Abstract
Hyperpolarized metabolic imaging is a growing field that has provided a tool for analyzing metabolism, particularly in cancer. Given the short life times of the hyperpolarized signal, fast and effective spectroscopic imaging methods compatible with dynamic metabolic characterizations are necessary. Several approaches have been customized for hyperpolarized 13C MRI, including CSI with a center-out k-space encoding, EPSI, and spectrally selective pulses in combination with spiral EPI acquisitions. Recent studies have described the potential of single-shot alternatives based on spatiotemporal encoding (SPEN) principles, to derive chemical-shift images within a sub-second period. By contrast to EPSI, SPEN does not require oscillating acquisition gradients to deliver chemical-shift information: its signal encodes both spatial as well as chemical shift information, at no extra cost in experimental complexity. SPEN MRI sequences with slice-selection and arbitrary excitation pulses can also be devised, endowing SPEN with the potential to deliver single-shot multi-slice chemical shift images, with a temporal resolution required for hyperpolarized dynamic metabolic imaging. The present work demonstrates this with initial in vivo results obtained from SPEN-based imaging of pyruvate and its metabolic products, after injection of hyperpolarized [1-13C]pyruvate. Multi-slice chemical-shift images of healthy rats were obtained at 4.7 T in the region of the kidney, and 4D (2D spatial, 1D spectral, 1D temporal) data sets were obtained at 7 T from a murine lymphoma tumor model.
Keywords: Ultrafast MRI, Chemical Shift Imaging, Spatiotemporal encoding, Hyperpolarized dynamic imaging, Hyperpolarized MRI, DNP, Spectroscopic Imaging, Cancer Diagnosis
1. Introduction
The importance of Magnetic Resonance Spectroscopic Imaging (MRSI) in pre-clinical and clinical studies has been enhanced by the emergence of dissolution dynamic nuclear polarization (d-DNP). Dissolution DNP has opened new horizons in dynamic metabolic imaging through 13C spectral observations of hyperpolarized compounds in vivo [1–2]. DNP can result in signal enhancements >10,000 for 13C; an unprecedented increase that is leading to new opportunities for imaging pathology and monitoring treatment [3–7]. Pyruvic acid has emerged as the principal 13C-labeled agent in d-DNP, but there is significant on-going research to develop other compounds for dynamic imaging of metabolism [8–10]. Despite this unique potential, in vivo d-DNP 13C NMR remains a challenge. The hyperpolarization decays irreversibly with T1, as well as following the spins’ excitation. Thus, fast schemes capable of delivering both spatial and spectral information from these transient signals, are required to exploit the opportunities opened by these experiments. A variety of strategies have been developed to deal with these demanding requirements; including multi-band pulses [11], variable-flip-angle excitations with pulse angles optimized for the injected hyperpolarized species and their products [12], spiral and blipped echo-planar imaging (EPI) acquisitions [13], and compressed sensing strategies [14]. Principal among the strategies in current d-DNP use are multi-scan chemical shift imaging (CSI) or IDEAL type sequences with a center-out phase-encoding scheme [15,16], and echo-planar spectroscopic imaging (EPSI) modules [17]. Both of these acquisition modes can provide practical spatial and spectral resolutions, albeit in multiple scans. EPI with suitable scan parameters can also be used for simultaneous imaging of two chemical shifts [18]. Each of these excitation and acquisition schemes makes some tradeoff in spatial, spectral and temporal resolution, and the most suitable approach depends on the application.
An alternative approach to single-shot imaging, which departs from traditional k-space concepts, has been developed over the last few years. First introduced for the acquisition of 2D NMR spectra in a single scan [19,20], spatiotemporal encoding (SPEN) utilizes a chirped pulse combined with a gradient for a sequential manipulation of the spins that can directly monitor their density in real space [21,22]. In an imaging context, SPEN uses such a manipulation along the low-bandwidth domain in combination with a conventional k-space readout, leading to a so-called hybrid acquisition mode [23,24]. Hybrid SPEN has a higher robustness against magnetic field inhomogeneities than EPI, reflecting the larger bandwidths it can access for the spatially sequential excitation and detection. This is aided by the possibility of refocusing all of the spin packets throughout acquisition [25,26]. Equally important is the fact that SPEN allows spectral-spatial imaging, at no additional expense over the basic imaging pulse sequence [27]. Further refinements arise from the use of super resolution (SR) and of other reconstruction methods to improve SPEN images by extracting the overlapping information included in the measured data points [28,29]. SR algorithms have also been extended to include chemical-shift information, and hence deliver improved high-resolution spectroscopic images [30]. Multi-slice implementations using 180° pulses for spatiotemporal encoding and a slice-selective excitation pulse with an arbitrary flip angle, have also been demonstrated [30,31].
In this study, we introduce and explore the performance of SPEN-based strategies for fast spectroscopic imaging of hyperpolarized compounds. Phantom experiments are described to illustrate the feasibility of SPEN-based 13C chemical-shift imaging in such a demanding context. Animal experiments are then presented, monitoring both healthy rats in the region of the kidneys, and mice with EL-4 murine lymphoma tumors as a function of time after injection of hyperpolarized [1-13C]pyruvate. Results are compared against multi-shot CSI sequences with center-out encoding for signal localization, and against a series of 1D spectra which were used to acquire dynamic information on the progressions of the pyruvate and lactate labeling. Overall, the results confirm the favorable properties of single-shot spatiotemporal encoding for hyperpolarized 13C imaging in vivo. Possible extensions of these experiments are discussed.
2. Theoretical Background
SPEN’s simultaneous spatial and spectral encoding
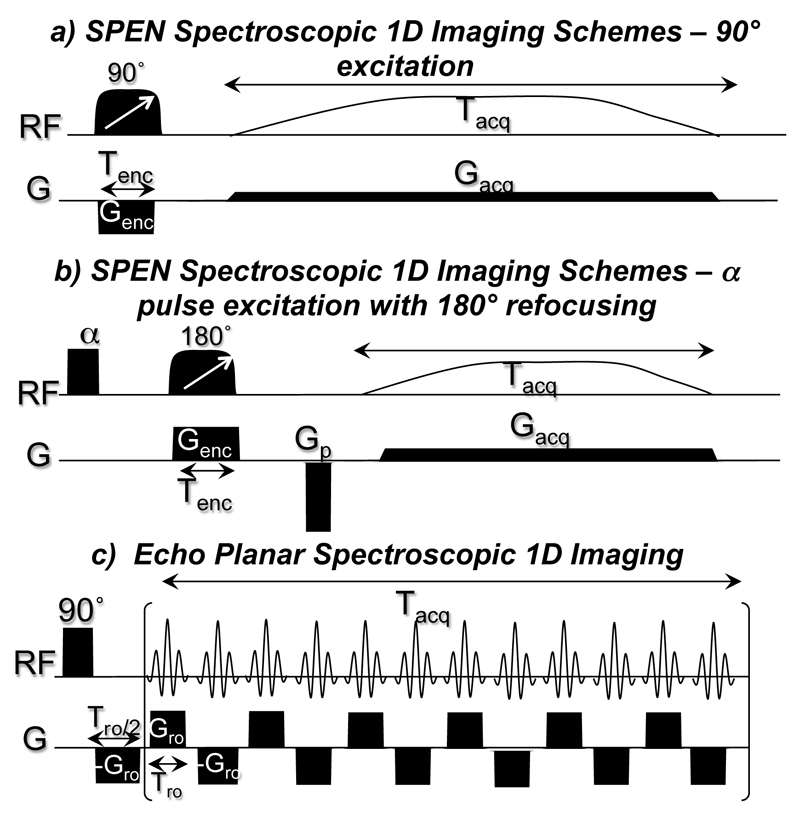
Spatiotemporal encoding relies on a manipulation involving the combined action of a frequency-swept pulse applied in the presence of an encoding gradient, followed by a sequential unraveling of the encoded imaging information using an acquisition gradient [20]. These manipulations can deliver an MR image without the use of a Fourier Transform (FT); when the encoding pulse duration is different from the acquisition duration and/or if no refocusing pulses are used, a built-in spectral encoding is also obtained [27]. This capability to extract both spatial and spectral information from the same Free Induction Decay (FID) can be understood from an analysis of the results arising in a model 1D experiment that uses a chirp pulse for either excitation (Fig. 1a) or inversion (Fig. 1b). Assuming that the ensuing FID arises from Q contributing chemical sites spanning a field-of-view FOV being addressed by the swept pulses, its expression can be written as [27]:
| (1) |
where and {αenc ,βenc }enc=90°,180° are coefficients depending on the magnitude and the duration of the encoding gradient (Genc, Tenc), as well as on the requested FOV: for the 90° chirp pulse case in Fig. 1a , for the 180° adiabatic sweep case in Fig. 1b , with β180° including a pre-phasing gradient. The appearing in eq. (1) is a wavenumber accrued during the acquisition time t, which over the course of its duration Tacq should fulfill an action kacq = Genc·Tenc in the case of a 90° chirp pulse and kacq =2·Genc·Tenc in the case of a 180° chirp pulse. Spectral information is contained in the -dependent phase terms appearing both in βenc and during the acquisition’s time-dependence of eq. (1). Once represented in a discrete ρq(yk) form, the spin density y-profile for each chemical site q can then be reconstructed by solving a set of equations with an extended SR method as described in Ref. [30]. It follows that the spin density 1D image of several chemical sites can be obtained simultaneously in this way using a constant acquisition gradient, with no need for the fast and strong oscillating gradients usually required in the classic EPSI sequence (Fig.1c).
Figure 1.
(a,b) SPEN spectroscopic 1D MRSI schemes based on excitation or refocusing chirp pulses. (c) Echo Planar Spectroscopic 1D imaging
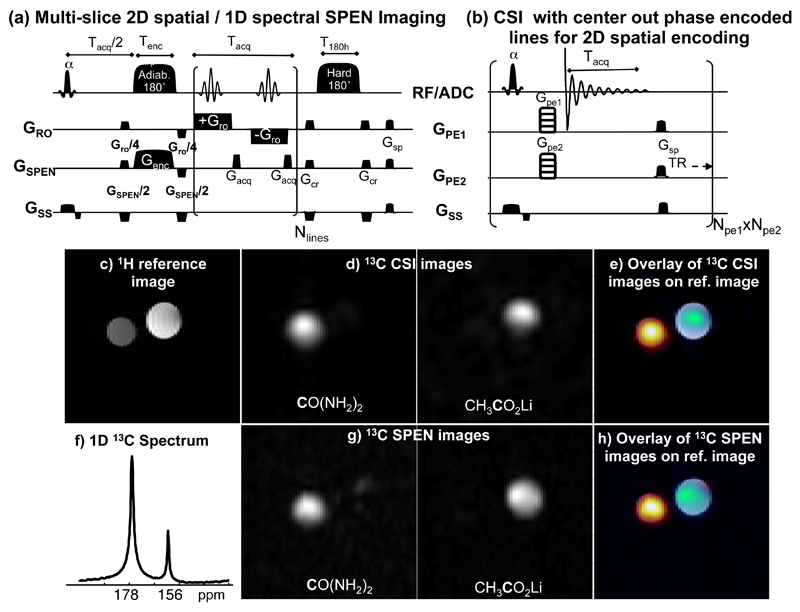
This 1D spatial / 1D spectral scheme can be extended to a single-shot 2D spatial/1D spectral sequence; for instance by encoding the readout dimension in a usual k-space fashion, and retaining the spatiotemporal procedure just described for encoding the low-bandwidth spatial/spectral dimensions. An echo-planar trajectory with appropriate gradients can then be used to explore a hybrid k- and SPEN-based 3D MRSI space in a single acquisition. To enable excitation with arbitrary flip angles, multi-slice 3D spatial imaging and the single-shot acquisition of kinetic data, this sequence was implemented here using a 180° adiabatic swept pulse for encoding as shown in Figure 1b, leading to the overall 1D spectral / 2D spatial multi-slice variant displayed in Figure 2a. Note that this sequence introduces a second inversion pulse after acquisition to restore the longitudinal magnetizations to +z, and thereby enable multiple shots from a single hyperpolarized injection.
Figure 2.
Comparison of SPEN and CSI results obtained upon 13C chemical shift imaging of a phantom. SPEN and CSI sequences are displayed in (a) and (b), respectively. (c) 1H reference image of two tubes containing [1-13C]acetate and [13C]urea. (f) 13C spectrum of the two tubes. (d, g) Urea and acetate 13C chemical-shift images obtained with CSI and SPEN acquisitions after suitable reconstruction, focusing on the labeled sites indicated in bold. (e, h) 13C chemical-shift images overlaid on the reference 1H image. See text for further experimental details.
Signal-to-noise ratio (SNR) considerations
The SNR available in single-shot SPEN spectroscopic images can be estimated theoretically and compared to that obtained with multi-shot CSI sequences of the kind usually employed in hyperpolarized MRSI (Fig. 2b), using an approach similar to that used in Ref. [32]. Given the similar T2* weighting in both sequences and the relatively long T2 of the samples, T2* and T2 effects can be neglected in these theoretical signal intensity estimates. The SNR of a 2D spatial/1D spectral CSI sequence can be estimated as:
| (2) |
where Sn is the signal arising from a given resonance for each of the multiple CSI scans, Nsum is the number of points in the frequency domain used to reconstruct the image of this specific peak, Nfreq is the number of points acquired in the FID dimension, Npe1 and Npe2 are the number of points for each phase-encoding dimension, sw is the spectral width and Δy, Δx are the image pixel dimensions in the scan plane. It should be noted that CSI images are defined for each peak as a sum, in the frequency dimension, over a range of frequencies centered on the peak maximum. In thermal equilibrium experiments all {sn}n = 1…Npe1·Npe2 will have similar S0 intensities, and the SNR becomes
| (3) |
For the single-shot hybrid SPEN sequence (Fig 2a) incorporating SR for the image spectral reconstruction, SNR can be estimated as:
| (4) |
where Ncs is the number of reconstructed chemical-shift images, Nro and NSPEN are the number of points acquired in the readout and SPEN dimensions, and BW·Tenc is the time-bandwidth product of the chirp pulse. SNRSPEN must be multiplied by if its results are averaged over Nrep repetitions.
Analyses of these expressions reveal that both approaches should yield comparable SNR’s if performed under optimized thermal equilibrium conditions. In hyperpolarized experiments, however, the SNR ratio between multi- and single-shot spectroscopic imaging sequences should shift in favor of the single-scan acquisition. This is due to a drop of the original signal during consecutive CSI shots as Sn ∝ sin (θ) · (co s(θ))n· e–nTR/T1, where n = 1, …, Npe1·Npe2 is the excitation step in the 2D phase encoding repetitions, θ is the (presumed constant) excitation flip angle, TR is the repetition time and T1 is the longitudinal relaxation time. Moreover, even if similar SNRs can be obtained, a single shot of the order of 100 ms can provide the full spectral and spatial information of interest, in contrast to the many seconds that are required to obtain the spatial information in CSI or EPSI sequences –and therefore could still prove to have practical advantages from a dynamics standpoint.
3. Materials and Methods
13C phantom thermal equilibrium experiments
Thermal equilibrium experiments were conducted on a 13C phantom consisting of two tubes, containing [13C]urea and lithium [1-13C]acetate, providing a chemical shift range of ∼1500 Hz. The aim of these experiments was to assess signal localization and to validate theoretical SNR estimates for the single- and multi-shot 13C imaging variants. These data were acquired on a 7T Varian preclinical, horizontal-bore scanner (Palo Alto, CA) using a 20 mm 1H/13C surface coil. The single-shot hybrid SPEN sequence was compared to a reference multi-shot CSI sequence with a FOV of 4x4 cm2 and a slice thickness of 1 cm. SPEN acquisitions used the sequence in Fig. 2a, with a 90° initial excitation pulse, sw = 50 kHz, Tenc = 5 ms, Genc 3.8 G/cm, Tacq = 46 ms, TE = 33..79 ms (depending on the spins’ positions), 16 x 96 acquired data points (effective resolution of 2.5 x 0.8 mm2), TR = 200 s, and 8 scans leading to a total experiment time of 0.5 hour. The CSI parameters were 90° flip angle pulses, sw = 6 kHz, Tacq = 85 ms, Nfreq = 512, Npe1 x Npe2 = 16 x 16 (effective resolution of 2.5 x 2.5 mm2), TR = 60 s and ≈ 4.3 hours total scan duration. In these SNR comparisons, the measured signal was calculated by drawing a region of interest centered on each tube, while the noise was calculated as the standard deviation in a signal-free region.
Hyperpolarized 13C experiments on phantoms
These basic SNR tests were followed by hyperpolarized experiments in vitro, where DNP-enhanced [1-13C]pyruvate was injected into a tube and a series of SPEN experiments were performed with various flip angles, to confirm that the signal decayed as expected given the known T1 value and repetition time. These experiments were performed on a 4.7 T MRI horizontal-bore magnet (Oxford Instruments, Oxford, UK) interfaced to a Varian scanner and equipped with a dual tuned 1H/13C linear volume transmit coil (Rapid Biomedical, Würzburg, Germany) for 13C excitation and with a 13C surface coil for acquisition. Hyperpolarization was carried out with a HyperSense polarizer (Oxford Instruments Molecular Biotools, Tubney Woods, UK). SPEN MRSI data were collected with the sequence shown in Figure 2a and the following parameters: ∼15° and ∼60° excitation angles α, sw = 31 kHz, Tenc = 8 ms, Genc = 0.6 G/cm, Tacq = 51 ms, TE = 39…90 ms, 20x64 acquired data points (effective resolution of 5 x 3.1 mm2), FOV of 10 x 10 cm2, slice thickness of 1 cm, single scan acquisition of ≈90 ms, TR of 1.1 s, and Nrep = 61 and 11, corresponding to each of the flip angles listed above.
Hyperpolarized [1-13C]pyruvate experiments in healthy rats
Hyperpolarized [1-13C]pyruvate was injected into healthy rats, and a region containing the animals’ kidneys was scanned. These experiments focused on the ability of single-shot SPEN to resolve the spectroscopic images of the four peaks –pyruvate, lactate, alanine and bicarbonate– expected to arise due to metabolism. These images were compared against results acquired using a CSI center-out phase encoding for the two spatial dimensions. The magnet and transmit coil setup in these experiments were the same as in the hyperpolarized phantom experiments, except that for the use of a 4-channel 13C phased-array coil (Rapid Biomedical) for data acquisition. The FOV was 6 x 6 cm2 and the slice thickness 2 cm. The SPEN parameters were: 90° flip angle α, sw = 31 kHz, Tenc = 8 ms, Genc = 1 G/cm, Tacq = 51 ms, TE = 39…90 ms, 20 x 64 acquired points (effective resolution of 3 x 3.75 mm2) and ≈ 90 ms total acquisition time. The CSI parameters were 10° flip angle, sw = 4 kHz, Tacq = 64 ms, Nfreq = 256, Npe1 x Npe2 − 16 x 16 (effective resolution of 3.75 x 3.75 mm2), TR = 70 ms and 16 s total scan duration.
Two male Sprague-Dawley rats (250-300 g) were anesthetized with isoflurane (0.8% isoflurane, 0.25 L/min oxygen, and 0.75 L/min air) and a tail vein catheter was inserted to allow administration of hyperpolarized [1-13C]pyruvate. In order to improve the comparison every animal was subjected to three injections, each of these followed by a different acquisition: first a SPEN acquisition, then a CSI scan, and finally a SPEN acquisition again. A 20 µL volume of [1-13C]pyruvic acid (Sigma Aldrich, Munich, Germany) containing 15 mM trityl radical OX063 (Oxford Instruments, Tubney Woods, UK) and 1.5 mM Dotarem (Guerbet, Villepinte, France) were inserted into the polarizer. This sample was polarized for approximately 45 min using 100 mW microwaves at 94.108 GHz. The 13C polarization was monitored by solid state NMR with an average 600 s build-up time constant. The hyperpolarized sample was dissolved in 4 mL of a dissolution medium made of 80 mM Tris, 100 mg/L EDTA, 50 mM NaCl and 80 mM NaOH, yielding 80 mM [1-13C]pyruvate at physiological pH. A volume of 1 mL was injected into the tail vein over a period of 10 s. The transfer time between dissolution and injection was 10 s on average, and MRS data acquisition was initiated 20 s after the start of injection. These measurements, as well as all associated animal-handling procedures, were performed in accordance with the guidelines for use and care of laboratory animals and were approved by the Danish Inspectorate of Animal Experiments.
Hyperpolarized [1-13C]pyruvate experiments in mice bearing an EL-4 murine lymphoma tumor model
A final set of in vivo experiments were carried out on tumor-implanted mice with the aims of (i) comparing the spectral resolutions and the degrees of spatial localization achievable in single-shot SPEN versus multi-scan CSI acquisitions (these data were collected on two different mice), and (ii) comparing the time evolutions of signals collected over 60 s using a train of low flip-angle SPEN MRSI repetitions versus signals collected using simple small flip-angle 1D NMR acquisition. These experiments were performed on a 7 T Varian preclinical scanner, using a dual-tuned 13C/1H volume coil for transmit and a 20 mm diameter surface coil for 13C receive (Rapid Biomedical). The FOV was 4 x 4cm2 and the slice thickness 1 cm. The SPEN parameters were: 20° flip angle, sw = 31 kHz, Tenc = 3 ms, Genc = 2.4 G/cm, Tacq = 41 ms, TE = 29…70 ms, 16 x 64 acquired points (effective resolution of 2.5 x 1.25 mm2), single scan acquisition of ≈70 ms, TR = 2.1 s; for an ≈ 60 sec time-progression Nrep = 30. The CSI parameters were 5° flip angles α, sw = 6 kHz, Tacq = 21 ms, Nfreq = 128, Npe1 x Npe2 − 32 x 32 (effective resolution of 1.25 x 1.25 mm2), TR = 30 ms and 31 seconds total duration for the 1D spectral/2D spatial scan. For the 1D spectral acquisition scan parameters were: 20° flip angle α, sw = 6 kHz, Tacq = 170 ms, Nfreq = 1024, TR = 2 s and Nrep = 30.
Experiments were performed on an EL-4 murine lymphoma model. EL-4 cells were grown to a density of ca. 5 x 107 cells/mL in RPMI 1640 medium supplemented with 10% (v/v) fetal-calf serum and 2 mM glutamine. C57/Blk6 female mice (10 weeks old) were injected subcutaneously with 100 µL of a suspension of 5 x 106 EL4 cells in the right flank and tumors were allowed to grow for 9 days (∼2 cm3 in volume). Prior to the imaging experiments, animals were anaesthetized by administration of a mixture containing O2 in medical air (25% / 75% v/v at 2 L/min) plus 3% isoflurane (Isoflo, Abbotts Laboratories Ltd) and subsequently 1-2% isoflurane in O2/medical air. They were then placed in a dual-tuned 13C/1H volume transmit coil, the 13C receive only surface coil was placed immediately over the tumor so that it detected signal that was principally from the latter. A cannula was inserted into the tail vein and its patency maintained through the use of heparin diluted in sterile saline (100 U/mL). 0.2 mL of hyperpolarized [1-13C]pyruvate solution (∼80 mM) were injected intravenously into the mouse. A maximum of three injections were given to each mouse. The above experiments were performed under the Animals (Scientific Procedures) Act of 1986 and were approved by local Cambridge University ethical review committees.
Pulse sequences and processing
For all SPEN experiments, radiofrequency pulses and gradient shapes were designed in Matlab® (The MathWorks Inc., Natick, MA) and uploaded onto the scanners. The reconstruction of SPEN and CSI images and spectra was performed in all instances using custom-written MATLAB packages, which included the possibility to process the SPEN-/k-space data with super-resolution along the spatiotemporal dimension and with FT along the k-dimension. Pre-SR data manipulations included minor realignments of positive and negative readout echoes and zero-filling to a 128 x 128 matrix size.
4. Results and Discussion
13C phantom experiments
While dissolution-DNP provides an unprecedented increase in NMR signal, applications to molecular imaging are still constrained by the available signal-to-noise ratio. Any increase in acquisition speed must then come with a well understood, and ideally minor, cost in SNR. In order to examine the SNR losses in single-shot SPEN MRSI, and to validate experimental SNR measurements with theoretical estimates, a 13C phantom with two distinct NMR peaks –13C-labeled urea and 13C1-acetate in separate tubes– was examined. Figures 2d – 2g show a comparison between chemical-shift images acquired in a single shot with a hybrid SPEN sequence, and reference images obtained with a multi-shot CSI sequence that used 2D phase encoding for the spatial dimensions. While other sequences provide shorter acquisition times [11, 12], CSI was selected here to provide a robust reference against which the SNR of single-shot SPEN could be compared. The SPEN images resolved for each of the 13C spectral peaks (Fig. 2), have good spectral definition vis-à-vis the CSI multi-scan counterparts. The SNR ratio between the two images produced by the different methods can be estimated from the scan parameters and compared to the measured SNR ratio, averaged over the acetate and urea peaks. Since the CSI raw data is three dimensional, including two spatial dimensions and a spectral one, the images of chemical sites were reconstructed and integrated over the relevant frequency range for each peak. In our example signals were summed for each peak over a range of ∼300 Hz (Nsum=27) around the peak maximum, to cover most of the resonance while avoiding signal contamination. Nsum and other relevant scan parameters from the SNR equations (2-4) for CSI and SPEN sequences were considered in the SNR estimation. The calculated SNRCSI/SNRSPEN for equal pixel size should be ∼13 for the selected scan parameters (which included larger pulse angles and many more scans in the CSI acquisitions), while the average SNRCSI/SNRSPEN measured per pixel was 9. These two values are comparable and within the errors expected from the various assumptions involved in the theoretical estimates. Possible sources for the small discrepancy between the calculated and measured SNRs could lie in our assumption that in CSI different frequency points contribute equally to the signal, or from the fact that longitudinal magnetization may not have been fully restored for the chosen TR. Notice that the CSI experiment took 4.3 hours to obtain a resolution of 2.5 x 2.5 mm2 (16 x 16 repetitions with a TR of 60s), while the SPEN experiment required only a single shot of 80 ms to obtain a resolution of 2.5 x 0.8 mm2 and 0.5 hour for 8 repetitions with a TR of 200 s. The time ratio between single-shot SPEN and CSI is thus of the order of 105, obtaining in this case an improvement in SNR per unit time of ∼104. Overall, these results confirm the successful implementation of the single-shot SPEN imaging and a good understanding of the relative sensitivities of the two sequences.
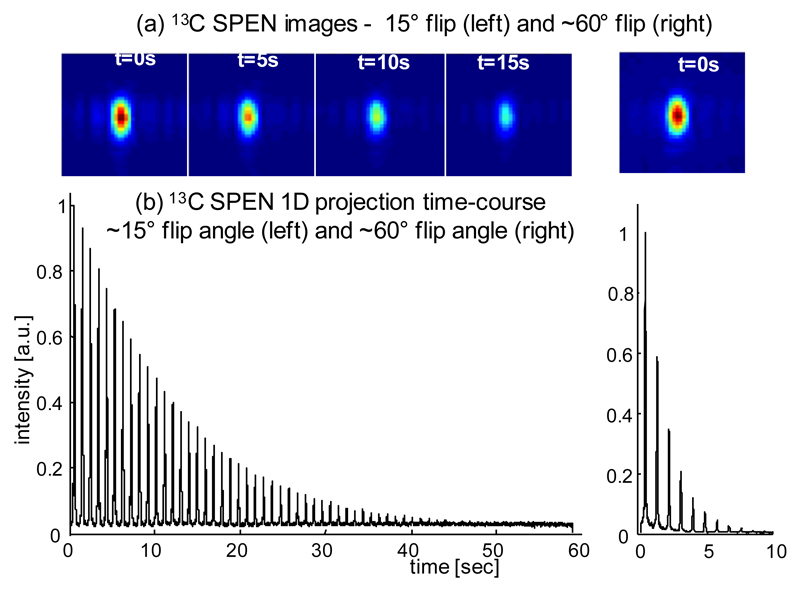
Figure 3 shows the results obtained immediately following the injection of hyperpolarized [1-13C]pyruvate into a tube, using a series of single-shot SPEN sequences. The figure shows the results obtained with two different excitation flip angles. The estimated T1 from the measured signal decay in both experiments was in the range of ∼40-60 s, which is as expected for such a compound.
Figure 3.
Measurements of signal decay following injection of hyperpolarized [1-13C]pyruvate into a tube using SPEN-based spectroscopic imaging. (a) Representative subset of pyruvate images acquired using the Hybrid SPEN sequence in Fig. 2a with flip angles α ∼ 15° (left) and ∼ 60° (right). (b) 1D profiles from the integrated images as a function of time, for the full train of repetitions.
Measurements with 13C hyperpolarized pyruvate in rats
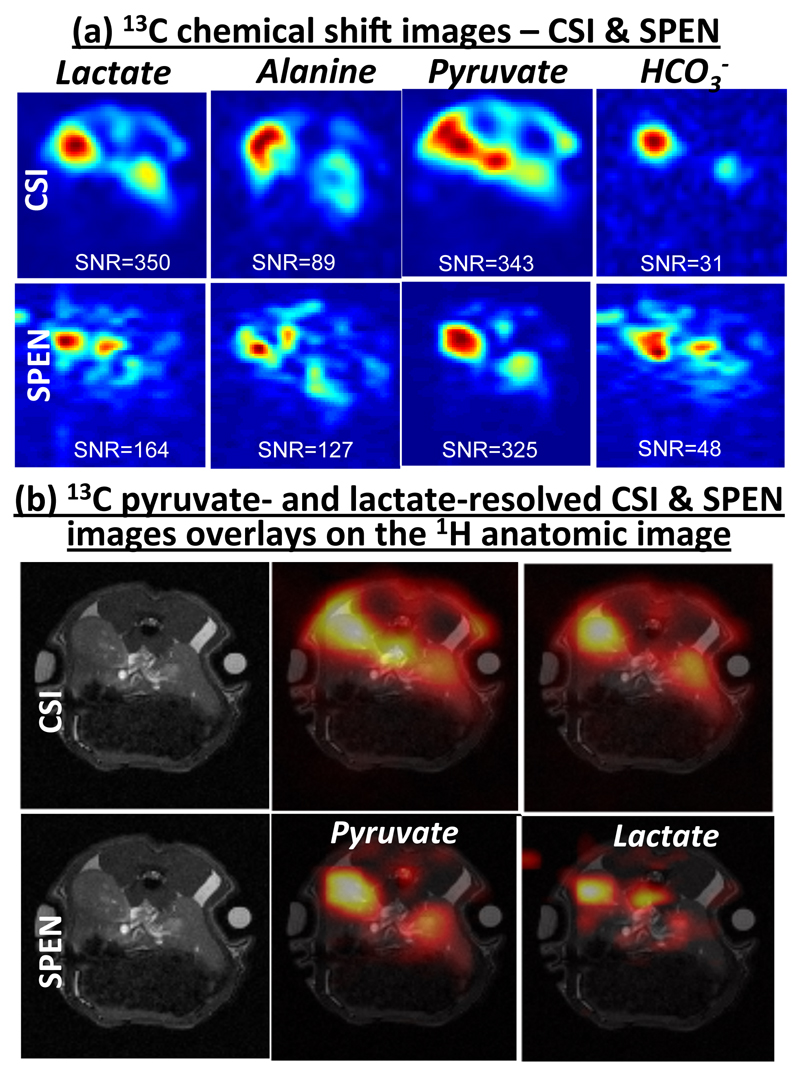
In vivo experiments were first performed on healthy rats. Figure 4 shows spectrally resolved images obtained for pyruvate and for its main metabolic products, lactate, alanine and bicarbonate. The two experiments compared here are single-shot SPEN-based spectroscopic imaging, and a reference multi-shot CSI sequence with a center-out sampling scheme. Overlays of the pyruvate and lactate images on the reference multi-scan 1H images are also shown. A comparison of the images obtained with SPEN and CSI shows more accurate signal localization for the single-shot technique. Interestingly, small but noticeable differences in signal can be observed between SPEN and CSI, especially for pyruvate. These differences could be due to the additional diffusion weighting of the SPEN sequence, which is based on a spin echo [33]. Such weighting can decrease significantly the signal from the vasculature, the suppression of which is usually desirable as it can interfere with the measurement of metabolism. This property of single-shot SPEN sequences could thus be useful for metabolic imaging. To further validate signal localization, three injections were performed consecutively on the same animal to acquire images with SPEN, CSI and then again with SPEN, with the two SPEN acquisitions showing comparable and consistent SNR and localization results (not shown).
Figure 4.
Representative metabolic images from a rat following injection of hyperpolarized [1-13C]pyruvate, comparing the results obtained with CSI and single-shot spectrally-resolved experiments. (a) 13C metabolic images obtained with CSI and SPEN for lactate, alanine, pyruvate and bicarbonate (the images are scaled independently - SNR values in the dominant peak regions of each image are displayed). (b) Pyruvate and lactate 13C images overlaid on their corresponding 1H anatomical images.
Sensitivity considerations are especially relevant for hyperpolarized experiments. The SNR ratio between CSI and SPEN pyruvate images was measured as SNRCSI/SNRSPEN∼0.95, while a calculation based on the scan parameters predicted a SNRCSI/SNRSPEN∼0.6 – note that the excitation flip angle was 90° for SPEN and 10° for CSI. The difference in the experimental and theoretical SNR ratio is reasonable, considering the variability involved in in vivo hyperpolarized injection experiments.
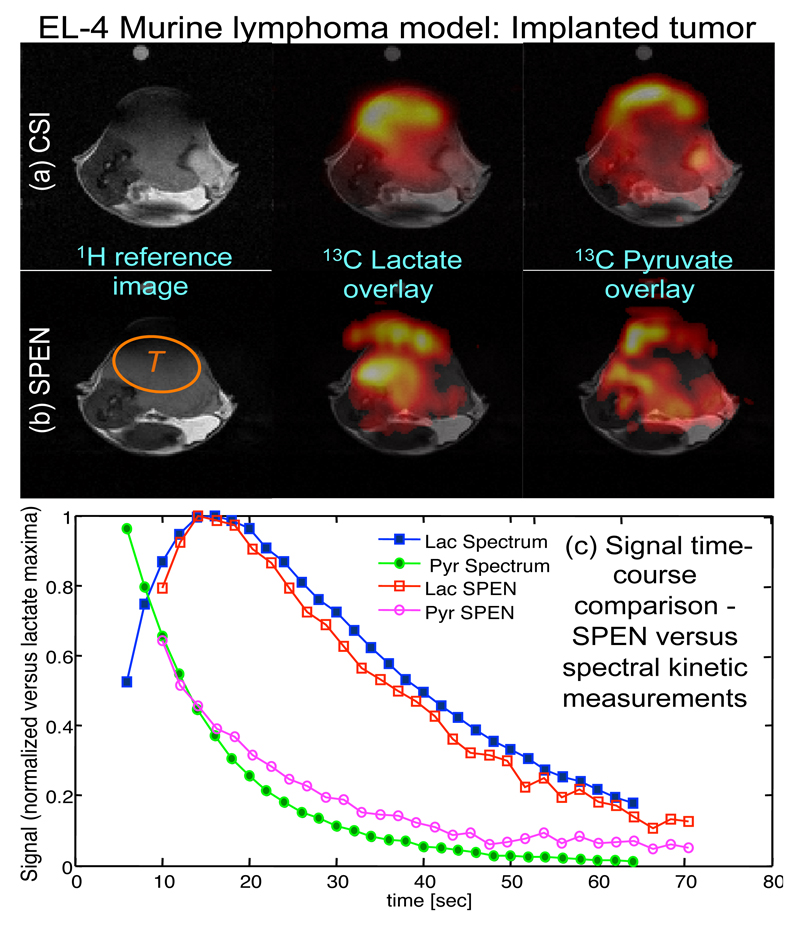
Since the measurement of metabolic rates using hyperpolarized [1-13C]pyruvate imaging could become an important diagnostic tool in tumor imaging, a set of such experiments was conducted. The performance of single-shot SPEN-based spectroscopic imaging was tested in such setting, using mice with implanted EL-4 lymphoma tumors. In these experiments, only pyruvate and lactate are present in significant concentrations; therefore only these two metabolites were analyzed. In a first stage we focused again on comparing the signal localization in the pyruvate and lactate images, spectrally-resolved by the single-shot hybrid SPEN sequence, versus images obtained with a CSI sequence (this comparison is shown in two different mice). The resulting images, displayed in Figures 5a and 5b, show a reasonable localization of the lactate and pyruvate signals with the SPEN-based sequence. In both cases, SPEN and CSI, the signal follows the tumor shape, as can be appreciated from the 1H proton reference images. An advantage afforded by single-shot MRSI sequences such as SPEN, is that they can be repeated numerous times after injection of a hyperpolarized substrate. The resulting data set can then be analyzed to obtain a time course for the metabolic conversion of the injected metabolite in pre-selected regions, thereby affording insight into the kinetics of utilization. Figure 5c shows such time course where a train of SPEN repetitions executed with a 20° flip angle, were used to obtain a time series of images for pyruvate and lactate; these signals were averaged over the major tumor region shown by the “T” in Fig. 5b. In this tumor a reference time course was also obtained with a series of low-flip-angle 1D spectral acquisitions, localized to a slice through the tumor. Figure 5c shows good agreement between the time courses obtained with SPEN and with 1D 13C spectral acquisitions. Overall, these results confirm that SPEN-based single-shot spectroscopic imaging methods can yield detailed spatial, spectral and temporal information, similar to that obtained from both multi-shot multidimensional spectroscopic imaging acquisitions and from 1D NMR spectroscopy. This offers new opportunities to investigate metabolic exchange rates in a spatially resolved manner.
Figure 5.
Representative 13C images acquired after injection of [1-13C]pyruvate in mice. (a, b) 1H anatomic images overlaid with 13C images of pyruvate and lactate obtained using CSI and SPEN. The region indicated by “T” marks the approximate tumor location. (c) Comparison of the pyruvate and lactate time courses obtained with SPEN-based spectroscopic imaging and with 1D experiments. The SPEN time-course was obtained by averaging over a region of interest where the signal was most intense (from “T”). A flip angle of 20° and a repetition time of 2 s were used in both experiments.
5. Conclusions
The first results obtained in vivo using single-shot SPEN for chemical-shift imaging in hyperpolarized 13C experiments have been demonstrated. This MRSI approach makes it possible to obtain, within 100 ms, 2D images for several compounds simultaneously. In healthy rats four metabolite peaks were observed; in mice with implanted tumors, a series of images could be obtained for pyruvate and lactate. Images obtained with single-shot SPEN were found to have comparable qualities to those obtained with a CSI sequence, despite the fact that the latter requires orders-of-magnitude longer signal acquisition durations due to the high number of excitation pulses. Although further optimizations could yield improved spectral and spatial resolution as well as added sensitivity, the current results already show that SPEN-based sequences can be utilized as a modality for fast dynamic imaging. Further developments are in progress to exploit additional benefits of spatiotemporal encoding, such as a built-in restricted FOV capability [24,26], multi-echo experiments and parallel imaging acquisition [34].
Highlights.
Novel spectroscopic imaging sequences for hyperpolarized studies are presented
These sequences use spatiotemporal-encoding and provide 3D MRSI data in ≤100 ms
Sequences are validated with 13C experiments on phantoms and in vivo
Acknowledgements
We are thankful to Sascha Gude, Piotr Dzien and Tiago Rodrigues for laboratory assistance. ERC Advanced Grant #246754, Marie Curie Action ITN Metaflux (project# 264780), DIP (project# 710907, Germany), The Danish Kidney Foundation, Helen and Ejnar Bjoernows Foundation and the COST Action TD-1103 EuroHyperPol grant, are gratefully acknowledged for financial support.
Abbreviations
- CSI
Chemical Shift Imaging
- EPI
Echo-Planar Imaging
- EPSI
Echo-Planar Spectroscopic Imaging
- d-DNP
Dissolution Dynamic Nuclear Polarization
- FOV
Field of View
- FT
Fourier Transform
- MRI
Magnetic Resonance Imaging
- NMR
Nuclear Magnetic Resonance
- PE
Phase Encode
- RF
Radio frequency
- RO
Read Out
- SAR
Specific Absorption Rate
- SPEN
SPatiotemporal ENcoding
- SR
Super resolution
- TE
Echo Time
References
- 1.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc Natl Acad Sci USA. 2003;100(18):10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golman K, Ardenkjaer-Larsen JH, Petersson JS, Mansson S, Leunbach I. Molecular imaging with endogenous substances. Proc Nat Acad Sci USA. 2003;100(18):10435–10439. doi: 10.1073/pnas.1733836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen AP, Albers MJ, Cunningham CH, Kohler SJ, Yen Y-F, Hurd RE, Tropp J, Bok R, Pauly JM, Nelson SJ, Kurhanewicz J, et al. Hyperpolarized C-13 spectroscopic imaging of the TRAMP mouse at 3T-initial experience. Magn Reson Med. 2007;58(6):1099–1106. doi: 10.1002/mrm.21256. [DOI] [PubMed] [Google Scholar]
- 4.Lau AZ, Chen AP, Ghugre NR, Ramanan V, Lam WW, Connelly KA, Wright GA, Cunningham CH. Rapid multislice imaging of hyperpolarized 13C pyruvate and bicarbonate in the heart. Magn Reson Med. 2010;64(5):1323–1331. doi: 10.1002/mrm.22525. [DOI] [PubMed] [Google Scholar]
- 5.Witney TH, Kettunen MI, Hu D-E, Gallagher FA, Bohndiek SE, Napolitano R, Brindle KM. Detecting treatment response in a model of human breast adenocarcinoma using hyperpolarized [1-13C]pyruvate and [1,4-13C2]fumarate. Br J Cancer. 2010;103(9):1400–1406. doi: 10.1038/sj.bjc.6605945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris T, Eliyahu G, Frydman L, Degani H. Kinetics of hyperpolarized 13 C1-pyruvate transport and metabolism in living human breast cancer cells. Proc Nat Acad Sci USA. 2009;106(43):18131–18136. doi: 10.1073/pnas.0909049106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Morze C, Larson PE, Hu S, Keshari K, Wilson DM, Ardenkjaer-Larsen J-H, Goga A, Bok R, Kurhanewicz J, Vigneron DB. Imaging of blood flow using hyperpolarized [(13)C]urea in preclinical cancer models. J Magn Reson Imaging. 2011;33(3):692–697. doi: 10.1002/jmri.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butt SA, Søgaard LV, Magnusson PO, Lauritzen MH, Laustsen C, Åkeson P, Ardenkjær-Larsen JH. Imaging cerebral 2-ketoisocaproate metabolism with hyperpolarized 13C Magnetic Resonance Spectroscopic Imaging. J Cereb Blood Flow Metab. 2012;32:1508–1514. doi: 10.1038/jcbfm.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher FA, Kettunen MI, Day SE, Hu DE, Ardenkjær-Larsen JH, ‘t Zandt R, Jensen PR, Karlsson M, Golman K, Lerche MH, Brindle KM. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labeled bicarbonate. Nature. 2008;453:940–943. doi: 10.1038/nature07017. [DOI] [PubMed] [Google Scholar]
- 10.Bohndiek SE, Kettunen MI, Hu D, Kennedy BWC, Boren J, Gallagher FA, Brindle KM. Hyperpolarized [1-13C]-ascorbic and dehydroascorbic acid: Vitamin C as a probe for imaging redox status in vivo. J Amer Chem Soc. 2011;133:11795–11801. doi: 10.1021/ja2045925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson PEZ, Bok R, Kerr AB, Lustig M, Hu S, Chen AP, Nelson SJ, Pauly JM, Kurhanewicz J, Vigneron DB. Investigation of tumor hyperpolarized [1-13C]-pyruvate dynamics using timeresolved multiband RF excitation echo-planar MRSI. Magn Reson Med. 2010;63(3):582–591. doi: 10.1002/mrm.22264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larson PE, Hu S, Lustig M, Kerr AB, Nelson SJ, Kurhanewicz J, Pauly JM, Vigneron DB. Fast dynamic 3D MR spectroscopic imaging with compressed sensing and multiband excitation pulses for hyperpolarized 13C studies. Magn Reson Med. 2011;65(3):610–9. doi: 10.1002/mrm.22650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayer D, Yen YF, Tropp J, Pfefferbaum A, Hurd RE, Spielman DM. Application of subsecond spiral chemical shift imaging to real-time multislice metabolic imaging of the rat in vivo after injection of hyperpolarized [1-13C]pyruvate. Magn Reson Med. 2009;62(3):557–564. doi: 10.1002/mrm.22041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu S, Lustig M, Balakrishnan A, Larson PE, Bok R, Kurhanewicz J, Nelson SJ, Goga A, Pauly JM, Vigneron DB. 3D compressed sensing for highly accelerated hyperpolarized (13)C MRSI with in vivo applications to transgenic mouse models of cancer. Magn Reson Med. 2010;63(2):312–321. doi: 10.1002/mrm.22233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin YS, Mayer D, Yen Y-F, Hurd RE, Spielman DM. Optimization of fast spiral chemical shift imaging using least squares reconstruction: Application for hyperpolarized 13C metabolic imaging. Magn Reson Med. 2007;58:245–252. doi: 10.1002/mrm.21327. [DOI] [PubMed] [Google Scholar]
- 16.Gordon JW, Niles DJ, Fain SB, Johnson KM. Joint spatial-spectral reconstruction and k-t spirals for accelerated 2D spatial/1D spectral imaging of 13C dynamics. Magn Reson Med. 2013 doi: 10.1002/mrm.24796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham CH, Vigneron DB, Chen AP, Xu D, Nelson SJ, Hurd RE, Kelley D, Pauly JM. Design of flyback echo-planar readout gradients for magnetic resonance spectroscopic imaging. Magn Reson Med. 2005;54:1286–1289. doi: 10.1002/mrm.20663. [DOI] [PubMed] [Google Scholar]
- 18.Reed GD, Larson PZ, von Morze C, Bok R, Lustig M, Kerr AB, Pauly JM, Kurhanewicz J, Vigneron DB. A method for simultaneous echo planar imaging of hyperpolarized 13C pyruvate and 13C lactate. J Magn Reson. 2012;217:41–47. doi: 10.1016/j.jmr.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frydman L, Scherf T, Lupulescu A. The acquisition of multidimensional nmr spectra within a single scan. Proceedings of the National Academy of Sciences. 2002;99(25):15858. doi: 10.1073/pnas.252644399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tal A, Frydman L. Single-scan multidimensional magnetic resonance. Prog Nucl Magn Reson Spectrosc. 2010;57:241–292. doi: 10.1016/j.pnmrs.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Kunz D. Use of frequency-modulated radiofrequency pulses in MR imaging experiments. Magn Reson Med. 1986;3:377–384. doi: 10.1002/mrm.1910030303. [DOI] [PubMed] [Google Scholar]
- 22.Pipe JG. Spatial encoding and reconstruction in MRI with quadratic phase profiles. Magn Reson Med. 1995;33:24–33. doi: 10.1002/mrm.1910330105. [DOI] [PubMed] [Google Scholar]
- 23.Shrot Y, Frydman L. Spatially-encoded NMR and the acquisition of 2D magnetic resonance images within a single scan. J Magn Reson. 2005;172:179–190. doi: 10.1016/j.jmr.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Chamberlain R, Park JY, Corum C, Yacoub E, Ugurbil K, Jack CR, Garwood M. RASER: a new ultrafast magnetic resonance imaging method. Magn Reson Med. 2007;58(4):794–799. doi: 10.1002/mrm.21396. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Eliezer N, Shrot Y, Frydman L. High-Definition Single-Scan 2D MRI in Inhomogeneous Fields Using Spatial Encoding Methods. Magn Reson Imag. 2010;28(1):77–86. doi: 10.1016/j.mri.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt R, Frydman L. New spatiotemporal approaches for fully refocused, multislice ultrafast 2D MRI. Magn Reson Med. 2013 doi: 10.1002/mrm.24714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tal A, Frydman L. Spectroscopic imaging from spatially-encoded single-scan multidimensional MRI data. J Magn Reson. 2007;189:46–58. doi: 10.1016/j.jmr.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Eliezer N, Irani M, Frydman L. Super-resolved spatially-encoded single-scan 2D MRI. Magn Reson Med. 2010;63(6):1594–1600. doi: 10.1002/mrm.22377. [DOI] [PubMed] [Google Scholar]
- 29.Cai C, Dong J, Cai S, Li J, Chen Y, Bao L, Chen Z. An efficient de-convolution reconstruction method for spatiotemporal-encoding single-scan 2D MRI. J Magn Reson. 2013;228:136–147. doi: 10.1016/j.jmr.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt R, Frydman L. In Vivo 3D Spatial / 1D Spectral Imaging by Spatiotemporal Encoding: A New Single-Shot Experimental and Processing Approach. Magn Reson Med. 2012 doi: 10.1002/mrm.24470. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Eliezer N, Frydman L. Spatiotemporal encoding as a robust basis for fast three-dimensional in vivo MRI. NMR Biomed. 2011;24:1191–1201. doi: 10.1002/nbm.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pohmann R, Kienlin M, Haase A. Theoretical Evaluation and Comparison of Fast Chemical Shift Imaging Methods. J Magn Reson. 1997;129:145–160. doi: 10.1006/jmre.1997.1245. [DOI] [PubMed] [Google Scholar]
- 33.Kettunen MI, Kennedy BWC, Hu D, Brindle KM. Spin echo measurements of the extravasation and tumor cell uptake of hyperpolarized [1-13C]Lactate and [1-13C]Pyruvate. Magn Reson Med. 2012 doi: 10.1002/mrm.24591. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt R, Baishya B, Ben-Eliezer N, Seginer A, Frydman L. Super-resolved Parallel MRI by Spatiotemporal Encoding. Magn Reson Imag. 2013 doi: 10.1016/j.mri.2013.07.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]