Abstract
Failed arthroscopic soft-tissue stabilization and anterior glenoid bone loss have been shown to have high failure rates after standard arthroscopic stabilization techniques. For patients with recurrent glenohumeral instability, the Bristow-Latarjet procedure is currently the standard of care. It is predominantly performed through an open deltopectoral approach but has recently been described arthroscopically. Although providing excellent clinical outcomes, the Bristow-Latarjet procedure violates the subscapularis muscle, has a steep learning curve with a high complication rate, and permanently changes the anterior shoulder anatomy, making any future revision surgery more challenging. We describe a technique for arthroscopic anterior glenoid augmentation using iliac crest bone graft that does not violate the subscapularis, by creating a far anterior-medial portal that traverses superior to the subscapularis and lateral to the conjoint tendon. The graft is passed through this portal and secured with rigid fixation. An arthroscopic Bankart capsulolabral repair is then performed, making the graft extra-articular. A remplissage can easily be added as indicated, allowing this procedure to arthroscopically address all 3 major components of structural instability: glenoid bone loss, capsulolabral tearing, and humeral bone loss.
Traumatic shoulder instability is typically associated with soft-tissue disruption in the form of a Bankart lesion with or without capsular involvement. Arthroscopic or open repair in this setting has shown very high success rates.1 Recently, interest has increased in patients with significant bone loss and failed soft-tissue Bankart repairs or patients with high-risk shoulders for recurrent dislocation.2, 3 In this setting bony augmentation to the anterior glenoid has become the treatment of choice. The evolution of anterior glenoid bony procedures has gone from the classic nonanatomic Bristow procedure to the more robust Latarjet procedure. Other authors have recommended a more anatomic reconstruction of the glenoid with distal tibial allograft, glenoid allograft, or tricortical iliac crest bone graft in an open manner.4, 5 The trend for minimally invasive surgery has followed, with many of these procedures being performed in an arthroscopic manner. Most recently, Lafosse and colleagues6 published their 5-year results of the arthroscopic Latarjet procedure. They reported good Western Ontario Shoulder Instability scores and a low rate of recurrent instability. Despite these encouraging findings, the procedure is technically challenging, placing vital neurovascular structures at risk. To date, the arthroscopic Bristow-Latarjet procedure has remained popular in the hands of only a few surgeons globally. Authors have also become concerned over the high rate of complications associated with the nonanatomic open Latarjet procedure. These include loss of motion, hardware failure, development of arthrosis, and resorption or nonunion of the bone graft. In addition, significant scarring associated with any form of violation of the subscapularis can lead to functional deficits of the subscapularis, making revision surgery quite challenging.7 For these reasons, we began to investigate an all-arthroscopic, subscapularis-sparing, anatomic anterior glenoid bony stabilization procedure with arthroscopic Bankart repair.
Surgical Technique
Positioning and Portal Establishment
The patient can be positioned in the lateral decubitus or beach-chair position. However, the lateral position allows better access to the anterior glenoid and posterior labrum. In the lateral position, a balanced suspension–traction system (Arthrex positioner and STAR sleeve; Arthrex, Naples, FL) or an articulated arm positioner can be used. The upper extremity is placed in the standard position of 70° of abduction and 15° of forward flexion. Draping must include the anterior skin medial to the nipple. A posterolateral (PL) viewing portal is established, slightly medial to the standard position, in an effort to be parallel to the glenoid surface. This position will aid in passage of the guide rod for the inside-out creation of the far anterior-medial portal (transpectoral portal). Next, the anterior midglenoid portal is created in an outside-in fashion with localization of a spinal needle ascertaining a good angle and access to the anteroinferior glenoid. The anterosuperior portal is created in an outside-in fashion through the rotator interval, staying posterior to the long head of the biceps and just anterior to the leading edge of the supraspinatus. The surgeon performs a complete diagnostic examination, being sure to evaluate for the presence of a Hill-Sachs lesion that may require treatment. If indicated, a standard remplissage technique can be used, at this time, through the posterior portal. The suture tails are left free outside the posterior cannula and tied at the end of the case.
Anterior Glenoid Preparation
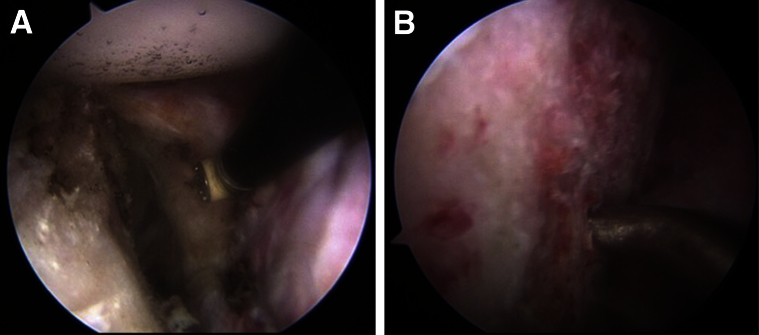
The camera is switched to the anterosuperior portal for viewing of the anterior glenoid defect. The size of the defect is estimated based on intraoperative measurement, computed tomography scanning, and/or magnetic resonance imaging scanning. After the anterior soft-tissue or bony defect is defined, the remaining anterior-superior labrum is transected at approximately the 1:30 clock-face position (right shoulder) to allow for exposure of the anterior glenoid neck and passage of the graft. The transected labrum is repaired at the end of the case with the remaining capsulolabral tissue. By use of a combination of blunt dissection, radiofrequency ablation, and mechanical shaving, the anterior capsulolabral tissue is peeled back off the anterior glenoid neck to expose the entire surface for placement of the bone graft (Fig 1A). The anterior glenoid neck is then prepared by light decortication, and bone marrow channels are created with a microfracture awl (Fig 1B). It is important to create a flat anterior glenoid neck surface that will accept and fit, in a flush manner, with the graft. Any additional anterior glenoid bone that is free in the soft tissue can be resected or incorporated into the soft-tissue repair at the end of the case depending on surgeon preference.
Fig 1.
View of left shoulder through anterior-superior portal in lateral decubitus position. (A) Radiofrequency device peeling back the capsulolabral tissue off the anterior glenoid neck. (B) Microfracture awl creating bone marrow channels in the anterior glenoid neck.
Attention is then turned to the preparation of the rotator cuff interval tissue. A complete resection of the interval tissue is necessary to adequately expose the coracoid, the conjoint tendon, and the upper border of the subscapularis. Resection of this tissue and complete exposure of the aforementioned structures are necessary to allow creation of the transpectoral portal and to enable passage of the graft. Occasionally, a small portion of the conjoint tendon or coracoacromial ligament may need to be released from the coracoid tip to allow for appropriate passage of the graft.
Far Anterior-Medial (Transpectoral) Portal Creation
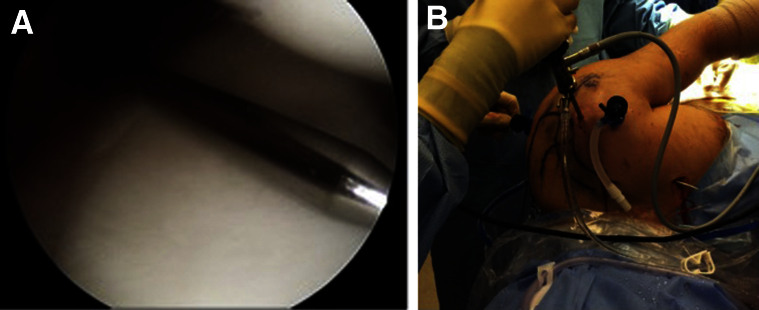
The far anterior-medial portal (transpectoral portal) is now created. This is accomplished in a safe inside-out manner by bringing a stiff Wissinger rod through the PL portal, parallel to the face of the glenoid (Fig 2A). The rod is then passed superior to the subscapularis and lateral to the conjoint tendon, with care taken to stay parallel to the glenoid face. It is often helpful to adduct the arm, medializing the conjoint tendon, if passage of the rod lateral to the conjoint tendon is difficult. The rod is then bluntly passed through the pectoralis muscle to exit the skin anteriorly about a handbreadth superior to the nipple (Fig 2B). By use of this technique, the axillary and musculocutaneous nerves, as well as the brachial vessels, are well protected. A vertical incision that is large enough to accommodate entrance of the graft (typically about 2 cm) is then created. Two hip arthroscopy half pipes (or a long Killian nasal dilator) are used to bluntly dilate through the pectoralis muscle to allow access to the joint. A proprietary dilator (DePuy Mitek, Raynham, MA) is then passed in and out to ensure ease of access.
Fig 2.
View of left shoulder through anterior-superior portal in lateral decubitus position. (A) Creation of the transpectoral portal in an inside-out manner with a stiff Wissinger rod being passed parallel to the glenoid through the posterolateral portal. (B) Stiff Wissinger rod exiting the skin after creation of the transpectoral portal.
Graft Preparation and Insertion
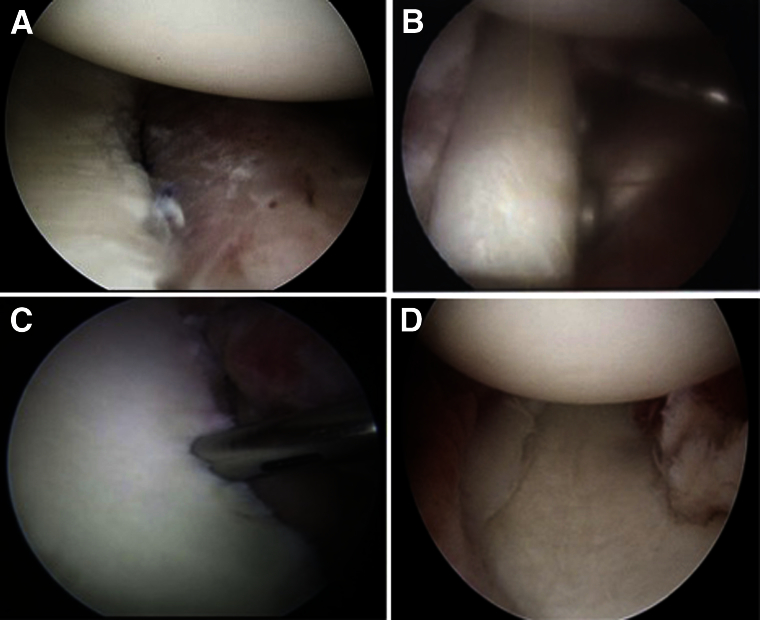
Attention is now turned to graft preparation. After confirmation of the bony defect, the tricortical iliac crest bone graft can be prepared or harvested in the standard manner. Allograft iliac crest bone block may also be used at the discretion of the surgeon, but our current choice is an ipsilateral autologous graft. On the back table, the tricortical graft is positioned such that the cancellous portion of the graft will rest against the anterior glenoid neck. Either the inner or the outer table of the graft can be placed laterally, depending on which side has the more anatomic concavity contour. The size of the block is typically no larger than 1 cm in the anterior-to-posterior dimension. It must be large enough to fully reconstitute the 20% or more of lost bone (typically 5 to 8 mm) and still allow for some resorption of the graft over time. The length of the graft is approximately 2 to 2.5 cm, based on the size and length of the defect. A double-cannula inserter handle system provided in the DePuy Mitek Bristow-Latarjet Instability Shoulder System allows for rigid fixation of the inserter handle to the graft. The graft, through the inserter handle, is then brought through the transpectoral portal into the joint and positioned along the anterior glenoid neck. During insertion of the graft, we have found it helpful to shift the humeral head anteriorly, thus putting slack on the anterior capsule, which will assist with positioning the graft on the anterior glenoid neck. The humeral head is then placed back into a reduced position to allow visualization of the graft-glenoid interface. It is important to critically analyze the position of the graft on the anterior glenoid neck. The graft should fit flush with the prepared anterior glenoid neck and sit 1 to 2 mm recessed off the glenoid surface cartilage (Fig 3A). The superior-inferior positioning is also assessed to allow for optimal stabilization effects with the goal of 25% to 50% of the graft being positioned above the glenoid equator.8 Once the graft is appropriately positioned, 2 guidewires are drilled anterior to posterior, exiting the skin of the posterior shoulder. These guidewires should exit parallel to each other and about 1 cm medial to the PL portal. The guidewires are then over-drilled, the depth measured, and the appropriate sized compression screw placed (Bristow-Latarjet Instability Shoulder System) (Fig 3B).
Fig 3.
View of left shoulder through anterior-superior portal in lateral decubitus position. (A) Positioning of the iliac crest autograft 1 to 2 mm recessed below the plane of the anterior glenoid. (B) Rigid fixation with the cannulated screw system (Bristow-Latarjet Instability Shoulder System). (C) Standard arthroscopic Bankart repair. (D) Final inspection shows a well-centered humeral head with anterior capsulolabral repair making the graft extra-articular.
Capsular and Labral Management
Once the graft has been fixed, a standard Bankart-type repair can still be performed in most cases (Fig 3C). The capsulolabral tissue, which had been mobilized and released earlier, is repaired back to the native glenoid using small soft suture anchors and standard labral repair technique. Care is taken to avoid the screws with the suture anchors. This reconstruction places the graft extra-articular while repairing any soft tissue that is still available to provide stability (Fig 1C). Any remplissage sutures are tied down at this time, and the shoulder is taken through a range of motion to ensure a stable humeral head. The shoulder and wounds are irrigated and a standard closure is performed, followed by application of a sterile dressing and placement of a sling with an abduction pillow (Video 1, Table 1).
Table 1.
Pearls and Pitfalls
| Pearls | Pitfalls |
|---|---|
| The lateral decubitus position allows improved visualization of the glenoid and improved access to the ipsilateral ICBG harvest site. | Decreased visualization will lead to difficulty in glenoid preparation and bone block application of the anterior glenoid and posterior labrum. |
| The PL portal is created parallel to the glenoid. | A PL portal that is not parallel to the glenoid will not allow appropriate creation of the far anterior-medial (transpectoral) portal and will lead to difficult graft positioning. |
| A bleeding bed is created on the anterior glenoid neck for optimal bone-to-bone fixation and healing. | Inadequate anterior glenoid preparation may lead to poor healing. |
| Dissection of the rotator interval is performed to expose key structures. | Inadequate dissection of the coracoid, conjoint tendon, and borders of the subscapularis will lead to difficulty in graft passage and increased risk to the musculocutaneous and axillary nerves. |
| The bone block is recessed by 1-2 mm from the glenoid face. | A proud bone block may lead to abnormal contact forces on the humeral head. |
| Revision Bankart repair is performed to create an extra-articular graft. | An intra-articular graft may lead to deleterious contact of the humeral head on the non-chondral surface of the ICBG. |
ICBG, iliac crest bone graft; PL, posterolateral.
Discussion
Recent evidence has questioned the efficacy of soft-tissue Bankart procedures for young male patients, contact athletes, patients with a history of multiple dislocations, and patients with failed soft-tissue procedures.2 In addition, a bony deficiency of the glenoid has been shown to lead to high failure rates with soft-tissue repair alone.2 Itoi et al.9 have suggested a threshold of 20% to 25% loss as an indication for a bony reconstructive procedure. Burkhart and colleagues10 have gone on to popularize the concept of the inverted pear–shaped glenoid as a risk factor for failed soft-tissue Bankart procedures. More recent work by Shaha et al.11 has questioned the 20% to 25% threshold. In their population of high-level patients, they went on to show that bone loss above 13.5% led to clinically unacceptable outcomes after arthroscopic soft-tissue reconstruction.
Given the improved understanding of instability and failure rates, bony procedures (Bristow-Latarjet) are on the rise. Although the results published by master surgeons are encouraging, there is concern over the potential complications associated with these nonanatomic procedures.6, 7 Another consideration associated with the Bristow-Latarjet procedure is violation of the subscapularis and subsequent anatomic distortion. Whether one performs a split or partial takedown of the subscapularis, for placement of the graft, there is potential for subscapularis dysfunction, axillary nerve injury, and distortion of normal anatomy that will make any revision surgery more challenging. In addition, it can be technically difficult to accurately place the bone block and repair the torn capsule as part of the open Bristow-Latarjet procedure, steps which can be easier with arthroscopic assistance.
Other free grafts have been described for glenoid reconstruction, namely the distal clavicle and fresh-frozen distal tibia.12, 13 Although the distal clavicle graft provides autologous cartilage, is readily available, and is cost-effective, its contour is not anatomic to the glenoid and can make for a difficult well-contoured fit. In addition, the cartilage may have chondral damage of its own that would significantly compromise the benefits of the chondral surface. Use of a fresh-frozen distal tibial allograft allows the benefits of a near anatomic chondral surface, but the long-term viability of this surface is not known. Moreover, this graft is expensive, may not be readily available, and has the inherent risks of graft rejection and infection.12, 13
We believe that an all-arthroscopic anterior glenoid bony augmentation procedure provides a promising and versatile option for those patients requiring a bony procedure in a minimally invasive anatomic manner. This procedure provides a safe, precise augmentation for glenoid bone loss while allowing for additional remplissage and arthroscopic Bankart repairs to be performed. This arthroscopic technique is challenging, and although we would not recommend this procedure to surgeons without advanced arthroscopic experience, it uses many of the techniques already familiar to arthroscopists. The far anterior-medial (transpectoral) portal can be created systematically to avoid both the axillary and musculocutaneous nerves, and there is no need for dissection around the brachial plexus. Anatomic graft placement is facilitated by arthroscopic visualization and fixed with the time-honored rigid screw technique.
As with any successful surgery, each successive step requires planning and precise application to limit risks and avoid pitfalls. In this technique it is paramount to create the posterior portal parallel to the glenoid surface. If the posterior portal is not parallel, it can be difficult to pass a switching stick, from posterior to anterior, to create the far anterior-medial (transpectoral) portal. The trajectory of these portals is crucial to allow parallel access of the graft to the anterior glenoid. Anteriorly, if the upper subscapularis, coracoid, and lateral conjoint tendon cannot be clearly dissected and safely retracted, graft placement will be difficult and may pose increased risk to the musculocutaneous nerve medial to the conjoint tendon and axillary nerve inferior to the subscapularis (Table 2).
Table 2.
Advantages, Risks, and Limitations
| Operation | Advantages | Risks | Limitations |
|---|---|---|---|
| Arthroscopic autologous ICBG to anterior glenoid with rigid fixation and no compromise of subscapularis | Arthroscopic anatomic bony procedure for anterior glenoid bone loss with readily available bone graft and no compromise of subscapularis | Increased risk to musculocutaneous and axillary nerves posed by inadequate dissection of rotator interval Potential for ICBG harvest-site complications |
Technically demanding |
ICBG, iliac crest bone graft.
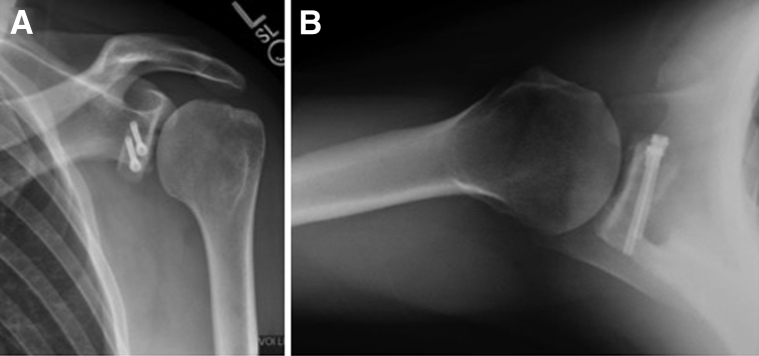
The early results are encouraging, but further research is demanded to evaluate the biomechanical strength, long-term outcomes, union rates, and degree of any bone resorption that may occur over time (Fig 4). The “sling effect” of a coracoid transfer is not available in the described procedure, but this procedure does allow for precise full restoration of the glenoid track, an anatomic intra-articular labral repair, remplissage, and other concomitant intra-articular procedures while sparing violation of the subscapularis and minimizing neurovascular risk.14, 15 Our hypothesis moving forward is that the benefits gained from this all-arthroscopic procedure will prove it to be a valuable addition to stabilization surgery.
Fig 4.
Postoperative radiographs of left shoulder. (A) Grashey view showing anatomic position of graft. (B) Axillary view showing anatomic position of bone graft.
Footnotes
The authors report the following potential conflict of interest or source of funding: J.P.B. receives support from ConMed.
Supplementary Data
Arthroscopic anterior glenoid augmentation with iliac crest bone graft, shown in a left shoulder in the lateral position. Standard posterolateral, anterior midglenoid, and anterosuperior working and viewing portals are created, and a far anterior-medial (transpectoral) portal is used for graft insertion.
References
- 1.Chalmers P.N., Mascarenhas R., Leroux T. Do arthroscopic and open stabilization techniques restore equivalent stability to the shoulder in the setting of anterior glenohumeral instability? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31:355–363. doi: 10.1016/j.arthro.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Porcellini G., Campi F., Pegreffi F., Castagna A., Paladini P. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537–2542. doi: 10.2106/JBJS.H.01126. [DOI] [PubMed] [Google Scholar]
- 3.Burkhart S.S., De Beer J.F. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: Significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16:677–694. doi: 10.1053/jars.2000.17715. [DOI] [PubMed] [Google Scholar]
- 4.Provencher M.T., Ghodadra N., LeClere L., Solomon D.J., Romeo A.A. Anatomic osteochondral glenoid reconstruction for recurrent glenohumeral instability with glenoid deficiency using a distal tibia allograft. Arthroscopy. 2009;25:446–452. doi: 10.1016/j.arthro.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Skendzel J.G., Sekiya J.K. Arthroscopic glenoid osteochondral allograft reconstruction without subscapularis takedown: Technique and literature review. Arthroscopy. 2011;27:129–135. doi: 10.1016/j.arthro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Dumont G.D., Fogerty S., Rosso C., Lafosse L. The arthroscopic Latarjet procedure for anterior shoulder instability: 5-Year minimum follow-up. Am J Sports Med. 2014;42:2560–2566. doi: 10.1177/0363546514544682. [DOI] [PubMed] [Google Scholar]
- 7.Warner J.P., Gill T.J., O'Hollerhan J.D., Pathare N., Millett P.J. Anatomical glenoid reconstruction for recurrent anterior glenohumeral instability with glenoid deficiency using an autogenous tricortical iliac crest bone graft. Am J Sports Med. 2006;34:205–212. doi: 10.1177/0363546505281798. [DOI] [PubMed] [Google Scholar]
- 8.Willemot L.B., Eby S.F., Thoreson A.R. Iliac bone grafting of the intact glenoid improves shoulder stability with optimal graft positioning. J Shoulder Elbow Surg. 2015;24:533–540. doi: 10.1016/j.jse.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itoi E., Lee S.B., Berglund L.J., Berge L.L., An K.N. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: A cadaveric study. J Bone Joint Surg Am. 2000;82:35–46. doi: 10.2106/00004623-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Lo I.K.Y., Parten P.M., Burkhart S.S. The inverted pear glenoid: An indicator of significant glenoid bone loss. Arthroscopy. 2004;20:169–174. doi: 10.1016/j.arthro.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 11.Shaha J.S., Cook J.B., Song D.J. Redefining “critical” bone loss in shoulder instability functional outcomes worsen with “subcritical” bone loss. Am J Sports Med. 2015;43:1719–1725. doi: 10.1177/0363546515578250. [DOI] [PubMed] [Google Scholar]
- 12.Tokish J.M., Fitzpatrick K., Cook J.B., Mallon W.J. Arthroscopic distal clavicular autograft for treating shoulder instability with glenoid bone loss. Arthrosc Tech. 2014;3:e475–e481. doi: 10.1016/j.eats.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Provencher M.T., Bhatia S., Ghodadra N.S. Recurrent shoulder instability: Current concepts for evaluation and management of glenoid bone loss. J Bone Joint Surg Am. 2010;92(suppl 2):133–151. doi: 10.2106/JBJS.J.00906. [DOI] [PubMed] [Google Scholar]
- 14.Shah A.A., Butler R.B., Romanowski J., Goel D., Karadagli D., Warner J.J. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495–501. doi: 10.2106/JBJS.J.01830. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto N., Muraki T., An K.N. The stabilizing mechanism of the Latarjet procedure: A cadaveric study. J Bone Joint Surg Am. 2013;95:1390–1397. doi: 10.2106/JBJS.L.00777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Arthroscopic anterior glenoid augmentation with iliac crest bone graft, shown in a left shoulder in the lateral position. Standard posterolateral, anterior midglenoid, and anterosuperior working and viewing portals are created, and a far anterior-medial (transpectoral) portal is used for graft insertion.