Abstract
BACKGROUND
Despite known risks of overdose and respiratory depression when treating opioid-naïve individuals with long-acting opioids, use of these potent agents may be common in nursing homes.
OBJECTIVES
To estimate prevalence of new initiation of long-acting opioids since national efforts to increase prescriber and public awareness on safe use of transdermal fentanyl patches.
DESIGN
Cross-sectional.
SETTING
US nursing homes.
PARTICIPANTS
22,253 Medicare-enrolled long-stay nursing home residents.
MEASUREMENTS
The Minimum Data Set 3.0 linked with Medicare enrollment, hospital claims, and prescription drug transaction data (January–December 2011) were used to determine the prevalence of new initiation among nursing home residents who were prescribed a long-acting opioid in the nursing home.
RESULTS
Of nursing home residents who were prescribed a long-acting opioid within 30 days of a nursing home admission (n = 12,278), 9.4% (95% confidence interval [CI]: 8.9–9.9%) lacked a prescription drug claim for a short-acting opioid in the previous 60 days. The most common initial prescriptions of long-acting opioids were fentanyl patch (51.9% of opioid-naïve nursing home residents), morphine sulfate (28.1%), and oxycodone (17.2%).
CONCLUSION
New initiation of long-acting opioids—especially fentanyl patches that have been the subject of safety communications—persists in nursing homes.
Keywords: nursing home, long-acting opioid, analgesics
INTRODUCTION
Opioid analgesics are essential treatment options for people who suffer from moderate to severe pain, for acute or chronic pain, and for end of life care. In nursing homes—medically supervised settings that have frequent use of medications for end-of-life care1—use of these potent agents is common. Previously, we reported that among nursing home residents with a cancer diagnosis, an estimated 36% of those with daily severe pain and 21% with daily moderate pain received a long-acting opioid during the first week of a nursing home stay.2 Despite their useful role in pain management, opioids are not without risk in this frail and vulnerable population. In the nursing home setting, opioids were among the top five drugs associated with overall adverse drug events and preventable adverse drug events.3 Long-acting opioids, in particular, may be associated with greater unintentional overdose injury than short-acting opioids.4
The US Food and Drug Administration (FDA) requires that “boxed” warnings appear on the labels or package inserts of long-acting opioids to call attention to their use in opioid-tolerant patients only. Long-acting opioids are potent drugs with prolonged time to elimination. Improper use of long-acting opioids is associated with substantial health risks, such as fatal overdose due to respiratory depression among patients not already tolerant to high doses of opioids. In July 2005 and December 2007, the FDA issued public health advisory warnings to alert health care providers, patients, and caregivers on the safe use of transdermal fentanyl systems (patches).5,6 Of particular concern is the use of long-acting opioids in patients who have not recently received any opioid analgesic, defined here as “new initiation.” The only study (to our knowledge) to estimate the prevalence of new initiation of long-acting opioids in the nursing home setting used Rhode Island Medicaid data from 2004 and 2005.7 In this geographically limited study, 39.3% of nursing home residents who received a long-acting opioid had not used any opioid in the previous 60 days.7 An update to our current understanding of the prescribing of long-acting opioids to opioid-naïve nursing home residents is needed.
Whether the prevalence of new initiation of long-acting opioid use in nursing homes has declined since the FDA advisories is unknown. FDA safety communications have been associated with reduced use of antipsychotics in older adults with dementia,8 long-acting β-agonists in patients with asthma,9 antidepressants in young adults with new-onset depression,10 and rosiglitazone for treatment of type 2 diabetes.11–13 We hypothesized that the prevalence of new initiation would have declined in light of efforts to increase prescriber and public awareness on the safe use of long-acting opioids. Therefore, we used recent national data of Medicare beneficiaries to estimate the prevalence of new initiation of long-acting opioids in nursing homes.
METHODS
We used four data sources: 1) Minimum Data Set (MDS) version 3.0, 2) Master Beneficiary Summary Files that determine Medicare enrollment, 3) MedPAR files containing hospital claims data, and 4) Medicare Part D prescription drug transaction data.
MDS 3.0 is a systematic and comprehensive assessment of care planning and resident health that consists of sociodemographic information; clinical items (e.g., falls and balance items, bladder and bowel, communication, behavior, signs, symptoms); active diagnoses; and treatments, procedures, and programs.14,15 Nursing home providers are required to perform full assessments on residents at admission and annually, and a subset of the MDS items are assessed quarterly or when a resident experiences a significant change in health status.14
MDS 3.0 is a revision of MDS 2.0 (used from 1999 to 2010) and was implemented in all Medicare- and Medicaid-certified nursing homes in October 2010. It is widely accepted for research purposes and, compared to earlier versions of the resident assessment instrument, offers improved quality and completeness of some data constructs, including symptoms and psychosocial experiences.16 The most significant conceptual departure from MDS 2.0 is the inclusion of direct resident interviews to assess key domains of health. Although resident interviews are the preferred method for completing the assessment, nursing home staff may answer alternative observation items on behalf of residents who cannot make themselves understood at least some of the time or who cannot complete an interview. Family members or significant others may answer items regarding resident preferences.
Study Sample
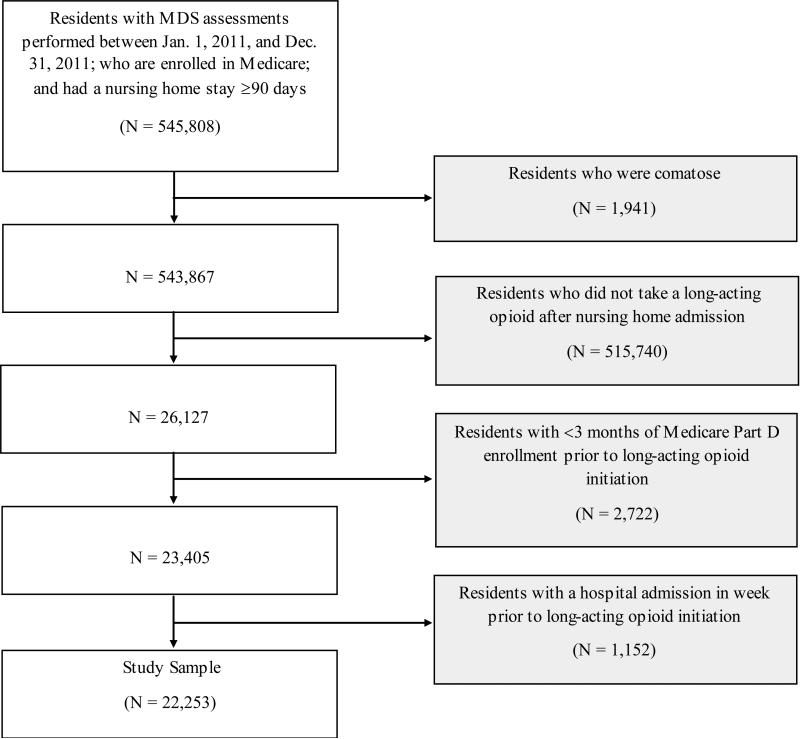
As shown in Figure 1, the sample frame for this study included Medicare-enrolled nursing home residents with an admission assessment performed between January 1, 2011, and December 31, 2011, and a nursing home stay ≥90 days (n = 545,808). Even though we focused on initiation of long acting opioids within the first months of nursing home admission, we required that residents have a long nursing home stay since short-stay residents are typically sicker that long-stay residents, and those admitted for a skilled nursing rehabilitation admission have medications covered by Medicare Part A and not Medicare Part D. We excluded residents who were comatose (n = 1,941); who did not initiate a long-acting opioid after nursing home admission (n = 517,740); or who had <3 months of continuous enrollment in Medicare Part D prior to initiation of a long-acting opioid in the nursing home (n = 2,722). The continuous enrollment restriction provided us with reassurance that if short acting opioids were obtained (either within the nursing home or prior to nursing home entry), we would have evidence in the Part D claims. We also excluded residents with a hospital admission in the seven days prior to initiation of a long-acting opioid in the nursing home to reduce the likelihood that we missed short acting opioid use initiated in the hospital setting (n = 1,152). We identified 22,253 nursing home residents who were prescribed a long acting opioid after their nursing home admission and met these eligibility criteria.
Figure 1.
Sample selection strategy.
Characteristics of Long-Stay Nursing Home Residents
Resident-level characteristics were drawn from MDS admission assessments. Key sociodemographic characteristics included age, sex, and race/ethnicity. Clinical characteristics included source of nursing home admission (acute hospital, community, other), life expectancy of <6 months at nursing home admission (indicated in MDS Section J, “Prognosis”), presence of parenteral feeding or feeding tube, difficulty chewing, difficulty swallowing, resident rejection of care “necessary to achieve the resident's goals for health and well-being,” active diagnoses that may impact analgesic treatment, functional status, cognitive status, and pain. Functional status was based on the Resource Utilization Groups (RUG)-III Activities of Daily Living (ADL) scale, with scores ranging from 4 (no impairment) to 18 (severe impairment).17 Cognitive status was based on the Cognitive Function Scale (CFS), with residents categorized as cognitively intact, mildly impaired, moderately impaired, or severely impaired.18 Section J of the MDS 3.0 defined pain as “pain or hurting at any time” during the five days preceding the assessment through either self-report or through staff assessment.19 Frequency (rarely, occasionally, frequently, almost constantly) and severity of pain using the Numeric Rating Scale (scale from 0-10 with 10 indicating horrible pain) was captured on the MDS. For staff assessed pain, other staff, direct observations and the medical record were used to evaluate indicators of pain indicators such as crying, moaning, and grimaces. The frequency of pain in the five days before the assessment was documented (none, occurring 1-2 days, 3-4 days, or daily).
Measurement of Opioid Use
We used Medicare Part D prescription drug transactions from January 1, 2011, to December 31, 2011. Data elements included brand and generic names of all prescription drugs dispensed to nursing home residents, product identification code (National Drug Code [NDC]), service date, days’ supply, quantity dispensed, drug strength, and drug formulation.
The Multum® drug database was used to code drug names and to map those names to therapeutic categories. We categorized opioid analgesics by duration of effect (i.e., long-acting, short-acting) according to recent clinical practice guidelines that consider pain management by level of opioid-tolerance.20–22 Long-acting opioids included controlled- or extended-release formulations of hydromorphone, morphine sulfate, oxycodone, oxymorphone, and tramadol, as well as any dose of fentanyl patch and buprenorphine patch. Short-acting opioids included immediate-release formulations of buprenorphine, butorphanol, codeine, fentanyl, hydrocodone, hydromorphone, meperidine, morphine sulfate, nalbuphine, opium, oxymorphone, oxycodone, pentazocine, tapentadol, and tramadol. Opioids combined with acetaminophen or non-steroidal anti-inflammatory drugs, which limit the maximum daily dose because of risks of liver and gastrointestinal toxicity, were also considered short-acting.23
The primary outcome of interest was new initiation of long-acting opioid within the first 30 days of a nursing home. Quality indicators from the Centers for Medicare and Medicaid Services (CMS) include the prevalence of uncontrolled moderate-to-severe pain occurring within the first 14 days of nursing home admission.24 We evaluated the first 30 days of a nursing home stay based on the expectation that initial provision of analgesic medications would occur shortly after admission.
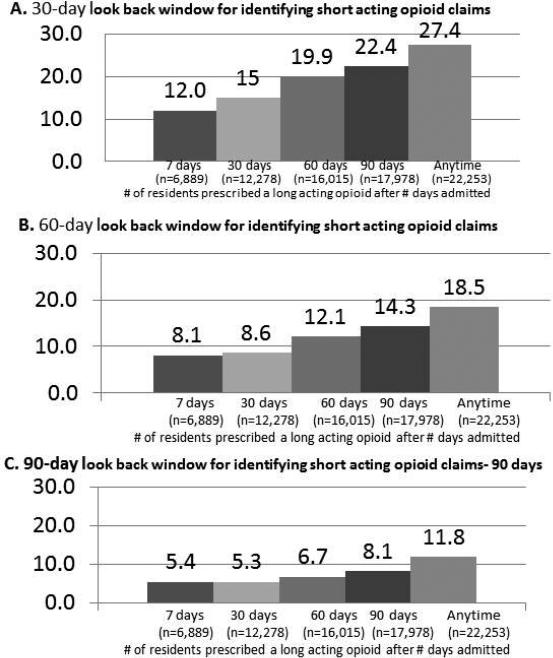
To facilitate comparison with published estimates of new initiation of long-acting opioids in nursing home residents,7 individuals were considered opioid-naïve if they had not used a short- or a long-acting opioid in the 60 days preceding initial receipt of a long-acting opioid after nursing home admission. Since some states limit prescriptions of controlled substances to a 30-day supply,25 we expected a 60-day look back period to capture intermittent use of opioid analgesics. We also evaluated initiation of long-acting opioids within different time periods after nursing home admission (7 days [n = 6,889]; 30 days [n = 12,278]; 60 days [n = 16,015]; 90 days [n = 17,978]; and anytime [n = 22,253]).
Analytic Approach
First, we used descriptive statistics to characterize nursing home residents who were prescribed a long-acting opioid during their nursing home stay. Second, we estimated the proportion of residents with new initiation of long-acting opioids by varying the look back period (30 days, 60 days, or 90 days) and the time since nursing home admission (7 days, 30 days, 60 days, 90 days, or anytime). We performed these sensitivity analyses to facilitate comparisons with previous work7 and to evaluate the impact of our look back window on our results. Finally, we describe initial long-acting opioid prescriptions and their strength, by presence of a short-acting opioid claim in the previous 60 days. With the sample size available, trivial differences in the distributions achieved statistical significance. As such, absolute differences in percentages of >5% were considered noteworthy.
RESULTS
Residents who were prescribed a long-acting opioid during their nursing home stay (n = 22,253) had a mean age of 75.0 ± 13.3 years, 71.4% were women, and 85.6% were non-Hispanic white (Table 1). The majority (64.4%) of residents were admitted to the nursing home from an acute hospital. Approximately 73.0% of residents had moderate to severe functional impairment and 19.3% had moderate to severe cognitive impairment. The most common diagnoses were arthritis (35.4%), pulmonary diseases (28.4%), heart failure (18.8%), osteoporosis (18.3%), and cancer (14.1%).
Table 1.
Characteristics of long-stay nursing home residents who were prescribed a long-acting opioid at any point after admission
| Samplea (n = 22,253) | |
|---|---|
| Age, years | |
| <65 | 18.9 |
| 65-74 | 23.4 |
| 75-84 | 30.4 |
| ≥85 | 27.3 |
| Women | 71.4 |
| Race/ethnicity | |
| Non-Hispanic white | 85.6 |
| Non-Hispanic black | 8.7 |
| Hispanic or Latino | 4.1 |
| Asian | 0.9 |
| American Indian or Alaskan Native | 0.4 |
| Native Hawaiian or Other Pacific Islander | 0.2 |
| Multiracial | 0.1 |
| Admitted fromb: | |
| Hospital | 64.4 |
| Community | 20.7 |
| Other | 14.9 |
| Life expectancy of <6 months | 6.0 |
| Degree of functional impairmentc | |
| Moderate | 43.8 |
| Severe | 29.2 |
| Degree of cognitive impairmentd | |
| Moderate | 15.5 |
| Severe | 3.8 |
| Parenteral feeding or feeding tube | 4.8 |
| Difficulty chewing | 2.0 |
| Difficulty swallowing | 4.9 |
| Key diagnoses | |
| Cancer | 14.1 |
| Arthritis | 35.4 |
| Osteoporosis | 18.3 |
| Hip fracture | 4.9 |
| Asthma/chronic obstructive pulmonary disease/chronic lung disease | 28.4 |
| Respiratory failure | 2.2 |
| Heart failure | 18.8 |
| Alzheimer disease | 5.3 |
| Stroke | 9.9 |
| Rejected care | 9.5 |
Percentages presented.
Residents were missing information on age (n = 6), race/ethnicity (n = 496), sex (n = 1), functional impairment (n = 13), cognitive impairment (n = 242), asthma (n = 7), cancer (n = 17), osteoporosis and respiratory failure (n = 11), stroke, heart failure, and arthritis (n = 10), other diagnoses (n = 12), rejected care (n = 46), feeding tube (n = 6), swallowing (n = 26), life expectancy (n = 70), pain (n = 808), pain frequency (n = 263), and pain severity (n = 593).
“Community” includes private home/apartment, board/care, assisted living, and group home. “Other” includes other nursing homes, psychiatric hospital, inpatient rehabilitation facility, mental retardation/developmental disabilities facility, hospice, and other.
Based on scores from 4 to 18: Resource Utilization Groups-III Activities of Daily Living score of 14 to 16 for moderate impairment, 17 or 18 for severe impairment.17
Based on the 4-level Cognitive Function Scale.18
Overall, 83.3% residents had documented pain in the five days preceding the assessment. Of residents able to self-report pain (n=16,970), 25.4% reported that pain was almost constant, 45.3% reported frequent pain, 25.6% reported occasional pain, 2.5% reported rarely experiencing pain and 1.0% were unable to answer. Most residents who self-reported used the numeric scale rating with 7.8% rating pain as 0 to 4, 53.0% rating pain as 5 or 6, 23.2% rating pain as 7 or 8 14.5% rating pain as 9 or 10 and 1.5% unable to answer. Among the 278 with staff assessed pain. 89.9% showed visual signs of pain and of those with documented pain, 26.8% had shown signs of pain for 1 or 2 days, 26.4% for 3 or 4 days and 46.8% daily.
Of residents who were prescribed a long-acting opioid within 30 days of admission (n = 12,278), 9.4% (95% CI: 8.9–9.9%) had not used an opioid analgesic in the previous 60 days. Figure 2 shows estimates of new long-acting opioid initiation using different look back periods and at different times since nursing home admission. Of the 22,253 residents who received a long-acting opioid in the nursing home, 6,889 (31.0%) initiated within the first week of admission and 12,278 (55.2%) within the first 30 days. The proportion with new initiation of long-acting opioids was 12.0– 27.4% using a 30-day look back period, 8.1–18.5% using a 60-day look back period, and 5.4–11.8% using a 90-day window. Regardless of look back period, estimates of new initiation were similar within 30 days of admission (e.g., 8.1% in those initiating a long-acting opioid in the first 7 days and 8.6% in the first 30 days of admission).
Figure 2.
Proportion of residents prescribed a long-acting opioid without a course of short-acting opioids, by look back window (Panel A-C) and days since admission.
Table 2 shows that the most common initial long-acting opioids were fentanyl patch (51.9% [opioid-naïve] and 56.4% [non-naïve]), morphine sulfate (28.1% [opioid-naïve] and 23.2% [non-naïve]) and oxycodone (17.2% [opioid-naïve] and 18.5% [non-naïve]). Differences in the strength of initial prescriptions for each long acting opioid are shown for opioid-naïve and non-naïve residents. Residents who had not previously received opioid therapy were more likely to receive lower doses of oxycodone (Table 2).
Table 2.
Long-acting opioids prescribed within 30 days of nursing home admission, by presence of short-acting opioid claim in prior 60 days
| Nursing home residents, % |
||
|---|---|---|
| Long-acting opioids prescribed without evidence of a short-acting opioid claim in prior 60 days (n = 1,052) | Long-acting opioids prescribed with evidence of a short-acting opioid claim in prior 60 days (n = 11,226) | |
| First long-acting opioid | Percentage | |
| Buprenorphine | 0.6 | 0.2 |
| Transdermal fentanyl | 51.9 | 56.4 |
| Hydromorphone | 0.1 | 0.0 |
| Morphine sulfate | 28.1 | 23.2 |
| Oxycodone | 17.2 | 18.5 |
| Oxymorphone | 0.8 | 0.6 |
| Tramadol | 1.3 | 1.0 |
| Percentage | ||
| Buprenorphine | (n = 6) | (n = 24) |
| 5 MCG/HR | 66.7 | 66.7 |
| 10 MCG/HR | 33.3 | 25.0 |
| 20 MCG/HR | 0.0 | 8.3 |
| Transdermal fentanyl | (n = 546) | (n = 6,327) |
| 12 MCG/HR | 26.7 | 25.9 |
| 25 MCG/HR | 37.8 | 38.6 |
| 50 MCG/HR | 20.5 | 19.7 |
| 75 MCG/HR | 8.8 | 8.8 |
| 100 MCG/HR | 6.4 | 7.0 |
| Hydromorphone | (n = 1) | (n = 5) |
| 8 MG | 0.0 | 40.0 |
| 12 MG | 0.0 | 20.0 |
| 16 MG | 100.0 | 40.0 |
| Morphine sulfate | (n = 296) | (n = 2,609) |
| ≤15 MG | 54.0 | 58.1 |
| 20 – 30 MG | 30.4 | 29.2 |
| >30 MG | 15.6 | 12.7 |
| Oxycodone | (n = 181) | (n = 2,080) |
| ≤15 MG | 65.0 | 58.7 |
| 20 – 30 MG | 20.0 | 27.5 |
| >30 MG | 15.0 | 13.8 |
| Oxymorphone | (n = 8) | (n = 71) |
| 5 MG | 50.0 | 11.3 |
| 7.5 – 10 MG | 12.5 | 18.3 |
| 15 MG | 0.0 | 9.9 |
| 20 – 30 MG | 12.5 | 43.7 |
| >30 MG | 25.0 | 16.9 |
| Tramadol | (n = 14) | (n = 110) |
| 100 MG | 71.4 | 70.0 |
| 200 MG | 28.6 | 25.5 |
| 300 MG | 0.0 | 4.6 |
a Represents the prescription that identified the nursing home resident as having received a long-acting opioid after admission.
DISCUSSION
This study reveals that more than 9% of nursing home residents who were prescribed a long-acting opioid in the first month of nursing home admission had no evidence of previous opioid therapy. These results are significantly decreased from those documented before large-scale changes to analgesic medication use in the nursing home setting. Our results are consistent with our hypothesis that prevalence of new initiation would decline after large-scale changes in analgesic medication use, including increased prescriber and public awareness on the safe use of long-acting opioids.
Dosa and colleagues estimated that approximately 39.3% of nursing home residents who received a long-acting opioid at any time during their nursing home stay may have been opioidnaïve.7 We evaluated long-acting opioids prescribed within the first 30 days of a nursing home stay, based on the expectation that initial provision of analgesic medications would occur shortly after admission. However, when using parameters similar to the prior study, we found that 18.5% of residents who received a long-acting opioid at any point after admission had no prior opioid prescription (Figure 2). Our results may also differ from the earlier estimate because of the previous study's small sample size, focus on nursing homes residents in one state (Rhode Island), and use of data that predated national changes to medication use in the nursing home setting and campaigns to increase awareness around safe opioid analgesic use.
We anticipated changes in the provision of long-acting opioids since the mid-2000s for several reasons. In March 2009, CMS revised surveyors’ interpretative guidelines for meeting compliance in the evaluation and management of pain in nursing home residents (F-Tag 309). Lapane and colleagues showed that these revisions improved nursing home providers’ recognition and management of pain, and also increased use of opioid analgesics among nursing home residents with documented non-cancer pain.26 Moreover, the January 2006 implementation of the Medicare Part D prescription drug benefit may have impacted use of opioids in the nursing home setting, specifically by instituting barriers to drugs that are expensive or that carry safety considerations.27
Few controlled studies have evaluated associations among specific long- and short-acting opioid regimens with morbidity and mortality.4 However, both the FDA and clinical guidelines for pain management in older adults strongly advise against the use of long-acting opioids to treat patients who are not already tolerant to high doses of opioid therapy.5,6,28 Serious adverse effects associated with improper use of long-acting opioids include unintentional overdose and respiratory depression. It is thus potential cause for concern that more than a quarter of nursing home residents who initiated long-acting opioids had a pulmonary condition, such as asthma, chronic obstructive pulmonary disease, and chronic lung disease. However, there are some individuals whose clinical needs require adjustments to the recommended course of stepped therapy. For example, the majority of nursing home residents who received a long-acting opioid during their nursing home stay had moderate-to-severe functional impairment. Those with feeding tubes may be unable to tolerate oral formulations or frequent dosing of short-acting analgesics. Some residents in our study also had cancer and limited life expectancy. The need to effectively control moderate-to-severe pain and provide patient comfort at the end of life may outweigh the risk of potential adverse drug effects. This is consistent with studies that have found increased polypharmacy–especially with medication for symptom control29,30–after referral to palliative care.29–32 Nursing home residents who are prescribed long-acting opioids may also benefit from less frequent medication administration, as well as decreased breakthrough pain because of stable opioid plasma levels.23
The landscape of opioid prescribing in long-term care continues to change. In July 2012, the FDA approved a class-wide Risk Evaluation and Mitigation Strategy (REMS) for long-acting opioids. The purpose of the REMS is to, “reduce serious adverse outcomes resulting from inappropriate prescribing, misuse, and abuse of extended-release or long-acting opioid analgesics while maintaining access to pain medications.”33 Through the REMS, the FDA requires pharmaceutical companies and distributors to provide education for medication prescribers and resources for counseling patients about the risks and benefits of long-acting opioid use. Failure to comply with these strategies may result in “misbranding” of the long-acting opioid and financial penalties of up to $10 million.34 Future research is needed to evaluate the extent to which these dramatic changes in medication safety strategies actually reach health care providers in nursing homes and, consequently, impact the quality of opioid therapy in this setting.
The present study has several strengths worth highlighting. First, we used national data from CMS to provide new evidence on long-acting opioid use in a large, national sample of Medicare beneficiaries residing in nursing homes. Second, we used nursing home residents assessments from the MDS 3.0, which emphasizes direct resident interviews and thus offers improved quality and completeness of information on symptoms and other subjective constructs.16 Third, our use of Medicare Part D prescription drug transactions allowed for evaluations of opioid use before and after nursing home admission. Finally, we included in our study sample nursing home residents who received any extended- or controlled-release opioid analgesic. Previous work evaluated only those who were prescribed a fentanyl patch, long-acting oxycodone, or long-acting morphine sulfate.
There were also some limitations. Due to our reliance on prescription drug transactions from a single payer, there is potential misclassification of nursing home residents as opioid-naïve. Specifically, we may have missed short-acting opioid prescriptions that were not paid by Medicare Part D. Therefore, the lack of all-payer drug dispensing records may have resulted in an overestimation of the proportion of nursing home residents who newly initiated a long-acting opioid. Moreover, absence of a prescription in a given time period does not necessarily mean absence of medication use. We may have misclassified nursing home residents who did not have an opioid prescription within our look-back windows, but who may have had access to opioids from previous prescriptions. Finally, our definition of naïve-tolerance is very liberal; we considered a nursing home resident to be opioid-tolerant if they used any dose of opioid analgesic prior to initiating a long-acting opioid.
CONCLUSION
This study demonstrates evidence of a decrease since the mid-2000s in the percentage of opioid-naïve long-stay nursing home residents who were prescribed long-acting opioids. Recent efforts to improve the quality of medication use and to increase awareness around the safe use of opioid analgesics may have had a positive effect on pain management in nursing homes. However, new initiation of long-acting opioids—especially fentanyl patches that have been the subject of FDA safety communications—appears to persist in this setting. This may indicate that educational efforts that target medication prescribers should also consider the important roles that non-prescribers (e.g., direct-care nursing staff) play in the provision of high-quality pain management for nursing home residents.
ACKNOWLEDGMENTS
Funding Sources: Dr. Pimentel is funded by the National Cancer Institute (R25CA172009). Dr. Lapane is funded by the National Cancer Institute (1R21CA198172 - 01).
Footnotes
Author Contributions: Dr. Pimentel had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Drs. Pimentel and Lapane. Acquisition of data: Drs. Tjia and Lapane. Analysis and interpretation of data: Drs. Pimentel, Gurwitz, Tjia, Hume, and Lapane. Preparation of manuscript: Drs. Pimentel and Lapane. Critical revision of manuscript for important intellectual content: Drs. Pimentel, Gurwitz, Tjia, Hume, and Lapane. Statistical analysis: Drs. Pimentel and Dr. Lapane. Obtained funding: Dr. Lapane. Study supervision: Dr. Lapane.
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Sponsor's Role: None
REFERENCES
- 1.Rigler SK, Shireman TI, Kallenbach L. Predictors of long-acting opioid use and oral versus transdermal route among older Medicaid beneficiaries. Am J Geriatr Pharmacother. 2007;5:91–99. doi: 10.1016/j.amjopharm.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Pimentel CB, Briesacher BA, Gurwitz JH, et al. Pain management in nursing home residents with cancer. J Am Geriatr Soc. 2015;63:633–661. doi: 10.1111/jgs.13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurwitz JH, Field TS, Judge J, et al. The incidence of adverse drug events in two large academic long-term care facilities. Am J Med. 2005;118:251–258. doi: 10.1016/j.amjmed.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med. 2015;175:608–615. doi: 10.1001/jamainternmed.2014.8071. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration Safety Warnings Regarding Use of Fentanyl Transdermal (Skin) Patches. 2005 Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm051739.htm.
- 6.U.S. Food and Drug Administration FDA Public Health Advisory: Important Information for the Safe Use of Fentanyl Transdermal System (Patch) 2007 Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm051257.htm.
- 7.Dosa DM, Dore DD, Mor V, et al. Frequency of long-acting opioid analgesic initiation in opioid-naïve nursing home residents. J Pain Symptom Manage. 2009;38:515–521. doi: 10.1016/j.jpainsymman.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Dorsey ER, Rabbani A, Gallagher SA, et al. Impact of FDA black box advisory on antipsychotic medication use. Arch Intern Med. 2010;170:96–103. doi: 10.1001/archinternmed.2009.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartung DM, Middleton L, Markwardt S, et al. Changes in long-acting β-agonist utilization after the FDA's 2010 drug safety communication. Clin Ther. 2015;37:114–123. doi: 10.1016/j.clinthera.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 10.Valluri S, Zito JM, Safer DJ, et al. Impact of the 2004 Food and Drug Administration pediatric suicidality warning on antidepressant and psychotherapy treatment for new-onset depression. Med Care. 2010;48:947–954. doi: 10.1097/MLR.0b013e3181ef9d2b. [DOI] [PubMed] [Google Scholar]
- 11.Aspinall SE, Zhao X, Good CB, et al. FDA warning and removal of rosiglitazone from VA national formulary. Am J Manag Care. 2013;19:748–758. [PubMed] [Google Scholar]
- 12.Shah ND, Montori VM, Krumholz HM, et al. Responding to an FDA warning--geographic variation in the use of rosiglitazone. N Engl J Med. 2010;363:2081–2084. doi: 10.1056/NEJMp1011042. [DOI] [PubMed] [Google Scholar]
- 13.Cohen A, Rabbani A, Shah N, et al. Changes in glitazone use among office-based physicians in the U.S., 2003-2009. Diabetes Care. 2010;33:823–825. doi: 10.2337/dc09-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Centers for Medicare and Medicaid Services MDS 3.0 RAI Manual. 2014 Available at: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/MDS30RAIManual.html.
- 15.Saliba D, Buchanan J. Making the investment count: Revision of the Minimum Data Set for nursing homes, MDS 3.0. J Am Med Dir Assoc. 2012;13:602–610. doi: 10.1016/j.jamda.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Mor V, Intrator O, Unruh MA, et al. Temporal and geographic variation in the validity and internal consistency of the Nursing Home Resident Assessment Minimum Data Set 2.0. BMC Health Serv Res. 2011;11:78. doi: 10.1186/1472-6963-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fries BE, Schneider DP, Foley WJ, et al. Refining a case-mix measure for nursing homes: Resource Utilization Groups (RUG-III). Med Care. 1994;32:668–685. doi: 10.1097/00005650-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Thomas KS, Dosa D, Wysocki A, et al. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care. 2015 Mar 11; doi: 10.1097/MLR.0000000000000334. [Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edelen MO, Saliba D. Correspondence of verbal descriptor and numeric rating scales for pain intensity: An item response theory calibration. J Gerontol A Biol Sci Med Sci. 2010;65:778–785. doi: 10.1093/gerona/glp215. [DOI] [PubMed] [Google Scholar]
- 20.Practice guidelines for cancer pain management A report by the American Society of Anesthesiologists Task Force on Pain Management, Cancer Pain Section. Anesthesiology. 1996;84:1243–1257. [PubMed] [Google Scholar]
- 21.Ripamonti CI, Santini D, Maranzano E, et al. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23:vii139–154. doi: 10.1093/annonc/mds233. [DOI] [PubMed] [Google Scholar]
- 22.NCCN Clinical Practice Guidelines in Oncology: Adult Cancer Pain. Available at: http://www.nccn.org/professionals/physician_gls/pdf/pain.pdf.
- 23.Argoff CE, Silvershein DI. A comparison of long- and short-acting opioids for the treatment of chronic noncancer pain: Tailoring therapy to meet patient needs. Mayo Clin Proc. 2009;84:602–612. doi: 10.1016/S0025-6196(11)60749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Centers for Medicare and Medicaid Services Design for Nursing Home Compare Five-Star Quality Rating System: Technical Users’ Guide. 2015 Available at: http://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/Downloads/usersguide.pdf.
- 25.U.S. Department of Justice Practitioner's Manual: An Informational Outline of the Controlled Substances Act. 2006 Available at: http://www.deadiversion.usdoj.gov/pubs/manuals/pract/pract_manual012508.pdf.
- 25.Rogers WH. Regression standard errors in clustered samples. Stata Tech Bull. 1993;3:19–23. [Google Scholar]
- 26.Lapane KL, Quilliam BJ, Chow W, et al. Impact of revisions to the F-Tag 309 surveyors’ interpretive guidelines on pain management among nursing home residents. Drugs Aging. 2012;29:385–393. doi: 10.2165/11599340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Stevenson DG, Keohane LM, Mitchell SL, et al. Medicare Part D Claims Rejections for Nursing Home Residents, 2006 to 2010. Am J Manag Care. 2012;18:647–654. [PMC free article] [PubMed] [Google Scholar]
- 28.American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57:1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- 29.Koh NY, Koo WH. Polypharmacy in palliative care: Can it be reduced? Singapore Med J. 2002;43:279–283. [PubMed] [Google Scholar]
- 30.Riechelmann RP, Krzyzanowska MK, O'Carroll A, et al. Symptom and medication profiles among cancer patients attending a palliative care clinic. Support Care Cancer. 2007;15:1407–1412. doi: 10.1007/s00520-007-0253-8. [DOI] [PubMed] [Google Scholar]
- 31.Currow DC, Stevenson JP, Abernethy AP, et al. Prescribing in palliative care as death approaches. J Am Geriatr Soc. 2007;55:590–595. doi: 10.1111/j.1532-5415.2007.01124.x. [DOI] [PubMed] [Google Scholar]
- 32.Nauck F, Ostgathe C, Klaschik E, et al. Drugs in palliative care: Results from a representative survey in Germany. Palliat Med. 2004;18:100–107. doi: 10.1191/0269216304pm852oa. [DOI] [PubMed] [Google Scholar]
- 33.U.S. Food and Drug Administration Extended-Release (ER) and Long-Acting (LA) Opioid Analgesic Risk Evaluation and Mitigation Strategy (REMS) 2014 Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM311290.pdf.
- 34.Mercadante S, Craig D, Giarratano A. U.S. Food and Drug Administration's Risk Evaluation and Mitigation Strategy for extended-release and long-acting opioids: Pros and cons, and a European perspective. Drugs. 2012;72:2327–2332. doi: 10.2165/11642230-000000000-00000. [DOI] [PubMed] [Google Scholar]