Abstract
Sulfur mustard (SM, bis- (2-chlororethyl) sulfide) is a highly reactive, potent warfare agent that has currently reemerged as a major threat to military and civilians. Exposure to SM is often fatal primarily due to pulmonary injuries and complications caused by its inhalation. Profound inflammation, hypercoagulation and oxidative stress are the hallmarks that define SM-induced pulmonary toxicities. Despite advances, effective therapies are still limited. This current review focuses on inflammatory and coagulation pathways that influence the airway pathophysiology of SM poisoning and highlights the complexity of developing an effective therapeutic target.
Keywords: sulfur mustard, pulmonary, coagulation, inflammation, extracellular RNA
Introduction
Sulfur mustard (SM, bis- (2-chlororethyl) sulfide) is a highly reactive, potent warfare agent that has currently reemerged as a major threat to military and civilians. Initial exposures of SM are not obvious due to lack of particular odor, however atmospheric accumulations of higher concentrations smell like mustard. Victims of SM exposure have a diverse variety of symptoms depending on the dose, duration and environment of SM exposure. SM-induced injuries are difficult to treat and cause many long-term complications. Skin, eye and lung are the immediate targets of SM where it causes quick irreversible reactions within the tissues. Profound injury to upper and lower conducting airways causes significant mortality at higher doses, and survivors of high dose exposure and those exposed to lower doses exhibit a host of clinical manifestations that include acute respiratory distress syndrome (ARDS). This review focuses on inflammatory and coagulation pathways that influence the airway pathophysiology of SM poisoning.
Sulfur mustard toxicity
Biochemical consequences
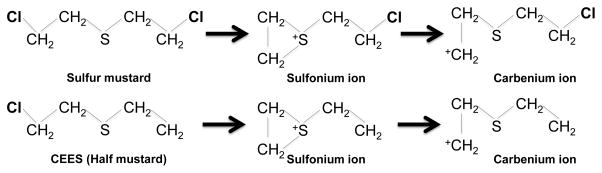
The chemical reactions caused by sulfur mustard and its analogs are fairly well characterized. Some of the mechanisms by which it causes injury are known. Despite this, effective therapies are still lacking and there is a need to better understand its pathogenesis. The actions of SM are mainly due to its two functional chlorine groups that can form highly reactive sulfonium and carbenium ions 1 (Figure 1). These ions are potent alkylating molecules that react with nucleotides, proteins and lipids. Due to its strong alkylating properties, primarily due to activity at guanine residues, SM forms DNA adducts and crosslinks 2, 3. SM is partially absorbed from the skin into the dermis where it can form DNA adducts or enter systemically and react with other organs including the lung 2, 4. SM-mediated DNA alkylation and crosslinking may cause DNA damage and activation of DNA reparative enzymes 5, 6, and may ultimately result in extensive cell death and lead to malignant transformations 7. The maximum toxicity of SM is primarily due to its modulation of DNA that specifically kills proliferative cells even at low doses. Further, SM is also highly reactive with sulfhydryl-containing compounds. Therefore, glutathione is depleted after SM exposure leading to intracellular accumulation of reactive oxygen species (ROS) 8. Antioxidant enzymes such as superoxide dismutase are also decreased after SM inhalation leading to increased reactive oxygen species levels 9. Reactive oxygen species cause apoptotic and necrotic cell death thereby contributing a secondary role in SM-induced toxicity 10. SM also reacts with lipids to cause lipid peroxidation. Oxidative stress and depletion of anti-oxidant are important players in SM-induced airway injury 11. Activation of poly (ADP-ribose) polymerase, MAPK and activating protein, AP-1 signaling pathways has been demonstrated after SM inhalation in the lung 12–14. Additionally, proteolytic enzymes and cytokines produced by cells and secondary inflammatory responses amplify SM toxicity. More recently involvement of chemosensory channels such as transient receptor potential (TRP) family has been identified as specific sensor of the vesicant SM in the lung 15. These effects of sulfur mustard are to a large extent mimicked by its analog, CEES (aka: half mustard; 2 chloroethyl ethyl sulfide). Analogs are frequently used as safer alternative to study vesicants in laboratories.
Figure 1.
Reactions of sulfur mustard and its analog CEES. Sulfur mustard and its analog 2-chloroethyl ethyl sulfide (CEES) can undergo internal cyclization to form the reactive sulfonium and carbenium ion intermediates. These intermediates react with functional groups in protein, carbohydrates and nucleic acids.
Pathophysiological consequences
Sulfur mustard causes a host of pathophysiological consequences. These findings are based on actual human exposures and exposure of animals to SM or CEES. The extent of injury depends on whether SM or its analogs are used. For instance, an LD50 of CEES is about 100 times less potent that SM. The extent of injury also depends on the animal model used, route, dose and duration of exposure 16. In general, larger animals like pigs and non-human primates mimic more closely human exposures. The most common organ systems affected by SM exposures are eyes, skin and lungs 17, 18. However sufficient evidence indicates dysfunction in brain, kidney, heart and bone marrow as well 17. High doses are fatal and may cause convulsions, coma and death during the exposure. With lower doses the effects are delayed and symptoms may appear slowly over time. Within 6h of exposure symptoms such as nausea, fatigue, headache, eye irritation, soreness of throat and difficulty in breathing occur. Conjunctivitis, edema and inflammation of the eyelids often lead to temporary blindness. Other ocular symptoms include lacrimation, photophobia, blepharospasm and corneal ulceration 16. In the next 24 h skin inflammation and blistering become apparent. Depending on exposure doses, SM also causes rhinorrhea, tracheobronchitis, airway hyperreactivity, vascular injuries, fibrin deposition followed by airway obstruction and fatalities from multi organ failure 16–19. In the lung most acute effects of SM in the lung occur in the upper respiratory tract, however, injury to the lower airways is not uncommon 20. Epithelial sloughing, pseudomembrane formation and airway occlusion has also been documented 16. Studies on cadavers of SM-related fatalities have revealed maximum chronic bronchitis, pulmonary fibrosis, pulmonary infections, cellular infiltration and aspergillioma 21. In individuals who survived there was a higher incidence of bronchiolitis obliterans and tissue biopsies revealed necrotic airways, dense cast formation, constrictive bronchiolitis, and respiratory and chronic cellular bronchiolitis 22. Increased airway remodeling due to transforming growth factor beta 1 (TGF-β1) has also been observed upon SM inhalation 23. Therefore, survivors of SM exposure present with a variety of respiratory anomalies including pulmonary fibrosis, bronchiectasis, acute respiratory distress syndrome (ARDS) and vascular injury and remodeling 24, 25, and are highly prone to chronic obstructive pulmonary disease (COPD) and cancers 26.
Role of coagulation in sulfur mustard poisoning
In acute lung injury and ARDS the coagulation cascade is often activated with an associated decrease in the fibrinolytic activities 27, 28. Activation of coagulation in these situations causes extravascular fibrin deposition that can promote pulmonary dysfunction and inflammation 28. In this context, SM exposure has great parallels with ARDS as both the coagulation and inflammatory pathways are activated 29–31. The earliest reported indications of the role of coagulation in SM-induced injuries were from individuals exposed during the Iran-Iraq conflict in 1980–88. Autopsy of one patient that died of airway obstruction indicated formation of bronchial casts 32, in conjunction with formation of fibrin rich pseudomembranes and inflammation of the trachea and bronchial tubes 32. These findings have also been reproduced in animal experiments with CEES or sulfur mustard 30, 31, 33, 34.
Exposure to SM/CEES causes hypercoagulation through both an increase in procoagulation factors and a decrease in the fibrinolytic factors. Veress et al. have demonstrated the formation of casts in the conducting airways of rats exposed to CEES and SM 31, 35. These fibrin rich casts cause obstruction and impaired gas exchange that is one of the principal causes of mortality following SM exposure 31, 32. Initiation of the procoagulatory pathways can occur through a number of different mechanisms. SM inhalation causes profound inflammation of the airways, apoptotic and necrotic cell death and vascular injuries, which can initiate coagulation via both intrinsic and extrinsic coagulation cascades. In the extrinsic pathway, tissue factor (TF) activation by SM/CEES appears to be the primary initiator. In addition to TF activation, SM/CEES exposures causes increases in fibrinogen, prothrombin and thrombin-antithrombin (TAT) complexes in the bronchoalveolar lavage fluid (BALF) of CEES-exposed rats 34. These exposures cause an increase in TF activity and an increase in Factor X (FX) expression 33. SM/CEES exposure also causes a parallel inhibition of the fibrinolytic pathway by increasing plasminogen activator inhibitor-1 (PAI-1), thrombin-activatable fibrinolysis inhibitor (TAFI) and α2-antiplasmin 33. In rats, treatment with tissue plasminogen activator (TPA) after SM inhalation prevented cast formation and mortality 35. Similarly, treatment with heparin and tissue factor pathway inhibitor (TFPI) also prevented mortality 34, 36. These studies underscore the importance of coagulation in SM/CEES-induced injuries. Our studies suggest a significant contribution of airway epithelium to TF and other components of the clotting cascade 37. Similarly, other studies have also shown that alveolar epithelium contributes significantly to coagulation 38. However, the mechanisms and factors responsible for SM-induced increased airway coagulation are yet to be addressed.
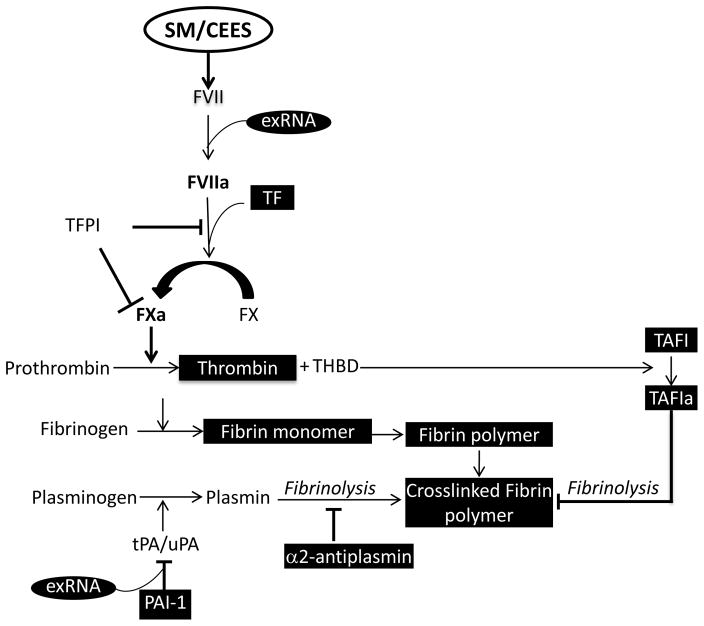
Several additional factors can also activate the clotting cascade following SM exposure. SM exposed individuals have increased serum levels of the proinflammatory IL-6, IL-8 and MMP9 5, 20. They also have increased levels of oxidants that include cholesterol, triglycerides and gamma-glutamyl transferase activity 39. Activation of the clotting cascade can also occur as a result of increased extracellular nucleic acids such as DNA and RNA that are actively released from the cells as a result of injury or necrosis. Extracellular DNA plays a dual role in both promoting and inhibiting fibrinolysis 40. Moreover, presence of nucleic acids and histones has been shown to increase the stability of fibrin clots 41. Growing evidence suggests that extracellular RNA (exRNA), which has been found in thrombi of mice subjected to carotid artery injury, can also provoke a significant procoagulant response 42, 43. While in vitro studies have shown that scavengers of exRNA can prolong clotting times, in vivo studies have demonstrated that these scavengers can prevent thrombus formation 42. Importantly, unlike most anticoagulants, these scavengers do not increase bleeding. Our studies have shown that there is increased exRNA in the plasma samples from rats exposed to CEES 44. Extracellular RNA can bind to PAI-1 and stabilize it 45. PAI-1 inhibits tissue plasminogen activator (TPA), which cleaves the proenzyme plasminogen to the active fibrinolytic plasmin (Figure 2). Interestingly PAI-1 secretion is also stimulated by pro-inflammatory cytokines. Given that PAI-1 is already increased in SM/CEES-exposed animals, further stabilization can result in prolonged inhibition of the fibrinolytic pathway. Additionally, pro-inflammatory cytokines and vascular injury expose TF and activate the extrinsic clotting pathway. Extracellular RNA from the blood can also initiate procoagulation events by activating Factor VII (FVII) 46. The activated FVII then binds to TF forming a complex that catalyzes the conversion of Factor X to Xa. FXa then converts prothrombin to thrombin. Thrombin cleaves fibrinogen to fibrin and can also activate platelets and endothelial cells. Fibrin in turn can enhance lung inflammation by increasing the expression of IL-1β, a specific marker of inflammation 47. Fibrin and its degradation products can also increase vascular permeability 48, enhance recruitment of neutrophils to the lung 49 and influence inflammatory cell proliferation and migration 50. These studies underscore the importance of extracellular nucleic acids in inflammation and coagulation and could have parallels with the SM-induced injuries. Therefore potential therapies against these targets are worth exploring.
Figure 2.
Schematic representation of the coagulation pathway in SM-induced injuries. Factors in black boxes are increased in SM/CEES-induced injuries. FVII: Factor VII, FVIIa: Activated Factor VII; exRNA: extracellular RNA; TF: Tissue factor; TFPI: Tissue factor pathway inhibitor; FX: Factor X; FXa: Activated Factor X; THBD: Thrombomodulin; TAFI: Thrombin activatable fibrinolysis Inhibitor; TAFIa: Activated Thrombin activatable fibrinolysis Inhibitor; PAI-1: Protease activated Inhibitor 1.
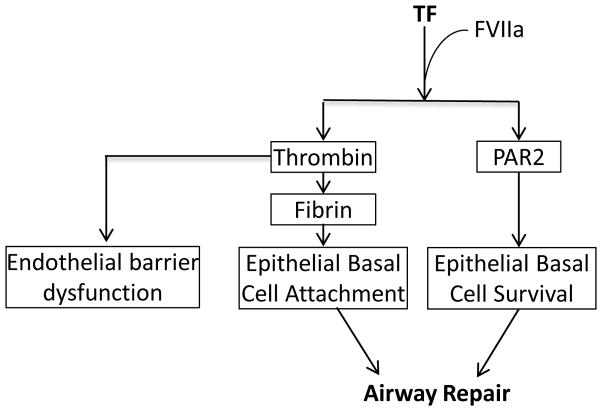
Activation of the coagulation cascade can also confer protection in a number of situations. In this context TF can play multiple roles. While the role of TF in activation of the coagulation cascade is well recognized, its role in repair is less well appreciated. Our previous studies have demonstrated that TF is critical to the survival of epithelial basal progenitor cell and required for repair of the damaged epithelium 37. We have also shown that these TF-mediated effects are modulated through PARs (protease activated receptors PAR-1 and PAR-2) 37. Therefore, therapies targeting TF can potentially impair the repair process and lead to comorbidities (Figure 3). A recent study in ARDS patients linked immune activation and expression of procoagulant proteins to survivability, suggesting that they provide compensatory survival responses 51. In several models of ARDS it has been demonstrated that prevention of fibrin formation protects against injury with improvements in oxygenation, lung compliance and inflammation 52. In spite of these promising studies there is little evidence that human patients benefit from such treatments, and in some cases there was even increase in mortality 53. This is conceivable since anticoagulation therapies in a number of cases did not correct inflammatory responses and were more prone to bleeding disorders 54. Apparently, blocking the procoagulation pathway alone may not be sufficient to offer long-term benefits. While other effective strategies are needed, the role of coagulation cannot be ignored.
Figure 3.
Schematic illustration of tissue factor-dependent pathways in repair an injury.
Role of inflammation in sulfur mustard poisoning
Pathways of inflammation and coagulation are interdependent and intrinsically linked. Significant inflammation is often associated with increased coagulation. Inflammation can influence coagulation by increasing cytokine levels of IL-6, IL-1 and IL-12, diminishing activated protein C (APC), decreasing fibrinolysis and increasing platelet activation 54. The role of inflammation in mediating TF activation and subsequent coagulation is increasingly being recognized 55. Bacterial lipopolysaccharide (LPS), a known inflammatory molecule, can promote coagulation by inducing TF activation and increasing thrombin generation 20, 55. Thrombin, in addition to its effect on coagulation, can promotes endothelial barrier dysfunction and further potentiate inflammation by its effects on cytokine production and activation of its receptor, protease activated receptors (PAR-1). In this context one would expect that by blocking thrombin generation or inhibition of PAR-1, inflammation could be suppressed. However, this may not always hold true. While the role of PAR-1 in promoting coagulation is unequivocal, its role as an anti-inflammatory molecule is equally important. Binding of APC to PAR-1 results in an anti-inflammatory phenotype 54. Additionally, PARs (PAR-1 and PAR-2) can confer pro-survival and pro-proliferative effects through a TF dependent mechanism 37. Taken together these studies point to the complexity of inflammatory and coagulation pathways involved in the pathogenesis of SM poisoning.
SM exposure causes severe blistering and inflammation of skin 56. Dermal exposure to SM caused immune activation and infiltration of CD4 and CD8 positive T cells along with a delayed type hypersensitivity response and inflammation in distal organs such as lungs 57. Dose dependent activation of mast cells and dermal neutrophil accumulation have been observed in SM-induced skin lesions 58. Dose and time dependent increases in expression of specific mRNA of inflammatory mediators such as IL-1β, IL-8 and IL-6, tumor necrosis factor alpha (TNFα), cyclooxygenase-2 (COX 2), macrophage inflammatory proteins and keratinocyte chemoattractants have also been observed 59, 60. Increased expression of adhesion molecules such as L-selectin and VCAM along with growth factors, granulocyte colony-stimulating factor and matrix metalloproteinases were also observed upon SM exposure of skin 61, 62. Some of these effects could be reversed using anti-inflammatory therapies 63.
Inflammatory pathways are also activated by ROS, generated as a result of exposure to SM or CEES. SM also causes a marked increase in proapoptotic proteins 64 and markers of oxidative stress, along with a decrease in antioxidants 20. Therefore it is conceivable that therapies like N-acetyl cysteine, that targets the antioxidant pathway, have shown greater promise in preventing mortality for up to 12 hours 65. Importantly, there was decreased neutrophil infiltration in these animals, indicative of reduced inflammation. It would also be interesting to know the effects of this therapy in prolonging survival and long-term morbidity.
Pulmonary airway mucosa is first to encounter inhaled SM. Epithelial cell death and release of cellular debris causes increased activation of the immune system. The markers of inflammation may persist in circulation of victims for a very long time after SM inhalation 66, 67. Serum levels of IL-6 and IL-8 were elevated in Iran war veterans 20. Similar increases in inflammatory markers like IL-6, TNF-α and myeloperoxidase activity have also been observed in animal models after CEES inhalation 20. Therapies targeting the inflammatory pathway have also shown efficacy in a number of studies. For instance, anti-TNF-α antibodies are protective against nitrogen mustard-induced injuries 68. Similarly, pentoxifylline (TNF-α inhibitor) can also mitigate effects on acute lung injury 69. Interestingly, TNF-α can also stimulate TF expression 70, suggesting that targeting pro-inflammatory molecules could also diminish coagulation.
SM exposure is accompanied by infiltration of inflammatory cell, mainly neutrophils, in to the airways 71. Similar increases in blood neutrophil counts were observed in Iranian SM victims several years post exposure. Analysis of blood samples of these victims demonstrated increased CD56/CD25 positive natural killer cells in patients with increased disease severity 20. Similarly, the BALF of these patients had increased proinflammatory cytokines and cytotoxic T cells 20. Activation of T cell mediated responses has also been reported in animal models of SM inhalation 20. However in one model, SM caused decreased T cell proliferation 72. The importance of inflammatory cells in CEES induced injuries is further supported by a study that showed protection in rats following neutrophil depletion 73. While these studies underscore the critical role of neutrophils in contributing towards the inflammatory phenotype, severe exposures lead to neutropenia, resulting in increased susceptibility to infections and increased mortality. In experimental settings, SM as well as nitrogen mustard exposures in non-human primates have been shown to cause neutropenia. In these animals administration of granulocyte colony stimulating factor (G-CSF) restored neutrophil counts much faster when compared to the untreated ones 74. Taken together, these studies underscore the importance of the inflammatory cells in CEES and SM-induced lung injuries and highlight the complexity of associated pathways. Therefore, molecules that interfere with the activation of these pathways can potentially alleviate SM-induced lung injury.
Challenges and future directions
SM exposures cause a host of clinical manifestations that involve multiple pathways, principal among them being the antioxidant, inflammatory and coagulation pathways. Although our current understanding of events leading to the pathogenesis of SM injuries has advanced significantly, effective therapies are still lacking. Therapies that prevent mortality against acute lethal exposures should perhaps be employed as the first line of defense. The choice of such therapies is still debatable and long-term effects should be carefully considered. Apparently in acute lethal exposures coagulation pathways are important as anticoagulant therapies in animal models can be used to prevent mortality. However, prolonged use can also lead to bleeding disorders and other comorbidities. Further, therapies that prevent hypercoagulation can potentially cause inhibition of repair pathways and lead to chronic effects. These confounding effects limit effective therapies. Evidently there is a need to develop drugs that target multiple pathways. Unfortunately, this may not come from a single wonder drug and may require combination therapies. Finally, safety and toxicity profiles of such therapies have to be looked into more carefully given the recent tragic incident with an experimental drug trial in France 75.
Acknowledgments
SA is supported by intramural funds from Department of Anesthesiology and Perioperative Medicine (UAB) and AA is supported by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD), the National Institute of Environmental Health Sciences (NIEHS), and the National Heart Lung and Blood Institute (NHLBI) Grant Numbers U01ES025069 and R01HL114933.
References
- 1.Wang QQ, et al. Sulfur, oxygen, and nitrogen mustards: stability and reactivity. Organic & biomolecular chemistry. 2012;10:8786–8793. doi: 10.1039/c2ob26482j. [DOI] [PubMed] [Google Scholar]
- 2.Yue L, et al. Distribution of DNA adducts and corresponding tissue damage of Sprague-Dawley rats with percutaneous exposure to sulfur mustard. Chemical research in toxicology. 2015;28:532–540. doi: 10.1021/tx5004886. [DOI] [PubMed] [Google Scholar]
- 3.Batal M, et al. A guanine-ethylthioethyl-glutathione adduct as a major DNA lesion in the skin and in organs of mice exposed to sulfur mustard. Toxicology letters. 2015;233:1–7. doi: 10.1016/j.toxlet.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Tang FR, Loke WK. Sulfur mustard and respiratory diseases. Critical reviews in toxicology. 2012;42:688–702. doi: 10.3109/10408444.2012.698405. [DOI] [PubMed] [Google Scholar]
- 5.Weinberger B, et al. Sulfur mustard-induced pulmonary injury: therapeutic approaches to mitigating toxicity. Pulmonary pharmacology & therapeutics. 2011;24:92–99. doi: 10.1016/j.pupt.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anand T, et al. Role of inflammatory cytokines and DNA damage repair proteins in sulfur mustard exposed mice liver. Toxicology mechanisms and methods. 2009;19:356–362. doi: 10.1080/15376510902903766. [DOI] [PubMed] [Google Scholar]
- 7.Ghanei M, Harandi AA. Lung carcinogenicity of sulfur mustard. Clinical lung cancer. 2010;11:13–17. doi: 10.3816/CLC.2010.n.002. [DOI] [PubMed] [Google Scholar]
- 8.Naghii MR. Sulfur mustard intoxication, oxidative stress, and antioxidants. Military medicine. 2002;167:573–575. [PubMed] [Google Scholar]
- 9.Mirbagheri L, et al. Downregulation of super oxide dismutase level in protein might be due to sulfur mustard induced toxicity in lung. Iranian journal of allergy, asthma, and immunology. 2013;12:153–160. [PubMed] [Google Scholar]
- 10.Vijayaraghavan R, et al. Dermal intoxication of mice with bis(2-chloroethyl)sulphide and the protective effect of flavonoids. Toxicology. 1991;69:35–42. doi: 10.1016/0300-483x(91)90151-p. [DOI] [PubMed] [Google Scholar]
- 11.Laskin JD, et al. Oxidants and antioxidants in sulfur mustard-induced injury. Annals of the New York Academy of Sciences. 2010;1203:92–100. doi: 10.1111/j.1749-6632.2010.05605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JH, Kagan E. Role of nicotinamide adenine dinucleotide phosphate oxidase in mediating vesicant-induced interleukin-6 secretion in human airway epithelial cells. American journal of respiratory cell and molecular biology. 2014;50:713–722. doi: 10.1165/rcmb.2012-0527OC. [DOI] [PubMed] [Google Scholar]
- 13.Malaviya R, et al. Attenuation of acute nitrogen mustard-induced lung injury, inflammation and fibrogenesis by a nitric oxide synthase inhibitor. Toxicology and applied pharmacology. 2012;265:279–291. doi: 10.1016/j.taap.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukhopadhyay S, et al. Role of MAPK/AP-1 signaling pathway in the protection of CEES-induced lung injury by antioxidant liposome. Toxicology. 2009;261:143–151. doi: 10.1016/j.tox.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Buch T, et al. Chemosensory TRP channels in the respiratory tract: role in toxic lung injury and potential as “sweet spots” for targeted therapies. Reviews of physiology, biochemistry and pharmacology. 2013;165:31–65. doi: 10.1007/112_2012_10. [DOI] [PubMed] [Google Scholar]
- 16.Balali-Mood M, Hefazi M. Comparison of early and late toxic effects of sulfur mustard in Iranian veterans. Basic Clin Pharmacol Toxicol. 2006;99:273–282. doi: 10.1111/j.1742-7843.2006.pto_429.x. [DOI] [PubMed] [Google Scholar]
- 17.Kehe K, Szinicz L. Medical aspects of sulphur mustard poisoning. Toxicology. 2005;214:198–209. doi: 10.1016/j.tox.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Sinclair DC. The clinical features of mustard-gas poisoning in man. Br Med J. 1948;2:290–294. doi: 10.1136/bmj.2.4570.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kehe K, et al. Acute effects of sulfur mustard injury--Munich experiences. Toxicology. 2009;263:3–8. doi: 10.1016/j.tox.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 20.Ghabili K, et al. Mustard gas toxicity: the acute and chronic pathological effects. Journal of applied toxicology: JAT. 2010;30:627–643. doi: 10.1002/jat.1581. [DOI] [PubMed] [Google Scholar]
- 21.Taghaddosinejad F, Fayyaz AF, Behnoush B. Pulmonary complications of mustard gas exposure: a study on cadavers. Acta medica Iranica. 2011;49:233–236. [PubMed] [Google Scholar]
- 22.Ghanei M, et al. Use of immunohistochemistry techniques in patients exposed to sulphur mustard gas. Pathology research international. 2011;2011:659603. doi: 10.4061/2011/659603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahriary A, et al. The footprint of TGF-beta in airway remodeling of the mustard lung. Inhalation toxicology. 2015;27:745–753. doi: 10.3109/08958378.2015.1116645. [DOI] [PubMed] [Google Scholar]
- 24.Ghanei M, et al. Bronchiolitis obliterans following exposure to sulfur mustard: chest high resolution computed tomography. Eur J Radiol. 2004;52:164–169. doi: 10.1016/j.ejrad.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 25.Emad A, Rezaian GR. The diversity of the effects of sulfur mustard gas inhalation on respiratory system 10 years after a single, heavy exposure: analysis of 197 cases. Chest. 1997;112:734–738. doi: 10.1378/chest.112.3.734. [DOI] [PubMed] [Google Scholar]
- 26.Steinritz D, et al. Epigenetic modulations in early endothelial cells and DNA hypermethylation in human skin after sulfur mustard exposure. Toxicology letters. 2015 doi: 10.1016/j.toxlet.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Gunther A, et al. Alveolar fibrin formation caused by enhanced procoagulant and depressed fibrinolytic capacities in severe pneumonia. Comparison with the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:454–462. doi: 10.1164/ajrccm.161.2.9712038. [DOI] [PubMed] [Google Scholar]
- 28.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med. 2003;31:S213–220. doi: 10.1097/01.CCM.0000057846.21303.AB. [DOI] [PubMed] [Google Scholar]
- 29.O’Neill HC, et al. Treatment with the catalytic metalloporphyrin AEOL 10150 reduces inflammation and oxidative stress due to inhalation of the sulfur mustard analog 2-chloroethyl ethyl sulfide. Free Radic Biol Med. 2010;48:1188–1196. doi: 10.1016/j.freeradbiomed.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rancourt RC, et al. Airway tissue factor-dependent coagulation activity in response to sulfur mustard analog 2-chloroethyl ethyl sulfide. American journal of physiology Lung cellular and molecular physiology. 2012;302:L82–92. doi: 10.1152/ajplung.00306.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veress LA, et al. Airway obstruction due to bronchial vascular injury after sulfur mustard analog inhalation. Am J Respir Crit Care Med. 2010;182:1352–1361. doi: 10.1164/rccm.200910-1618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenmenger W, et al. Clinical and morphological findings on mustard gas [bis(2-chloroethyl)sulfide] poisoning. J Forensic Sci. 1991;36:1688–1698. [PubMed] [Google Scholar]
- 33.Rancourt RC, et al. Antifibrinolytic mechanisms in acute airway injury after sulfur mustard analog inhalation. American journal of respiratory cell and molecular biology. 2014;51:559–567. doi: 10.1165/rcmb.2014-0012OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rancourt RC, et al. Tissue factor pathway inhibitor prevents airway obstruction, respiratory failure and death due to sulfur mustard analog inhalation. Toxicology and applied pharmacology. 2013;272:86–95. doi: 10.1016/j.taap.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veress LA, et al. Airway tissue plasminogen activator prevents acute mortality due to lethal sulfur mustard inhalation. Toxicological sciences: an official journal of the Society of Toxicology. 2015;143:178–184. doi: 10.1093/toxsci/kfu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houin PR, et al. Intratracheal heparin improves plastic bronchitis due to sulfur mustard analog. Pediatric pulmonology. 2015;50:118–126. doi: 10.1002/ppul.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmad S, et al. Tissue factor signals airway epithelial basal cell survival via coagulation and protease-activated receptor isoforms 1 and 2. American journal of respiratory cell and molecular biology. 2013;48:94–104. doi: 10.1165/rcmb.2012-0189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bastarache JA, et al. The alveolar epithelium can initiate the extrinsic coagulation cascade through expression of tissue factor. Thorax. 2007;62:608–616. doi: 10.1136/thx.2006.063305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keramati MR, et al. Biochemical and hematological findings of Khorasan veterans 23 years after sulfur mustard exposure. Journal of research in medical sciences: the official journal of Isfahan University of Medical Sciences. 2013;18:855–859. [PMC free article] [PubMed] [Google Scholar]
- 40.Komissarov AA, Florova G, Idell S. Effects of extracellular DNA on plasminogen activation and fibrinolysis. The Journal of biological chemistry. 2011;286:41949–41962. doi: 10.1074/jbc.M111.301218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Longstaff C, et al. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. The Journal of biological chemistry. 2013;288:6946–6956. doi: 10.1074/jbc.M112.404301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain S, et al. Nucleic acid scavengers inhibit thrombosis without increasing bleeding. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12938–12943. doi: 10.1073/pnas.1204928109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kannemeier C, et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Husain M, Zafar I, Christiaans SC, Pittet JF, Ahmad S, Ahmad A. Extracellular RNA in 2-chloroethyl ethyl sulfide (CEES)-induced lung injury. Presented at 9th Annual NIH Countermeasures Against Chemical Threats (CounterACT) Network Research Symposium; New York, NY. June 15–17, 2015.2015. [Google Scholar]
- 45.Wygrecka M, et al. Plasminogen activator inhibitor-1 is an inhibitor of factor VII-activating protease in patients with acute respiratory distress syndrome. The Journal of biological chemistry. 2007;282:21671–21682. doi: 10.1074/jbc.M610748200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakazawa F, et al. Extracellular RNA is a natural cofactor for the (auto-)activation of Factor VII-activating protease (FSAP) The Biochemical journal. 2005;385:831–838. doi: 10.1042/BJ20041021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perez RL, Roman J. Fibrin enhances the expression of IL-1 beta by human peripheral blood mononuclear cells. Implications in pulmonary inflammation. J Immunol. 1995;154:1879–1887. [PubMed] [Google Scholar]
- 48.Dang CV, et al. Disorganization of cultured vascular endothelial cell monolayers by fibrinogen fragment D. Science. 1985;227:1487–1490. doi: 10.1126/science.4038818. [DOI] [PubMed] [Google Scholar]
- 49.Leavell KJ, Peterson MW, Gross TJ. The role of fibrin degradation products in neutrophil recruitment to the lung. American journal of respiratory cell and molecular biology. 1996;14:53–60. doi: 10.1165/ajrcmb.14.1.8534486. [DOI] [PubMed] [Google Scholar]
- 50.Ciano PS, et al. Macrophage migration in fibrin gel matrices. Laboratory investigation; a journal of technical methods and pathology. 1986;54:62–70. [PubMed] [Google Scholar]
- 51.Bhargava M, et al. Proteomic profiles in acute respiratory distress syndrome differentiates survivors from non-survivors. PloS one. 2014;9:e109713. doi: 10.1371/journal.pone.0109713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welty-Wolf KE, et al. Tissue factor in experimental acute lung injury. Semin Hematol. 2001;38:35–38. doi: 10.1053/shem.2001.29505. [DOI] [PubMed] [Google Scholar]
- 53.Standiford TJ, Ward PA. Therapeutic targeting of acute lung injury and acute respiratory distress syndrome. Translational research: the journal of laboratory and clinical medicine. 2016;167:183–191. doi: 10.1016/j.trsl.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petaja J. Inflammation and coagulation. An overview. Thrombosis research. 2011;127(Suppl 2):S34–37. doi: 10.1016/S0049-3848(10)70153-5. [DOI] [PubMed] [Google Scholar]
- 55.van der Poll T. Tissue factor as an initiator of coagulation and inflammation in the lung. Crit Care. 2008;12(Suppl 6):S3. doi: 10.1186/cc7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith KJ, et al. Sulfur mustard: its continuing threat as a chemical warfare agent, the cutaneous lesions induced, progress in understanding its mechanism of action, its long-term health effects, and new developments for protection and therapy. Journal of the American Academy of Dermatology. 1995;32:765–776. doi: 10.1016/0190-9622(95)91457-9. [DOI] [PubMed] [Google Scholar]
- 57.Mishra NC, et al. Sulfur mustard induces immune sensitization in hairless guinea pigs. International immunopharmacology. 2010;10:193–199. doi: 10.1016/j.intimp.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kehe K, et al. Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering. Toxicology. 2009;263:12–19. doi: 10.1016/j.tox.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 59.Balszuweit F, et al. Development of a co-culture of keratinocytes and immune cells for in vitro investigation of cutaneous sulfur mustard toxicity. Chemico-biological interactions. 2014;223C:117–124. doi: 10.1016/j.cbi.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Ricketts KM, et al. Inflammatory cytokine response in sulfur mustard-exposed mouse skin. Journal of applied toxicology: JAT. 2000;20(Suppl 1):S73–76. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat685>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 61.Shohrati M, et al. Serum matrix metalloproteinase levels in patients exposed to sulfur mustard. Iranian Red Crescent medical journal. 2014;16:e15129. doi: 10.5812/ircmj.15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sabourin CL, et al. Cytokine, chemokine, and matrix metalloproteinase response after sulfur mustard injury to weanling pig skin. Journal of biochemical and molecular toxicology. 2002;16:263–272. doi: 10.1002/jbt.10050. [DOI] [PubMed] [Google Scholar]
- 63.Chang YC, et al. Therapeutic potential of a non-steroidal bifunctional anti-inflammatory and anti-cholinergic agent against skin injury induced by sulfur mustard. Toxicology and applied pharmacology. 2014;280:236–244. doi: 10.1016/j.taap.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ray R, et al. Sulfur mustard induces apoptosis in lung epithelial cells via a caspase amplification loop. Toxicology. 2010;271:94–99. doi: 10.1016/j.tox.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 65.Jugg B, et al. N-acetyl-L-cysteine protects against inhaled sulfur mustard poisoning in the large swine. Clin Toxicol (Phila) 2013;51:216–224. doi: 10.3109/15563650.2013.780208. [DOI] [PubMed] [Google Scholar]
- 66.Pourfarzam S, et al. Serum levels of IL-8 and IL-6 in the long term pulmonary complications induced by sulfur mustard: Sardasht-Iran Cohort Study. International immunopharmacology. 2009;9:1482–1488. doi: 10.1016/j.intimp.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 67.Mohammadhoseiniakbari H, et al. Delayed effects of sulfur mustard poisoning on CD4+ and CD8+ lymphocytes in Iranian veterans 25 years after exposure. Medical science monitor: international medical journal of experimental and clinical research. 2008;14:CR580–583. [PubMed] [Google Scholar]
- 68.Malaviya R, et al. Attenuation of Nitrogen Mustard-Induced Pulmonary Injury and Fibrosis by Anti-Tumor Necrosis Factor-alpha Antibody. Toxicological sciences: an official journal of the Society of Toxicology. 2015;148:71–88. doi: 10.1093/toxsci/kfv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sunil VR, et al. Pentoxifylline attenuates nitrogen mustard-induced acute lung injury, oxidative stress and inflammation. Experimental and molecular pathology. 2014;97:89–98. doi: 10.1016/j.yexmp.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van der Poll T, et al. Activation of coagulation after administration of tumor necrosis factor to normal subjects. N Engl J Med. 1990;322:1622–1627. doi: 10.1056/NEJM199006073222302. [DOI] [PubMed] [Google Scholar]
- 71.Malaviya R, et al. Inflammatory effects of inhaled sulfur mustard in rat lung. Toxicology and applied pharmacology. 2010;248:89–99. doi: 10.1016/j.taap.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mei YZ, et al. The injury progression of T lymphocytes in a mouse model with subcutaneous injection of a high dose of sulfur mustard. Military Medical Research. 2014;1:28. doi: 10.1186/s40779-014-0028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McClintock SD, et al. Protection from half-mustard-gas-induced acute lung injury in the rat. Journal of applied toxicology: JAT. 2002;22:257–262. doi: 10.1002/jat.856. [DOI] [PubMed] [Google Scholar]
- 74.Anderson DR, et al. Sulfur mustard-induced neutropenia: treatment with granulocyte colony-stimulating factor. Military medicine. 2006;171:448–453. doi: 10.7205/milmed.171.5.448. [DOI] [PubMed] [Google Scholar]
- 75.Butler D, Callaway E. Scientists in the dark after French clinical trial proves fatal. Nature. 2016;529:263–264. doi: 10.1038/nature.2016.19189. [DOI] [PubMed] [Google Scholar]