein mix. The imidazole was removed by applying the protein mix to a preparative desalting column (HiPrep_26/10, GE Healthcare) equilibrated with phosphate buffered saline (PBS). The protein was filtered, 0.02% NaN3 plus Trypsin were added to it, and the mixture was subsequently incubated overnight at 37 °C in order to digest the reductase. The ensuing polypeptide mix was then concentrated on a Centricon with a 10 kDa molecular weight cut off (Millipore, Billerica, MA). The flow-through contained peptides with a Mw <10 kDa which were subsequently removed from a Resource column (GE Healthcare) with 90% Acetonitrile and 0.1% TFA. The resulting mixture of polypeptides was frozen and lyophilized to obtain a dry powder. An ~11mg/mL solution was prepared by dissolving the powder in 97% D2O buffer (25 mM KH2PO4, 50mM NaCl) and its pD was adjusted to ~10 with NaOH to ensure rapid hydrogen exchange.
NMR spectroscopy
NMR experiments were conducted in an 11.7 T Magnex magnet (Abingdon, Oxfordshire, UK) run by a Varian iNova console (Palo Alto, CA) and equipped with a QNP Bruker (Karlsruhe, Germany) 10 mm probe. NMR experiments were triggered upon dissolution and injection of the hyperpolarized water sample into the NMR tubes waiting with their samples inside the magnet bore. All NMR data were processed using Matlab® software (The MathWorks Inc, Natick, MA) using an exponential decay as a line-broadening function, and when needed peaks were fitted as Lorentzians using Dmfit (The Comfit Consortium, Orleans, France).28
3. Results
Hyperpolarizing water
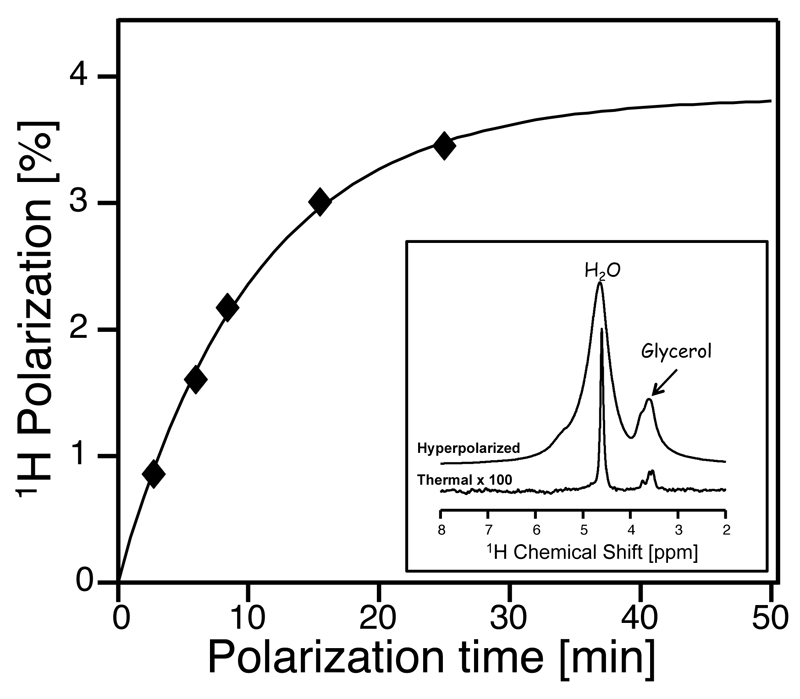
Dissolution DNP studies have shown that water samples containing 10-40 mM of a nitroxide radical mixed with the appropriate proportion of glassing agent can be efficiently polarized when irradiated by microwaves at T ≤ 1.5 K in high magnetic fields.18,29,30 Figure 1 illustrates the build-up behavior for this microwave-driven water polarization, as measured by the liquid state enhancement observed after dissolving a sample polarized in a 3.35T Hypersense, with 3 mL D2O. This curve evidences a 10±2 min characteristic buildup time for the solid-state polarization; in terms of the achievable post-dissolution enhancement, such optimized hyperpolarization conditions led to signals decaying ≈1000-fold as they reach thermal equilibrium in the 11.7 T NMR used in this study (Fig. 1, inset).
Figure 1.
DNP-enhanced 1H signal buildup observed for water, as a function of the polarization time under cryogenic conditions. The experimental points arise from independent dissolution experiments, where the water signal enhancement was compared to the thermal counterpart after returning to equilibrium. Samples consisted of 30 µL H2O:Glycerol 3:2 (v/v) hyperpolarized at 1.5 K and 94.1 GHz using 25 mM TEMPO as polarizing agent, and subsequently dissolved with 3 mL D2O. Comparison of the resulting data to its thermal counterpart (inset) indicates a plateauing 1H polarization at these conditions of (3.9±0.3)% and a buildup time constant of (10±2) min. Alternative polarization and dissolution conditions (cf. Fig. 2) can elevate the former figure beyond 5 %.
Although very promising, such enhancement figures are deceptively high. Comparisons between a hyperpolarized and a thermal signal measure relative enhancements, but ignore the 1H signal reduction due to the dilution of the hyperpolarized water with the glassing agent needed for an effective cryogenic DNP process, or the substantial dilution with non-polarized solvent that the hyperpolarized sample undergoes upon melting and flushing it across the two magnets. In order to address the first of these concerns, we used glycerol as water’s co-glassing agent. Glycerol was chosen over other possible co-solvents, given this compound’s relatively high concentration of exchangeable protons. These will be polarized as well by the solids DNP process, and eventually contribute to the pool of exchangeable protons whose hyperpolarization one aims to transfer to the biomolecule. At a 3:2 water:glycerol v/v ratio the ensuing sample polarized efficiently, and still delivered ~76% of the exchangeable protons expected from a pure water counterpart. To address the second concern, we attempted to decrease the dilution factor by increasing the volume of hyperpolarized sample –without a concomitant increase in the volume of the dissolution solvent. While the water’s dilution could be reduced by a factor of ≈10 in this fashion, this came at the cost of severely reducing the T1 of the hyperpolarized water. This penalty reflects the fact that all efforts aimed at reducing a pellet’s dilution, will de facto increase the nitroxide’s post-dissolution concentration; since this radical efficiently polarizes the protons but is also an effective water T1 relaxation agent, particularly in the low magnetic fields experienced by H2O during its transfer from the polarizer to the NMR magnet,31,32 the net hyperpolarization achievable from these reduced-volume solutions actually drops. In order to reduce water’s post-DNP dilution without decreasing the T1 of the hyperpolarized 1HS, a number of alternatives were tested. Most efficient among these ended up being the combined use of immiscible organic and aqueous solvents to melt and transfer the hyperpolarized water pellet.30 This method reduces the dilution factor thanks to the phase separation that the immiscible organic solvent will undergo after the sample is transfered, as it settles outside the NMR observation coil region. At the same time, a suitable organic phase can efficiently extract the organic co-polarizing TEMPO radical over the course of the sample-transfer process, thereby decreasing the aqueous phase relaxivity. Heptane was found as a useful co-solvent for achieving these dual goals, without introducing substantial susceptibility-derived distortions in the ensuing lineshapes. Typically, 150 µL of hyperpolarized water samples were thus dissolved and transferred with a 1.5/3 mL mix of water/heptane, leading to a net dilution factor of ≈8. Further reductions in the aqueous’ phase dissolution volumes did not significantly reduce the hyperpolarized pellet’s dilution factor.
Given the importance of maximizing T1s for the sake of minimizing polarization losses in the case of fast-relaxing nuclei like 1Hs, two additional provisions were adopted. First, in all our experiments D2O was used as the aqueous dissolution phase; by relying on this deuterated solvent, a ca. four-fold increase in the T1 of the hyperpolarized water protons was observed. In addition, the tube transferring the dissolved hyperpolarized water between the DNP and the NMR magnets, as well as the NMR probe itself, were preheated to ca. 40-50 °C; this lead to an additional lengthening of the hyperpolarized 1H’s lifetimes. Numerous other precautions were assessed in the hope of further increasing the protons’ T1s –including the surrounding of the transfer line with ≈1000 G magnets along its ca. 2 m route, and solvent degassing. Yet these lead to negligible enhancements, and their use was thus discontinued. Table 1 in the Supplementary Information gives further quantitative data on how each of the processes described in this paragraph, assisted in achieving an enhanced water hyperpolarization at the NMR probe position.
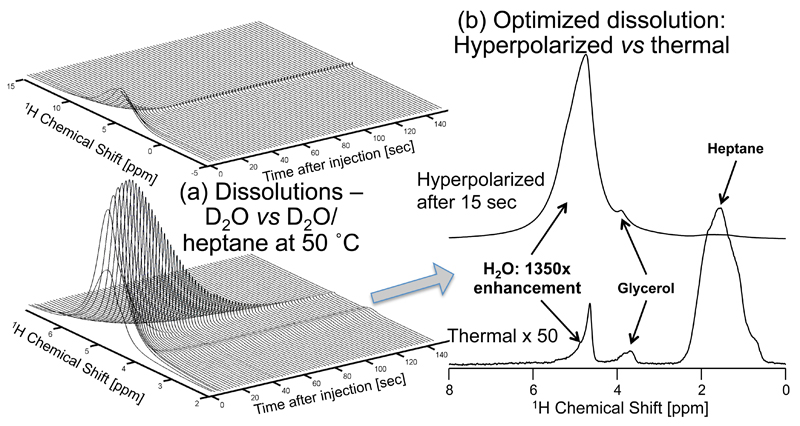
The outcome of these efforts is summarized by the post-dissolution traces in Figure 2. This compares results obtained for a dissolution employing solely D2O, with those stemming from a joint D2O/heptane dissolution mix incorporating heating of the transfer line. The stronger, longer-lasting enhancements afforded by all the aforementioned steps are clearly evidenced; unfortunately, so are the significant radiation damping effects that highly polarized water at these fields and concentrations are bound to lead to. These are reflected in both severe shifts and broadenings immediately upon dissolution, which decay as the hyperpolarization dies down. Still, judging by the areas of these small pulse-angle (<1°) experiments, optimal cases led to a polarization ≥5.2% and a T1 ≥ 18 sec. When contemplating the use of such polarization as source for enhancing the sensitivity of additional molecules, this performance should be further scaled by a ≈1/8 dilution factor associated with the sample’s dissolution, and a 0.76 factor reflecting the decrease in labile protons owing to the use of the glycerol. All these factors combined still lead to a ≥120× enhancement over the polarization that is present in a pure water tube polarized in a 1 GHz NMR spectrometer –not an insignificant gain, that compares favorably to absolute 1H water enhancements of approximately 15× obtained at 4.7 T by conventional dissolution DNP,29 and of approximately -10× obtained at 1.5 T by liquid state continuous-flow DNP.33
Figure 2.
Improving water’s hyperpolarized signal by co-dissolution with heptane. (a) Water signal evolution following hyperpolarization of a 150 µL 3:2 (v/v) mixture of H2O:Glycerol with 25 mM TEMPO, and dissolution in either 3 mL at ~35°C D2O (top), or in a mixture of 1.5 mL D2O and 3mL heptane with transport and measurement at ca. 50°C (bottom). The relaxation time T1 of the water resonance is extended from 3.6 sec (top) to 18.2 sec (bottom), and the absolute enhancement at t = 0 is increased by a factor of 4.5. (b) Comparison between the hyperpolarized 1D 1H NMR arising from the D2O/heptane dissolution 15 seconds after it has reached the NMR magnet, and a thermal spectrum of the same sample. All spectra were obtained by acquiring 28 k complex data points using a small (~1°) flip-angle pulse excitation and a carrier frequency set to 2.9 ppm; time zero corresponds to the conclusion of the sample flushing from the DNP polarizer.
Sensitivity enhancement of exchangeable protons in small biomolecules
With these gains at hand, the use of DNP-enhanced water protons towards the magnification of NMR signals arising from labile biomolecular protons, was explored. To this end we targeted protons possessing solvent exchange rates kex that are sufficiently slow in the NMR time scale to give distinct peaks in the ensuing 1H spectrum, and at the same time sufficiently fast to accommodate significant gains for the above-mentioned hyperpolarized water T1 times. As the ultimate goal is to exploit these exchange processes in biomolecules with significantly shorter T1s than those of the hyperpolarized water, the investigated paradigm explored the gains in polarization achieved by biomolecules that were waiting in the NMR magnet/probe, and exchanged their labile protons with those of water that was suddenly injected following dissolution DNP. This approach would have the advantage that during the transfer process the polarization will decay with the longer T1 of the water protons, and significant polarizations could be imparted even on species with short proton T1s. To investigate under what conditions would this approach be beneficial, basic calculations were performed on the extent by which protons Hex that are initially thermally polarized, will enhance their z-magnetizations 〈Hex〉z by chemical exchange with hyperpolarized water. Assuming that the injected water hyperpolarization is much higher than its thermal (Th) counterpart (i.e., that 〈H2O〉z(0) ≫ 〈H2O〉z(Th)) and that [H2O]≫[Hex], these calculations follow from modified Bloch-McConnell equations34 and predict a time-dependent exchangeable proton magnetization:
| (1) |
where the T1‘s denote the spin-lattice relaxation delay of the species and kex their mutual exchange rate.
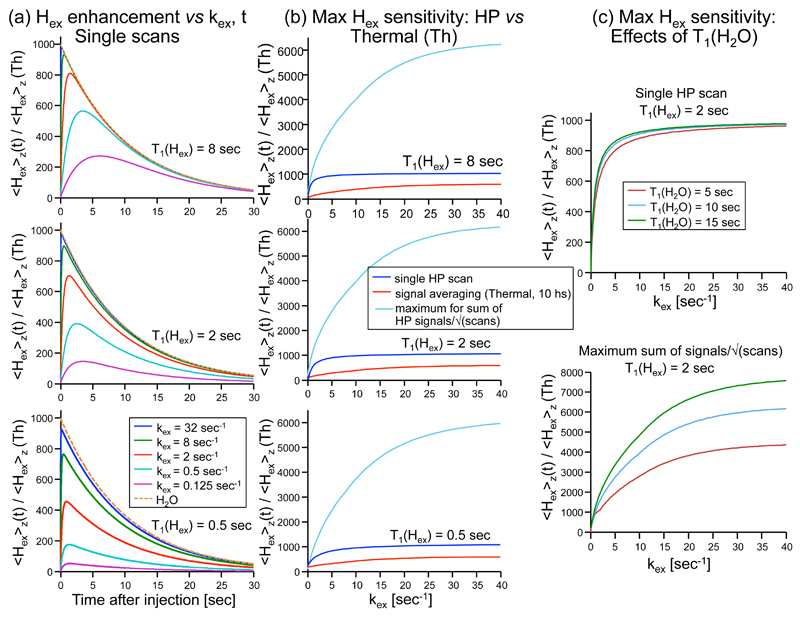
Plots of this equation for a variety of conditions are given in Fig. 3a. These show that the maximum magnetization achievable by the exchangeable protons will be relatively insensitive to or even to the water T1, but will be sensitive to the product. Importantly, not only can high levels of single-shot polarization be achieved in this manner for Hex relative to the polarization arriving with the water protons, but by applying selective pulses on the exchangeable proton sites, the water polarization can be preserved and multiple scans with enhanced signal can be acquired from a single dissolution. For a 90° selective pulsing taking place at a constant repetition time TR, the polarization contributions to the exchangeable proton signals will be described by
| (2) |
As shown in Figure 3b for a variety of instances, substantial sensitivity increases can then be obtained for the exchangeable protons. This can also be important if attempting to acquire multidimensional spectra, or to follow a dynamic process. It follows as well from the last expression that although an increase in the water T1 only leads to a slight increase in the initial Hex magnetization, the achievable polarization enhancement of the exchangeable sites in a multi-scan experiment can be significantly increased by prolonging (Figure 3c).
Figure 3.
Calculations of the relative 1H magnetization enhancements of protons Hex, due to exchange with hyperpolarized water protons. Unless stated otherwise the relaxation of water was kept constant at = 10 sec, and the initial relative enhancement of water was = 1000. (a) Enhancement as a function of time since the hyperpolarized (HP) water injection, for the different exchange rate(kex) values indicated in the bottom panel. Calculations are given for three different values of T1(Hex) (top, middle and bottom panels); the dashed orange line in all panels represents the decay of the water polarization with time. (b) Enhancement achievable by Hex as a function of kex, calculated assuming that: a single scan was measured by a 90° pulse at an optimal time after injection of hyperpolarized water (blue lines), that thermal signal averaging was performed over the course of 10 hours with optimal conditions (ie., with 90° pulses and recycle delays given by kex and not solely by T1(Hex); red lines), or that multiple hyperpolarized scans were done on Hex at optimum times assuming minimal TR of 100 ms (cyan lines; in this latter case we display the sum of signals collected with 90° pulses divided by square root of the number of scans). Other parameters are the same as in (a). (c) The effect of T1(H2O) on the Hex enhancement, shown for an optimized single scan acquisition (top), or for multiple scans seeking maximum SNR as a function of kex. T1(Hex) = 2 sec and the T1(H2O) is varied – 5, 10 and 15 sec (red, cyan and green lines, respectively).
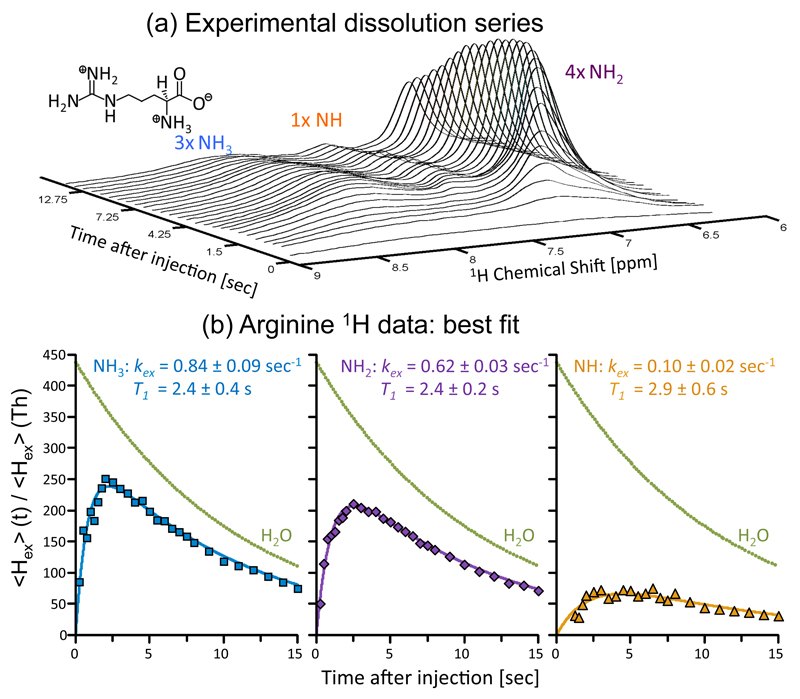
With these expectations as background, Figure 4 illustrates the gains that this procedure afforded when applied to arginine, a small molecule possessing multiple exchanging sites. This compound exhibits different H2O ←→ HN- exchange rates kex for the non-equivalent NH, NH2 and NH3 groups in the molecule, with strong pH and temperature dependencies.35,36 The polarization buildup is thus different for each group but is in all cases significant. A train of acquisitions following a water-based dissolution DNP experiment allows one to obtain insight into the rates of hydrogen exchange of these sites with the solvent (Fig. 4a-b).
Figure 4.
Transferring water hyperpolarization to the resonance of arginine’s exchangeable protons. (a) Progression of arginine’s 1H NMR spectrum upon sudden dissolution of hyperpolarized water into 3 mL of a 1M arginine sample (pD ~ 3) dissolved in D2O, and waiting in the 500 MHz spectrometer used to collect the data. Each trace involved the acquisition of 4k complex points, arising from a small flip-angle (~1°) excitation (carrier at 7.3 ppm) with a 0.25 sec TR. The different types of protons in the arginine sample (inset - molecular formula) are indicated above their corresponding peaks. (b) Peak intensities arising from the experimental time course, together with fits to Eq. (2) for each arginine site (solid lines), lead to the indicated relaxation times T1 and exchange rates kex. These fits revealed an initial water polarization enhancement of (438±3)×, and a characteristic decay T1(H2O) of 10.9 ± 0.1 sec; the ensuing decay curve is presented in the figure as green dots).
Heteronuclei signal enhancement
Interestingly, not only can exchangeable protons be polarized, but also heteronuclei directly bound to such exchangeable protons are spontaneously polarized by injection of hyperpolarized water. This is illustrated in Figure 5a, which demonstrates how polarization from DNP-enhanced water 1Hs migrates to urea’s 15N, without the need for any 1H pulsing. A train of low flip angle pulses on the 15N channel evidences the slow buildup of urea’s 15N polarization, reaching a maximum at ≈40 sec. The decay of this polarization is also slow, reflecting a that for urea in a partly deuterated solution like the one arising in this case, is on the order of minutes. A number of factors are involved in this build-up/decay function, including the rate of amide/water 1H exchange kex, the rate of 1H-15N cross-relaxation kNOE driving the heteronuclear polarization transfer within urea, and the rates of polarization decay given by the 1H T1’s of the water and urea sites as well as by the 15N’s own T1. Three-site exchange simulations (Supporting Information) show that the magnitude of the 15N enhancement will depend in a complex fashion on these multiple factors. Still, fits of the experimental data based on this model reveal that the heteronuclear Overhauser transfer kNOE is the rate determining step of this polarization transfer process. With this knowledge at hand, one can propose a simpler two-site model whereby the observable 15N magnetization only arises from a 1H reservoir made available by the DNP experiment. As this is left unperturbed apart from its relaxation back to equilibrium, the 15N polarization’s time evolution can be described by37:
| (3) |
where k 1H→15N summarizes the average effects of the H2O→15N process, and is a decay time factoring both the natural T1 of the 15N as well as the depleting effects of the pulses used to interrogate the signal.
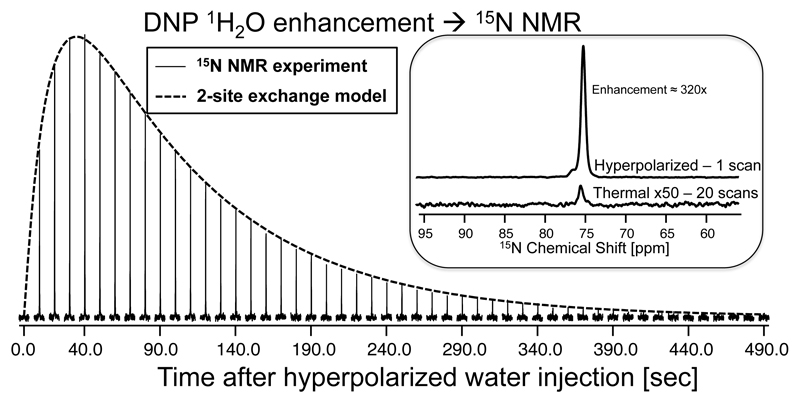
Figure 5.
15N NMR enhancement achieved in 15N-urea via heteronuclear polarization transfer from hyperpolarized water. Spectra were collected using a small flip-angle (9°) single-pulse irradiating on the 15N channel at 76 ppm, and acquiring 9 k data points (4 sec acquisition time). The dashed line is a fit to the two-site exchange model in eq. (3). The inset compares a single-scan DNP-enhanced 15N spectrum collected using a 90° pulse applied at an optimal post-injection delay of ≈40 sec, against a thermal equilibrium 15N NMR spectrum measured for the same sample by signal averaging 20 fully-relaxed scans, over a total of 13:50 hours. All measurements were done at 50 °C.
The enveloping line in Figure 5 shows a fit of this simplified model to traces arising from this kind of experiment, leading to an effective rate k 1H→15N = 0.29±0.02 sec-1, and times . By setting to zero, this model also let’s one find the approximate time leading to the maximal 15N enhancement: tmax = 34 sec. For an initial degree of maximal 1H polarization injected in the reservoir the solution of eq. (3) also predicts a maximum achievable 15N polarization that from the parameters fitted in Figure 5 should be ≈344×, close to the experimentally observed value of 320× (Figure 5, inset).
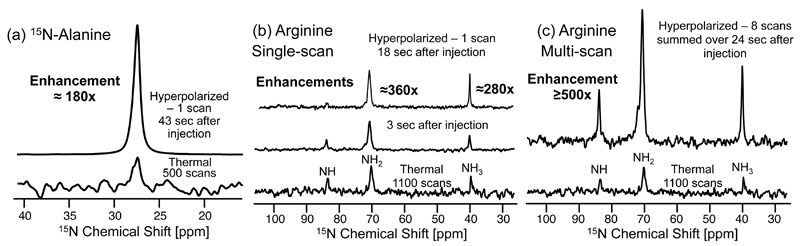
Figure 6 illustrates an application of this strategy to the enhancement of 15N sites in alanine and arginine. For alanine, a similar analysis as the one just described suggests a maximal 15N sensitivity enhancement ca. 40 sec after sample injection, although with a polarization enhancement of ≈180×. A similar experiment on a 0.7 M D2O solution of natural abundance arginine at pD≈3 shows a maximum enhancement at ≈20 sec with the 15NH2 and 15NH3 sites showing: ≈360× and ≈280× levels of enhancement, respectively. Much lower enhancements (≈50x) are observed for the NH site, due to its slower rate of hydrogen exchange. Noteworthy, as the hydrogen exchange with water for the former two arginine sites is fairly rapid, it is not necessary to wait a relatively long T1 delay to obtain the optimum enhancement: multiple scans collected at times ≈(kex)-1 lead to significantly enhanced signals which can be averaged over several repeated scans (Fig. 6c). A similar approach could proof useful in the acquisition of Hadamard-encoded or sparsely-sampled 2D NMR spectra.
Figure 6.
Enhancement vis-à-vis thermal counterparts of the 15N signals of 15N-alanine (a) and of natural abundance arginine ((b) and (c)), by polarization transfers from hyperpolarized water. The optimal delay in each case was extracted from simulations of the kind given in Figure 5. Hyperpolarized 15N NMR spectra in (a) and (b) were detected in single-scan experiments using a 90° 15N pulse, applied 43 and 18 sec after the injection of the water, respectively. Thermal acquisitions in (a) and (b/c) took ca. 2 and 14 hours, respectively. (c) Sum of the first 8 scans collected after injection of hyperpolarized water to an arginine sample, over a total time of 24 sec. An effective average enhancement ≥500× is observed. All measurements were done at ≈50 °C under conditions akin to those in Fig. 5. Notice that the NH of arginine has significantly lower enhancement than the other two peaks due to its slow kex at pH≈3.
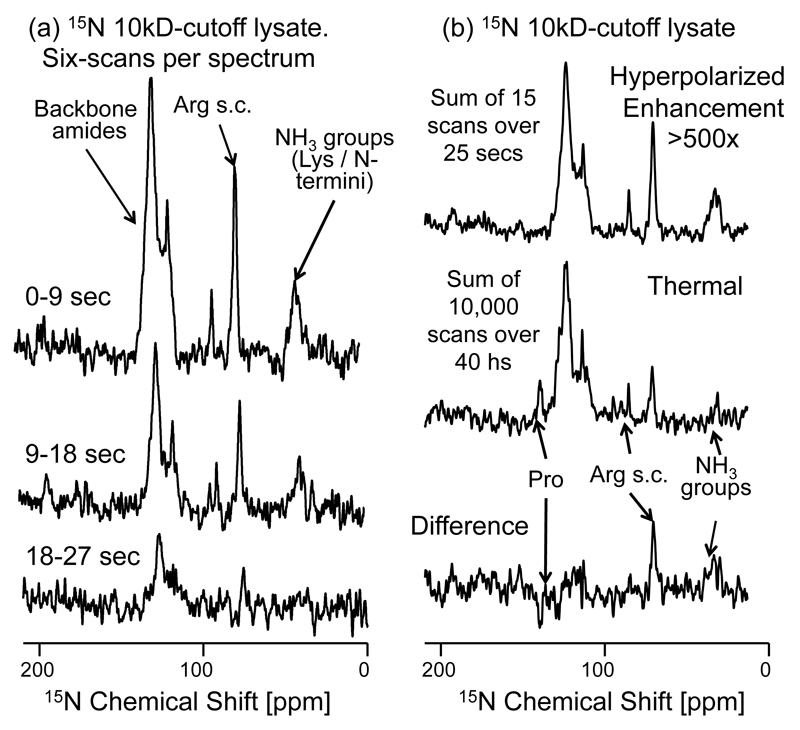
In order to investigate whether these initial observations can be extended to larger biomolecules, the heteronuclear transfer experiment was applied on a lysate of 15N-labeled aldehyde reductase. Trypsin-based lysis reduced the original 40 kDa MW of this well-folded protein into an array of peptides of mostly MW ≈ 3 kDa, with some 5% reaching up to the 10 kDa molecular weight cut-off used. It can be assumed that the peptides in this mixture do not contain residual structure and that their chains are fully extended. These conditions should favor a rapid exchange of their amine and amide NH’s with the hyperpolarized water protons. To examine what kind of effective 15N signal enhancement this could lead to, a series of 15N NMR spectra where collected using 90° excitation pulses following the injection of hyperpolarized water. These results are shown in Figure 7a, and confirm a sensitivity enhancement of both backbone amide and side-chain amine resonances. The build-up of these signals is relatively rapid, as expected for the high kex rates characterizing these unfolded peptides. The apparent decay of the signal enhancement by contrast, 20±1 sec, is much slower than the overall relaxation of the backbone amides, whose global is 1.8±0.8 sec. This relatively slow decay reflects the T1 of the hyperpolarized water protons, that support the 15N repolarization process between consecutive 15N scan. These long lifetimes allows one to achieve an 15N enhancement beyond what would be possible with a single acquisition; comparing a sum of scans collected over a 25 sec period (Figure 7b, upper trace) against a thermal equilibrium 15N spectrum (Figure 7b, middle trace), indicates that most peaks in the amide backbone region can be enhanced in this multi-scan fashion by > 500×. A similar enhancement characterizes 15N sites in the NH3 region, as well as arginine’s guanidine 15N sites in the lysate. The only amide nitrogens that do not appear enhanced are those belonging to proline groups, owing to their lack of exchangeable protons.
Figure 7.
Enhancement of 15N signals in a 15N-labeled polypeptide lysate with molecular weight cut-off of 10kDa, via polarization transfer from hyperpolarized water. (a) 15N NMR spectra arising from 90° 15N pulses applied over the indicated post-dissolution times, reflecting the 17.9 sec T1 we detect for the water protons onto the 15N signals enhanced by the exchangeable protons. The sum of six consecutive scans from this time series are displayed. (b) Sum of first 15 scans (upper trace) following the injection of hyperpolarized water, compared vs a thermal equilibrium 15N spectrum (middle trace), measured by signal averaging 10000 scans over the course of ca. 40 hours. The difference between these spectra is displayed in the bottom trace, highlighting the over-enhancement of the arginine side-chains (NH2) and lysine’s (NH3) groups, and the under-enhancement of the proton-less proline backbone nitrogens. The average signal enhancement of the 15N backbone amides is >500× relative to 15N thermal equilibrium signal. Other acquisition parameters are as in Figure 5.
4. Discussion and Conclusions
Bringing the benefits of DNP to bear onto the study of biomolecules in solution, is an important challenge of contemporary NMR. The present work investigated a way of bypassing the T1 bottleneck that slowly-tumbling biomacromolecules will face upon transferring from the DNP to the NMR fields, based on an ex situ hyperpolarization of water and subsequent exchange-driven transfers of polarization to labile biomolecular sites. Although addressing a small subset of all sites, such an efficient enhancement of exchangeable protons –and of their bonded nitrogens - could facilitate a wide variety of studies currently supported, inter alia, by 1H-15N 2D correlations. This strategy’s success depends on maximizing the absolute polarization of the H2O achieved in the cryogenic solid, minimize the dilution that the cryogenic pellet will undergo upon melting and shuttling, and reduce the 1H relaxation losses that limit the time over which the H2O polarization can be exploited in the biomolecular analyses. The present study placed an emphasis on optimizing the last two of these aspects, with dilution and relaxation losses minimized by a combination of dissolution and transfer precautions. With these provisions dilution penalties were limited to ≈90% –still a non-negligible factor liable to improvement – and relaxation times reached 15 - 20 sec. All this lead to a nearly ≈100× enhancement over the polarization characterizing a tube of pure water placed in a high field system. Even further room for enhancement remains in terms of optimizing the cryogenic solid-state polarization, as witnessed by the fact that electrons are over 90% polarized in the DNP setup used whereas the 1H polarization hardly cleared the 5% mark.
Even with these limitations, an interesting aspect of the examined approach lies in its ability to spontaneously enhance the resonances of 15N attached to labile protons, by factors in the 100-1000× range. These results are particularly promising for 15N sites that undergo rapid 1H exchange, e.g. Lysine side chains and amide positions in unstructured proteins at high pH; these sites cannot be efficiently enhanced by INEPT-like sequences, while thermal equilibrium 1H→15N NOE methods are inefficient in macromolecules. The spontaneous nature of the transfer is also promising for human-oriented NMR imaging setups, which are rarely equipped with full double-resonance irradiation capabilities. It is conceivable, however, that a more active INEPT-like transfer might be more effective for N-H sites undergoing intermediate proton exchange than the spontaneous transfer assayed in this study. We have carried out such tests, but preliminary results indicate that this strategy is challenged if attempting to leave the radiation-broadened reservoir of hyperpolarized H2O untouched for the sake of performing multiple 15N acquisitions. Further efforts aimed at clarifying these issues are ongoing.
The enhanced biomolecular sensitivity experiments demonstrated in this work were carried out on intrinsically unfolded systems liable to fast hydrogen exchange of their backbone protons. Additional potential targets could include structured polypeptides which are kept artificially unfolded in the NMR tube where their measurement will take place, until the arrival of hyperpolarized water triggers their sudden folding. Even in folded systems, sensitivity gains should arise from water accessible side-chains whose protons are rapidly exchanging with those of the hyperpolarized water. A different kind of experimental window that might be opened by the hyperpolarized experiments hereby described, involves measuring the rates of water exchange and/or water accessibility in biomolecules.38 Two kinds of water-proton exchange experiments are commonly used, depending on the range of exchange rates to be accessed. Slower exchange processes are usually determined by isotope dilution methods whereby the volumes of proton peaks in proteins whose exchangeable sites were fully deuterated, are monitored in real time as the sample is diluted by fully protonated water (or conversely, whereby peak decays are quantified as a fully protonated protein is diluted in deuterated water).39,40 Another method, better suited for studying more rapid exchange processes, relies on observing the decrease in the intensities of the labile peaks upon solvent water saturation/inversion. In this method the signal of the individual exchangeable proton sites will depend on kex as well as the site’s T1 value, and hence is limited to kex on the order of the site’s T1 (i.e., kex ≈ 1 s-1).41 Studying hydrogen exchange processes using the hyperpolarized water principles described in this paper, has many features that complement both of these methods – both due to its real-time nature, and by virtue of the various timescales that the hyperpolarization lifetime enables one to probe. In particular, the fact that a high signal contrast is not governed solely by the intrinsic T1 of the exchanging sites but rather by T1(H2O) (Fig. 3C, bottom), means that it should be possible to characterize slower rates of kex than in conventional magnetization transfer methods. This intriguing research avenue is currently being investigated.
Supplementary Material
Acknowledgements
We are grateful to K. Zibzener for technical assistance, and to Dr. Shira Albeck (ISPC, Weizmann Institute) for the preparation of the reductase lysate. Financial support from ERC Advanced Grant #246754, EU’S BioNMR Grant #261863, DIP Project 710907 (Ministry of Education and Research, Germany), the Clore Foundation, and the generosity of the Perlman Family Foundation, are also acknowledged.
References
- (1).Abragam A, Goldman M. Rep Prog Phys. 1978;41:395. [Google Scholar]
- (2).Wind RA. eMagRes. John Wiley & Sons Ltd; 2007. [Google Scholar]
- (3).Barnes AB, De Paëpe G, van der Wel PCA, Hu KN, Joo CG, Bajaj VS, Mak-Jurkauskas ML, Sirigiri JR, Herzfeld J, Temkin RJ, Griffin RG. Appl Magn Reson. 2008;34:237. doi: 10.1007/s00723-008-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Maly T, Debelouchina GT, Bajaj VS, Hu K-N, Joo C-G, MakJurkauskas ML, Sirigiri JR, Wel PCAvd, Herzfeld J, Temkin RJ, Griffin RG. J Chem Phys. 2008;128:052211. doi: 10.1063/1.2833582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Prisner T, Köckenberger W, editors. Appl Magn Reson (special issue) 2008;34:213. [Google Scholar]
- (6).Griffin RG, Prisner TF, editors. Phys Chem Chem Phys (special issue) 2010;12:5737. doi: 10.1039/c0cp90019b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Bennati M, Tkach I, Turke MT. Electron Paramagnetic Resonance: Volume 22. Vol. 22. The Royal Society of Chemistry; 2011. p. 155. [Google Scholar]
- (8).Rossini AJ, Zagdoun A, Hegner F, Schwarzwälder M, Gajan D, Copéret C, Lesage A, Emsley L. J Am Chem Soc. 2012;134:16899. doi: 10.1021/ja308135r. [DOI] [PubMed] [Google Scholar]
- (9).Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Proc Natl Acad Sci U S A. 2003;100:10158. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, DeBerardinis RJ, Green GG, Leach MO, Rajan SS, Rizi RR, et al. Neoplasia. 2011;13:81. doi: 10.1593/neo.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Wolber J, Ellner F, Fridlund B, Gram A, Jóhannesson H, Hansson G, Hansson LH, Lerche MH, Månsson S, Servin R, Thaning M, et al. Nucl Instrum Methods Phys Res A. 2004;526:173. [Google Scholar]
- (12).Day SE, Kettunen MI, Gallagher FA, Hu DE, Lerche M, Wolber J, Golman K, Ardenkjaer-Larsen JH, Brindle KM. Nat Med. 2007;13:1382. doi: 10.1038/nm1650. [DOI] [PubMed] [Google Scholar]
- (13).Mieville P, Jannin S, Helm L, Bodenhausen G. Chimia. 2011;65:260. [PubMed] [Google Scholar]
- (14).Bornet A, Melzi R, Jannin S, Bodenhausen G. Appl Magn Reson. 2012;43:107. [Google Scholar]
- (15).Bornet A, Melzi R, Perez Linde AJ, Hautle P, van den Brandt B, Jannin S, Bodenhausen G. J Chem Phys Lett. 2012;4:111. doi: 10.1021/jz301781t. [DOI] [PubMed] [Google Scholar]
- (16).Hurd RE, Yen Y-F, Chen A, Ardenkjaer-Larsen JH. J Magn Reson Imaging. 2012;36:1314. doi: 10.1002/jmri.23753. [DOI] [PubMed] [Google Scholar]
- (17).Jannin S, Bornet A, Melzi R, Bodenhausen G. Chem Phys Lett. 2012;549:99. [Google Scholar]
- (18).Mishkovsky M, Eliav U, Navon G, Frydman L. J Magn Reson. 2009;200:142. doi: 10.1016/j.jmr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- (19).Lerche MH, Meier S, Jensen PR, Hustvedt S-O, Karlsson M, Duus JØ, Ardenkjær-Larsen JH. NMR Biomed. 2011;24:96. doi: 10.1002/nbm.1561. [DOI] [PubMed] [Google Scholar]
- (20).Ragavan M, Chen H-Y, Sekar G, Hilty C. Anal Chem. 2011;83:6054. doi: 10.1021/ac201122k. [DOI] [PubMed] [Google Scholar]
- (21).Kay LE, Torchia DA, Bax A. Biochemistry. 1989;28:8972. doi: 10.1021/bi00449a003. [DOI] [PubMed] [Google Scholar]
- (22).Yao S, Babon JJ, Norton RS. Biophys Chem. 2008;136:145. doi: 10.1016/j.bpc.2008.06.002. [DOI] [PubMed] [Google Scholar]
- (23).Ferrage F, Dutta K, Shekhtman A, Cowburn D. J Biomol NMR. 2010;47:41. doi: 10.1007/s10858-010-9409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Leggett J, Hunter R, Granwehr J, Panek R, Perez-Linde AJ, Horsewill AJ, McMaster J, Smith G, Kockenberger W. Phys Chem Chem Phys. 2010;12:5883. doi: 10.1039/c002566f. [DOI] [PubMed] [Google Scholar]
- (25).Bowen S, Hilty C. Angew Chem Int Ed. 2008;47:5235. doi: 10.1002/anie.200801492. [DOI] [PubMed] [Google Scholar]
- (26).Bowen S, Hilty C. Phys Chem Chem Phys. 2010;12:5766. doi: 10.1039/c002316g. [DOI] [PubMed] [Google Scholar]
- (27).Barb AW, Hekmatyar SK, Glushka JN, Prestegard JH. J Magn Reson. 2011;212:304. doi: 10.1016/j.jmr.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Massiot D, Fayon F, Capron M, King I, Le Calvé S, Alonso B, Durand J-O, Bujoli B, Gan Z, Hoatson G. Magn Reson Chem. 2002;40:70. [Google Scholar]
- (29).Ardenkjaer-Larsen JH, Laustsen C, Pullinger B, Kadlecek S, Emami K, Rizi R. Proc Intl Soc Mag Reson Med 19. Montreal: 2011. p. 3534. [Google Scholar]
- (30).Harris T, Bretschneider C, Frydman L. J Magn Reson. 2011;211:96. doi: 10.1016/j.jmr.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Polnaszek CF, Bryant RG. J Chem Phys. 1984;81:4038. [Google Scholar]
- (32).Bennati M, Luchinat C, Parigi G, Turke M-T. Phys Chem Chem Phys. 2010;12:5902. doi: 10.1039/c002304n. [DOI] [PubMed] [Google Scholar]
- (33).McCarney ER, Armstrong BD, Lingwood MD, Han S. Proc Natl Acad Sci USA. 2007;104:1754. doi: 10.1073/pnas.0610540104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).McConnell HM. J Chem Phys. 1958;28:430. [Google Scholar]
- (35).Henry GD, Sykes BD. J Biomol NMR. 1995;6:59. doi: 10.1007/BF00417492. [DOI] [PubMed] [Google Scholar]
- (36).Liepinsh E, Otting G. Magn Reson Med. 1996;35:30. doi: 10.1002/mrm.1910350106. [DOI] [PubMed] [Google Scholar]
- (37).Krishna NR, Goldstein G, Glickson JD. Biopolymers. 1980;19:2003. doi: 10.1002/bip.1980.360191106. [DOI] [PubMed] [Google Scholar]
- (38).Armstrong BD, Han S. J Am Chem Soc. 2009;131:4641. doi: 10.1021/ja809259q. [DOI] [PubMed] [Google Scholar]
- (39).Englander SW, Mayne L. Annu Rev Biophys Biomol Struct. 1992;21:243. doi: 10.1146/annurev.bb.21.060192.001331. [DOI] [PubMed] [Google Scholar]
- (40).Englander SW. Annu Rev Biophys Biomol Struct. 2000;29:213. doi: 10.1146/annurev.biophys.29.1.213. [DOI] [PubMed] [Google Scholar]
- (41).Waelder S, Lee L, Redfield AG. J Am Chem Soc. 1975;97:2927. doi: 10.1021/ja00843a066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.