Abstract
Triclosan (TCS) is a synthetic antimicrobial agent used in many consumer goods at millimolar concentrations. As a result of exposure, TCS has been detected widely in humans. We have recently discovered that TCS is a proton ionophore mitochondrial uncoupler in multiple types of living cells. Here we present novel data indicating that TCS is also a mitochondrial uncoupler in a living organism: 24 hour post fertilization zebrafish embryos. These experiments were conducted using a Seahorse Bioscience XFe 96 Extracellular Flux Analyzer modified for bidirectional temperature control, using the XF96 spheroid plate to position and measure one zebrafish embryo per well. Using this method, following acute exposure to TCS, basal oxygen consumption rate (OCR) increases, without a decrease in survival or heartbeat rate. TCS also decreases ATP-linked respiration and spare respiratory capacity and increases proton leak: all indicators of mitochondrial uncoupling. Our data indicate, that TCS is a mitochondrial uncoupler in vivo, which should be taken into consideration when assessing the toxicity and/or pharmaceutical uses of TCS. This is the first example of usage of a Seahorse Extracellular Flux Analyzer to measure bioenergetic flux of a single zebrafish embryo per well in a 96 well assay format. The method developed in this study provides a high-throughput tool to identify previously-unknown mitochondrial uncouplers in a living organism.
Introduction
Triclosan (5-chloro-2-(2,4-dichlorophenoxy)phenol; TCS) is a synthetic antimicrobial agent (Lyman and Furia 1968) that has been used in hospitals since the 1970’s. TCS can be found in soaps, deodorants, mouthwashes, toothpastes, household cleaners, and more products typically at concentrations up to several mM (Jones, et al. 2000; Rodricks, et al. 2010). Most U.S. citizens are exposed to triclosan (Calafat, et al. 2008). Chronic exposure of zebrafish embryo/larvae to TCS has been found to cause delayed development, malformations, delayed hatching and inhibition of growth (Oliveira, et al. 2009).
Recently, it has been published that TCS is a proton ionophore mitochondrial uncoupler in living rat (RBL-2H3) and human (HMC-1) mast cells, mouse fibroblasts (NIH-3T3), and primary human keratinocytes (Weatherly, et al. 2015). Without cytotoxicity, TCS reduces ATP production and increases oxygen consumption rate (OCR), while its non-protonated sister compound TCS-methyl causes no such effects (Weatherly, et al. 2015). Triclosan has also been shown to impair mitochondrial function in isolated rat liver mitochondria (Ajao, et al. 2015; Newton, et al. 2005). Additionally, in vitro studies suggest that TCS inhibits mitochondrial membrane potential (Attene-Ramos, et al. 2015), an indicator of proton ionophore mitochondrial uncoupling (Kuznetsov, et al. 2011; Suzuki, et al. 2006).
In the present study, we investigate whether TCS is a mitochondrial uncoupler in an intact living organism, the 24 hour post fertilization (hpf) zebrafish embryo. Carbonyl cyanide p-triflouromethoxyphenylhydrazone (FCCP) is an ionophore proton ion carrier which disrupts ATP synthesis by transporting hydrogen ions across the inner mitochondrial membrane, thus bypassing the proton channel of ATP synthase (Heytler and Prichard 1962). In this study, we utilized FCCP, a known mitochondrial uncoupler, as a reference compound to compare the effects of TCS on mitochondrial fidelity. This study is the first demonstration of TCS’s mitochondrial effects in vivo, and our results indicate that the previous in vitro findings are valid.
Recently, Seahorse Bioscience XF-24 Extracellular Flux Analyzers have been used successfully to assess the bioenergetics of zebrafish (Bestman, et al. 2015; Gibert, et al. 2013; Stackley, et al. 2011). One of the studies measured OCR and bioenergetics of zebrafish from 3 to 48 hpf (Stackley, et al. 2011). They also validated the XFe 24 measurements with the Clark electrode method (Stackley, et al. 2011). Another XF-24 study analyzed the bioenergetics effects of the known mitochondrial uncoupler 2,4-dinitrophenol (DNP) on 3–72 hpf zebrafish (Bestman et al., 2015). However, these published studies have utilized relatively low-throughput 24-well plates, time-consuming placement of islet capture screens, and multiple fish per well (required to attain suitable signal-to-noise ratios). The present study is the first instance of using chorionated zebrafish in a custom-modified Seahorse Bioscience XFe 96 Extracellular Flux Analyzer, which allows temperature-controlled, high-throughput (96-well) bioenergetic measurements with a single embryo per well, without a need of an islet capture screen.
Materials and Methods
Animals and Ethics Statement
The fertilized eggs of wild-type zebrafish (Danio rerio, AB strain) were obtained from the Zebrafish Facility at the University of Maine by natural crosses. Embryos were maintained in egg water (60μg/mL Instant Ocean sea salts [Aquarium Systems, Mentor, OH, USA]) at 28 °C with a 14/10 hr light/dark cycle. All experiments were performed with 24 hpf zebrafish, which remain in the chorion at this stage. Animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Maine and performed in accordance with the defined guidelines for use of zebrafish embryos.
TCS preparation
Triclosan (TCS) was purchased from Sigma-Aldrich (St. Louis, MO, USA) (“irgasan,” ≥97% by HPLC; CAS no. 3380-34-5). A starting TCS stock (22.5 or 45 μM) was freshly prepared for each experimental day by dissolving TCS in egg water, without use of organic solvents, following our published protocol (Weatherly, et al. 2013). TCS concentration was determined by UV-Vis spectrophotometry (Weatherly, et al. 2013).
Mitochondrial oxygen consumption assay
A modified XFe 96 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA, USA; details of the commercially-available instrument are found at http://www.seahorsebio.com/products/how-xf-works.php) was used to measure oxygen consumption rate (OCR). Three modifications of published methods were used for this study: 1.) instrument re-configuration to allow for bi-directional temperature control; 2.) use of chorionated zebrafish embryos to obviate the need for islet capture screens; and 3.) the use of one embryo per well rather than the two or more embryos required per well in previous studies. A novel modification of this instrument was made to allow for bi-directional temperature control, in order to allow temperature stabilization at the moderate levels (28–29°C) favored by zebrafish. Prior to each run, calibration was performed using XF Calibrant solution (200 μL per well) (Seahorse Bioscience) in a XFe 96 Extracellular Flux assay kit (Seahorse Bioscience) using an automated process by the analyzer. Live zebrafish embryos (24 hpf) were arrayed in XFe 96 Spheroid microplate (Seahorse Bioscience) with 50 μL of egg water (1 embryo/well). These chorionated embryos were briefly checked to confirm that they had settled into the center of the wells. We found that the chorion provides enough mass to weigh down the embryos to keep them settled at the bottom centers of the wells, where they remained for the duration of the assays, thus obviating the need for time-consuming placement of islet capture screens. In contrast to the previously published zebrafish work utilizing the XF-24 Seahorse equipment (Bestman et al., 2015; Gibert et al., 2013; Stackley et al., 2011), only one embryo per well (rather than multiple fish) were required to attain a robust signal-to-noise ratio. The XF-24 system’s micro-chambers utilize a measurement volume of 7 μL, whereas the XFe 96’s flux measurements occur in a volume of <2 μL. This ~4X reduction in volume translates to improved sensitivity which allows for the use of a single embryo per well. OCR values resulting from one 24 hpf zebrafish embryo/well were 100–150 pmol/min, and OCR values resulting from background control wells which contained egg water but no fish were close to 0 pmol/min, ± 9–11 pmol/min (standard deviation). Thus, the signal from one chemically-untreated 24 hpf embryo is 10–15 times the noise, a robust signal-to-noise level. Next, 100 μL of either 22.5 μM or 45 μM of TCS were added to the appropriate wells, for final concentrations of 15 and 30 μM TCS, respectively. FCCP (provided in the XF Mito Stress Test Kit, Seahorse Bioscience), at a final concentration of 0.5μM, was also added (100 μL) as a positive control to the appropriate wells. Measurements of total zebrafish OCR were started immediately and performed according to the manufacturer’s instructions. Thirteen basal readings were performed, each with 2 minutes of mixing and 3 minutes of measuring. When the readings were completed, the mortality of each embryo was determined by heartbeat measurement manually by eye under the light microscope for 30 seconds per embryo (Nesan and Vijayan 2012; Oliveira, et al. 2009; Pylatiuk, et al. 2014).
XF Cell Mito Stress Test Kit OCR assay
The XF Cell Mito Stress Test kit (Seahorse Bioscience) contains three inhibitors, oligomycin, FCCP, and rotenone/antimycin-A, which target the functions of ATP synthase, mitochondrial uncoupling, and complex I/III, respectively. Oligomycin is used to distinguish the percentage of oxygen consumption devoted to ATP synthesis and the percentage of oxygen consumption required to overcome the natural proton leak across the inner mitochondrial membrane. The OCR stimulated by FCCP treatment can be used to calculate the spare respiratory capacity of cells, which is highly effective for determining mitochondrial fidelity and function. Rotenone/antimycin-A shuts down mitochondrial respiration and enables the delineation of mitochondrial versus non-mitochondrial OCR fractions contributing to respiration.
All drugs were prepared following the manufacturer’s recommendations and were dissolved in egg water. The inhibitors were then loaded into XFe 96 extracellular flux assay plate (Seahorse Bioscience). Titrations were performed to determine the optimal concentration of each inhibitor (data not shown). Before successive injections of these three drugs, basal OCR (± TCS) was measured for ~1 hour (12 cycles, each consisting of 1.5 min mixing and 3 min measurement). Then, sequential injections of 12.5 μM oligomycin, 2 μM FCCP, and 0.5 μM rotenone/antimycin-A were performed, and OCR measurements were recorded for 3 minutes (following 2 minutes of mixing) for 14, 3, and 14 times, respectively. Proton leak (which is defined as [lowest OCR measurement following oligomycin addition] - [OCR following rotenone/antimycin-A treatment]), ATP-linked respiration (which is defined as [final basal OCR measurement before oligomycin addition] - [lowest OCR measurement following oligomycin addition]), and spare respiratory capacity (which is defined as [maximal respiration following FCCP injection] - [final basal OCR measurement before oligomycin addition)] were calculated by the XF Cell Mito Stress Test Report Generator (Seahorse Bioscience). The applied concentrations of oligomycin and FCCP were previously found not to cause zebrafish embryo death (Stackley, et al. 2011).
Statistical Analysis
OCR was calculated by the Seahorse Wave software (Seahorse Bioscience). For Figure 1A, data from 16–22 embryo replicates per treatment group per experimental day were averaged at each time point. These averages were then normalized to the last time point of the 30 μM TCS sample average. These results were then further normalized by comparing them to the 0 μM TCS control from the corresponding experimental day and by multiplying by 100% to get the percent of control. Next, these normalized data from 3 independent biological replicate experiments performed on different days were averaged together and were plotted as mean ± standard error of the mean (SEM).
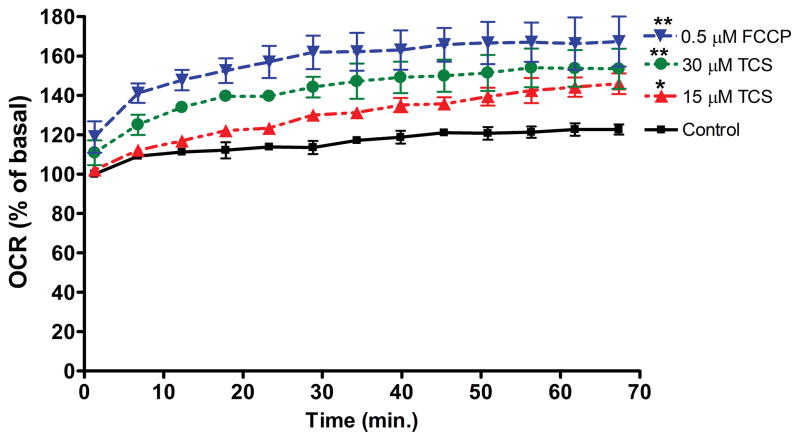
Figure 1. Measurement of basal OCR of living zebrafish embryos exposed to TCS.
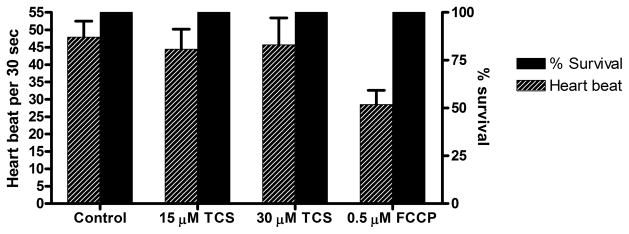
A) Basal OCR was measured for ~1 hour (13 readings), immediately after 24 hpf embryos were exposed to 0, 15, 30 μM TCS or 0.5 μM FCCP in egg water. At each timepoint, measurements were normalized to the first reading from the 0 μM control for that experimental day. Values represent means ± SEM for three independent experiments, where 16–22 embryos per treatment group were tested for each experiment. One-way ANOVA with Dunnett’s post-tests (compared to the 0 μM control) were performed using Graphpad Prism software; *p< 0.05, **p< 0.01. B) Immediately after each basal OCR assay was completed, measurements of heartbeat of individual embryos were taken for 30 seconds, and the survival rate was determined. No significance was determined by one-way ANOVA with Dunnett’s post-tests (compared to the 0 μM control).
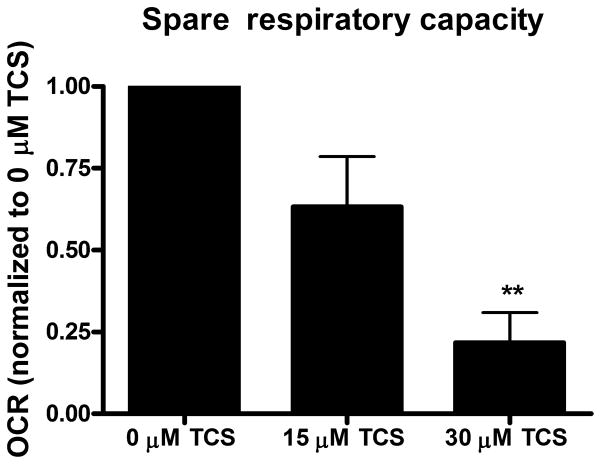
The data representing proton leak, ATP-linked respiration, and spare respiratory capacity from 4 independent biological replicate experiments performed on different days (each experiment consisted of 14–16 embryo replicates per treatment group per experimental day) were analyzed by the XF Cell Mito Stress Test Report Generator (Seahorse Bioscience). Next, each set of experimental data was normalized to either the 0 μM control or 30 μM TCS sample. These normalized data (4 experiments) were averaged together to generate the Figure 2 bar charts.
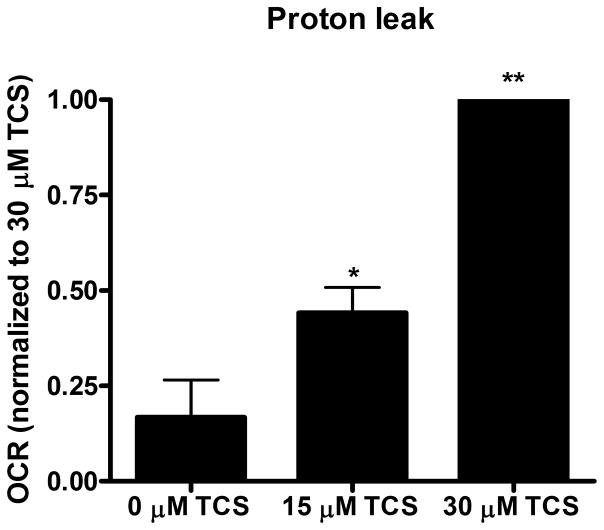
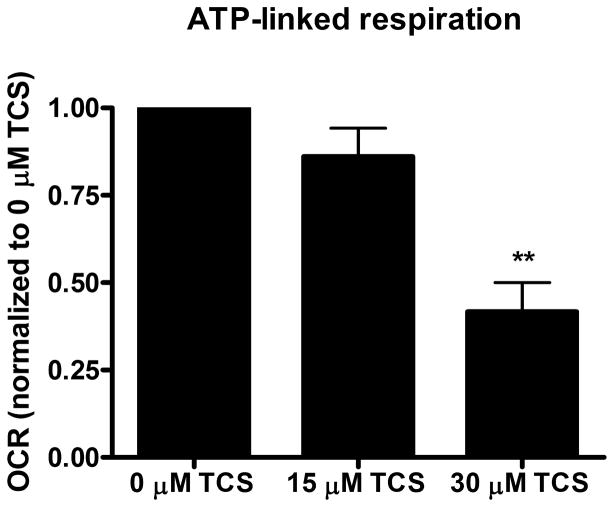
Figure 2. TCS as a mitochondrial uncoupler in living zebrafish.
Zebrafish embryos (24 hpf) were exposed to TCS for ~1 hr (12 measurements of basal OCR), then the Mito Stress Test kit (Seahorse Bioscience) was performed. A) Proton leak, B) ATP-linked respiration, and C) Spare respiratory capacity were calculated using the Mito Stress Test Report Generator V2 (Seahorse Bioscience). Data from individual experimental days were normalized either to 30 μM TCS (A) or to 0 μM TCS (B and C) and values represent means ± SEM for four independent experiments, where 14–16 embryos per treatment group were tested in each experiment. One-way ANOVA with Dunnett’s post-tests (compared to the 0 μM TCS) were performed using Graphpad Prism software; *p< 0.05, **p< 0.01
The significance was assessed via one-way analysis of variance (ANOVA) with Tukey’s or Dunnett’s post-hoc test, using Prism software (Graphpad, San Diego, CA, USA).
Results
Triclosan increases oxygen consumption rate in living zebrafish
One embryo at 24hpf was placed in each well with egg water. Various concentrations of TCS or FCCP were added right before the assay plate was placed in the analyzer for immediate OCR measurements for ~1 hour. The first OCR measurement of each experiment was recorded ~5 minutes after TCS or FCCP exposures began. Both 15 μM and 30 μM TCS cause significantly higher basal OCR compared to control (Fig. 1A). FCCP (0.5 μM) also displays higher OCR compared to the control group (Fig. 1A). Also, ANOVA with Tukey’s post-test analysis indicates that the 15 μM TCS sample is different from the 0.5 μM FCCP sample (p < 0.001) and that the 30 μM TCS sample is different from the 0.5 μM FCCP sample (p < 0.05).
For each experiment, 4–6 wells were assigned as background wells, that contained egg water ± TCS, but no embryos. Background OCR levels were not significantly altered by 30 μM TCS compared to control (data not shown), indicating that the effect on OCR is due to a biological response to TCS.
When the assay was completed, the heartbeat of each embryo was measured for 30 seconds under a light microscope. No mortality was observed in any of the groups (Fig. 1B). Also, TCS exposure did not affect heartbeat rate; however, 0.5 μM FCCP seemed to slow heartbeat rate compared to control, although this effect was not statistically significant (Fig 1B).
Triclosan causes uncoupled mitochondrial respiration in living zebrafish
To further assess whether non-lethal doses of TCS disrupt mitochondrial function, the XF Cell Mito Stress Test Kit, which contains oligomycin, FCCP, and rotenone/antimycin-A, was utilized. Immediately following TCS or 0 μM control exposure, 12 measurements of basal OCR were taken before sequential injection of oligomycin (12.5 μM), FCCP (2 μM) and rotenone/antimycin-A (0.5 μM). The proton leak, ATP-linked respiration and spare respiratory capacity were calculated as described before using the XF Cell Mito Stress Test Report Generator (Seahorse Bioscience). Both 15 and 30 μM TCS cause significant increases in proton leak, compared to 0 μM TCS control (Fig. 2A). High proton leak values indicate high levels of mitochondrial uncoupling and inefficient ATP production (Brand and Nicholls 2011). ATP-linked respiration (that is, the respiration that is correlated with mitochondrial production of ATP) is inhibited by TCS (Fig. 2B). Spare respiratory capacity also decreases significantly at 30 μM TCS (Fig. 2C), indicating mitochondrial dysfunction during conditions of increased energy demand (Brand and Nicholls 2011).
Discussion
Assessment of chemicals that can cause adverse human health and environmental effects needs to be conducted robustly and in a cost-effective manner. Zebrafish offer an effective, high-throughput low-cost alternative to mammalian testing. Zebrafish embryos have become a useful model in the field of toxicology (Braunbeck, et al. 2005; Langheinrich 2003; McGrath and Li 2008; Nagel 2002; Parng 2005). This method we have developed could be a potential high-throughput in vivo assay to screen a variety of toxicants or drugs in order to identify novel mitochondrial uncoupler or other effectors of bioenergetic function.
Using zebrafish embryos, here we show that TCS is a mitochondrial uncoupler in vivo. We show that basal OCR increases with an increase in TCS and does not decrease survival or heartbeat rate at these concentrations. We also show TCS inhibition of ATP-linked respiration and spare respiratory capacity and TCS stimulation of proton leak. Triclosan’s inhibition of ATP-linked respiration indicates that TCS inhibits mitochondrial ATP production. The increase in proton leak indicates mitochondrial uncoupling (Brand and Nicholls 2011) caused by TCS. Spare respiratory capacity is a measure of the organism’s ability to respond to an increase in energy requirement (Yadava and Nicholls 2007), and its inhibition by TCS indicates mitochondrial dysfunction (Brand and Nicholls 2011). Decrease in ATP production and increase in OCR, both of which are shown here to be caused by TCS in 24 hpf zebrafish, are two of the main hallmarks of a mitochondrial uncoupler (Brand and Nicholls 2011; Hanstein 1976; Poe, et al. 1967).
Zebrafish embryos (24–72 hpf) were previously used to assess the bioenergetics of a known mitochondrial uncoupler, 2,4-dinitrophenol (DNP), as an in vivo model (Bestman, et al. 2015). This earlier study utilized the XF-24 Extracellular Flux Analyzer (Seahorse Bioscience). An islet capture screen was utilized to keep the zebrafish in place. As in a previous study, two embryos per well were necessary at the 7–30 hpf stages, and 3 embryos per well were required at the 3 hpf stages, in order to achieve sufficient OCR levels. With 3 hr exposure to 0.5 μM DNP, beginning at 3hpf, basal OCR levels were significantly increased in zebrafish embryos (Bestman, et al. 2015). In comparison, we found that basal respiration of 24 hpf embryos is also increased due to TCS exposure for 1 hour at 15 and 30 μM (Fig. 1A). Proton leak in 24 hpf zebrafish embryos that had been treated with 0.5 μM DNP for total of 21 hours was increased (Bestman, et al. 2015). Similar to this, at 24 hpf, 15 and 30 μM TCS exposure for 1 hour cause significant increases in proton leak (Fig. 2A). At 24–72 hpf, zebrafish embryos’ ATP-linked respiration was decreased after exposure to 0.5 μM DNP for 21–69 hrs (Bestman et al, 2015). Similarly, 1 hour of TCS exposure inhibits ATP-linked respiration (Fig. 2B). Our finding that TCS causes similar effects on the key bioenergetic parameters basal OCR, ATP-linked respiration, and proton leak, compared to the known mitochondrial uncoupler DNP, further support the idea that TCS is a mitochondrial uncoupler in vivo.
The robust effects of TCS (by immersion) on zebrafish bioenergetics that we report (Fig. 1A and Fig. 2) strongly suggest that TCS passes through the chorion. However, no complete assessment of the differential permeability of the chorion, which remains intact in 24 hpf embryos, has been performed, but the chorion has been shown to act as a barrier to various compounds (Braunbeck, et al. 2005; Carlsson, et al. 2013). The chorion discriminates against certain compounds depending on chemical structure (Braunbeck, et al. 2005; Harvey, et al. 1983; Kais, et al. 2013; Sachidanandan, et al. 2008). However, the exact nature of which compounds pass more easily through the chorion is contradictory. When TCS is in egg water (pH 7.0), ~90% of TCS molecules are protonated and, thus, lipophilic. The lipophilicity of the compound does seem to be a factor in the permeability across the chorion, but whether or not more lipophilic compounds increase or decrease the permeability remains up for debate (Braunbeck et al., 2005; Sachidanandan et al., 2008), and lipophilicity is likely only one of many structural factors determining chorion permeability of a particular chemical. A study found that exposure to 1.1 μM fipronil (a broad-use insecticide), starting at 1 hpf and ending at 48 hpf, caused notochord degeneration in zebrafish (Stehr, et al. 2006). The exposure and subsequent notochord degeneration occurred while the embryos were still in the chorion, indicating that the fipronil most likely crossed the chorion barrier. Fipronil and TCS have similar two-ring organohalogen structures. Thus, along with the fact that TCS caused extensive bioenergetic effects on the zebrafish, it is highly likely that at least some fraction of the applied TCS crossed the chorion during the treatment period. Size of the compound of interest also seems to be a factor in chorion permeability. A study found that 3,000 Da fluorescent dextrans were able to pass through the chorion while 10,000 Da dextrans could not permeate the chorion (Creton 2004). Because triclosan’s molecular weight is ~289 Da, its size is likely not too large to penetrate the chorion. However, given the published data relating to the ability of the chorion to present a barrier to various xenobiotics (Braunbeck et al., 2005), it is probable that TCS’s potency is greater in dechorionated embryos.
The present investigation demonstrates that the ubiquitous antimicrobial TCS causes mitochondrial uncoupling, with no mortality, in a living organism at concentrations ~1000-fold lower than those used in personal care products. TCS at 30 μM is nearly as potent as 0.5 μM FCCP in 24 hpf zebrafish (Figure 1A), and TCS’s uncoupling effect is a biochemical mechanism that likely underlies many organismal health effects. Because triclosan’s toxicity is currently being reviewed by U.S. and other government agencies, these live animal, mechanistic data are critical. This study was performed using a novel method, utilizing live, single, chorionated zebrafish embryos in a 96-well high-throughput format with bidirectional temperature control.
Acknowledgments
Funding information: Support was provided by the National Institute of Environmental Health Sciences of the National Institute of Health under the Award Number R15ES24593.
The authors thank Erik Gerson, Logan Gerchman, Hina Hashmi, Mark Nilan, and Audrey Bergeron for help with equipment, zebrafish, and lab assistance. We also thank Dr. Kristy L. Townsend and Magdalena Blaszkiewicz for their advice and arrangement of preliminary experiments.
References
- Ajao C, Andersson MA, Teplova VV, Nagy S, Gahmberg CG, Andersson LC, Hautaniemi M, Kakasi B, Roivainen M, Salkinoja-Salonen M. Mitochondrial toxicity of triclosan on mammalian cells. Toxicology Reports. 2015;2:624–637. doi: 10.1016/j.toxrep.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attene-Ramos MS, Huang R, Michael S, Witt KL, Richard A, Tice RR, Simeonov A, Austin CP, Xia M. Profiling of the Tox21 Chemical Collection for Mitochondrial Function to Identify Compounds that Acutely Decrease Mitochondrial Membrane Potential. Environ Health Perspect. 2015;123:49–56. doi: 10.1289/ehp.1408642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestman JE, Stackley KD, Rahn JJ, Williamson TJ, Chan SS. The cellular and molecular progression of mitochondrial dysfunction induced by 2,4-dinitrophenol in developing zebrafish embryos. Differentiation. 2015;89:51–69. doi: 10.1016/j.diff.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/bj20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunbeck T, Boettcher M, Hollert H, Kosmehl T, Lammer E, Leist E, Rudolf M, Seitz N. Towards an alternative for the acute fish LC(50) test in chemical assessment: the fish embryo toxicity test goes multi-species -- an update. Altex. 2005;22:87–102. [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environ Health Perspect. 2008;116:303–307. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson G, Patring J, Kreuger J, Norrgren L, Oskarsson A. Toxicity of 15 veterinary pharmaceuticals in zebrafish (Danio rerio) embryos. Aquat Toxicol. 2013;126:30–41. doi: 10.1016/j.aquatox.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Creton R. The calcium pump of the endoplasmic reticulum plays a role in midline signaling during early zebrafish development. Brain research. Developmental brain research. 2004;151:33–41. doi: 10.1016/j.devbrainres.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Gibert Y, McGee SL, Ward AC. Metabolic profile analysis of zebrafish embryos. Journal of visualized experiments : JoVE. 2013 doi: 10.3791/4300:e4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanstein WG. Uncoupling of oxidative phosphorylation. Biochim Biophys Acta. 1976;456:129–148. doi: 10.1016/0304-4173(76)90010-0. [DOI] [PubMed] [Google Scholar]
- Harvey B, Kelley RN, Ashwood-Smith MJ. Permeability of intact and dechorionated zebra fish embryos to glycerol and dimethyl sulfoxide. Cryobiology. 1983;20:432–439. doi: 10.1016/0011-2240(83)90033-0. [DOI] [PubMed] [Google Scholar]
- Heytler PG, Prichard WW. A new class of uncoupling agents--carbonyl cyanide phenylhydrazones. Biochem Biophys Res Commun. 1962;7:272–275. doi: 10.1016/0006-291x(62)90189-4. [DOI] [PubMed] [Google Scholar]
- Jones RD, Jampani HB, Newman JL, Lee AS. Triclosan: A review of effectiveness and safety in health care settings. American Journal of Infection Control. 2000;28:184–196. [PubMed] [Google Scholar]
- Kais B, Schneider KE, Keiter S, Henn K, Ackermann C, Braunbeck T. DMSO modifies the permeability of the zebrafish (Danio rerio) chorion-implications for the fish embryo test (FET) Aquat Toxicol. 2013;140–141:229–238. doi: 10.1016/j.aquatox.2013.05.022. [DOI] [PubMed] [Google Scholar]
- Kim KT, Tanguay RL. The role of chorion on toxicity of silver nanoparticles in the embryonic zebrafish assay. Environmental health and toxicology. 2014;29:e2014021. doi: 10.5620/eht.e2014021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov AV, Margreiter R, Amberger A, Saks V, Grimm M. Changes in mitochondrial redox state, membrane potential and calcium precede mitochondrial dysfunction in doxorubicin-induced cell death. Biochim Biophys Acta. 2011;1813:1144–1152. doi: 10.1016/j.bbamcr.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Langheinrich U. Zebrafish: a new model on the pharmaceutical catwalk. BioEssays : news and reviews in molecular, cellular and developmental biology. 2003;25:904–912. doi: 10.1002/bies.10326. [DOI] [PubMed] [Google Scholar]
- Lyman FL, Furia TE. Toxicology of 2, 4,4′-trichloro-2′-hydroxyphenyl ether. IMS Ind Med Surg. 1968;37:546. [PubMed] [Google Scholar]
- McGrath P, Li CQ. Zebrafish: a predictive model for assessing drug-induced toxicity. Drug discovery today. 2008;13:394–401. doi: 10.1016/j.drudis.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Nagel R. DarT: The embryo test with the Zebrafish Danio rerio--a general model in ecotoxicology and toxicology. Altex. 2002;19(Suppl 1):38–48. [PubMed] [Google Scholar]
- Nesan D, Vijayan MM. Embryo exposure to elevated cortisol level leads to cardiac performance dysfunction in zebrafish. Mol Cell Endocrinol. 2012;363:85–91. doi: 10.1016/j.mce.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Newton AP, Cadena SM, Rocha ME, Carnieri EG, Martinelli de Oliveira MB. Effect of triclosan (TRN) on energy-linked functions of rat liver mitochondria. Toxicol Lett. 2005;160:49–59. doi: 10.1016/j.toxlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Oliveira R, Domingues I, Koppe Grisolia C, Soares AM. Effects of triclosan on zebrafish early-life stages and adults. Environ Sci Pollut Res Int. 2009;16:679–688. doi: 10.1007/s11356-009-0119-3. [DOI] [PubMed] [Google Scholar]
- Parng C. In vivo zebrafish assays for toxicity testing. Current opinion in drug discovery & development. 2005;8:100–106. [PubMed] [Google Scholar]
- Poe M, Gutfreund H, Estabrook RW. Kinetic studies of temperature changes and oxygen uptake in a differential calorimeter: the heat of oxidation of NADH and succinate. Archives of biochemistry and biophysics. 1967;122:204–211. doi: 10.1016/0003-9861(67)90140-3. [DOI] [PubMed] [Google Scholar]
- Pylatiuk C, Sanchez D, Mikut R, Alshut R, Reischl M, Hirth S, Rottbauer W, Just S. Automatic zebrafish heartbeat detection and analysis for zebrafish embryos. Zebrafish. 2014;11:379–383. doi: 10.1089/zeb.2014.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodricks JV, Swenberg JA, Borzelleca JF, Maronpot RR, Shipp AM. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Critical reviews in toxicology. 2010;40:422–484. doi: 10.3109/10408441003667514. [DOI] [PubMed] [Google Scholar]
- Sachidanandan C, Yeh JR, Peterson QP, Peterson RT. Identification of a novel retinoid by small molecule screening with zebrafish embryos. PLoS One. 2008;3:e1947. doi: 10.1371/journal.pone.0001947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackley KD, Beeson CC, Rahn JJ, Chan SS. Bioenergetic profiling of zebrafish embryonic development. PLoS One. 2011;6:e25652. doi: 10.1371/journal.pone.0025652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehr CM, Linbo TL, Incardona JP, Scholz NL. The developmental neurotoxicity of fipronil: notochord degeneration and locomotor defects in zebrafish embryos and larvae. Toxicol Sci. 2006;92:270–278. doi: 10.1093/toxsci/kfj185. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Yoshimaru T, Inoue T, Ra C. Mitochondrial Ca2+ flux is a critical determinant of the Ca2+ dependence of mast cell degranulation. Journal of leukocyte biology. 2006;79:508–518. doi: 10.1189/jlb.0705412. [DOI] [PubMed] [Google Scholar]
- Weatherly LM, Kennedy RH, Shim J, Gosse JA. A microplate assay to assess chemical effects on RBL-2H3 mast cell degranulation: effects of triclosan without use of an organic solvent. Journal of visualized experiments : JoVE. 2013 doi: 10.3791/50671:e50671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherly LM, Shim J, Hashmi HN, Kennedy RH, Hess ST, Gosse JA. Antimicrobial agent triclosan is a proton ionophore uncoupler of mitochondria in living rat and human mast cells and in primary human keratinocytes. J Appl Toxicol. 2015 doi: 10.1002/jat.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadava N, Nicholls DG. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J Neurosci. 2007;27:7310–7317. doi: 10.1523/jneurosci.0212-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]