Abstract
Aims
In regenerating liver, hepatic progenitor cells (HPCs) are recruited in response to injury; however, few highly specific human HPC markers exist for the hepatocyte lineage. LGR5, a Wnt-associated stem cell marker, has been extensively studied in intestinal stem cells, but little is known about its expression in human liver. We hypothesized that LGR5+ HPCs are induced in the regenerative response to pediatric liver injury.
Methods and Results
Immunohistochemistry was used to characterize LGR5 expression in pediatric liver explants (n = 36). We found cytoplasmic LGR5 expression in all cases; although, much less was observed in acute hepatic necrosis compared to chronic liver diseases. In the latter cases, >50% of hepatocytes were LGR5+, signifying a robust regenerative response mainly in the periphery of regenerative nodules. Only weak LGR5 staining was noted in bile ducts, suggesting hepatocyte-specific expression at the interface.
Conclusions
Although we observed some degree of regenerative response in all cases, LGR5 was highly expressed in chronic liver disease, possibly due to alternate regeneration and reprogramming pathways. LGR5 is predominant in periseptal hepatocytes rather than EpCAM+ ductular reactions in chronic pediatric liver diseases, and may represent a transitional HPC phenotype for the hepatocyte lineage. These studies are the first to support a unique role for LGR5 in human hepatocyte regeneration and as a potential predictive biomarker for recovery of liver function in children. Future work will also investigate the molecular mechanisms behind LGR5 expression.
Keywords: alpha-1 antitrypsin deficiency, biliary atresia, hepatic progenitor cell, PFIC, reprogramming, transdifferentiation
Introduction
Liver regeneration is a carefully orchestrated event, as demonstrated in rodents following 2/3 partial hepatectomy (reviewed in(1)). If the proliferative capacity of hepatocytes is overwhelmed, such as in massive hepatocyte loss from severe acute liver injury or in chronic liver damage from cirrhosis, resident hepatic progenitor cells (HPCs) are recruited to regenerate and repair the liver (2). In the rodent model, HPCs appear in the peri-portal area and bear resemblance to cells of small bile ductules (canals of Hering). These “oval cells” arise from the biliary compartment when hepatocyte proliferation is suppressed, acting as facultative adult stem cells that can transdifferentiate into hepatocytes (3). Recent experiments in zebrafish demonstrated that after near-total loss of hepatocytes, cholangiocytes can dedifferentiate and re-differentiate to completely replace lost hepatocytes (4, 5). Additional studies have shown that terminally differentiated hepatocytes are capable of spontaneously reprogramming in response to biliary injury, thereby preserving cellular plasticity (6). Similar transdifferentiation events have been reported in human liver failure (7, 8). Specifically, ductular reactions in adult humans are analogous to oval cells in the rat, with intermediate histology and phenotype (9). We have previously described EpCAM+ regenerative clusters of mixed cholangiocyte-hepatocyte differentiation in adult human fulminant hepatic failure; these clusters show promiscuous expression of hepatocyte- or cholangiocyte-associated transcription factors in either of the two cell types (10, 11).
Despite extensive research on experimentally induced models of transdifferentiation (reviewed in (12)), much less is known about parallel pathways in human pediatric liver diseases. Furthermore, discrepancies exist between rodents and humans about the relative contribution of HPCs, mature hepatocyte proliferation, and biliary cell transdifferentiation to liver regeneration. In humans with acute liver failure, >50% hepatocyte loss is required for significant activation of HPCs, and the number of HPCs is also inversely correlated with the number of Ki67+ hepatocytes, suggesting that unlike some animal models, human HPC activation depends on both hepatocyte loss and decreased proliferation (13, 14). Furthermore, in cirrhotic liver, the microenvironment plays a significant role in determining the proliferation response of hepatocytes, as demonstrated by cell transplantation and repopulation studies in rodents (15-17). Understanding the spatiotemporal expression of these signals may provide histologic clues into the regenerative capacity of hepatocytes in response to liver damage.
There is also a paucity of highly specific human markers that can demonstrate HPCs contributing to the hepatocyte lineage. Markers used to detect HPCs, such as EpCAM, are not only activated and expressed after liver injury, but are also present in cholangiocytes of healthy liver (reviewed in(14)). The LGR5/R-spondin1 axis has gained recent attention for its role in multipotent adult stem cell lineages (reviewed in(18)). LGR5, an evolutionary conserved leucine-rich repeat-containing G-protein-coupled receptor, binds to its ligand R-spondin1 (RSPO1) to form a complex analogous to Wnt/frizzled. Internalization of this complex strongly potentiates canonical pcatenin signaling. Structurally, LGR5 is described as a “G-protein coupled receptor,” but it is unclear precisely how G-proteins link to Wnt/p-Catenin downstream. LGR5+ stem cells, extensively studied in the intestinal crypts as a cycling pool of intestinal stem cells, have also been implicated in regeneration of tissues requiring high turnover (gut, skin, hair) (18). In contrast, few studies exist exploring these cells in the liver. With the exception of hepatocellular carcinoma, basal expression of LGR5 is not a feature found in resting liver (19). LGR5+ HPCs appear to be induced. Huch et al. found that following CCl4-induced liver injury in mice, LGR5+ cells acted as “first responders”, appearing as small cells near bile ducts, and lineage tracing revealed that these bipotent cells could generate hepatocytes and cholangiocytes in vivo (20). Furthermore, clonal expansion and differentiation of isolated LGR5+ cells into 3D liver organoid cultures generated functional hepatocytes when transplanted into FAH-/- mice, resulting in partial rescue of the type 1 tyrosinemia phenotype.
Although LGR5 is expressed by HPCs in injured murine liver, little is known about how endogenous LRG5+ cells may influence regeneration in human liver diseases. Identifying more specific markers would be clinically useful in assessing regenerative capacity in liver biopsy specimens. This is especially intriguing in pediatric liver disease, since the majority of children who develop chronic fibrosis have not yet progressed to tumor formation. LGR5+ cells may signify a facultative stem cell intermediate, representing transdifferentiation between cholangiocytes and hepatocytes. We hypothesized that LGR5+ cells, normally not active in resting liver, are induced as a regenerative response in pediatric liver disease, potentially to rescue dysfunctional or otherwise stressed hepatocytes that are incapable of proliferating. In the present study, we characterized LGR5 expression in liver explants primarily from pediatric patients with A1AT deficiency (A1ATD), since preliminary studies in our lab identified the novel association of LGR5 with both a progenitor-cell like phenotype and the clearance of ATZ globules in the PiZ mouse model of A1ATD (Khan et al., manuscript in preparation). We also compare LGR5 expression in A1ATD to pediatric progressive familial intrahepatic cholestasis (PFIC), biliary atresia (BA), and acute liver failure (ALF).
Materials and methods
Patients and Tissue Samples
With approval from the institutional review board of the University of Pittsburgh (PRO09030166), pediatric pathology databases were queried and a total of 36 cases were selected, consisting of A1ATD (n = 15), biliary atresia (n = 4), progressive familial intrahepatic cholestasis (PFIC, n = 10), and acute liver failure (ALF, n = 7). Four of the A1ATD cases consisted of diagnostic liver biopsies and liver explant pairs from the same patient, providing comparison of early (neonatal) and late time points. A tissue microarray of non-neoplastic cholestatic liver disease and acute hepatic necrosis explants was used for comparison with the A1ATD livers. Normal human liver controls from autopsy material were tested for staining purposes. A limited number of adult explanted livers were also available.
Histology and Immunohistochemistry
Livers were fixed in 10% buffered formalin for 24 hours and embedded into paraffin blocks and sectioned. Details on antibodies and conditions are included in Supplementary Table 1. Immunohistochemistry for LGR5 was performed using the Vectastain Elite ABC kit according to the manufacturer's protocol (Vector Laboratories, Burlingame, CA). In brief, after deparaffinization and rehydration of sections, endogenous peroxidase activity was quenched 5 minutes in dH2O containing 3% H2O2, and 10 minutes of antigen retrieval was performed in boiling 10 mmol/L citrate buffer (pH 6.0) with slow cooling. Sections were blocked 5 minutes at room temperature with Ultra V block (Thermo Fisher, Pittsburgh, PA) and then incubated with primary antibody overnight at 4°C or 30 minutes at RT. Primary-deleted negative controls for background were treated with the antibody diluent alone. After incubation for 30 minutes at room temperature with affinity-purified biotinylated secondary antibody, sections were treated with ABC reagent followed by diaminobenzidine chromagen. All sections were counterstained in hematoxylin, dehydrated, and coverslipped with Cytoseal (Richard-Allan Scientific, Kalamazoo, MI). Periodic acid-Schiff/diastase (PAS/d) staining was performed according to manufacturer's instructions (Sigma-Aldrich, St. Louis, MO). Normal intestine served as the positive control for LGR5. Immunolocalization was scored semi-quantitatively based on staining intensity and cellular distribution. The expression in hepatocytes versus bile ducts was assessed using a semi-quantitative scoring system: 1+ (<10% of cells), 2+ (10-50% of cells), and 3+ (>50% of cells).
Immunohistochemistry for cyclin D1, Epcam, HEPPAR1, β-catenin, and glutamine synthetase were performed on a Ventana Benchmark Ultra automated staining platform, using HIER (heat induced epitope retrieval) with a proprietary buffer from Ventana (Tucson, AZ). Detection of these was performed using the ultraview DAB detection kit from Ventana followed by counterstaining with hematoxylin. LGR5 immunohistochemistry was also tested on the Ventana system for reproducibility, and the overall staining pattern was preserved.
Results
LGR5 is highly expressed in pediatric A1ATD
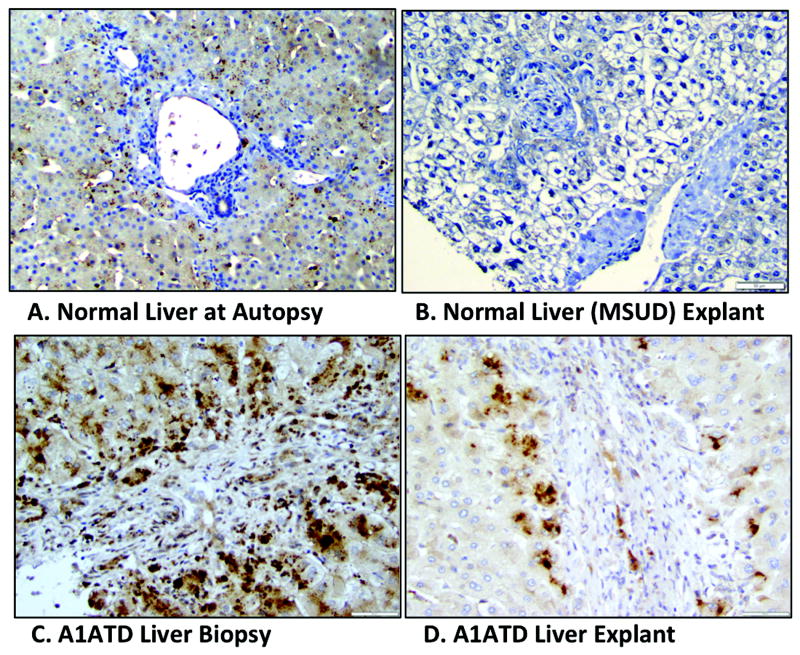
To investigate LGR5 expression is present in human A1ATD, we used immunohistochemistry to analyze human (pediatric) liver explants from A1ATD patients (n = 15), with examples shown in Fig. 1. In normal (non-diseased) liver from autopsy specimens (Fig. 1A), there was negative LGR5 expression in peri-portal hepatocytes, which is where regeneration is expected to occur. In some normal liver from autopsy cases (not shown), we did note LGR5 expression in centrilobular hepatocytes, as well as lipofuscin pigment. Fig. 1B shows a liver explant from an MSUD (maple syrup urine disease) patient, which would be the best example of freshly isolated and fixed normal pediatric liver parenchyma. We found cytoplasmic LGR5 overexpression in all 15 A1ATD pediatric cases examined. By comparing human liver biopsies (obtained in the newborn period) to explanted livers from the same patients (n = 4, of which 3 were transplanted 5, 6, and 12 years, after biopsy) with A1ATD we found robust peri-portal LGR5 expression at time of biopsy compared to transplant (Fig. 1C and D). This suggests the first evidence of a predominantly LGR5+ regenerative response earlier in the disease process, followed by decreased and likely ineffective regenerative capacity in the failing liver requiring transplant.
Figure 1. Example of LGR5 expression over time in human A1 AT deficiency.
Immunolocalization of human LGR5 in normal liver from autopsy patient (A), normal liver from an MSUD explant with no parenchymal disease (B), as well as in liver biopsy (C) and explant (D). Note that C and D are from the same A1ATD patient. The normal liver in B highlights negative LGR5 staining in the peri-portal region, which is the expected location for HPC-like cells. Magnification 100x (A), 200x (B-D).
LGR5 expression is characteristic of several chronic human liver diseases
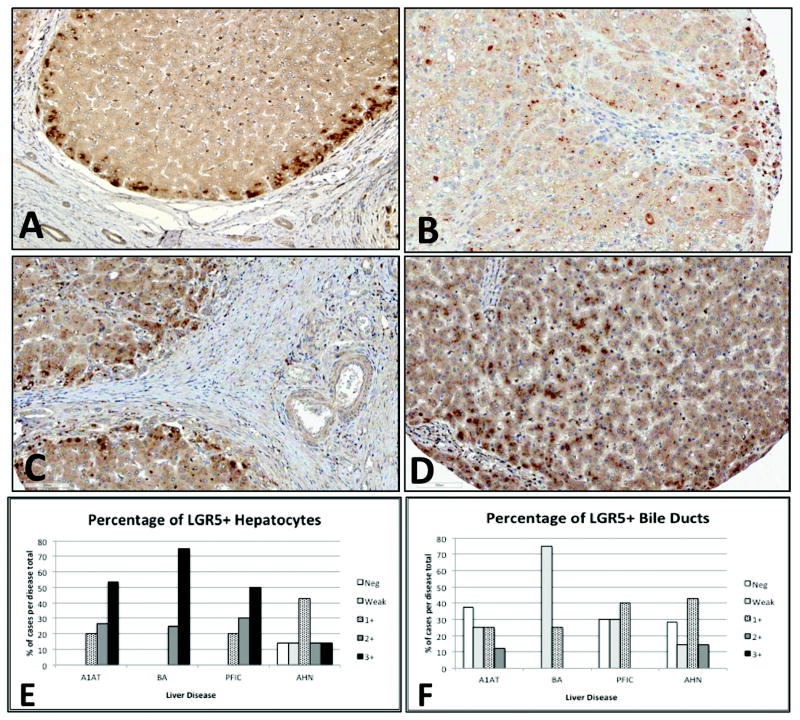
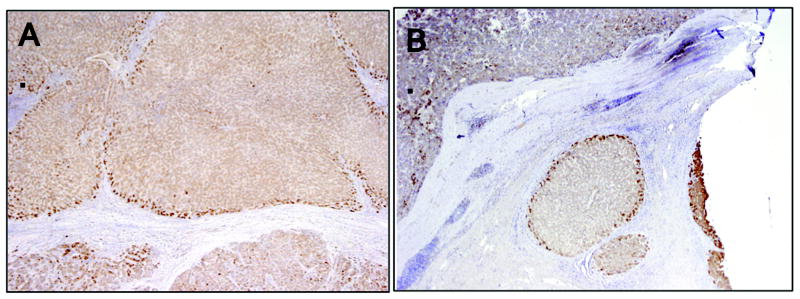
To further investigate whether LGR5 expression plays a global role in human liver injury, we used immunohistochemistry to analyze human (pediatric) liver explants from PFIC (n = 10), BA (n = 4), and ALF (n = 7). As shown in Fig. 2, we found that the majority of LGR5 expression was adjacent to areas of chronic fibrosis, even in BA (Fig 2C), where fibro-obliteration of the bile ducts is the predominant mechanism rather than direct proteotoxic injury to hepatocytes. In contrast to advanced cirrhosis, reduced LGR5 expression was observed in ALF (Fig 2B), where hepatic regenerative capacity is overwhelmed by severe acute hepatocyte necrosis. Similar peri-septal expression patterns were observed in adult cases of advanced cirrhosis secondary to either HCV infection or iron accumulation from hereditary hemochromatosis (Fig. 3), further implicating induction of LGR5+ cells as a conserved response. Intense proliferation of EpCAM+ biliary cells and hepatocytes (ductular reactions) was observed in ALF (Fig. 4A), despite reduced expression of LGR5 in hepatocytes, suggesting failing hepatocyte regeneration, with compensation by biliary cells in severe acute injury. Regenerative clusters contained LGR5+ hepatocytes surrounded by EpCAM+ biliary cells at the interface (Fig. 4, insets). Together, these results suggest that although LGR5+ cells may play a role in hepatocyte regeneration in chronic liver disease, this small subset of cells may be insufficient for adequate regeneration to occur after a massive acute insult. In the latter cases, EpCAM+ biliary cells are activated as well.
Figure 2. LGR5 expression in pediatric liver diseases.
LGR5 immunolocalization in alpha-1 antitrypsin deficiency (A), acute hepatic necrosis (B), biliary atresia (C), and progressive intrahepatic familial cholestasis (D). Note intense periseptal and peri-portal distribution in regenerative nodules (A) and markedly reduced expression in acute hepatic necrosis (B). Semi-quantitative scoring is shown for LGR5+ hepatocytes (E) versus biliary epithelial cells (F) in alpha-1 antitrypsin deficiency (A1ATD, n = 15), biliary atresia (BA, n = 4), progressive familial intrahepatic cholestasis (PFIC, n = 10), and acute hepatic necrosis (AHN, n = 7).
Figure 3. LGR5 expression in adult cases of human cirrhosis.
Peri-septal localization of LGR5 is also a feature of adult chronic liver diseases, as shown here in cases of HCV (A) and hereditary hemochromatosis (B).
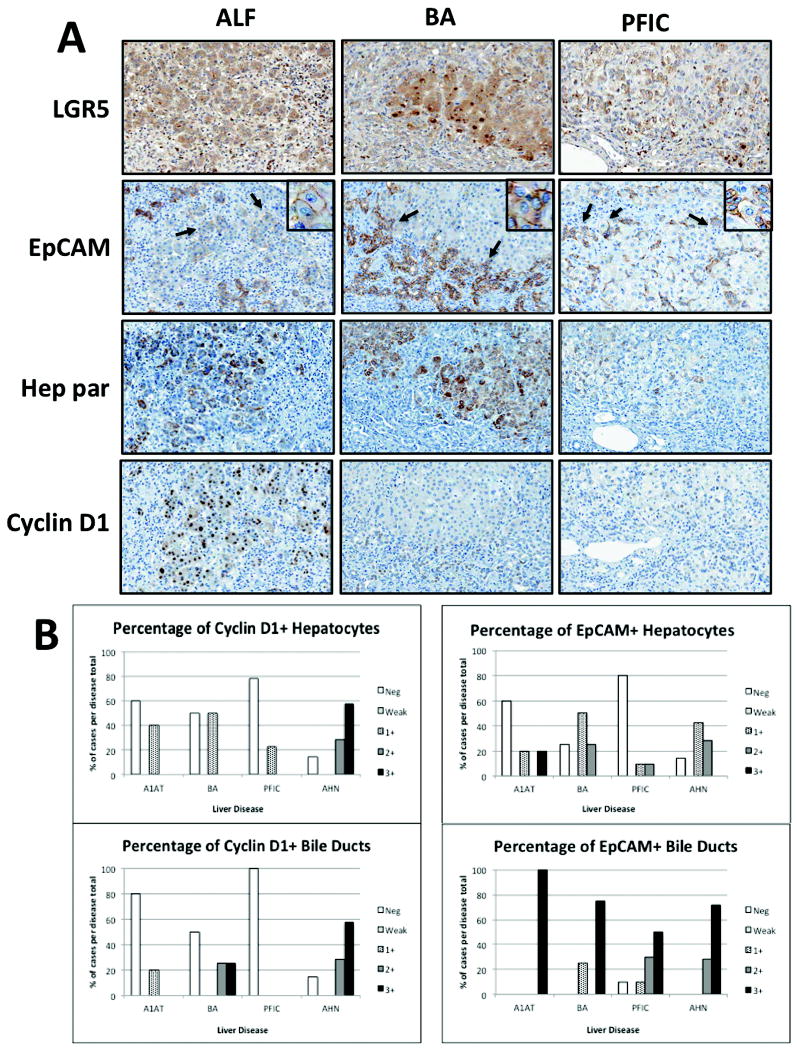
Figure 4. Liver cell reprogramming in other pediatric liver diseases.
Panel A shows comparison of immunolocalization of LGR5, EpCAM, Hep Par, and Cyclin D1 in ALF, BA, and PFIC. In ALF, a robust proliferative response (ductular reactions) to acute hepatocyte necrosis can be seen in repopulated clusters. In contrast, less Cyclin D1 staining was observed in PFIC; although, repopulated clusters of EpCAM+ hepatocytes were still present. In these examples, LGR5+ staining is most intense in biliary atresia. Evidence of transdifferentiation from EpCAM+ biliary cells to hepatocytes can be identified (arrows, insets) in all cases. Magnification 200x. Semi-quantitative scoring of EpCAM and cyclin D1 are compared for all cases in panel B for hepatocytes versus bile ducts. These results illustrate that LGR5+ cells comprise a small subset of EpCAM+ biliary cells.
LGR5 is highly expressed in peri-septal hepatocytes compared to bile ducts in pediatric liver disease
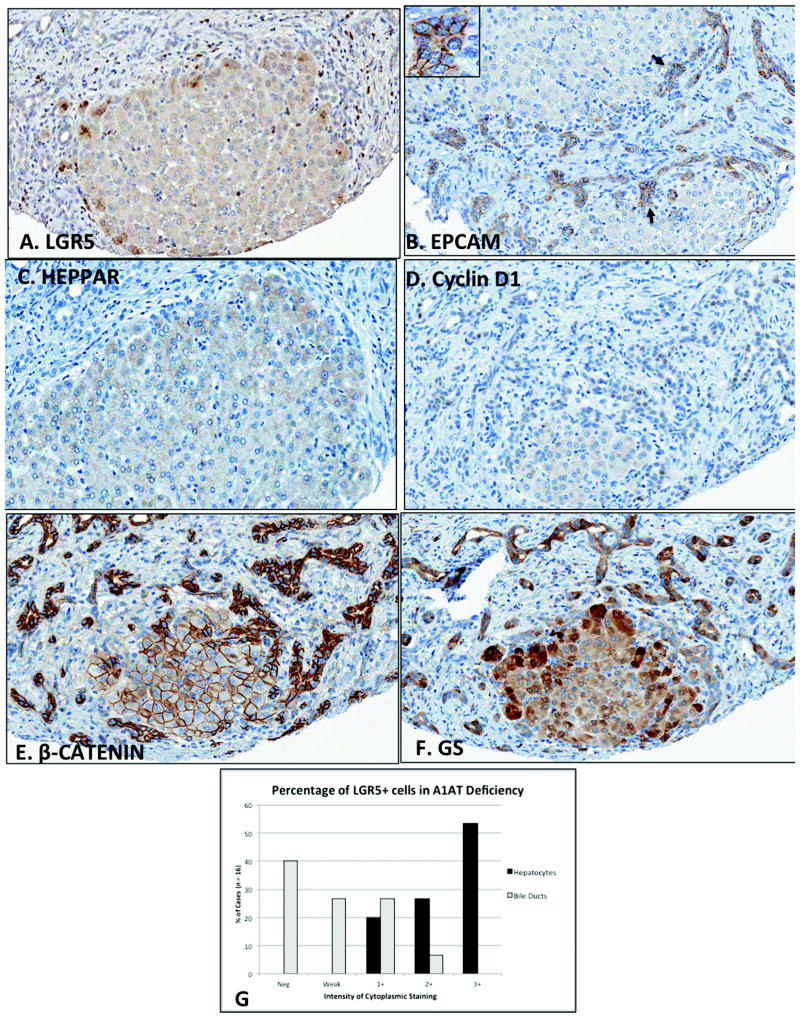
In response to liver injury, newly regenerated hepatocytes can arise directly from HPCs, or from mature hepatocytes (10, 11, 21). This altered differentiation state could represent a reprogrammed “transitional” cell population, typically with intermediate or mixed hepatocyte/cholangiocyte features. To explore our interesting findings of LGR5 versus EpCAM in pediatric liver disease, we used immunohistochemistry to analyze the expression of LRG5 in hepatocytes and cholangiocytes (Fig. 4 and 5). As shown in Fig. 5A, C, and D, marked periseptal expression was prominent in regenerative nodules, consistent with known patterns of regeneration in children and adults (as described above). Given these findings, we analyzed expression of several known HPC markers in pediatric regenerative nodules. Since LGR5 can potentiate Wnt/β-catenin signaling, we performed immunohistochemistry for β-catenin. We found strong membranous β-catenin staining in the center of the nodule, with cytoplasmic β-catenin staining at the periphery. Since β-catenin protein is susceptible to high turnover from proteosomal degradation, and we did not observe strong β-catenin nuclear localization, we further confirmed our findings with glutamine synthetase, a known Wnt target gene in the liver recently shown to be an early activated human HPC marker (22, 23). GS+ cells also stained more intensely at the periphery of the nodules (Fig. 5F). In contrast to LGR5, EpCAM, β-catenin, and GS are strongly expressed in both ductular reactions as well as hepatocytes. Finally, as tabulated in Fig. 5G, 8 of the 15 A1ATD cases (53%) showed intense 3+ LGR5 staining in the majority of hepatocytes. In contrast, 3+ staining was not observed in the bile ducts, and the majority of cases showed negative (n = 6, 40%), weak (n = 4, 27%), or 1+ (n = 4, 27%) intensity of LGR5 in biliary cells. Taken together, these results show that LGR5 is predominant in peri-septal hepatocytes rather than EpCAM+ biliary cells in chronic pediatric liver diseases, and may represent a transitional HPC phenotype for the hepatocyte lineage.
Figure 5. Peri-septal localization of LGR5 in human A1ATD.
LGR5+ cells (A) stain intensely at the perimeter of regenerative nodules, with majority of staining localizing to hepatocytes (labeled by Hep Par staining in C) compared to bile ducts. EPCAM+ biliary cells can be seen extending into regenerative clusters as fine tubules intercalating with small hepatocyte buds (B, arrows and inset). Cyclin D1 staining identifies actively proliferating biliary cells in areas of fibrosis (D). Strong membranous β-catenin staining is observed in the center of the nodule, with cytoplasmic β-catenin staining at the periphery (E). Glutamine synthetase-positive cells also stain more intensely at the periphery of the nodules (F). Unlike LGR5, EpCAM, β-catenin, and GS are strongly expressed in both ductular reactions as well as hepatocytes (magnification 200x, all sections are from the same case). Panel G shows semiquantitative scoring of LGR5+ hepatocytes versus biliary epithelial cells in A1ATD (n = 15). Scoring system is defined as negative, weak, 1+ (<10% cells), 2+ (10-50% cells), and 3+ (>50% cells).
Discussion
LGR5 is a well-known adult stem cell marker involved in the self-renewal of many organs with high-turnover. Although its role as a Wnt-associated progenitor cell marker has been extensively characterized in the intestinal epithelium, recent reports have described its expression in mouse HPCs, especially in response to liver injury (20). In contrast, studies in reporter mice have failed to show hepatic expression of LGR5 under normal physiological conditions (24). In the present study, we investigated the expression of the HPC marker LGR5 in pediatric liver diseases. Our primary focus was A1ATD, which we compared to PFIC, BA, and ALF to assess LGR5 expression in different mechanisms of liver disease (acute hepatic necrosis in ALF, chronic cholestasis without misfolded protein accumulation in PFIC, and extrahepatic biliary obstruction in BA). In liver explants, we observed strong cytoplasmic expression of LGR5 in hepatocytes, with relatively weak staining in EpCAM+ bile ducts. Unlike LGR5, EpCAM, β-catenin, and GS were strongly expressed in ductular reactions as well as peri-septal hepatocytes, suggesting a role for LGR5 as a hepatocyte-specific regeneration marker. This would be consistent with previous reports, which identified LGR5+ cells as only a small subset of EpCAM+ biliary cells isolated from adult and fetal human liver; although, the isolated cells themselves may not be comparable to those found in pediatric liver diseases (25, 26). Since some LGR5 staining is still observed in biliary cells, these regions may represent a zone of transition, where both peri-septal hepatocytes and cholangiocytes take on a progenitor cell phenotype, possibly due to transdifferentiation of proliferating bile ducts into early hepatocytes at the interface (10, 22, 27). Surprisingly, we found there was decreased expression of cyclin D1 in the chronic cholestatic liver diseases (ATD, PFIC, BA) compared to highest expression in ALF, when the liver is battling an acute hepatic necrosis, signifying that expansion of proliferating cyclin D1+ hepatocytes and EpCAM+ biliary cells are inefficient to generate functional hepatocytes once a critical mass of hepatocytes is obliterated. These findings are consistent with other reports that human HPC activation depends on both hepatocyte loss and decreased proliferation (13, 14). Of note, similar HPC incapacitation was recently reported in liver explants from patients with severe alcoholic hepatitis compared to those with cirrhosis (28). Previous studies in rodents have also demonstrated that the regenerative capacity of cirrhotic hepatocytes can be restored if they are transplanted into healthy livers, suggesting that the microenvironment impairs effective proliferation in response to chronic injury (15-17). When we further compared neonatal liver biopsies and childhood explants from the same patients with A1ATD, we found increased expression of LGR5 at time of biopsy, suggesting recruitment of a predominantly LGR5+ regenerative response early on, before end-stage cirrhosis. Interestingly, HPCs have been identified in pediatric liver diseases prior to the development of significant fibrosis, suggesting an early response to liver injury (29, 30). Recently, Kou et al. showed by immunohistochemistry that the regenerative compartment is expanded in patients undergoing liver transplantation for BA, compared to patients with an earlier stage of disease undergoing Kasai hepatoportoenterostomy (31). Specifically, they found an increase in the biliary markers CK7, CK19, and CD56 in ductular reactions at time of transplant, which also corroborates that children with advanced disease recruit more HPCs from the biliary compartment (31). Collectively, these observations support the immunophenotypic heterogeneity of HPCs in relation to disease progression and severity. Our data also point to an early progenitor cell response involving LGR5, and may favor a therapeutic window of recovery where interventions (e.g., autophagy-inducing drugs, targeted gene/protein correction, cell transplantation) may be more beneficial to the patient.
Several lines of evidence have shown that in times of stress, mature hepatocytes and biliary epithelial cells can undergo interconversion (transdifferentiation) to take on progenitor cell characteristics in an extensive and dramatic regenerative response to escape hepatocyte specific injuries (2, 4-6, 21, 32-37). Interestingly, liver cell transdifferentiation is reminiscent of the process of iPS cell reprogramming (38, 39). The emergence of this progenitor cell-like phenotype appears to be closely linked with a transitional facultative stem cell intermediate. Our data did support the presence of transitional cells with mixed cholangiocyte-hepatocyte features, confirming previous reports that transdifferentiation is a common and active process within regenerative clusters of hepatocytes in pediatric liver disease (40, 41).
Liver cells are clearly not identical to intestinal cells, and their progenitor cell response should not be expected to behave the same as well. Unlike intestine or hair or skin, there is no known rapidly cycling LGR5+ stem cell component in the liver, as there are several layers of “protection” and regeneration when the liver is damaged. Furthermore, there are considerable microenvironmental differences within the liver (zonation, oxygenation, glucose/nutrient metabolism). Endogenous LGR5+ cells must be induced, as in chronic toxic injury (20, 42). Though not shown here, recent work by our laboratory on the PiZ transgenic mouse, which recapitulates the human liver disease of A1ATD, has shown the induction of globule-free LGR5+ cells in response to stressed hepatocytes containing misfolded ATZ protein globules (Khan, Stolz, and Michalopoulos; manuscript in preparation). These LGR5+ cells are distinct and do not co-localize with ATZ globules.
We propose that LGR5+ cells in human liver disease may be analogous to the progenitor cells (“oval cells”) found in regenerating rodent liver (2, 3). In fact, the concept of a progenitor cell phenotype in pediatric liver disease is not unfamiliar. Crosby et al. first described the immunolocalization of the rat oval cell marker OV6 in human pediatric liver tissues, including four A1ATD patients (43). Unlike CK19 and HEA125, which were expressed in ductular proliferative cells, OV6 immunolocalized intensely to peri-septal hepatocytes as well as in the liver lobule, similar to our and others' findings of LGR5 labeling only a subset of EpCAM+ cells. Due to the diffuse parenchymal OV6 staining and lack of OV6 expression in normal human controls, the authors concluded that CK19-/HEA125-/OV6+ hepatocytes could represent a less differentiated “progenitor stem cell-like phenotype,” consisting of newly regenerated or transitional hepatocytes. More recently, Huch et al. demonstrated that EpCAM+ cells could be isolated from the bile ducts of adult patients with A1ATD, expanded in long-term organoid cultures, and differentiated for in vitro disease modeling (25). These bipotent EpCAM+ sorted HPCs readily expressed the stem cell markers LGR5 and PROM1/CD133 in some but not all cells, as well as biliary (SOX9, OC2) and hepatocyte (HNF4α) markers. The two aforementioned studies implicate a role for endogenous HPCs induced in human liver regeneration, and once again support the principle of immunophenotypic heterogeneity of HPCs depending on disease stage.
Collectively, our data support the initial findings of Crosby et al. in pediatric liver tissues, and open up the possibility of LGR5 as a potential predictive marker for hepatic regenerative capacity. In relation to this, a recent study by Saigusa et al. investigated LGR5 expression in biopsies of human liver damaged by chemotherapy for metastatic colorectal cancer (42). In these regenerating livers, they also observed cytoplasmic and membranous LGR5 expression in ductular reactions, but not mature bile ducts, further suggesting that immunolocalization of LGR5 is specific for a hepatocyte stress-induced progenitor cell response. Similar to our findings, they also noted increased LGR5 expression in fibrotic areas compared to normal adjacent liver, and even confirmed these results in a small number of cases of hepatitis C and BA. Increased LGR5 expression (and less expression of the biliary marker NCAM) was localized to these fibrotic areas as well, consistent with our data.
Interestingly, the regulatory mechanisms behind LGR5 structure and function are still being elucidated. For example, structurally LGR5 is described as a “G-protein coupled receptor”, and the binding of LGR5 and its high affinity ligand RSPO1 is analogous to Wnt-Frizzled, but it is not completely clear how G-proteins link with LGR5 on the membrane and the canonical Wnt/ β-catenin downstream. One possibility is co-stimulation by Wnt3a (16); however, we were unable to detect Wnt3a in our liver samples. Interestingly, Carmon et al. showed that although internalization of LGR5 is mediated through clathrin-dependent endocytosis, inhibition of this process had no effect on LGR5 signaling or potentiation of the canonical Wnt pathway (44). RSPO1 and ZNF3/RNF43 act to inversely module Wnt receptor turnover at them membrane, and these are potential mechanisms to explore in liver (15, 45). Of note, recent studies in human and mouse livers also suggest a role for both RSPO1 and RSPO2 in hepatic stellate cell activation and liver fibrosis (27, 46).
In general, after binding with their ligands, many plasma membrane receptors are internalized in the cytoplasm. The more ligand they bind, the more they are internalized. Many receptors (e.g., EGFR, LDL-R) are returned to the plasma membrane after internalization. Frizzled receptor stains are very often seen in the cytoplasm (47). Most often the receptor protein is inside the cell. In vitro live cell data by Snyder et al. demonstrated that LGR5 constitutively internalizes from the plasma membrane and retrograde traffics to the trans-Golgi network, while glucocorticoids were found to potently increase LGR5 expression at the plasma membrane (48-50). Morgan et al. have recently shown that in colorectal cancer cell lines overexpressing LGR5, glucose deprivation alters the glycosylation status of LGR5, leading to reduced cell surface expression and less robust Wnt signaling (22). Using sophisticated in vitro techniques, they concluded that in cancer, a tumor-initiating cell, when stressed by a glucosedeprived microenvironment, could obtain a competitive advantage through reduced LGR5 surface levels, by directing the cell into a pro-survival mode rather than the proliferative response associated with Wnt signaling promotion. Similar microenvironmental stress may influence the post-translational modification of LGR5 in diseased hepatocytes, which would also support our findings of predominantly cytoplasmic LGR5 localization in the liver.
There are very few published studies on LGR5 in human liver disease, with no large series in children. None of our pediatric liver explants had tumors, but they did show ductular reactions, a common feature of liver injury. Of note, Saigusa et al. found both membranous and cytoplasmic staining in ductular reactions and endothelial cells of otherwise healthy adult livers affected by chemotherapy (42). Nevertheless, pediatric liver cells (and patients) are not the same as cancer cells. As an example, we have previously shown that unlike in liver tumors, the transcription factor hypoxia-inducible factor-1α (HIF-1α) translocates from the cytoplasm to peroxisomes rather than the nucleus when “normal” non-diseased hepatocytes are exposed to hypoxic conditions (51). This may represent a mechanism to keep aberrant hypoxia-induced gene expression in check in resting liver, where a physiologic oxygen gradient provides a constant stimulus. Similarly, both membranous and cytoplasmic staining of LGR5 has been shown by others, and this may represent minimal membrane expression at baseline (when less ligand is present in “resting” conditions), but if over-expressed, the receptor could translocate to the cytoplasm. Intense nuclear localization of LGR5 can suggest a transcriptional link to the Wnt pathway once LGR5 is over-expressed. Unfortunately, most of the original work by Clevers and others on LGR5 in rodents relied on either in situ hybridization, or expression of “knock-in” reporters downstream of the LGR5 promoter (e.g. LacZ, GFP), rather than immunohistochemistry; thus, the exact surface expression of LGR5 is not consistently shown but is presumed to be membranous.
Cirrhosis is also a common feature of chronic cholestatic liver diseases. In humans, cirrhotic regenerative nodules can originate from monoclonal expansion of HPCs attempting to rescue the end-stage liver (52). Initially, CK19+ reactive bile ductules extend from peri-portal regions as delicate tubules continuous to broad septa with round rosette-like clusters of intermediate cells and immature hepatocytes (27). This mechanism has also been shown to be active in acute liver failure, providing additional reserves to the regenerative capacity in overwhelming hepatocyte loss (10, 31). Our human data suggest a similar mechanism in several pediatric liver diseases, with a peri-septal zone of transitional cells where both hepatocytes and cholangiocytes take on progenitor cell characteristics, and this may involve transdifferentiation of proliferating bile ducts into early hepatocytes. Along these lines, human and rat hepatocytes isolated from cirrhotic livers showed significant regenerative potential and metabolic function when transplanted into healthy rodent livers, indicating that the microenvironment has significant “bystander” effects on the cells' regenerative capacity (24, 53). Taken together, these studies corroborate our findings in pediatric liver diseases and highlight the role of liver cell reprogramming and plasticity as mechanisms of hepatocyte survival in cellular stress.
In conclusion, we present the first evidence of an early LGR5+ regenerative response in pediatric liver diseases. LGR5 may represent a useful regenerative marker for a progenitor cell-like phenotype specifically associated with the hepatocyte lineage. Future experiments will aim to identify critical intercellular pathways that can induce LGR5+ cells and support hepatocyte rescue by exploiting the liver's inherent regenerative capacity. Prospective studies in patients, such as in biopsies obtained to help diagnosis the etiology of evolving liver dysfunction, will help establish a role for LGR5 as a potential predictive biomarker for liver failure versus recovery in acute and chronic liver diseases.
Supplementary Material
Acknowledgments
We acknowledge Lori A. Schmitt for her excellent technical assistance. Funding for this research was provided in part by NIH grants PHS 1T32HD071834 (ZK), PHS K12HD052892 (ZK), and NIDDK P01DK096990 (GKM). This work was also partially supported by the Alpha-1 Foundation (ZK) and the Hillman Foundation (ZK).
Grant support: ZK: T32HD0718344, K12HD052892, the Alpha-1 Foundation, and the Hillman Foundation. GKM: NIH/NIDDK P01DK096990.
List of abbreviations
- A1ATD
alpha-1 antitrypsin deficiency
- AFP
alpha fetoprotein
- ALF
acute liver failure
- AHN
acute hepatic necrosis
- BA
biliary atresia
- EpCAM
epithelial cell adhesion molecule
- GS
glutamine synthetase
- HPC
hepatic progenitor cell
- LGR5
leucine-rich repeat-containing G-protein coupled receptor 5
- MSUD
maple syrup urine disease
- PFIC
progressive familial intrahepatic cholestasis
- RSPO1
R-spondin1
Footnotes
Disclosures/Conflict of interest: The authors do not have any disclosures or conflicts of interest.
References
- 1.Michalopoulos GK. Principles of liver regeneration and growth homeostasis. Compr Physiol. 2013;3:485–513. doi: 10.1002/cphy.c120014. [DOI] [PubMed] [Google Scholar]
- 2.Michalopoulos GK, Khan Z. Liver Stem Cells: Experimental Findings and Implications for Human Liver Disease. Gastroenterology. 2015;149:876–882. doi: 10.1053/j.gastro.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tatematsu M, Ho RH, Kaku T, Ekem JK, Farber E. Studies on the proliferation and fate of oval cells in the liver of rats treated with 2-acetylaminofluorene and partial hepatectomy. Am J Pathol. 1984;114:418–430. [PMC free article] [PubMed] [Google Scholar]
- 4.Choi TY, Ninov N, Stainier DY, Shin D. Extensive conversion of hepatic biliary epithelial cells to hepatocytes after near total loss of hepatocytes in zebrafish. Gastroenterology. 2014;146:776–788. doi: 10.1053/j.gastro.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He J, Lu H, Zou Q, Luo L. Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology. 2014;146:789–800 e788. doi: 10.1053/j.gastro.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 6.Yanger K, Zong Y, Maggs LR, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719–724. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roskams T, De Vos R, van den Oord JJ, Desmet V. Cells with neuroendocrine features in regenerating human liver. APMIS Suppl. 1991;23:32–39. [PubMed] [Google Scholar]
- 8.Roskams T, van den Oord JJ, De Vos R, Desmet VJ. Neuroendocrine features of reactive bile ductules in cholestatic liver disease. Am J Pathol. 1990;137:1019–1025. [PMC free article] [PubMed] [Google Scholar]
- 9.Demetris AJ, Seaberg EC, Wennerberg A, Ionellie J, Michalopoulos G. Ductular reaction after submassive necrosis in humans. Special emphasis on analysis of ductular hepatocytes. Am J Pathol. 1996;149:439–448. [PMC free article] [PubMed] [Google Scholar]
- 10.Hattoum A, Rubin E, Orr A, Michalopoulos GK. Expression of hepatocyte epidermal growth factor receptor, FAS and glypican 3 in EpCAM-positive regenerative clusters of hepatocytes, cholangiocytes, and progenitor cells in human liver failure. Hum Pathol. 2013;44:743–749. doi: 10.1016/j.humpath.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Limaye PB, Alarcon G, Walls AL, et al. Expression of specific hepatocyte and cholangiocyte transcription factors in human liver disease and embryonic development. Lab Invest. 2008;88:865–872. doi: 10.1038/labinvest.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michalopoulos GK. Liver regeneration: alternative epithelial pathways. Int J Biochem Cell Biol. 2011;43:173–179. doi: 10.1016/j.biocel.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katoonizadeh A, Nevens F, Verslype C, Pirenne J, Roskams T. Liver regeneration in acute severe liver impairment: a clinicopathological correlation study. Liver Int. 2006;26:1225–1233. doi: 10.1111/j.1478-3231.2006.01377.x. [DOI] [PubMed] [Google Scholar]
- 14.Shin S, Kaestner KH. The origin, biology, and therapeutic potential of facultative adult hepatic progenitor cells. Curr Top Dev Biol. 2014;107:269–292. doi: 10.1016/B978-0-12-416022-4.00010-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Yannam GR, Nishikawa T, et al. The microenvironment in hepatocyte regeneration and function in rats with advanced cirrhosis. Hepatology. 2012;55:1529–1539. doi: 10.1002/hep.24815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishikawa T, Bell A, Brooks JM, et al. Resetting the transcription factor network reverses terminal chronic hepatic failure. J Clin Invest. 2015;125:1533–1544. doi: 10.1172/JCI73137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suemizu H, Nakamura K, Kawai K, et al. Hepatocytes buried in the cirrhotic livers of patients with biliary atresia proliferate and function in the livers of urokinase-type plasminogen activator-NOG mice. Liver Transpl. 2014;20:1127–1137. doi: 10.1002/lt.23916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koo BK, Clevers H. Stem Cells Marked by the R-Spondin Receptor LGR5. Gastroenterology. 2014;147:289–302. doi: 10.1053/j.gastro.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Ekins S, Williams AJ. Finding promiscuous old drugs for new uses. Pharm Res. 2011;28 doi: 10.1007/s11095-011-0486-6. [DOI] [PubMed] [Google Scholar]
- 20.Huch M, Dorrell C, Boj SF, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Espanol-Suner R, Carpentier R, Van Hul N, et al. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology. 2012;143:1564–1575 e1567. doi: 10.1053/j.gastro.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Fleming KE, Wanless IR. Glutamine synthetase expression in activated hepatocyte progenitor cells and loss of hepatocellular expression in congestion and cirrhosis. Liver Int. 2013;33:525–534. doi: 10.1111/liv.12099. [DOI] [PubMed] [Google Scholar]
- 23.Cadoret A, Ovejero C, Terris B, et al. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene. 2002;21:8293–8301. doi: 10.1038/sj.onc.1206118. [DOI] [PubMed] [Google Scholar]
- 24.Huch M, Dolle L. The plastic cellular states of liver cells: Are EpCAM and Lgr5 fit for purpose? Hepatology. 2016 doi: 10.1002/hep.28469. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huch M, Gehart H, van Boxtel R, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 2009;174:715–721. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin X, Yi H, Wu W, Shu J, Wu X, Yu L. R-spondin2 activates hepatic stellate cells and promotes liver fibrosis. Dig Dis Sci. 2014;59:2452–2461. doi: 10.1007/s10620-014-3208-1. [DOI] [PubMed] [Google Scholar]
- 28.Cardinale V, Carpino G, Gentile R, et al. Transplantation of human fetal biliary tree stem/progenitor cells into two patients with advanced liver cirrhosis. BMC Gastroenterol. 2014;14:204. doi: 10.1186/s12876-014-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunt EM, Blomenkamp K, Ahmed M, Ali F, Marcus N, Teckman J. Hepatic progenitor cell proliferation and liver injury in alpha-1-antitrypsin deficiency. J Pediatr Gastroenterol Nutr. 2010;51:626–630. doi: 10.1097/MPG.0b013e3181e7ff55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macarron R, Banks MN, Bojanic D, Burns DJ, Cirovic DA, Garyantes T. Impact of high-throughput screening in biomedical research. Nat Rev Drug Discov. 2011;10 doi: 10.1038/nrd3368. [DOI] [PubMed] [Google Scholar]
- 31.Kuo FY, Huang CC, Chen CL, et al. Immunohistochemical characterization of the regenerative compartment in biliary atresia: a comparison between Kasai procedure and transplant cases. Hum Pathol. 2015 doi: 10.1016/j.humpath.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Sone M, Nishikawa Y, Nagahama Y, et al. Recovery of mature hepatocytic phenotype following bile ductular transdifferentiation of rat hepatocytes in vitro. Am J Pathol. 2012;181:2094–2104. doi: 10.1016/j.ajpath.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 33.Limaye PB, Bowen WC, Orr A, Apte UM, Michalopoulos GK. Expression of hepatocytic- and biliary-specific transcription factors in regenerating bile ducts during hepatocyte-to-biliary epithelial cell transdifferentiation. Comp Hepatol. 2010;9:9. doi: 10.1186/1476-5926-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Limaye PB, Bowen WC, Orr AV, Luo J, Tseng GC, Michalopoulos GK. Mechanisms of hepatocyte growth factor-mediated and epidermal growth factor-mediated signaling in transdifferentiation of rat hepatocytes to biliary epithelium. Hepatology. 2008;47:1702–1713. doi: 10.1002/hep.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41:535–544. doi: 10.1002/hep.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarlow BD, Pelz C, Naugler WE, et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605–618. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krause P, Unthan-Fechner K, Probst I, Koenig S. Cultured hepatocytes adopt progenitor characteristics and display bipotent capacity to repopulate the liver. Cell Transplant. 2014;23:805–817. doi: 10.3727/096368913X664856. [DOI] [PubMed] [Google Scholar]
- 38.Yanger K, Stanger BZ. Liver cell reprogramming: parallels with iPSC biology. Cell Cycle. 2014;13:1211–1212. doi: 10.4161/cc.28381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhave VS, Paranjpe S, Bowen WC, et al. Genes inducing iPS phenotype play a role in hepatocyte survival and proliferation in vitro and liver regeneration in vivo. Hepatology. 2011;54:1360–1370. doi: 10.1002/hep.24507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan J, Hytiroglou P, Wieczorek R, et al. Immunohistochemical evidence for hepatic progenitor cells in liver diseases. Liver. 2002;22:365–373. doi: 10.1034/j.1600-0676.2002.01622.x. [DOI] [PubMed] [Google Scholar]
- 41.Xiao JC, Ruck P, Kaiserling E. Small epithelial cells in extrahepatic biliary atresia: electron microscopic and immunoelectron microscopic findings suggest a close relationship to liver progenitor cells. Histopathology. 1999;35:454–460. doi: 10.1046/j.1365-2559.1999.035005454.x. [DOI] [PubMed] [Google Scholar]
- 42.Saigusa S, Tanaka K, Toiyama Y, et al. Leucine-rich repeat-containing G protein-coupled receptor 5 expression in ductular reactions after chemotherapy for metastatic colorectal cancer. Hepatol Res. 2013;43:84–90. doi: 10.1111/j.1872-034X.2012.01048.x. [DOI] [PubMed] [Google Scholar]
- 43.Crosby HA, Hubscher SG, Joplin RE, Kelly DA, Strain AJ. Immunolocalization of OV-6, a putative progenitor cell marker in human fetal and diseased pediatric liver. Hepatology. 1998;28:980–985. doi: 10.1002/hep.510280412. [DOI] [PubMed] [Google Scholar]
- 44.Carmon KS, Lin Q, Gong X, Thomas A, Liu Q. LGR5 interacts and cointernalizes with Wnt receptors to modulate Wnt/beta-catenin signaling. Mol Cell Biol. 2012;32:2054–2064. doi: 10.1128/MCB.00272-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hao HX, Xie Y, Zhang Y, et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485:195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- 46.Xinguang Y, Huixing Y, Xiaowei W, Xiaojun W, Linghua Y. R-spondin1 arguments hepatic fibrogenesis in vivo and in vitro. J Surg Res. 2015;193:598–605. doi: 10.1016/j.jss.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Kadzik RS, Cohen ED, Morley MP, Stewart KM, Lu MM, Morrisey EE. Wnt ligand/Frizzled 2 receptor signaling regulates tube shape and branch-point formation in the lung through control of epithelial cell shape. Proc Natl Acad Sci U S A. 2014;111:12444–12449. doi: 10.1073/pnas.1406639111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snyder JC, Rochelle LK, Lyerly HK, Caron MG, Barak LS. Constitutive internalization of the leucine-rich G protein-coupled receptor-5 (LGR5) to the trans-Golgi network. J Biol Chem. 2013;288:10286–10297. doi: 10.1074/jbc.M112.447540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snyder JC, Pack TF, Rochelle LK, et al. A rapid and affordable screening platform for membrane protein trafficking. BMC Biology. 2015;13:1–10. doi: 10.1186/s12915-015-0216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snyder JC, Rochelle LK, Marion S, Lyerly HK, Barak LS, Caron MG. Lgr4 and Lgr5 drive the formation of long actin-rich cytoneme-like membrane protrusions. J Cell Sci. 2015;128 doi: 10.1242/jcs.166322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wermuth CG. Selective optimization of side activities: the SOSA approach. Drug Discov Today. 2006;11 doi: 10.1016/S1359-6446(05)03686-X. [DOI] [PubMed] [Google Scholar]
- 52.Lin WR, Lim SN, McDonald SA, et al. The histogenesis of regenerative nodules in human liver cirrhosis. Hepatology. 2010;51:1017–1026. doi: 10.1002/hep.23483. [DOI] [PubMed] [Google Scholar]
- 53.Morgan RG, Molnar E, Jones RF, et al. Nutrient stress alters the glycosylation status of LGR5 resulting in reduced protein stability and membrane localisation in colorectal tumour cells: implications for targeting cancer stem cells. Br J Cancer. 2015;112:714–719. doi: 10.1038/bjc.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.