Abstract
The U.S. FDA issued several announcements related to potential risk of bisphosphonates including osteonecrosis of the jaw (2005), atrial fibrillation (2007) and atypical femur fracture (2010). We aimed to evaluate the impact of three FDA drug safety announcements on the use of bisphosphonates in patients with hip fracture using claims data from a U.S. commercial health plan (2004–13). We calculated the proportion of patients in each quarter who received a bisphosphonate or other osteoporosis medication in the 6 months following hospitalization for hip fracture. Segmented logistic regression models examined the time trends. Among 22,598 patients with hip fracture, use of bisphosphonate decreased from 15% in 2004 to 3% in the last quarter of 2013. Prior to the 2007 announcement, there was a 4% increase in the odds of bisphosphonate use every quarter (OR 1.04, 95%CI 1.02–1.07). After the 2007 announcement, there was a 4% decrease in the odds of bisphosphonate use (OR 0.96, 95%CI 0.93–0.99) every quarter. The announcement in 2007 was associated with a significant decline in the rate of change of bisphosphonate uses over time (p<0.001), but no impact on other osteoporosis medication use (p=0.2). After the 2010 announcement, the odds of bisphosphonate use continued to decrease by 4% (OR 0.96. 95%CI, 0.94–0.98) each quarter and the odds of other osteoporosis medication use remained stable over time (OR 0.99, 95%CI 0.96–1.02). The FDA safety announcement related to atrial fibrillation in 2007 was significantly associated with a decrease in bisphosphonate use among patients with hip fracture.
INTRODUCTION
Bisphosphonates are the first-line therapy for treatment of osteoporosis. While current guidelines recommend use of pharmacologic treatment including bisphosphonates for the secondary prevention of hip fracture,[1] the rate of post-fracture osteoporotic treatment has been suboptimal and decreasing.[2 3] Patients’ and clinicians’ concerns over potential side effects of bisphosphonates may explain this decline, at least in part. Over the past decade, the U.S. Food and Drug Administration (FDA) has issued several safety alerts or announcements related to potential risk of bisphosphonates including osteonecrosis of the jaw (ONJ) in May 2005, atrial fibrillation in October 2007, and atypical femur fracture in March 2010.[4 5]
While it is important to recognize potential side effects of bisphosphonates, the benefits of bisphosphonates outweigh harms in post-osteoporotic fracture patients. The occurrence of ONJ was rare and mostly noted in patients using high dosages of bisphosphonates for cancer, rather than osteoporosis.[6 7] The risk of atrial fibrillation was initially reported to be greater in the zoledronic acid group versus placebo (1.3% vs.0.5%, p<0.001) in the HORIZON-PFT trial,[8] but the risk does not appear to be increased in oral bisphosphonate users based on a recent meta-analysis of 26 randomized controlled trials.[9] The risk of atypical femur fracture is increased with use of bisphosphonates, particularly the long-term use, but it is also rare; current estimates suggest that it occurs in 3 to 50 cases per 100,000 person-years of bisphosphonate users.[10 11] Since it is likely that these side effects of bisphosphonates received more media attention after the FDA safety announcements compared with the known benefits of these drugs in patients who sustained osteoporotic fracture, we hypothesized that the FDA safety announcements would have a negative impact on the use of bisphosphonates among patients with hip fracture in the U.S.
The objectives of this study were 1) to evaluate the trend in the use of bisphosphonates and other osteoporosis medications in patients following hospitalization for hip fracture over the past decade and 2) to examine whether the three aforementioned FDA drug safety announcements had an impact on the trend in use of bisphosphonates and other osteoporosis medications in the post-hip fracture patient population.
MATERIALS AND METHODS
Data Source
Data were collected from a large U.S. commercial health plan, United HealthCare, between 2004 and 2013. This plan insures primarily working adults and their family members. The database contains demographic information, health plan enrollment status and longitudinal claims information including medical diagnoses and procedures from inpatient and outpatient medical encounters -- coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM), and Current Procedural Terminology, Fourth Edition - and pharmacy dispensing on its approximately 14 million subscribers across the U.S. on a yearly basis. The quality of these data on inpatient diagnoses, procedures, health care utilization and drug dispensing as well as some outpatient diagnoses is known to be high.[12]
Study Cohort
We identified all adults aged ≥50 years who had a hospitalization for hip fracture based on a combination of ICD-9 and procedure codes.[3] This claims-based algorithm was shown to have a positive predictive value over 95% for hip fracture. [13] The date of hospitalization for hip fracture was defined as the index date. The primary outcome of interest was the proportion of patients in each quarter who received an oral or intravenous bisphosphonate (i.e., alendronate, risedronate, ibandronate, or zoledronic acid) in the 6 months after the index date. The secondary outcome of interest was the proportion of patients in each quarter who received any non-bisphosphonate osteoporosis medication (i.e., calcitonin, hormone replacement therapy, raloxifen, teriparatide, or denosumab) in the 6 months after the index date. Because of the long duration of treatment effect, we excluded patients who received zoledronic acid prior to the index date.
Data Analysis
We assessed a number of variables related to the risk of hip fracture and use of osteoporosis medication data from the 180-day baseline period before the index date. These variables included demographic characteristics, presence of comorbidities, comorbidity index, [14] use of bisphosphonate or other osteoporosis medication at baseline, and health care utilization factors (Table 1 for details). We compared these variables between patients who received a bisphosphonate and those who did not in the 6 months following hospitalization for hip fracture. We calculated the proportion of patients who died within a year following hospitalization for hip fracture. During the 10-year study period, we calculated the proportion of patients who received a bisphosphonate or other osteoporosis medications in the 6 months following hospitalization for hip fracture over time. We used segmented logistic regression to examine the impact of three FDA announcements on use of bisphosphonates over calendar time. The model included an initial intercept, four slope terms that described the trend in use of bisphosphonates before and after each FDA announcement, and 3 terms for step changes at the time of the announcements. As a negative control, these steps were repeated for the secondary outcome of interest defined as the proportion of patients who received other osteoporosis medications in the 6 months following hospitalization for hip fracture each quarter. All analyses were performed using SAS 9.3 and Stata 13. The study protocol was approved by the Institutional Review Boards of the Brigham and Women’s Hospital. Patient informed consent was not required, as the dataset was de-identified.
Table 1.
Patient characteristics in 180 days prior to hospitalization for hip fracture
| Total | Received a bisphosphonate following hip fracture | P-value | ||
|---|---|---|---|---|
|
| ||||
| Yes | No | |||
|
|
||||
| N | 22,598 | 3,273 | 19,325 | |
| Demographics | ||||
| Age (mean ± SD), yr | 72.4 ± 10.8 | 70.9 ± 10.6 | 72.6 ± 10.8 | <0.0001 |
| Female | 67.9% | 86.2% | 64.8% | <0.0001 |
|
| ||||
| Comorbidities | ||||
| Prior fall | 6.2% | 6.2% | 6.2% | 1.00 |
| Prior fracture | 25.9% | 30.1% | 25.2% | <0.0001 |
| Osteoporosis diagnosis | 10.8% | 22.4% | 8.8% | <0.0001 |
| Comorbidity indexa (mean ± SD) | 5.4 ± 20.3 | 7.3 ± 24.6 | 5.0 ± 19.5 | <0.0001 |
|
| ||||
| Medications | ||||
| Any bisphosphonates | 10.7% | 48.1% | 4.4% | <0.0001 |
| Alendronate | 6.6% | 29.7% | 2.7% | <0.0001 |
| Other osteoporosis drugs | 16.9% | 55.2% | 10.4% | <0.0001 |
|
| ||||
| Health care utilization | ||||
| Acute hospitalization | 17.6% | 16.6% | 17.7% | 0.12 |
| No. of outpatient visits | 4.5 ± 4.8 | 5.3 ± 5.5 | 4.3 ± 4.6 | <0.0001 |
The range of combined comorbidity score is −2 to 26.
RESULTS
There were a total of 22,598 patients who were hospitalized with hip fracture. The mean (SD) age was 72.4 (10.8) years and 68% were female. Of these, 3,273 (14.5%) received a bisphosphonate in the 6 months after the index date. Patients who received a bisphosphonate after the index date were more likely to be older and female and have a diagnosis of osteoporosis and a greater number of comorbidities at baseline compared with those who did not receive a bisphosphonate. Prior to the index date, 26% had at least one fracture at any location and 11% received at least 1 dispensing for bisphosphonates. Table 1 describes patient characteristics at baseline. During the year following hospitalization for hip fracture, a total of 3,930 (17%) patients, 10% of those who received a bisphosphonate after hip fracture and 19% of those who did not, died.
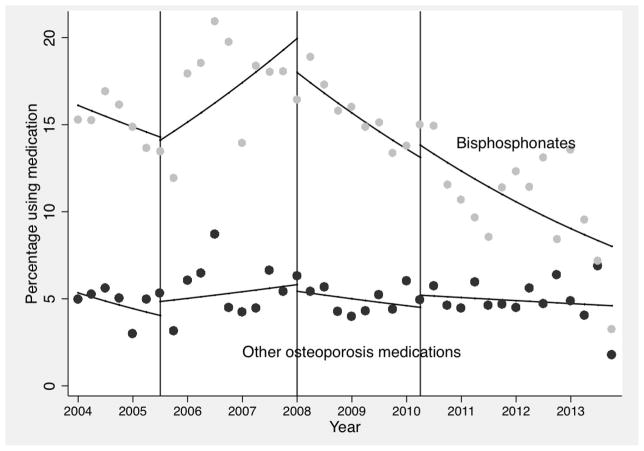
Over the study time period, the proportion of patients who filled at least one prescription for a bisphosphonate or received at least one intravenous administration following hip fracture decreased from 15% in 2004 to 3% in the last quarter of 2013, while the proportion of other osteoporosis medication use was stable around 5% over time (Figure 1). Until the last quarter of 2007 when the FDA issued the 2nd announcement related to the risk of atrial fibrillation, there was a 4% increase in the odds of bisphosphonate use after hip fracture every quarter [odds ratio (OR) 1.04, 95% CI 1.02–1.07]. Between 2008 and 2010, there was a 4% decrease in the odds of bisphosphonate use (OR 0.96, 95% CI 0.93–0.99) every quarter. The 2nd announcement in 2007 was associated with a significant change in the trend of bisphosphonate uses over time (p<0.001), but it had no impact on the trend of other osteoporosis medication use over time (p=0.2). After the 1st quarter of 2010 following the 3rd FDA announcement related to risk of atypical femur fracture, the odds of bisphosphonate use following hip fracture continued to decrease by 4% (OR 0.96, 95% CI, 0.94–0.98) each quarter but the odds of other osteoporosis medication use continued to have a non-significant trend over time (OR 0.99, 95% CI 0.96–1.02).
Figure 1. Impact of FDA safety announcements on the use of osteoporosis medications following hip fracture.
Dots represent the observed values. The solid lines are the predicted values from the regression models fit to the person-level data. Three vertical lines indicate the quarters immediately after the three FDA announcements (far left for osteonecrosis of the jaw, middle for atrial fibrillation and far right for atypical femur fracture) were released.
DISCUSSION
This population-based longitudinal study showed that use of bisphosphonate decreased from 15% in 2004 to 6% in 2013 in patients following hospitalization for hip fracture. We also found that the FDA safety announcement related to the risk of atrial fibrillation in 2007 was associated with a significant decline in the use of bisphosphonates over the study period.
The results of this study call attention to several issues related to management of osteoporosis in general as well as secondary prevention of osteoporotic fracture. First, it is concerning that not even 10% of patients receive a bisphosphonate for the secondary prevention of osteoporotic fracture in more recent years. It is well-known that patients who sustain a hip fracture are at an increased risk for recurrent hip or other osteoporotic fractures.[15] An Italian study showed that over 4% of patients with a hip fracture develop a fracture in the contralateral femur with a 1-year refracturing rate of 2.4%.[16] In another study of 549 elderly patients with hip fracture, the rate of any subsequent self-reported fracture after the initial hip fracture was 10.4 per 100 person-years.[17] Starting from such a low level of post-fracture treatment, any reduction in osteoporosis treatment rates exacerbates an existing public health concern.
Second, despite well-established evidence on the effectiveness of these agents, the FDA safety announcements appear to have a negative impact on the use of oral or intravenous bisphosphonates in clinical scenarios with very strong indication for treatment.[18 19] While the FDA is responsible for timely updates of the public with potential safety issues related to previously approved drugs, their safety communications must be considered in the appropriate public health context. The FDA’s mandate is for assuring the safe use of drugs, but a more balanced approach may have recognized that osteoporosis treatments post-fracture are under-utilized despite strong recommendations. One hopes that this consideration might have led to a more balanced approach to discussing large benefits versus rare risks of these agents.
Furthermore, when the FDA or others describe potential side effects of a drug, it is critical to report an absolute risk as well as a relative risk. For example, a study that analyzed three randomized controlled trials of bisphosphonates nicely summarized their findings that treating 1,000 women with osteoporosis for 3 years with a bisphosphonate will prevent approximately 100 vertebral or non-vertebral fractures (number needed to treat: 10) while it will cause 0.3 to 1.4 atypical femur fractures assuming the relative risk ranges from 1.5 to 3.0 (number needed to harm: 714–3333).[20] This type of information allows for making a well-informed and balanced treatment decision.
The current study has limitations. First, we were unable to study reasons why patients did not receive a bisphosphonate following hospitalization for hip fracture. Several factors such as patient or physician preference, contraindications to bisphosphonates or frailty may have affected the treatment decision not to start a bisphosphonate. Second, there is a potential for misclassification of hip fracture. While we use a previously validated algorithm to identify patients with hip fracture, the algorithm was not validated in this study database. Third, because this study is based on a commercially insured patient cohort, our results may not be generalizable to uninsured or underinsured patients. The underuse of bisphosphonates for the secondary prevention of osteoporosis fracture may be worse in other populations. Fourth, our study cannot imply causal association between the FDA safety announcements and a decrease in the use of bisphosphonates.
In conclusion, over the past decade, use of bisphosphonates following hospitalization for hip fracture has substantially decreased. Our study found that the FDA safety announcement related to atrial fibrillation in 2007 was significantly associated with a decrease in trend of bisphosphonate use in patients with hip fracture. Given the clinical importance of the secondary prevention of hip fracture, these results highlight the need to weigh benefits versus harms of bisphosphonates and to improve the communication of drug safety information with both clinicians and patients.
Acknowledgments
Acknowledgements/Disclosures:
SC Kim was supported by the NIH grant K23 AR059677 at the time of this study. She received research grants to the Brigham and Women’s Hospital from Pfizer, AstraZeneca, Genentech, and Lilly.
DH Kim is supported by the Paul B. Beeson Clinical Scientist Development Award in Aging K08 AG051187 from the NIH, American Federation for Aging Research, the John A. Hartford Foundation, and the Atlantic Philanthropies. DH Kim provides paid consultative services on geriatrics care to the Alosa Foundation, a non-profit educational organization with no relationship to any drug or device manufacturers.
Mogun, Eddings, Polinski, and Franklin have nothing to disclose.
Solomon is supported by the NIH grants K24 AR055989, P60 AR047782, and R01 AR056215. Solomon receives salary support through research grants to the Brigham and Women’s Hospital from CORRONA, Astra Zeneca, Amgen, Genentech, Pfizer, and Lilly. He serves in an unpaid role on a trial sponsored by Pfizer unrelated to the current study. He serves on the multi-specialty board of the American Orthopaedic Association’s Own the Bone Program and on the Governing Board of the National Bone Health Alliance.
This study had no specific funding source. SCK was supported by the NIH grant K23 AR059677 at the time of this study. DHK is supported by the Paul B. Beeson Clinical Scientist Development Award in Aging K08 AG051187 from the NIH, American Federation for Aging Research, the John A. Hartford Foundation, and the Atlantic Philanthropies. DHS is supported by the NIH grants K24 AR055989, P60 AR047782, and R01 AR056215.
SCK is supported by the NIH grant K23 AR059677. DHK is supported by the Paul B. Beeson Clinical Scientist Development Award in Aging K08 AG051187 from the NIH. DHS is supported by the NIH grants K24 AR055989, P60 AR047782, and R01 AR056215.
Footnotes
- Study design: All authors
- Study conduct: SCK, HM, WE
- Data collection: SCK, HM, WE
- Data analysis: SCK, HM, WE
- Data interpretation: All authors
- Drafting manuscript: SCK
- Revising manuscript content: All authors
- Approving final version of manuscript: All authors
- SCK takes responsibility for the integrity of the data analysis.
Potential Conflict of Interest
SCK receives research grants to the Brigham and Women’s Hospital from Pfizer, AstraZeneca, Genentech, and Lilly.
DHK provides paid consultative services on geriatrics care to the Alosa Foundation, a non-profit educational organization with no relationship to any drug or device manufacturers.
JMP is currently an employee of CVS Caremark.
DHS has received research grants to the Brigham and Women’s Hospital from CORRONA, Astra Zeneca, Amgen, Genentech, Pfizer, and Lilly. He serves in unpaid roles on studies sponsored by Pfizer. Solomon serves on the multi-specialty board of the American Orthopaedic Association’s Own the Bone Program and on the Governing Board of the National Bone Health Alliance.
Authors’ Contributions: SCK had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. SCK is the guarantor of this work. SCK is responsible for the concept and design of the study, the acquisition of data and the analysis and interpretation of data. SCK drafted and finalized the manuscript. DHK, JMP and DHS are responsible for the concept and design of the study, the interpretation of data and critical revision of the manuscript. HM is responsible for the design of the study, the analysis of data and critical revision of the manuscript. WE and JMF are responsible for the design of the study, the analysis and interpretation of data, and critical revision of the manuscript. All authors approved the final version of the manuscript.
Statement of Informed Consent: Patient informed consent was not required as the dataset was de-identified to protect subject confidentiality.
Conflict of Interest: SCK receives research grants to the Brigham and Women’s Hospital from Pfizer, AstraZeneca, Genentech, and Lilly. DHK provides paid consultative services on geriatrics care to the Alosa Foundation, a non-profit educational organization with no relationship to any drug or device manufacturers. HM, WE, JMF, and JMP have nothing to disclose. DHS receives salary support through research grants to the Brigham and Women’s Hospital from CORRONA, Astra Zeneca, Amgen, Genentech, Pfizer, and Lilly. He serves in an unpaid role on a trial sponsored by Pfizer unrelated to the current study. He serves on the multi-specialty board of the American Orthopaedic Association’s Own the Bone Program and on the Governing Board of the National Bone Health Alliance.
References
- 1.National Osteoporosis Foundation. Cinician’s guide to prevention and treatment of osteoporosis Secondary Cinician’s guide to prevention and treatment of osteoporosis. 2013 http://nof.org/files/nof/public/content/resource/913/files/580.pdf.
- 2.Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis Medication Use after Hip Fracture in U.S. Patients between 2002 and 2011. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2014 doi: 10.1002/jbmr.2202. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SC, Kim MS, Sanfelix-Gimeno G, et al. Use of osteoporosis medications after hospitalization for hip fracture: a cross-national study. The American journal of medicine. 2015;128(5):519–26e1. doi: 10.1016/j.amjmed.2015.01.014. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Safety Alerts for Human Medical Products - Aredia (pamidronate disodium), Zometa (zoledronic acid) Secondary Safety Alerts for Human Medical Products - Aredia (pamidronate disodium), Zometa (zoledronic acid) 2005 http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm150676.htm.
- 5.Bisphosphonates (marketed as Actonel, Actonel+Ca, Aredia, Boniva, Didronel, Fosamax, Fosamax+D, Reclast, Skelid, and Zometa) Information. Secondary Bisphosphonates (marketed as Actonel, Actonel+Ca, Aredia, Boniva, Didronel, Fosamax, Fosamax+D, Reclast, Skelid, and Zometa) Information. 2015 Jul 8; http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm101551.htm.
- 6.Ulmner M, Jarnbring F, Torring O. Osteonecrosis of the jaw in Sweden associated with the oral use of bisphosphonate. Journal of oral and maxillofacial surgery: official journal of the American Association of Oral and Maxillofacial Surgeons. 2014;72(1):76–82. doi: 10.1016/j.joms.2013.06.221. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 7.Solomon DH, Mercer E, Woo SB, Avorn J, Schneeweiss S, Treister N. Defining the epidemiology of bisphosphonate-associated osteonecrosis of the jaw: prior work and current challenges. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013;24(1):237–44. doi: 10.1007/s00198-012-2042-6. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 8.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. The New England journal of medicine. 2007;356(18):1809–22. doi: 10.1056/NEJMoa067312. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 9.Kim DH, Rogers JR, Fulchino LA, Kim CA, Solomon DH, Kim SC. Bisphosphonates and risk of cardiovascular events: a meta-analysis. PloS one. 2015;10(4):e0122646. doi: 10.1371/journal.pone.0122646. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2012;27(12):2544–50. doi: 10.1002/jbmr.1719. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 11.Gedmintas L, Solomon DH, Kim SC. Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: a systematic review and meta-analysis. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2013;28(8):1729–37. doi: 10.1002/jbmr.1893. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strom BL. Overview of Automated Databases in Pharmacoepidemiology. In: Strom BL, Kimmel SE, editors. Textbook of Pharmacoepidemiology. Philadelphia: John Wiley & Sons, Ltd; 2006. pp. 167–72. [Google Scholar]
- 13.Ray W, Griffin M, Fought R, Adams M. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45(7):703–14. doi: 10.1016/0895-4356(92)90047-q. [DOI] [PubMed] [Google Scholar]
- 14.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–59. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisman JA, Bogoch ER, Dell R, et al. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2012;27(10):2039–46. doi: 10.1002/jbmr.1698. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 16.Scaglione M, Fabbri L, Di Rollo F, Bianchi MG, Dell’omo D, Guido G. The second hip fracture in osteoporotic patients: not only an orthopaedic matter. Clinical cases in mineral and bone metabolism: the official journal of the Italian Society of Osteoporosis, Mineral Metabolism, and Skeletal Diseases. 2013;10(2):124–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Colon-Emeric C, Kuchibhatla M, Pieper C, et al. The contribution of hip fracture to risk of subsequent fractures: data from two longitudinal studies. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2003;14(11):879–83. doi: 10.1007/s00198-003-1460-x. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 18.Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic Acid in Reducing Clinical Fracture and Mortality after Hip Fracture. The New England journal of medicine. 2007;357:nihpa40967. doi: 10.1056/NEJMe074941. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bondo L, Eiken P, Abrahamsen B. Analysis of the association between bisphosphonate treatment survival in Danish hip fracture patients-a nationwide register-based open cohort study. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013;24(1):245–52. doi: 10.1007/s00198-012-2024-8. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 20.Black DM, Kelly MP, Genant HK, et al. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. The New England journal of medicine. 2010;362(19):1761–71. doi: 10.1056/NEJMoa1001086. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]