Abstract
Background
It is unknown if physical activity and good diet quality modify the risk of poor outcomes, such as mortality, among older adults with sarcopenia.
Aim
To examine if physical activity and good diet quality modify the risk of poor outcomes, such as mortality, among older adults with sarcopenia
Methods
A population-based cohort study among 1,618 older-adults with sarcopenia from the Third National Health and Nutrition Survey (NHANES III; 1988-1994). Sarcopenia was defined by the European Working Group on Sarcopenia in Older People. Physical activity was self-reported, and classified as sedentary (0 bouts per week), physically inactive (1-4 bouts per week), and physically active (≥5 bouts per week). Diet quality was assessed with the healthy eating index (a scale of 0-100 representing adherence to federal dietary recommendations), and classified as poor (<51), fair (51-80), and good (>80) diet quality.
Results
Compared to participants who were sedentary, those who were physically inactive were 16% less likely to die [HR: 0.84 (95% CI: 0.64-1.09)], and those who were physically active were 25% less likely to die [HR: 0.75 (95% CI: 0.59-0.97); Ptrend=0.026]. Compared to participants with poor diet quality, those with fair diet quality were 37% less likely to die [HR: 0.63 (95% CI: 0.47-0.86)], and those with good diet quality were 45% less likely to die [HR: 0.55 (95% CI: 0.37-0.80); Ptrend=0.002].
Conclusions
Participation in physical activity and consumption of a healthy diet correspond with a lower risk of mortality among older adults with sarcopenia. Randomized trials are needed in this population.
Keywords: exercise, lifestyle, population-based, energy balance, behavior
INTRODUCTION
Sarcopenia is recognized as a syndrome characterized by critically low levels of muscle mass and muscle strength or muscle performance that predisposes individuals to adverse health outcomes [1, 2]. The prevalence of sarcopenia may be as high as 36.5% among older adults [3]. Older adults with sarcopenia are prone to higher rates of disability [4], longer hospital lengths of stay, more frequent readmissions [5], and earlier mortality [3], compared to those without sarcopenia. Consequently studies are needed to identify interventions that may improve outcomes among this large and vulnerable population of older adults.
Prior studies have shown that modifiable health behaviors, such as the participation in physical activity [6-11], and consumption of a healthy diet [6, 7, 12] (i.e., high vegetable and protein intake) may be associated with the delay or prevention of sarcopenia. However, among older adults with sarcopenia, it is unknown if participation in physical activity and consumption of a healthy diet delays or reduces the risk of poor outcomes, such as mortality. This study sought to determine if participation in physical activity and consumption of a healthy diet influence the risk of mortality among a population-based sample of 1,618 older-adults (aged ≥65 years) with sarcopenia.
METHODS
Study Population and Design
The Third National Health and Nutrition Examination Survey, 1988–1994 (NHANES III) was a stratified multistage study designed to provide health information on a nationally-representative sample of U.S. civilians. The NHANES III sample does not include persons residing in nursing homes, members of the armed forces, institutionalized persons, or U.S. nationals living abroad. Participants provided written informed consent prior to completing any study-related activities. Participants in this analysis included adults of age ≥65 years.
Definition of Sarcopenia
Sarcopenia was operationalized using gait speed as a measure of muscle function and bioimpedance analysis (BIA) as a measure of muscle mass following the criteria defined by the European Working Group on Sarcopenia in Older People [1, 2].
Gait speed was assessed using a four-meter walk. Participants completed two walks, and the faster of the two trials were used in this analysis. Gait speed was calculated as the quotient of four-meters and time (in seconds) required for the walk and expressed as meters per second (m/s). Participants with a gait speed ≤0.8 m/s were considered to have a slow gait. Gait speed ≤0.8 m/s is associated with adverse health outcomes and is recommended to identify sarcopenia [1].
BIA was assessed using a bioresistance body composition analyzer (Valhalla 1990B, Valhalla Medical, San Diego, California) [13]. Whole-body BIA measurements were obtained between the right wrist and ankle while lying in the supine position [13, 14]. All subjects fasted for a minimum of six-hours. Muscle mass was calculated using a validated equation, where skeletal muscle mass (in kilograms) equals: [(height2/BIA resistance × 0.401) + (gender × 3.825) + (age × −0.071)] + 5.102; where height is reported in centimeters; BIA resistance is reported in ohms; gender is equal to 1 for men and 0 for women; and age is reported in years [15]. This equation is correlated with muscle mass quantified using magnetic resonance imaging (r=0.93) [15]. The skeletal muscle index (SMI) is the absolute muscle mass (kg) indexed for height2 (in meters) [13]. Males with an SMI <10.76 kg/m2 and females with an SMI <6.75 kg/m2 were defined to have low muscle mass. These SMI thresholds are associated with an increased risk of disability [13], and are recommended to identify sarcopenia [1]. Participants with slow gait speed (≤0.8 m/s) and low muscle mass (SMI <10.76 kg/m2 for men and <6.75 kg/m2 for women) were classified as having sarcopenia [1].
Physical Activity
Participants were asked to report if they engaged in any of the following leisure-time physical activities during the past month: jogging or running (≥1 mile), riding a bicycle, swimming, aerobic or other dance, callisthenic or floor exercise, gardening or yard work, and weight lifting. For each affirmative response, participants were asked how frequently they engaged in the physical activity. Duration of each bout of physical activity was not collected. Participants who reported 0, 1-4, or ≥5 bouts per week of physical activity were classified as sedentary, physically inactive, and physically active, respectively [16].
Diet Quality
The Healthy Eating Index (HEI) was designed as a measure that integrates ten components of diet quality to assess conformance to federal dietary recommendations [17]. The ten dietary components include the number of servings of grains, fruits, vegetables, meats, and dairy products; intake of total fat, saturated fat, cholesterol, and sodium; and dietary variety. The HEI was derived from a single 24-hour dietary recall using an automated interview process [17, 18]. The HEI score ranges from 0 to 100, with higher scores indicating higher conformance with dietary recommendations. Participants with HEI scores <51, 51-80, and >80 were classified has having a poor, fair, or good diet quality, respectively [19].
Mortality Outcome
Vital status was identified using the National Death Index (NDI) database through December 31, 2011 Participants were linked to the NDI database using probabilistic matching that included 12 identifiers such as Social Security Number, sex, and date of birth [20]. The National Center for Health Statistics found that 96.1% of deceased participants and 99.4% of living participants were correctly classified using the probabilistic matching algorithm [21]. The National Center for Health Statistics removed select subject characteristics in the file to prevent re-identification of study participants. The publically released survival data are nearly identical to the restricted-use NHANES III mortality-linked file [22].
Covariates
Demographic information including date of birth, sex, and race were self-reported with a standardized questionnaire [23]. Height (meters), body mass (kilograms) were measured by study technicians and used to calculate body mass index (kg/m2; BMI). Cognitive function was quantified using the short portable version of the Mini Mental Status Exam to form a score that ranges from 0 to 17, with higher scores indicating better cognition [24]. The presence of comorbid health conditions including hypertension, diabetes, hyperlipidemia, COPD, arthritis, myocardial infarction, stroke, congestive heart failure, or kidney disease were self-reported. Behavioral and clinical information including smoking status, hospitalization, falls, and self-rated health were reported using a standardized questionnaire [23]. Albumin, c-reactive protein, glycated hemoglobin, insulin, glucose, and creatinine were quantified using standardized laboratory assay procedures that have been described in detail [25, 26].
Statistical Analysis
Continuous variables are presented as means (standard error) or medians (interquartile 25-75% range), and categorical variables are presented as percentages (%). We used Cox proportional hazards regression models to estimate the hazard ratio (HR) and 95% confidence interval (95% CI) between physical activity or diet quality and mortality. We tested for a dose-response effect using linear contrasts. To determine if participation in physical activity and consumption of a healthy diet interact with one-another to jointly influence the risk of mortality, we included a statistical interaction term in the Cox proportional hazards regression models. Due to the known limitations in statistical power when examining interactions [27], the a priori threshold for statistical significance for interactions was P<0.10, and the threshold for statistical significance for all other analyses was P<0.05. Sample weights were incorporated into the statistical analyses to account for nonresponse bias, and multistage sampling probabilities were used to provide estimates generalizable to the U.S. population [28]. Stata/SE v.14.1 statistical software was used for all analyses.
RESULTS
Cohort Characteristics
The prevalence of sarcopenia from the source cohort was 36.5%, yielding 1,618 study participants for this analysis [3]. The mean age of study participants was 73.1 years (range: 65-90), 46.7% were female, and 32.6% of participants reported ≥3 comorbid health conditions (Table 1). The average skeletal muscle index was 7.8 kg/m2 (range: 3.7-10.7), and gait speed was 0.63 (range: 0.27-0.80). During a median of 9.0 years [interquartile range: 4.8-14.8], 1,323 participants died (81.8%).
Table 1.
Characteristics of study participantsa
| Characteristic | Overall (N=1,618) |
|---|---|
| Demographic Characteristics | |
| Age, yrs | 73.1 (0.28) |
| Sex, % | |
| Male | 53.3 |
| Female | 46.7 |
| Race, % | |
| White | 87.3 |
| Black | 9.7 |
| Other | 3.0 |
| Anthropometric Characteristics | |
| Body Mass Index, kg/m2 | 24.7 (0.14) |
| Muscle Mass, kg | 21.6 (0.25) |
| Skeletal Muscle Index, kg/m2 | 7.8 (0.06) |
| Clinical Characteristics | |
| Cognitive Function, sp-MMSE score | 12.6 (0.13) |
| Comorbid Health Conditions, % | |
| Hypertension | 41.7 |
| Diabetes | 10.0 |
| Hyperlipidemia | 37.1 |
| COPD | 14.7 |
| Cancer | 11.1 |
| Arthritis | 45.2 |
| Myocardial Infarction | 13.0 |
| Stroke | 6.8 |
| Heart Failure | 6.9 |
| Kidney Disease | 20.3 |
| Self-Rated Health, % | |
| Excellent | 9.7 |
| Very Good | 23.3 |
| Good | 35.0 |
| Fair | 22.7 |
| Poor | 9.3 |
| Hospitalization (≥1/year), % | 17.3 |
| Falls (≥1/year), % | 23.6 |
| Gait Speed, meters/second | 0.63 (0.005) |
| Laboratory Characteristics | |
| Hemoglobin, g/dL | 14.0 (0.05) |
| Albumin, g/dL | 4.1 (0.01) |
| C-Reactive Protein, mg/dL | 0.57 (0.03) |
| Glycated Hemoglobin, % | 5.7 (0.04) |
| Insulin, pmol/L | 66.6 (2.07) |
| Glucose, mmol/L | 6.0 (0.07) |
| Creatinine, mg/dL | 1.2 (0.01) |
| Behavioral Characteristics | |
| Smoking Status, % | |
| Never | 38.7 |
| Former | 41.2 |
| Current | 20.1 |
| Physical Activity, % | |
| Sedentary (0 bouts/week) | 33.2 |
| Inactive (1-4 bouts/week) | 27.8 |
| Active (≥5 bouts/week) | 39.0 |
| Diet Quality, % | |
| Poor (<51) | 12.9 |
| Fair (51-81) | 65.1 |
| Good (>81) | 22.1 |
| Follow-Up Characteristics | |
| Died, % | 81.8 |
| Follow-Up, Yearsb | 9.0 [4.8-14.8] |
Values are means (standard error) or percentages (%) except where noted.
bMedian [Interquartle: 25-75% Range]. sp-MMSE: short-portable version of the Mini Mental Status Exam.
Physical Activity
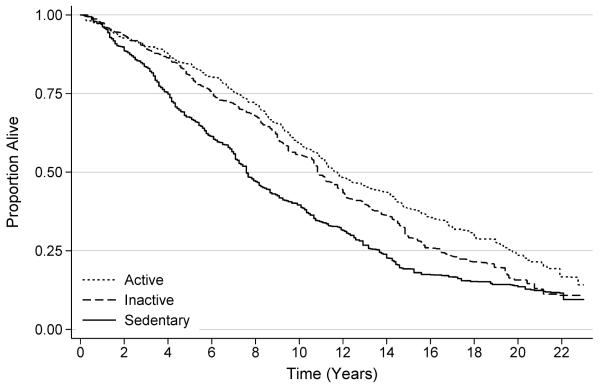
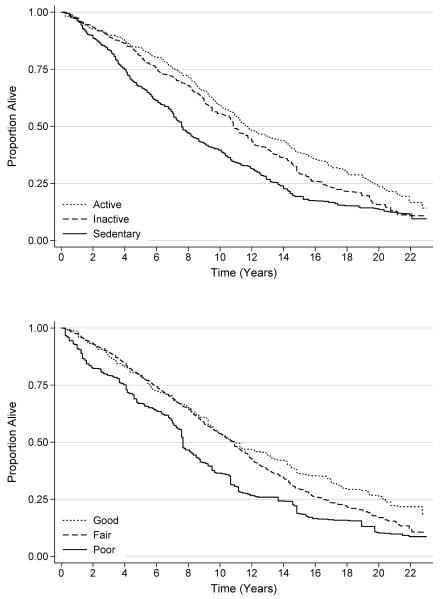
The median frequency of physical activity among all participants was two bouts per week [interquartile range: 0-8]; 33.2%, 27.8%, and 39.0% were classified as sedentary (0 bouts per week), physically inactive (1-4 bouts per week), and physically active (≥5 bouts per week), respectively. Compared to participants who were sedentary, those who were physically inactive were 16% less likely to die [HR: 0.84 (95% CI: 0.64-1.09)], and those who were physically active were 25% less likely to die [HR: 0.75 (95% CI: 0.59-0.97); Ptrend=0.026; Table 2; Figure 1]. This relationship did not change after adjustment for diet quality (Ptrend=0.047).
Table 2.
Association between physical activity and healthy eating index classification and mortality
| Model 1a | Model 2b | Model 3c | ||
|---|---|---|---|---|
|
| ||||
| Median Survival | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
|
Physical Activity Classification
|
||||
| Sedentary | 7.6 [4.0-13.7] | 1.00 -- Reference | 1.00 -- Reference | 1.00 -- Reference |
| Inactive | 10.9 [6.1-16.5] | 0.80 (0.65-0.98) | 0.84 (0.64-1.09) | 0.84 (0.65-1.09) |
| Active | 11.8 [7.2-19.8] | 0.65 (0.54-0.80) | 0.75 (0.59-0.97) | 0.78 (0.61-0.99) |
| Plog-rank<0.001 | Ptrend<0.001 | Ptrend=0.026 | Ptrend=0.047 | |
|
| ||||
|
Diet Quality
|
||||
| Poor | 7.7 [4.1-13.7] | 1.00 -- Reference | 1.00 -- Reference | 1.00 -- Reference |
| Fair | 10.8 [5.8-16.5] | 0.59 (0.46-0.74) | 0.63 (0.47-0.86) | 0.64 (0.48-0.87) |
| Good | 11.0 [5.6-20.4] | 0.53 (0.40-0.70) | 0.55 (0.37-0.80) | 0.53 (0.38-0.82) |
| Plog-rank=0.006 | Ptrend<0.001 | Ptrend=0.002 | Ptrend=0.003 | |
Note: Model 1 is adjusted for for age and sex.
Model 2 is adjusted for the covariates in model 2, and race, body mass index, smoking status, cognitive function, hypertension, hyperlipidemia, COPD, cancer, arthritis, myocardial infarction, stroke, heart failure, kidney disease, self-rated health, hospitalization, falls, hemoglobin, c-reactive protein, glycated hemoglobin, insulin, glucose, and creatinine.
Model 3 is adjusted for the covariates in model 2, and either health eating index classification (for physical activity model) or physical activity classification (for healthy eating index model).
Figure 1.
Survival of study participants, stratified by physical activity
Diet Quality
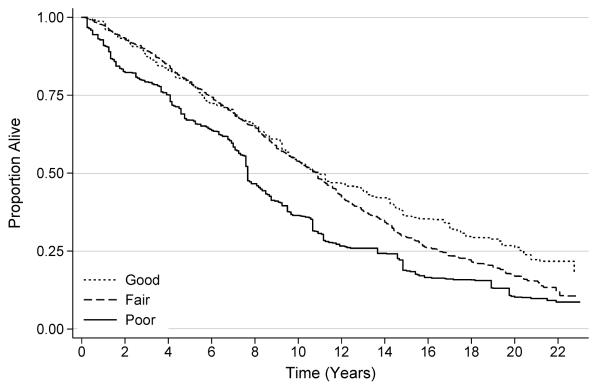
The median diet quality score among all participants was 62.8 [interquartile range: 53.6-72.1]; 12.9%, 65.1%, and 22.1% were classified with a poor (<51), fair (51-80), and good (>80) diet quality, respectively. Compared to participants with poor diet quality, those with fair diet quality were 37% less likely to die [HR: 0.63 (95% CI: 0.47-0.86)], and those with good diet quality were 45% less likely to die [HR: 0.55 (95% CI: 0.37-0.80); Ptrend=0.002; Figure 2]. This relationship did not change after adjustment for physical activity (Ptrend=0.003).
Figure 2.
Survival of study participants, stratified by diet quality
Physical Activity and Diet Quality
Physical activity and diet quality did not interact with one-another to jointly influence the risk of mortality (Pinteraction=0.640; Table 3).
Table 3.
Interaction between classification of physical activity and diet quality
| Hazard Ratio (95% Confidence Interval) | |||
|---|---|---|---|
|
| |||
| Diet Quality |
|||
| Physical Activity Classification | Poor | Fair | Good |
| Sedentary | 1.00 – Reference | 0.56 (0.39-0.79) | 0.65 (0.42-1.01) |
| Inactive | 0.70 (0.44-1.14) | 1.20 (0.70-2.05) | 0.90 (0.47-1.76) |
| Active | 0.72 (0.44-1.16) | 1.03 (0.60-1.76) | 0.72 (0.37-1.38) |
|
|
|||
| Pinteraction=0.640 | |||
Note: Adjusted for age and sex.
DISCUSSION
The major findings of this study are that participation in physical activity and the consumption of a healthy diet are both independently associated with a lower risk of mortality among older adults with sarcopenia. These data provide evidence to substantiate the importance of modifiable lifestyle behaviors among older adults with sarcopenia and advance the understanding and management of sarcopenia among community-dwelling older adults [29].
In this population-based sample of older adults, 39.0% of older adults were physically active (≥5 bouts per week), 27.8% were insufficiently physically active (1-4 bouts per week), and 33.2% participated in no physical activity (0 bouts per week). These findings are consistent with prior reports of objectively-measured physical activity which indicate that the majority of older adults with sarcopenia do not meet the recommended levels of physical activity [9, 11]. For example, among 114 men and women aged ≥60 years with sarcopenia, only 9.7% engaged in regular moderate-intensity physical activity [11]. While prior studies have focused on the benefits of participation in physical activity to delay the onset of sarcopenia and premature mortality [10, 11], our study suggests that physical activity may provide ongoing health benefits among older adults who have become sarcopenic and at greater risk of adverse health outcomes. Compared to those who were sedentary, physically inactive and physically active sarcopenic older adults were 16% and 25% less likely to die, respectively.
Our study also considered the contribution of nutrition to health outcomes among sarcopenic older adults. In this population-based sample, 22.1% of sarcopenic older adults consumed a diet that was of good quality when compared to federal dietary recommendations, 65.1% consumed a diet of fair quality, and 12.9% consumed a diet of poor quality. Our data are consistent with prior reports that older adults with sarcopenia consume a diet of poorer diet quality when compared to non-sarcopenic persons [11, 12]. For example, among 2,176 mid-age and older women, those who consumed a diet that was high in alcoholic drinks, desserts, and starchy vegetables, tended to have higher rates of sarcopenia compared to women who consumed a healthy diet pattern [12]. Prior studies suggest that a high daily intake of vegetables and protein is associated with a lower risk of developing sarcopenia [6, 7, 11]. Our data build upon these prior findings and indicate that consumption of a healthy diet may also provide health benefits among older adults who have already developed sarcopenia. Compared to those with a poor diet quality, those that consumed a diet of fair and good quality were 37% and 45% less likely to die, respectively.
These data may be useful in the design of randomized trials. Preliminary data from randomized phase-two studies are necessary to demonstrate that lifestyle modification, such as increasing physical activity and modifying diet are feasible among this population of older adults, and to better understand thresholds of activity and diet modification that are associated with improved outcomes [30]. Subsequently, randomized phase-three trials are necessary to demonstrate that modifying lifestyle behaviors such as increasing physical activity and improving diet quality improve distal health outcomes such as falls, hospitalization, and mortality that are common among older adults with sarcopenia. However, these trials will also need to demonstrate that lifestyle modification preserves or improves meaningful surrogate endpoints related to sarcopenia, such as lean body mass quantified using dual-energy absorptiometry [31]. Other important secondary endpoints may include cardiovascular fitness, muscle fiber composition, muscular strength or power, and hormonal, nutritional, and inflammatory biomarkers. The prevalence of sarcopenia among older adults and the propensity for poor outcomes underscores the importance of identifying efficacious interventions for this population [30].
There are several limitations to this study that must be acknowledged. The main limitation of this study is the observational design which limits causal inference. The assessment of physical activity and diet quality were cross-sectional measures. It is unknown if increasing physical activity or improving dietary patterns would improve health outcomes among older adults with sarcopenia. A randomized trial is necessary to examine this hypothesis. Our definition of sarcopenia did not include muscle strength, relaying on gait speed as a measure of muscle function. The physical activity questionnaire did not quantify duration of physical activity. Consequently we were unable to examine if the duration or volume of physical activity influenced the risk of mortality. We were not able to examine the specific benefits of weight lifting or muscle strengthening exercise, as a minority of older adults in this sample (<5%) reported regular recent participation in these types of activities. We were unable to examine specific dietary constituents such as protein intake. Ascertainment of mortality endpoint data was based on a probabilistic matching algorithm that was not based on direct reporting. In our interaction analyses of participation in physical activity and adherence to a healthy dietary pattern our statistical power was limited. Comorbidities in this sample were self-reported.
There are several strengths to this study. The main strength of this study is the large sample size that, based on the sampling design, is representative of the US population of community-dwelling older adults. Our sample included participants that ranged in age from 65 to 90 years. Our definition of sarcopenia was based on valid and reliable assessments of muscle mass and muscle function [1, 3]. The cohort had an extensive length of follow up (median 9.0 years). Our multivariable-adjusted regression analysis accounted for variables that are known to influence the relationship between physical activity or diet quality and mortality.
In conclusion, among older adults with sarcopenia, participation in physical activity and consumption of a healthy diet are strongly associated with a lower risk of mortality. Many older adults with sarcopenia do not achieve the recommended levels of physical activity and/or do not consume a healthy diet, as defined by federal dietary recommendations. Lifestyle modification is positioned as a potentially efficacious intervention for older adults with sarcopenia. However, a randomized controlled trial is necessary to demonstrate the feasibility and efficacy of lifestyle modification in this vulnerable population.
ACKNOWLEDGMENTS
Funding Source: Research reported in this publication was supported by the National Cancer Institute (F31-CA192560, R21-CA182726), National Heart, Lung, And Blood Institute (F31-HL127947) and the National Institute of Diabetes and Digestive and Kidney Diseases (F32-DK096758) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding: Research reported in this publication was supported by the National Cancer Institute (F31-CA192560, R21-CA182726), National Heart, Lung, and Blood Institute (F31-HL127947) and the National Institute of Diabetes and Digestive and Kidney Diseases (K23-DK105207) of the National Institutes of Health.
Footnotes
On behalf of all authors, the corresponding author states that there is no conflict of interest.
REFERENCES
- [1].Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. Journal of the American Medical Directors Association. 2011;12:249–56. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Brown JC, Harhay MO, Harhay MN. Sarcopenia and mortality amonf a population-based sample of community dwelling older adults. J Cachexia Sarcopenia Muscle. 2015 doi: 10.1002/jcsm.12073. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Janssen I. Influence of sarcopenia on the development of physical disability: the Cardiovascular Health Study. J Am Geriatr Soc. 2006;54:56–62. doi: 10.1111/j.1532-5415.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- [5].Gariballa S, Alessa A. Sarcopenia: prevalence and prognostic significance in hospitalized patients. Clinical Nutrition. 2013;32:772–6. doi: 10.1016/j.clnu.2013.01.010. [DOI] [PubMed] [Google Scholar]
- [6].Morris MS, Jacques PF. Total protein, animal protein and physical activity in relation to muscle mass in middle-aged and older Americans. Br J Nutr. 2013;109:1294–303. doi: 10.1017/S0007114512003133. [DOI] [PubMed] [Google Scholar]
- [7].Kim J, Lee Y, Kye S, Chung Y, Kim K. Association Between Healthy Diet and Exercise and Greater Muscle Mass in Older Adults. J Am Geriatr Soc. 2015;63:886–92. doi: 10.1111/jgs.13386. [DOI] [PubMed] [Google Scholar]
- [8].Shephard RJ, Park H, Park S, Aoyagi Y. Objectively measured physical activity and progressive loss of lean tissue in older Japanese adults: longitudinal data from the Nakanojo study. J Am Geriatr Soc. 2013;61:1887–93. doi: 10.1111/jgs.12505. [DOI] [PubMed] [Google Scholar]
- [9].Park H, Park S, Shephard RJ, Aoyagi Y. Yearlong physical activity and sarcopenia in older adults: the Nakanojo Study. Eur J Appl Physiol. 2010;109:953–61. doi: 10.1007/s00421-010-1424-8. [DOI] [PubMed] [Google Scholar]
- [10].Ryu M, Jo J, Lee Y, Chung YS, Kim KM, Baek WC. Association of physical activity with sarcopenia and sarcopenic obesity in community-dwelling older adults: the Fourth Korea National Health and Nutrition Examination Survey. Age Ageing. 2013;42:734–40. doi: 10.1093/ageing/aft063. [DOI] [PubMed] [Google Scholar]
- [11].Kim S, Kim T, Hwang H. The relationship of physical activity (PA) and walking with sarcopenia in Korean males aged 60 years and older using the Fourth Korean National Health and Nutrition Examination Survey (KNHANES IV-2, 3), 2008–2009. Arch Gerontol Geriatr. 2013;56:472–7. doi: 10.1016/j.archger.2012.12.009. [DOI] [PubMed] [Google Scholar]
- [12].Fanelli Kuczmarski M, Mason MA, Beydoun MA, Allegro D, Zonderman AB, Evans MK. Dietary patterns and sarcopenia in an urban African American and white population in the United States. Journal of nutrition in gerontology and geriatrics. 2013;32:291–316. doi: 10.1080/21551197.2013.840255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413–21. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- [14].Lukaski HC, Johnson PE, Bolonchuk WW, Lykken GI. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am J Clin Nutr. 1985;41:810–7. doi: 10.1093/ajcn/41.4.810. [DOI] [PubMed] [Google Scholar]
- [15].Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol (1985) 2000;89:465–71. doi: 10.1152/jappl.2000.89.2.465. [DOI] [PubMed] [Google Scholar]
- [16].Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–59. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- [17].Kennedy ET, Ohls J, Carlson S, Fleming K. The healthy eating index: design and applications. J Am Diet Assoc. 1995;95:1103–8. doi: 10.1016/S0002-8223(95)00300-2. [DOI] [PubMed] [Google Scholar]
- [18].Kappeler R, Eichholzer M, Rohrmann S. Meat consumption and diet quality and mortality in NHANES III. Eur J Clin Nutr. 2013;67:598–606. doi: 10.1038/ejcn.2013.59. [DOI] [PubMed] [Google Scholar]
- [19].Ervin RB. Healthy Eating Index scores among adults, 60 years of age and over, by sociodemographic and health characteristics: United States, 1999–2002. Adv Data. 2008;395:1–16. [PubMed] [Google Scholar]
- [20].Rogot E, Sorlie P, Johnson NJ. Probabilistic methods in matching census samples to the National Death Index. J Chronic Dis. 1986;39:719–34. doi: 10.1016/0021-9681(86)90155-4. [DOI] [PubMed] [Google Scholar]
- [21].National Center for Health Statistics The Third National Nutrition and Health Survey Linked Mortality File: Matching Methodology. 2006;2014 [Google Scholar]
- [22].Wilper AP, Woolhandler S, Lasser KE, McCormick D, Bor DH, Himmelstein DU. Health insurance and mortality in US adults. Am J Public Health. 2009;99:2289–95. doi: 10.2105/AJPH.2008.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].National Center for Health Statistics NHANES III Questionnaires, Datasets and Related Documentation.
- [24].Obisesan TO, Obisesan OA, Martins S, Alamgir L, Bond V, Maxwell C, et al. High blood pressure, hypertension, and high pulse pressure are associated with poorer cognitive function in persons aged 60 and older: the Third National Health and Nutrition Examination Survey. J Am Geriatr Soc. 2008;56:501–9. doi: 10.1111/j.1532-5415.2007.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].National Center for Health Statistics Laboratory Procedures Used for the Third National Health and Nutrition Exam Survey (NHANES III), 1988-1994.
- [26].Lacher DA, Hughes JP, Carroll MD. Estimate of biological variation of laboratory analytes based on the third national health and nutrition examination survey. Clin Chem. 2005;51:450–2. doi: 10.1373/clinchem.2004.039354. [DOI] [PubMed] [Google Scholar]
- [27].Greenland S. Tests for interaction in epidemiologic studies: a review and a study of power. Stat Med. 1983;2:243–51. doi: 10.1002/sim.4780020219. [DOI] [PubMed] [Google Scholar]
- [28].Korn EL, Graubard BI. Epidemiologic studies utilizing surveys: accounting for the sampling design. Am J Public Health. 1991;81:1166–73. doi: 10.2105/ajph.81.9.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Filippin LI, de Oliveira Teixeira, Vivian Nunes, da Silva, Magali Pilz Monteiro, Miraglia F, da Silva FS. Sarcopenia: a predictor of mortality and the need for early diagnosis and intervention. Aging clinical and experimental research. 2015;27:249–54. doi: 10.1007/s40520-014-0281-4. [DOI] [PubMed] [Google Scholar]
- [30].Cruz-Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age Ageing. 2014;43:748–59. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chumlea WC, Cesari M, Evans WJ, Ferrucci L, Fielding RA, Pahor M, et al. Sarcopenia: designing phase IIB trials. J Nutr Health Aging. 2011;15:450–5. doi: 10.1007/s12603-011-0092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]