Brain plasticity, defined as the capability of cerebral neurons to change in response to experience, is fundamental for behavioral adaptability, learning, memory, functional development, and neural repair. The visual cortex is a widely used model for studying neuroplasticity and the underlying mechanisms. Plasticity is maximal in early development, within the so-called critical period, while its levels abruptly decline in adulthood [1]. Recent studies, however, have revealed a significant residual plastic potential of the adult visual cortex by showing that, in adult humans, short-term monocular deprivation alters ocular dominance by homeostatically boosting responses to the deprived eye [2–4]. In animal models, a reopening of critical period plasticity in the adult primary visual cortex has been obtained by a variety of environmental manipulations, such as dark exposure, or environmental enrichment, together with its critical component of enhanced physical exercise [5–8]. Among these non-invasive procedures, physical exercise emerges as particularly interesting for its potential of application to clinics, though there has been a lack of experimental evidence available that physical exercise actually promotes visual plasticity in humans. Here we report that short-term homeostatic plasticity of the adult human visual cortex induced by transient monocular deprivation is potently boosted by moderate levels of voluntary physical activity. These findings could have a bearing in orienting future research in the field of physical activity application to clinical research.
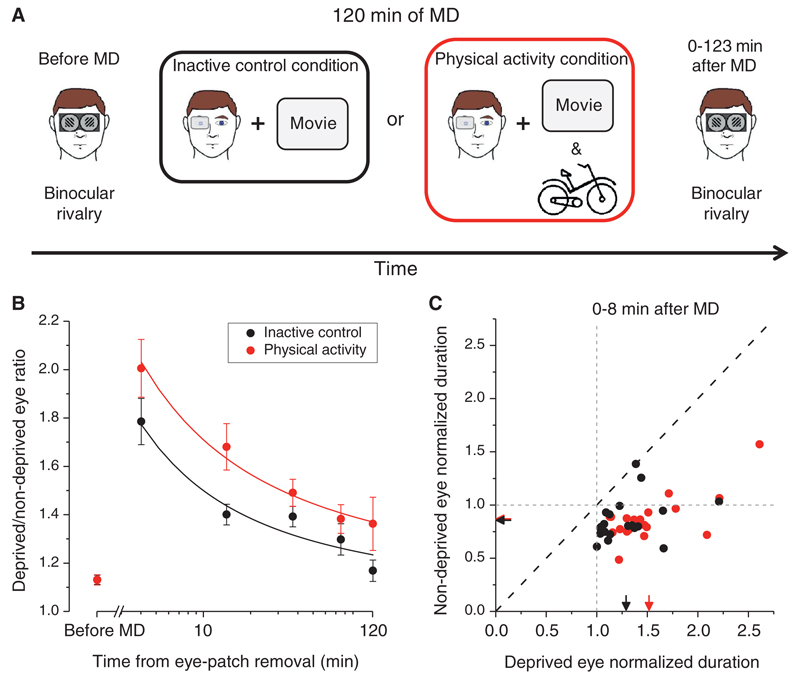
When incompatible images are simultaneously presented to the two eyes, the brain is faced with ambiguity resulting in perceptual bistability, in which conscious perception continuously oscillates between alternative monocular images. This phenomenon, called binocular rivalry [9], displays short-term plasticity in response to transient monocular deprivation [2–4]. We tested binocular rivalry in twenty adults before and after 120 minutes of monocular deprivation achieved by having subjects wear a translucent eye-patch over the dominant eye. Each subject performed the experiment two times in two different conditions (Figure 1): an inactive control condition, in which, during the deprivation period, the subject watched a movie while sitting on a chair; and a physical activity condition, in which, during the deprivation period, the subjects watched a movie while intermittently cycling on a stationary bicycle (10 minutes of exercise and 10 minutes of rest alternated for a total of 120 minutes). In order to keep the physical effort comparable across subjects, participants were required to maintain a heart rate of 120 beats per minute during the activity. At the end of the monocular deprivation period, binocular rivalry between orthogonal gratings was measured for 120 minutes at different time intervals (Figure 1A). In order to obtain an index of eye dominance, we computed the ratio between the mean phase duration (the average time of reported perceptual dominance of one of the rivalrous images) of the deprived and non-deprived eye for each time interval tested.
Figure 1. Schematic diagram of the experimental paradigm and results.
(A) Binocular rivalry between orthogonally oriented gratings was tested before monocular deprivation and served as a behavioral baseline index for ocular dominance. During 120 minutes of monocular deprivation subjects wore a translucent eyepatch over the dominant eye. Two experimental conditions were tested for each subject: an inactive control condition in which, during monocular deprivation, the subject watched a movie while sitting still, and a physical activity condition in which, while watching a movie, the subject intermittently cycled on a stationary bike. After the end of the monocular deprivation period, binocular rivalry was tested for 120 minutes in 3-minute blocks at different time intervals. (B) The ratio between the mean phase duration of the deprived and that of the non-deprived eye plotted as a function of time from removal of the eye-patch. The effect of monocular deprivation (increase in deprived eye dominance) was higher for the physical activity condition (red symbols) compared to the inactive control condition (black symbols). A power decay function of the form given by equation 1 in the Supplemental Experimental Procedures section well fitted to the decay of the effect (inactive control condition: R2 = 0.79; physical exercise condition: R2= 0.97). (C) Scatter plot showing the mean phase durations of the deprived and non-deprived eyes recorded during the first 8 minutes after monocular deprivation and normalized for each subject to their baseline mean phase duration (inactive control condition: black symbols; physical exercise condition: red symbols). The arrows on the x and y axes represent average mean phase durations. The dashed grey lines indicate the point of no difference in mean phase duration between pre- and post-deprivation measurements. Error bars show ± 1 s.e.m.
Consistent with previous reports [2,3], in the inactive control condition (Figure 1B, black symbols), the deprived eye strongly dominated visual rivalrous perception immediately after eye-patch removal (deprived/non-deprived eye mean phase ratio: 1.78 ± 0.1) and the effect of deprivation decayed over time following a power decay profile (that is, a power function well fitted to the data). In the physical activity condition (Figure 1B, red symbols), the effect of monocular deprivation appeared robustly enhanced compared to the inactive control condition: soon after eye-patch removal the deprived eye mean phase duration was twice as long as the non-deprived eye (deprived/non-deprived eye mean phase ratio: 2.00 ± 0.12), and the effect of deprivation remained higher than the inactive control condition, while following a similar power decay.
A two-way (condition x time) repeated measures ANOVA revealed a significant main effect of both the factor condition (F(1,19) = 9.58, p = 0.006) and time (F(5,19) = 47.9, p < 0.0001), but no significant interaction between the two (F(1,19) = 1.75, p = 0.2), indicating similar time-courses for both conditions (a bootstrap sign-test on the parameters of the power decay fit for the two conditions confirmed no significant difference between them; Table S1 in the Supplemental Information). Mean phase durations of the deprived and non-deprived eye measured during the first 8 minutes following monocular deprivation and normalized for each observer to the baseline durations are shown in Figure 1C: mean phase durations decreased for the non-deprived eye and increased for the deprived eye in both conditions (see Figure S1 for normalized phase-duration distributions and Table S2 for raw phase durations). A two-way (condition x time) repeated measures ANOVA on the deprived eye normalized mean phase durations revealed a main effect of both the factor condition (F(1,19) = 5.38, p = 0.03) and time (F(4,19) = 7.98, p < 0.001). A two-way (condition x time) repeated measures ANOVA on the non-deprived eye normalized mean phase duration revealed a main effect of the factor time (F(4,19) = 15.65, p < 0.0001) but no effect of the factor condition (F(1,19) = 0.02, p = 0.88). This indicates that the enhanced effect of deprivation on binocular rivalry induced by physical activity was mainly attributable to an increase in the deprived eye phase durations compared to the inactive control condition.
The results presented here demonstrate that physical activity enhances short-term plasticity of the adult visual cortex by boosting the homeostatic response of the visual system to transient monocular deprivation. This homeostatic response could represent the initial adaptive reaction of the visual system to visual deprivation, potentially evolving into long-term plasticity with prolonged deprivation times. In animal models, physical exercise promotes visual cortical plasticity by altering the excitation/inhibition balance in the primary visual cortex, either by decreasing the release of GABA [5], or by inhibiting the activity of somatostatin GABAergic interneurons via activation of vasoactive positive (VIP+) cells in the primary visual cortex [7]. Strikingly, recent evidence has shown that, in humans, short-term homeostatic plasticity induced by transient monocular deprivation is also mediated by a change in GABAergic inhibition [4]. Thus, we propose a model in which the enhancement of short-term visual cortical plasticity induced by physical activity is likely mediated by a change in the primary visual cortex excitation/inhibition balance.
Enhancing neuroplasticity in adult subjects is fundamental for the treatment of amblyopia, a major developmental visual disorder caused by abnormal visual experience during early development [10]. Successful therapies are currently not available for adult amblyopic patients, due to the dramatic decline in adult visual cortex plasticity. By providing the first evidence that physical activity enhances short-term adult visual cortical plasticity in humans, our results suggest a possible application of physical exercise therapies, in combination with eye occlusion, to visual function recovery in adult amblyopic subjects. More generally, enhancing neuroplasticity through motor activity might emerge as a suitable strategy to increase neuroplasticity under a number of different conditions, with application to recovery after brain injury and prevention of pathological brain aging.
Supplemental Information
Supplemental Information includes experimental procedures, one figure and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2015.10.026
Acknowledgements
This research has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FPT/2007-2013), under grant agreement n.338866, ECSPLAIN. We are thankful to Maria Concetta Morrone, David C. Burr and Paola Binda for helpful comments throughout the research and to Luca Lo Verde for help during the data collection.
References
- 1.Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30:14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lunghi C, Burr DC, Morrone C. Brief periods of monocular deprivation disrupt ocular balance in human adult visual cortex. Curr Biol. 2011;21:R538–R539. doi: 10.1016/j.cub.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Lunghi C, Burr DC, Morrone MC. Long-term effects of monocular deprivation revealed with binocular rivalry gratings modulated in luminance and in color. J Vis. 2013;13:1. doi: 10.1167/13.6.1. [DOI] [PubMed] [Google Scholar]
- 4.Lunghi C, Emir UE, Morrone MC, Bridge H. Short-term monocular deprivation alters GABA in the adult human visual cortex. Curr Biol. 2015;25:1496–1501. doi: 10.1016/j.cub.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baroncelli L, Bonaccorsi J, Milanese M, Bonifacino T, Giribaldi F, Manno I, Cenni MC, Berardi N, Bonanno G, Maffei L, et al. Enriched experience and recovery from amblyopia in adult rats: impact of motor, social and sensory components. Neuropharmacology. 2012;62:2388–2397. doi: 10.1016/j.neuropharm.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 6.He HY, Ray B, Dennis K, Quinlan EM. Experience-dependent recovery of vision following chronic deprivation amblyopia. Nat Neurosci. 2007;10:1134–1136. doi: 10.1038/nn1965. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko M, Stryker MP. Sensory experience during locomotion promotes recovery of function in adult visual cortex. eLife. 2014;3:e02798. doi: 10.7554/eLife.02798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sale A, Maya Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R, Maffei L. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10:679–681. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- 9.Blake R, Logothetis NK. Visual competition. Nat Rev Neurosci. 2002;3:13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- 10.Wu C, Hunter DG. Amblyopia: diagnostic and therapeutic options. Am J Ophthalmol. 2006;141:175–184. doi: 10.1016/j.ajo.2005.07.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.