Abstract
Background
Evidence suggests that ejaculation frequency may be inversely related to the risk of prostate cancer (PCa), a disease for which few modifiable risk factors have been identified.
Objective
To incorporate an additional 10 yr of follow-up into an original analysis and to comprehensively evaluate the association between ejaculation frequency and PCa, accounting for screening, clinically relevant disease subgroups, and the impact of mortality from other causes.
Design, setting, and participants
A prospective cohort study of participants in the Health Professionals Follow-up Study utilizing self-reported data on average monthly ejaculation frequency. The study includes 31 925 men who answered questions on ejaculation frequency on a 1992 questionnaire and followed through to 2010. The average monthly ejaculation frequency was assessed at three time points: age 20–29 yr, age 40–49 yr, and the year before questionnaire distribution.
Outcome measurements and statistical analysis
Incidence of total PCa and clinically relevant disease subgroups. Cox models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs).
Results and limitations
During 480 831 person-years, 3839 men were diagnosed with PCa. Ejaculation frequency at age 40–49 yr was positively associated with age-standardized body mass index, physical activity, divorce, history of sexually transmitted infections, and consumption of total calories and alcohol. Prostate-specific antigen (PSA) test utilization by 2008, number of PSA tests, and frequency of prostate biopsy were similar across frequency categories. In multivariable analyses, the hazard ratio for PCa incidence for ≥21 compared to 4–7 ejaculations per month was 0.81 (95% confidence interval [CI] 0.72–0.92; p < 0.0001 for trend) for frequency at age 20–29 yr and 0.78 (95% CI 0.69–0.89; p < 0.0001 for trend) for frequency at age 40–49 yr. Associations were driven by low-risk disease, were similar when restricted to a PSA-screened cohort, and were unlikely to be explained by competing causes of death.
Conclusions
These findings provide additional evidence of a beneficial role of more frequent ejaculation throughout adult life in the etiology of PCa, particularly for low-risk disease.
Patient summary
We evaluated whether ejaculation frequency throughout adulthood is related to prostate cancer risk in a large US-based study. We found that men reporting higher compared to lower ejaculatory frequency in adulthood were less likely to be subsequently diagnosed with prostate cancer.
Keywords: Epidemiology, Ejaculation, Behavioral risk factors
1. Introduction
Prostate cancer (PCa) accounts for approximately 15% of all new cancer diagnoses among men worldwide, and the burden of disease continues to increase globally [1]. While diet and physical activity may provide some promise for secondary prevention [2–5], there are no evidence-based recommendations to offer healthy adult men to reduce PCa risk. The few established disease risk factors—age, race, family history, and germline polymorphisms—are not modifiable [6].
Sexual behaviors represent potential modifiable risk factors and may influence PCa development through a variety of specific mechanisms. One biological mechanism involves prostatic accumulation of potentially carcinogenic secretions, which may create more opportunity for PCa development, sometimes referred to as the prostate stagnation hypothesis [7,8]. On the basis of this premise, a prospective report from the Health Professionals Follow-up Study (HPFS) cohort published in 2004 found a statistically significant inverse association between monthly ejaculation frequency and PCa risk based on 8 yr of follow-up [8]. Compared to men reporting an average of 4–7 ejaculations per month (EPM), the risk of PCa among men reporting ≥21 EPM in middle age was 50% lower. Although these initial findings were intriguing, the strongest reduction in risk was noted for ejaculation frequency in the time period immediately before questionnaire administration, raising concerns about the potential influence of undiagnosed PCa on the results.
To confirm and build on these results [8], we conducted an updated study within the HPFS cohort with an additional 10 yr of follow-up and 3839 PCa cases, more than double the number included in the original report. This updated analysis allows us to address possible reverse causation, investigate the potential impact of PSA screening, and determine whether the association between ejaculation frequency and PCa differed according to the clinical disease characteristics, as has been observed for other PCa risk factors [9]. Finally, because ejaculation frequency maybe an indicator of health status and could be related to mortality from multiple causes, the current analysis considers the impact of competing causes of death on our findings. Thus, this updated analysis represents a comprehensive evaluation of the association between ejaculation frequency and PCa in a large US-based prospective cohort.
2. Patients and methods
2.1. Study population
The HPFS is an ongoing prospective cohort study among 51 529 US male health professionals [8]. In brief, cancer-free, predominantly Caucasian (>91%) health professionals aged 40–75 yr were recruited in 1986 and have been followed with biennial questionnaires on medical history and lifestyle, including known or suspected cancer and chronic disease risk factors, diet, use of supplements, and preventive behaviors. Ejaculation frequency was assessed in the 1992 questionnaire, which was completed by 46 213 men. Men with a diagnosis of cancer before 1992 (excluding non-melanoma skin cancer) were excluded from the analysis, leaving 41 201 men. Of these, 9276 did not complete all three questions on ejaculation frequency, leaving 31925 men in the study population for the current analysis. Nonresponders who provided information on weight, physical activity, and diet appeared to be similar to the responders. Among participants who were alive in 2010, follow-up was 96% complete. All participants provided informed consent and the study was approved by the human subjects committee of the Harvard T.H. Chan School of Public Health, Boston.
2.2. Exposure and covariate assessment
In 1992, participants were asked the following question: “On average, how many ejaculations did you have per month during these ages?: ages 20–29; ages 40–49; past year.” The frequency at each time point was reported in the categories none, 1–3, 4–7, 8–12, 13–20, and >20 EPM. To limit the burden for participants and because the question was designed specifically to address the prostate stagnation hypothesis, no information on the specific type of activity leading to ejaculation was requested. Information on potential confounders was ascertained in the 1992 questionnaire and most were updated on the biennial questionnaires throughout follow-up. PSA testing was first assessed in the 1994 questionnaire; starting in 1994, men were also asked if they had an elevated PSA level and whether they had undergone a prostate biopsy or rectal ultrasound.
2.3. Outcome assessment
For men reporting a diagnosis of PCa, we retrieved medical records and pathology reports to confirm the diagnosis and to obtain information on age at diagnosis, PSA level, and tumor stage and grade. Cases were followed through biennial questionnaires to collect information on the clinical course, including the development of metastases and treatments. Deaths were ascertained through repeated mailings and telephone calls to participants, as well as periodic searches of the National Death Index. Cause of death was assigned following a review of death certificates, information from the family, and medical records.
Total PCa incidence was the primary endpoint of interest. Men diagnosed with stage T1a cancers were excluded from analyses. To determine whether the association between ejaculation frequency and PCa differed according to the clinical disease characteristics, we also used clinical information to group PCa diagnoses into four risk categories according to National Comprehensive Cancer Network (NCCN) guidelines [10]. Locally advanced and metastatic disease categories were combined owing to limited numbers. Men were assigned to the highest category for which they were eligible: low risk = T1/T2 tumor, PSA< 10 ng/ml, Gleason score 6; intermediate risk = T1/T2 tumor, PSA 10–<20 ng/ml, Gleason score 7; high risk = T3 tumor, PSA 20–<50 ng/ml, Gleason score 8; and regional or distant metastases = T4/N1/M1 tumor, PSA ≥50 ng/ml. To more carefully explore differences in risk for indolent and aggressive disease, we also considered the following subgroups as secondary analyses: lethal disease (defined as PCa death or metastases to bone or other organs before the end of follow-up), advanced disease (stage T3b, T4 or N1 or M1 at diagnosis or lethal disease during follow-up), organ-confined disease (low-grade stage T1 or T2 and N0, M0 at diagnosis and no progression to metastasis or death during follow-up); and categories of Gleason score based on prostatectomy or biopsy pathology reports (Gleason ≤ 3 + 4 and Gleason ≥ 4 + 3).
2.4. Statistical analyses
Person-time was calculated from the return date for the 1992 questionnaire to the date of PCa diagnosis, death, or the end of the follow-up period (January 31, 2010). Actuarial curves for PCa-free survival were generated according to the ejaculation frequency category for age 40–49 yr using the Kaplan-Meier method. Cox proportional hazards models were used to estimate the hazard ratio (HR) and 95% confidence intervals (CI) for total PCa and for each of the clinical subgroups for each ejaculation frequency category. As in the 2004 report [8], 4–7 EPM was selected as the reference category as relatively few men reported an average of 0–3 EPM. The top two categories were combined for some analyses owing to small numbers of men in the ≥21 EPM group. Age-adjusted and multivariable models were evaluated. Age-adjusted models are adjusted for age in months (as the time scale) and for calendar time. Multivariable models were additionally adjusted for: race (Caucasian, African-America, Asian, other ancestry, missing); family history of PCa (yes/no); vigorous physical activity (quintiles); body mass index (BMI; <21, 21–<23, 23–<25, 25–<27.5, 27.5–<30, >30kg/m2, missing); height (quintiles); diabetes (yes/no); marital status (married, divorced, other); intake of energy, processed meat, tomato sauce, calcium, alcohol, and a-linolenic acid (all quintiles); multivitamin use (yes, no, missing); smoking (never, quit >10 yr ago, quit <10 yr ago, current, missing); history of vasectomy (yes/no); and history of PSA testing (yes/no in the previous 2-yr questionnaire cycle). Statistical significance was evaluated based on a p trend estimated by assigning the minimum frequency for each category. The missing indicator method was used for missing data on most covariates [11]. All participants had baseline questionnaire data for food frequency, and missing data on nutrients were carried forward from previous reported values. For activity, missing data were assumed to be in the lowest reference category. For height, missing data were assumed to be in the middle category.
2.5. Sensitivity analyses and effect modification
If men with erectile dysfunction have lower ejaculatory frequency and serious comorbidities associated with a higher risk of premature death from other causes, a spurious association between less frequent ejaculation and reduced risk of PCa could result. To address this issue, we performed a sensitivity analysis that excluded men who reported a history of erectile dysfunction, defined as poor or very poor ability to maintain an erection without treatment during the period 1990–1994, as assessed in the 2000 questionnaire. To eliminate a possible effect of undiagnosed disease on ejaculation frequency reported, we also performed analyses that excluded cases diagnosed within the first 4 yr of follow-up. Finally, to address the fact that diagnostic intensity may vary according to ejaculation frequency, we conducted a sensitivity analysis restricted to a PSA-screened subset of men who reported a PSA test before 1994 with follow-up from 1994 to 2010. Stratified analyses were performed to evaluate potential effect modifications by age, BMI, and vasectomy status. The statistical significance of effect modifications was tested using likelihood ratio tests to compare models with and without interaction terms between the potential effect modifier and ejaculation frequency. All aforementioned analyses were performed using SAS statistical software (release 9.3; SAS Institute, Cary, NC, USA).
2.6. Competing risks analysis
PCa has a long natural history [12], is very sensitive to diagnostic intensity [13], and is often indolent [14]. Thus, to better understand the interplay between PCa and deaths due to other causes, we modeled these events jointly using a multistate model in a semi-competing risks framework [15], as shown in Supplementary Figure 1. Specifically, we modeled PCa diagnoses as intermediate states and deaths due to PCa or other causes as final states. We grouped men into the four NCCN risk categories described above. Each transition hazard was modeled assuming that hazards are proportional across ejaculation frequency levels. We present results for a simple model using age as the time scale and considering event occurrence by levels of ejaculation frequency reported at age 40–49 yr. We also investigated causes of death across ejaculation frequency categories to better understand the different mortality rates. Analyses were run in R using the mstate package [16]. For all analyses, p < 0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
Ejaculation frequency declined with age. The proportion of men reporting average frequency of ≥13 EPM was 57% at age 20–29 yr but dropped to 32% at age 40–49 yr. The Spearman correlation between ejaculation frequency as an ordinal variable and ages 20–29 and 40–49 yr was 0.66. Some 40% of men were in the same frequency category for ages 20–29 and 40–49 yr, and 47% of men moved down a single category from age 20–29 yr to age 40–49 yr.
The baseline age-standardized characteristics of the study population (n = 31925) according to average monthly ejaculation frequency at age 40–49 yr are presented in Table 1. Having had a PSA test by 1994 or by 2008 was not monotonically associated with ejaculation frequency at age 40–49 yr, and the total number of PSA tests was similar across frequency categories. Among 17 093 men who reported an initial elevated PSA, the percentage who subsequently reported prostate biopsy was similar across ejaculation categories. Men reporting ≥21 EPM who were subsequently diagnosed with PCa were somewhat less likely to undergo radical prostatectomy and more likely to report radiation compared to men reporting lower frequencies.
Table 1.
Age-standardized characteristics at baseline in 1992 for the 31 925 men from the Health Professionals Follow-up Study according to reported ejaculation frequency at age 40–49 yra
| Ejaculation frequency | |||||
|---|---|---|---|---|---|
| 0–3 EPM | 4–7 EPM | 8–12 EPM | 13–20 EPM | ≥21 EPM | |
| Cases (n) | 1713 | 7812 | 12147 | 7440 | 2813 |
| Age (yr) | 59.9 (10.4) | 59.1 (9.6) | 58.9 (9.1) | 58.5 (8.9) | 58.0 (8.9) |
| Height (cm) | 178 (7) | 178 (7) | 178 (7) | 178 (7) | 178 (7) |
| Body mass index in 1992 (kg/m2) | 25.6 (3.6) | 25.5 (3.3) | 25.8 (3.4) | 26.0 (3.4) | 26.3 (3.7) |
| Total activity (MET h/wk) | 31 (35) | 33 (38) | 37 (39) | 41 (47) | 42 (46) |
| Total calorie intake (kcal/d) | 1847 (583) | 1890 (561) | 1923 (576) | 1968 (590) | 2013 (619) |
| Alcohol intake (g/d) | 8.4 (12.9) | 9.4 (13.3) | 10.4 (14.2) | 11.3 (15.3) | 12.2 (17.0) |
| Processed meat intake (servings/d) | 0.3 (0.4) | 0.3 (0.4) | 0.3 (0.4) | 0.3 (0.4) | 0.3 (0.4) |
| Tomato sauce intake (servings/wk) | 0.9 (0.9) | 0.9 (0.9) | 0.9 (0.9) | 1.0 (1.0) | 1.0 (1.0) |
| Calcium intake (mg/d) | 908 (396) | 916 (391) | 909 (389) | 908 (404) | 927 (417) |
| α-Linolenic acid intake (g/d) | 1.0 (0.4) | 1.1 (0.4) | 1.0 (0.4) | 1.0 (0.4) | 1.0 (0.4) |
| Divorced | 4.9 | 4.1 | 5.1 | 7.8 | 11.8 |
| Smoked in the past 10 yr | 33.5 | 37.5 | 38.8 | 39.3 | 39.6 |
| Self-reported history of syphilis or gonorrhea | 1.2 | 2.6 | 3 | 3.3 | 4.6 |
| Vasectomy history | 18.2 | 25 | 27.6 | 27.2 | 27.9 |
| Race | |||||
| White | 93.4 | 96.3 | 96.7 | 96.9 | 96.2 |
| African-American | 0.6 | 0.6 | 0.8 | 0.6 | 0.8 |
| Asian | 4 | 1.7 | 1.3 | 1.2 | 1.1 |
| Other | 2.1 | 1.3 | 1.2 | 1.4 | 2.0 |
| Screening behavior | |||||
| Had physical examination in past 2 yr | 67.8 | 71.3 | 71.1 | 70.7 | 68.9 |
| Had rectal examination in past 2 yr | 65.4 | 67.4 | 68.1 | 67.1 | 66.3 |
| Had PSA test by 1994 | 48.2 | 54.4 | 55.6 | 53.8 | 50.4 |
| Had PSA test by 2008 | 90.9 | 92.7 | 93.1 | 92.2 | 90.6 |
| Total periods with PSA test by 2008 (n)b | 4.6 (2.6) | 5.1 (2.5) | 5.1 (2.5) | 4.9 (2.6) | 4.8 (2.6) |
| Had prostate biopsy after first report of elevated PSAc | 47.2 | 47.4 | 48 | 46.7 | 47.2 |
| Primary treatment among 3839 cases | |||||
| Cases (n) | 192 | 1041 | 1509 | 807 | 290 |
| Radical prostatectomy | 38.4 | 41.8 | 40.8 | 41.8 | 36.2 |
| Radiation therapy | 30.2 | 31.9 | 34.2 | 34.3 | 39.3 |
| Hormone therapy | 8.3 | 5.9 | 5.7 | 6.6 | 8.4 |
| Active surveillance/none | 8.4 | 8.2 | 8.3 | 6.7 | 5.1 |
| Other | 2.2 | 1.6 | 1.8 | 2.9 | 0.7 |
| Missing treatment information | 12.6 | 10.6 | 9.3 | 7.7 | 10.2 |
EPM = ejaculations per month; MET = metabolic equivalent of task; PSA = prostate-specific antigen.
Data are presented as the age-standardized mean (standard deviation) for continuous variables and the age-standardized percentage for categorical variables. All characteristics except age and primary treatment are age-standardized to the distribution for the full cohort in 1992. Primary treatment is standardized to the age distribution for all prostate cancer cases.
Number of 2-yr questionnaire cycles in which a PSA test was reported in the previous 2 yr (maximum = 8).
Among 17 093 men who reported “elevated PSA”; based only on first report of elevated PSA.
Overall, associations between the covariates investigated and ejaculation frequency were similar for frequency at age 20–29 yr and in the year before the questionnaire (Supplementary Table 1 and Supplementary Table 2). Although the associations were not monotonic, there was some evidence that men in the highest ejaculation frequency category in the year before the questionnaire were less likely to have had a PSA screening test by 1994 (45.4% vs 52.6%) and by 2008 (87.5% vs 91.8%). However, the associations between covariates and ejaculatory frequency remained similar to those for the overall cohort when analysis was restricted to the subset of 13 405 screened men who reported having had a PSA test in 1994 (Supplementary Table 3).
3.2. Ejaculation frequency and PCa risk
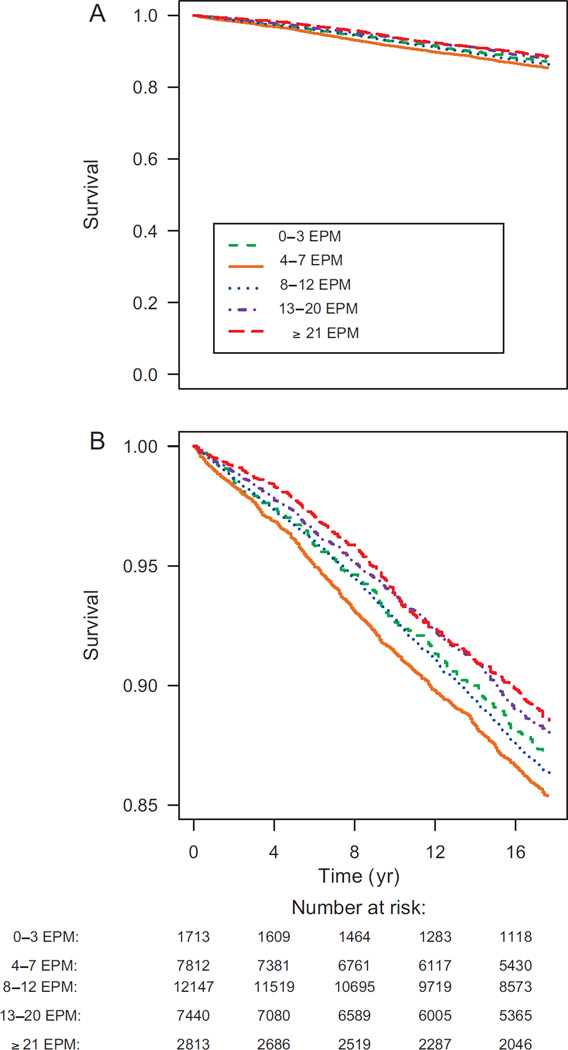
During 480 831 person-years of follow-up, a total of 3839 incident PCa cases were diagnosed. As shown in Figure 1, PCa was less frequently diagnosed among men in the higher ejaculation frequency categories. The age-adjusted and multivariable-adjusted HRs according to average monthly ejaculation frequency are presented in Table 2. We also present results excluding 10 103 men who reported erectile dysfunction, leaving 21 822 men and 2704 total cases.
Fig. 1.
Kaplan-Meier curve for prostate cancer-free survival according to ejaculation frequency category for age 40–49 yr (1992–2010). (A) Plot over the full prostate cancer–free survival range. (B) Magnified plot for a restricted survival range. EPM = ejaculations per month.
Table 2.
Hazard ratio for incidence of total prostate cancer among 31 925 men in the Health Professionals Follow-up Study according to reported ejaculation frequency in different time periodsa
| Ejaculation frequency | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0–3 EPM | 4–7 EPM | 8–12 EPM | 13–20 EPM | ≥21 EPM | p trend | ≥13 EPM | p trend | |
| Age 20–29 yr | ||||||||
| Cases (n) | 137 | 438 | 1208 | 1303 | 753 | 2056 | ||
| Age-adjusted HR (95% CI) | 0.97 (0.80–1.17) | 1.00 | 1.03 (0.92–1.15) | 0.92 (0.83–1.03) | 0.80 (0.71–0.90) | <0.0001 | 0.87 (0.79–0.97) | 0.0001 |
| Age-adjusted no-ED HR (95% CI) | 0.90 (0.70–1.15) | 1.00 | 0.98 (0.86–1.12) | 0.89 (0.78–1.01) | 0.78 (0.67–0.89) | <0.0001 | 0.84 (0.75–0.96) | 0.0009 |
| MV HR (95% CI) | 0.99 (0.81–1.19) | 1.00 | 1.03 (0.92–1.14) | 0.92 (0.82–1.02) | 0.81 (0.72–0.92) | <0.0001 | 0.88 (0.79–0.97) | 0.0002 |
| MV no-ED HR (95% CI) | 0.91 (0.71–1.17) | 1.00 | 0.99 (0.86–1.13) | 0.90 (0.78–1.02) | 0.80 (0.69–0.92) | <0.0001 | 0.86 (0.76–0.97) | 0.002 |
| Age 40–49 yr | ||||||||
| Cases (n) | 192 | 1041 | 1509 | 807 | 290 | 1097 | ||
| Age-adjusted HR (95% CI) | 0.87 (0.74–1.01) | 1.00 | 0.91 (0.84–0.98) | 0.80 (0.72–0.87) | 0.77 (0.67–0.87) | <0.0001 | 0.79 (0.72–0.86) | <0.0001 |
| Age-adjusted no-ED HR (95% CI) | 0.91 (0.75–1.11) | 1.00 | 0.93 (0.85–1.02) | 0.80 (0.72–0.89) | 0.80 (0.68–0.93) | <0.0001 | 0.80 (0.72–0.88) | <0.0001 |
| MV HR (95% CI) | 0.88 (0.76–1.03) | 1.00 | 0.90 (0.83–0.98) | 0.80 (0.73–0.88) | 0.78 (0.69–0.89) | <0.0001 | 0.80 (0.73–0.87) | <0.0001 |
| MV no-ED HR (95% CI) | 0.91 (0.75–1.11) | 1.00 | 0.93 (0.84–1.02) | 0.81 (0.72–0.90) | 0.82 (0.70–0.96) | 0.0006 | 0.81 (0.73–0.90) | 0.0002 |
| Year before questionnaire (1991) | ||||||||
| Cases (n) | 1293 | 1299 | 831 | 322 | 94 | 416 | ||
| Age-adjusted HR (95% CI) | 1.03 (0.95–1.11) | 1.00 | 0.95 (0.87–1.04) | 0.90 (0.80–1.02) | 0.73 (0.59–0.90) | 0.0004 | 0.86 (0.76–0.96) | 0.002 |
| Age-adjusted no-ED HR (95% CI) | 1.06 (0.96–1.17) | 1.00 | 0.96 (0.87–1.06) | 0.91 (0.80–1.05) | 0.71 (0.56–0.90) | 0.0005 | 0.86 (0.76–0.97) | 0.002 |
| MV HR (95% CI) | 1.05 (0.97–1.13) | 1.00 | 0.96 (0.87–1.05) | 0.93 (0.82–1.05) | 0.76 (0.61–0.94) | 0.001 | 0.89 (0.79–0.99) | 0.004 |
| MV no-ED HR (95% CI) | 1.07 (0.97–1.19) | 1.00 | 0.96 (0.87–1.06) | 0.94 (0.82–1.08) | 0.74 (0.58–0.94) | 0.002 | 0.89 (0.78–1.01) | 0.007 |
EPM = ejaculations per month; HR = hazard ratio; CI = confidence interval; ED = erectile dysfunction; MV = multivariate.
Age-adjusted models are adjusted for age in months and calendar time. Multivariate models are additionally adjusted for: race; family history of prostate cancer; vigorous physical activity (quintiles); body mass index (six categories); height (quintiles); diabetes; marital status; intake of energy, processed meat, tomato sauce, calcium, alcohol, and α-linolenic acid (all quintiles); multivitamin use; smoking (never, quit >10 yr ago, quit ≤10 yr ago, current); history of vasectomy; and history of PSA testing. No-ED models exclude men who reported poor or very poor ability to have and maintain an erection in the time period 1990–1994, for which analyses include 21 822 participants and 2704 prostate cancer events.
Results for total PCa were similar for the age-adjusted and multivariable analyses for all three time points at which ejaculation frequency was assessed, and for the sensitivity analysis excluding men with erectile dysfunction (Table 2). Compared to men with an average monthly frequency of 4–7 ejaculations, men reporting ≥21 EPM at ages 20–29 and 40–49 yr and in 1991 had a significantly lower risk of total PCa, with a multivariable-adjusted HR of 0.81 (95% CI 0.72– 0.92), 0.78 (95% CI 0.69–0.89), and 0.76 (95% CI 0.61–0.94), respectively. Trend tests at each time point when excluding men in the lowest ejaculation frequency category, who may be more likely to have serious comorbidities, were similar to results when considering all five categories (p < 0.0001 for ages 20–29 and 40–49 yr, p = 0.06 for 1991).
The absolute PCa incidence rate for frequency at age 20–29 yr was 6.56 cases/1000 person-years for ≥21 EPM and 8.95 cases/1000 person-years for 4–7 EPM (incidence rate difference [IRD] 2.39 cases/1000 person-years). For frequency at age 40–49 yr, the absolute rate was 6.74 cases/ 1000 person-years for ≥21 EPM and 8.94 cases/1000 person-years for 4–7 EPM (IRD 2.20 cases/1000 person-years). For frequency in the year before the questionnaire, the rate was 4.49 cases/1000 person-years for ≥21 EPM and 8.35 cases/1000 person-years for 4–7 EPM (IRD 3.89 cases/ 1000 person-years).
Men who reported an average frequency of ≥21 EPM at both age 20–29 yr and age 40–49 yr experienced the same risk reduction for total PCa as men in the highest EPM category at age 40–49 yr (HR 0.78, 95% CI 0.68–0.90). The association appeared to be driven by frequency at age 40–49 yr when frequency at both time points was included in the same model. Compared to men with 4–7 EPM, the HR for men with ≥13 EPM at age 40–49 yr was 0.85 (95% CI 0.76–0.94; p = 0.005 for trend) after adjusting for frequency at age 20–29 yr. The HR for >13 EPM compared to 4–7 EPM at age 20–29 yr was 0.95 (95% CI 0.83–1.08; p = 0.30 for trend) after adjusting for frequency at age 40–49 yrs. However, the correlation between frequencies at different time points makes it challenging to completely disentangle the associations.
According to the four NCCN risk groups, 1585 cases had localized low-risk PCa, 1493 had localized intermediate-risk disease, 604 had localized high-risk PCa, and 157 patients had evidence of regional or distant metastases at diagnosis. Information on clinical disease characteristics was missing for 434 (11%) men, who were classified in the two lowest risk categories depending on whether their PCa diagnosis occurred after a PSA test (n = 336; 21.2% of the localized low-risk group) or in the absence of a PSA test (n = 98; 6.6% of the localized intermediate-risk group). For all three time periods, ≥13 EPM was associated with a significantly lower risk (25–28%) of low-risk PCa in comparison to 4–7 EPM (Table 3). Ejaculation frequency at age 20–29 yr was also significantly associated with intermediate-risk PCa (p = 0.0003), with a 27% reduction for ≥13 versus 4–7 EPM. Ejaculation frequency at any time point was not significantly associated with diagnosis of high-risk PCa or regional/distant metastases. However, for age 20–29 yr there was a suggestion of an inverse association between ejaculation frequency and local/distant metastases (HR 0.89, 95% CI 0.68–1.15; p = 0.07 for >13 vs 4–7 EPM).
Table 3.
Hazard ratio for prostate cancer by disease severity among 31 925 men in the Health Professionals Follow-up Study according to reported ejaculation frequency at different timesa,b
| 0–3 EPM | 4–7 EPM | 8–12 EPM | ≥13 EPM |
p value for trend |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (n) |
MV HR (95% CI) |
Cases (n) |
MV HR (95% CI) |
Cases (n) |
MV HR (95% CI) |
Cases (n) |
MV HR (95% CI) |
||
| Age 20–29 yr | |||||||||
| Low-risk disease | 40 | 0.84 (0.59–1.18) | 160 | 1.00 (Ref) | 407 | 0.91 (0.75–1.09) | 687 | 0.75 (0.63–0.89) | 0.0006 |
| Intermediate-risk disease | 39 | 0.85 (0.60–1.20) | 156 | 1.00 (Ref) | 390 | 0.88 (0.73–1.06) | 664 | 0.73 (0.61–0.88) | 0.0003 |
| High-risk disease | 43 | 0.99 (0.70–1.39) | 142 | 1.00 (Ref) | 435 | 1.13 (0.93–1.36) | 775 | 1.00 (0.84–1.20) | 0.46 |
| Regional/distant metastases | 28 | 1.20 (0.77–1.87) | 70 | 1.00 (Ref) | 186 | 1.01 (0.77–1.33) | 320 | 0.89 (0.68–1.15) | 0.07 |
| Age 40–49 yr | |||||||||
| Low-risk disease | 65 | 0.95 (0.72–1.23) | 347 | 1.00 (Ref) | 502 | 0.88 (0.77–1.01) | 335 | 0.72 (0.61–0.83) | <0.0001 |
| Intermediate-risk disease | 52 | 0.70 (0.52–0.94) | 363 | 1.00 (Ref) | 579 | 0.99 (0.87–1.13) | 401 | 0.83 (0.72–0.96) | 0.11 |
| High-risk disease | 39 | 1.13 (0.80–1.61) | 160 | 1.00 (Ref) | 217 | 0.85 (0.69–1.04) | 188 | 0.89 (0.72–1.11) | 0.17 |
| Regional/distant metastases | 7 | 0.61 (0.27–1.35) | 44 | 1.00 (Ref) | 55 | 0.85 (0.57–1.27) | 51 | 0.96 (0.64–1.45) | 0.65 |
| Year before | |||||||||
| questionnaire (1991) | |||||||||
| Low-risk disease | 365 | 1.05 (0.91–1.21) | 446 | 1.00 (Ref) | 303 | 0.94 (0.81–1.09) | 135 | 0.75 (0.62–0.92) | 0.002 |
| Intermediate-risk disease | 432 | 1.01 (0.89–1.16) | 481 | 1.00 (Ref) | 325 | 1.00 (0.87–1.15) | 157 | 0.89 (0.74–1.07) | 0.27 |
| High-risk disease | 232 | 1.18 (0.96–1.45) | 180 | 1.00 (Ref) | 129 | 1.20 (0.95–1.50) | 63 | 1.12 (0.84–1.51) | 0.94 |
| Regional/distant metastases | 72 | 0.94 (0.64–1.38) | 48 | 1.00 (Ref) | 15 | 0.59 (0.33–1.07) | 22 | 1.82 (1.07–3.10) | 0.19 |
EPM = ejaculations per month; MV = multivariate; HR = hazard ratio; CI = confidence interval; PSA = prostate-specific antigen.
Risk groups are based on National Comprehensive Cancer Network guidelines. Men were assigned to the highest category for which they were eligible: low risk = T1/T2 tumor, PSA <10 ng/ml, Gleason score 6; intermediate risk = T1/T2 tumor, PSA 10–<20 ng/ml, Gleason score 7; High risk = T3 tumor, PSA level 20–<50 ng/ml, Gleason score 8; regional or distant metastases =T4/N1/M1 tumor, PSA >50 ng/ml.
Age-adjusted models are adjusted for age in months and calendar time. Multivariate models are additionally adjusted for: race; family history of prostate cancer; vigorous physical activity (quintiles); body mass index (six categories); height (quintiles); diabetes; marital status; intake of energy, processed meat, tomato sauce, calcium, alcohol, and α-linolenic acid (all quintiles); multivitamin use; smoking (never, quit >10 yrs ago, quit ≤10 yrs ago, current); history of vasectomy; and history of PSA testing.
The risk of both organ-confined and low-grade PCa was significantly lower for ≥13 compared to 4–7 EPM for all three time periods (Table 4). For high-grade PCa, there was a suggestion of higher risk for men in the lowest frequency category at age 20–29 yr (HR 1.32, 95% CI 0.91–1.92) and age 40–49 yr (HR 1.38, 95% CI 1.03–1.86). However, there was some evidence that higher ejaculation frequency in the year before questionnaire distribution is associated with higher risk of advanced PCa (HR 1.37, 95% CI 1.00–1.86) or lethal PCa (HR 1.48, 95% CI 1.02–2.15), but the trend tests were only marginally significant (p values 0.11 for advanced and 0.05 for lethal PCa). When we excluded men diagnosed within the first 4 yr of follow-up to address the impact of undiagnosed disease or early symptoms on the results, the associations for ≥13 versus 4–7 EPM were attenuated for both advanced PCa (HR 1.15, 95% CI 0.79–1.69; p = 0.36 for trend) and lethal PCa (HR 1.19, 95% CI 0.73–1.94; p = 0.33 for trend; data not shown).
Table 4.
Multivariable prostate cancer incidence by disease severity among 31 925 men in the Health Professionals Follow-up Study according to reported ejaculation frequency at different timesa
| Ejaculation frequency | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–3 EPM | 4–7 EPM | 8–12 EPM | ≥13 EPM |
p value for trend |
|||||
| Cases (n) |
MV HR (95% CI) |
Cases (n) |
MV HR (95% CI) |
Cases (n) |
MV HR (95% CI) |
Cases (n) |
MV HR (95% CI) |
||
| Age 20–29 yr | |||||||||
| Low grade (GS ≤ 3 + 4) | 76 | 0.88 (0.68–1.13) | 283 | 1.00 (Ref) | 785 | 1.00 (0.87–1.15) | 1364 | 0.86 (0.76–0.98) | 0.006 |
| High grade (GS ≥ 4 + 3) | 39 | 1.32 (0.91–1.92) | 93 | 1.00 (Ref) | 244 | 1.00 (0.78–1.27) | 451 | 0.93 (0.74–1.16) | 0.08 |
| Organ-confined | 98 | 1.04 (0.82–1.30) | 309 | 1.00 (Ref) | 877 | 1.04 (0.91–1.18) | 1508 | 0.89 (0.78–1.00) | 0.0008 |
| Advanced | 21 | 0.79 (0.48–1.29) | 72 | 1.00 (Ref) | 156 | 0.87 (0.65–1.15) | 268 | 0.80 (0.62–1.05) | 0.23 |
| Lethal | 14 | 0.63 (0.35–1.14) | 57 | 1.00 (Ref) | 113 | 0.83 (0.60–1.14) | 200 | 0.82 (0.60–1.10) | 0.71 |
| Age 40–49 yr | |||||||||
| Low grade (GS ≤ 3 + 4) | 106 | 0.76 (0.62–0.93) | 689 | 1.00 (Ref) | 1010 | 0.90 (0.82–0.99) | 703 | 0.76 (0.68–0.85) | <0.0001 |
| High grade (GS ≥ 4 + 3) | 57 | 1.38 (1.03–1.86) | 197 | 1.00 (Re) | 318 | 1.01 (0.85–1.21) | 255 | 0.99 (0.82–1.19) | 0.23 |
| Organ-confined | 132 | 0.86 (0.71–1.03) | 749 | 1.00 (Ref) | 1126 | 0.93 (0.84–1.02) | 785 | 0.78 (0.71–0.86) | <0.0001 |
| Advanced | 33 | 0.98 (0.67–1.44) | 146 | 1.00 (Ref) | 185 | 0.83 (0.66–1.03) | 153 | 0.85 (0.67–1.07) | 0.16 |
| Lethal | 24 | 0.95 (0.61–1.49) | 107 | 1.00 (Ref) | 133 | 0.83 (0.64–1.08) | 120 | 0.95 (0.73–1.24) | 0.78 |
| Year before | |||||||||
| questionnaire (1991) | |||||||||
| Low grade (GS ≤ 3 + 4) | 778 | 1.04 (0.94–1.15) | 869 | 1.00 (Ref) | 587 | 0.97 (0.87–1.08) | 274 | 0.82 (0.72–0.95) | 0.003 |
| High grade (GS ≥ 4 + 3) | 284 | 1.08 (0.91–1.29) | 264 | 1.00 (Ref) | 180 | 1.07 (0.88–1.30) | 99 | 1.13 (0.89–1.43) | 0.68 |
| Organ-confined | 901 | 1.08 (0.98–1.18) | 952 | 1.00 (Ref) | 648 | 1.00 (0.90–1.11) | 291 | 0.82 (0.72–0.94) | 0.0008 |
| Advanced | 211 | 0.99 (0.79–1.22) | 157 | 1.00 (Ref) | 88 | 0.98 (0.75–1.28) | 61 | 1.37 (1.00–1.86) | 0.11 |
| Lethal | 169 | 0.95(0.74–1.22) | 114 | 1.00 (Ref) | 60 | 1.01 (0.74–1.39) | 41 | 1.48(1.02–2.15) | 0.05 |
EPM = ejaculations per month; MV = multivariable; HR = hazard ratio; CI = confidence interval; GS = Gleason score.
Organ-confined disease: stage T1/2N0M0 at diagnosis that did not progress to metastasis or death during the follow-up period. Advanced disease: stage T3b/4 or N1 or M1 at diagnosis or prostate cancer death or distant metastasis during follow-up. Lethal disease: prostate cancer death or metastases to bone or other organs before the end of the follow-up period.
Sensitivity analyses, including analyses excluding men diagnosed with PCa in the first 4 yr of follow-up and analyses restricted to a screened cohort, produced results similar to the overall findings (Supplementary Material and Supplementary Table 4 and Supplementary Table 5). Several stratified analyses were conducted to explore potential effect modifiers. Results stratified by age at baseline, age at diagnosis, BMI at diagnosis, and history of vasectomy provided no evidence that any of these factors modified the association between ejaculation frequency and PCa risk (data not shown).
3.3. Competing causes of death
Because ejaculation frequency may be an indicator of health status, we fitted the multistate model in Supplementary Figure 1 to examine PCa incidence over time across the four NCCN risk groups and the cumulative incidence of lethal PCa in light of other causes of death. None of the ejaculation categories was significantly associated with changes in PCa-specific survival after diagnosis, but categories for the lowest (0–3 EPM) and highest (≥13 EPM) frequency had a higher risk of other-cause mortality (Supplementary Table 6 and Supplementary Table 7). However, the predicted probability of events over time for men who were cancer-free at age 50 yr (Supplementary Fig.) shows that the reduction in PCa risk in the highest category cannot be fully explained by death from other causes. Additional details on the results from this analysis are included in the Supplementary Material.
4. Discussion
The results of this prospective cohort study involving 31 925 men, 18 yr of follow-up, and 3839 PCa cases offer additional evidence of a role for ejaculation frequency in the etiology of PCa, particularly for low-risk disease. The absolute difference in PCa rate between ≥21 and 4–7 EPM was 2.39 cases/1000 person-years for frequency at age 20–29 yr, 2.20 cases/1000 person-years for frequency at age 40–49 yr, and 3.89 cases/1000 person-years for frequency in the year before questionnaire distribution.
An initial report published in 2004 for this cohort found that more frequent ejaculation was related to a lower risk of total PCa, with strongest associations for higher frequency in the year before questionnaire distribution [8]. With an additional decade of follow-up, we demonstrate that ejaculation frequency at three different time points during adulthood is associated with statistically significant modest reductions in risk of total PCa. The association with frequency at age 20–29 yr became more pronounced with additional follow-up, while the associations with frequency at age 40–49 yr and in the year before the questionnaire remained statistically significant but were somewhat attenuated. Taken together with the fact that strong inverse associations remained after excluding men diagnosed in the first 4 yr of follow-up, the updated results are unlikely to be strongly influenced by the effects of undiagnosed disease on ejaculation frequency.
Importantly, our findings were robust to adjustment for time-varying factors such as BMI, physical activity, and diet that differed with ejaculation frequency and that have also been associated with PCa and its progression [5], as well as other factors associated with PCa risk in this cohort. Because men in the higher ejaculation frequency categories had some exposure patterns that might put them at higher risk of morbidity and mortality due to other causes—higher BMI, greater alcohol consumption, and more frequent history of smoking and sexually transmitted infections—we were concerned that the reduction in PCa risk we observed in this group might be attributable to premature death from other causes among men who may have had undiagnosed PCa. Thus, a strength of our study is the consideration of a model for semi-competing risks. From this model, the increase in risk of death by age 80 yr among men with the lowest ejaculation frequency is 3.8%, while the reduction in PCa risk is 2.2%. By comparison, men reporting ≥13 EPM have an increase of only 1.8% in the risk of dying from other causes by age 80 yr, while their decrease in PCa risk is 3.8%. Thus, in both cases the reduction in PCa risk may be partly explained by premature death due to other causes, but the reduction among men reporting high ejaculation frequency seemingly cannot be explained by this effect alone.
Several important limitations of our study should be noted. Ascertainment of the exposure relied on reporting of sexual activity in the past. This may introduce measurement error, particularly in the reporting of frequency at age 20–29 yr. However, the use of an anonymized questionnaire may have resulted in more accurate reporting of sexual behaviors than a one-on-one interview [17]. Previous studies suggest that the validity of data on sensitive information is further improved by: (1) an understanding among participants that their data will be kept confidential, most likely true in this large ongoing cohort in which men had already responded to at least one and potentially two previous questionnaires; and (2) the avoidance of implying a “normal” response category [18]. Most importantly, however, the data were collected prospectively, preventing differential misclassification (ie, recall bias). Thus, our results can be considered conservative estimates of the true association.
While the utilization of a large prospective study has numerous advantages, the clinical information available for men at the time of their PCa diagnosis is more limited than in clinical settings. Thus, we are not able to distinguish very low-risk disease from low-risk disease in subgroup analyses. The abundance of data on potential confounders is an advantage of working within the well-annotated HPFS cohort, but we still cannot rule out residual confounding by other lifestyle factors. Furthermore, our cohort consisted primarily of Caucasian men and the frequency of ejaculation may vary across populations. However, the results may still be generalizable to other men, as we would not expect a true biological association between ejaculation frequency and PCa to differ by race or ethnicity.
The literature exploring the role of sexual activity in the etiology of PCa is inconsistent [7,19–30]. Previous studies are primarily retrospective case-control studies, raising concerns about recall bias, especially given that erectile dysfunction, ejaculatory dysfunction, and decreased libido are common consequences of both PCa and its treatment [31,32]. Moreover, few previous studies have considered ejaculation frequency per se, with most utilizing proxies of sexual activity, such as age at first marriage, marital status, number of sexual partners, and number of children. Few previous studies have examined associations according to tumor grade or stage despite their particular importance for PCa; spurious associations with more favorable disease may result from confounding by early detection. We do, in fact, find that the inverse association with overall PCa is driven by low-risk disease, which could indicate that more sexually active men might undergo less screening and follow-up testing. This alternative explanation for our findings is especially plausible given the potential resulting side effects of PCa and its treatment on sexual function. However, PSA screening history and biopsy utilization after elevated PSA were quite similar across the ejaculation frequency categories. Moreover, the results were consistent even when we restricted the analysis to a screened cohort and PSA history was taken into account. Nonetheless, we cannot rule out residual confounding by screening or post-screening biopsy behaviors.
Our results identified suggestive but not statistically significant associations between higher ejaculation frequency in the year before the questionnaire and both advanced and lethal PCa. However, the findings appear to be driven by men diagnosed in the period immediately following the questionnaire. The attenuated association in sensitivity analyses excluding men diagnosed in the first 4 yr of follow-up, together with the fact that these suggestive positive associations were only found for ejaculation frequency in the year before the questionnaire distribution and not at younger ages, is consistent with men with undiagnosed aggressive PCa experiencing symptoms that promoted more frequent ejaculation. While we are not aware of any literature supporting ejaculation for relief of PCa symptoms, it nonetheless seems unlikely that these suggestive associations with advanced and lethal disease reflect causality.
In addition to the prostate stagnation hypothesis [7], a number of mechanisms have been proposed to explain an inverse association between ejaculation frequency and PCa. More frequent ejaculation may influence the function of peripheral-zone epithelial cells, hindering the metabolic switch from citrate secretion to citrate oxidation known to occur early in prostate tumorigenesis [33]. Alternatively, more frequent ejaculation may reduce the development of prostatic intraluminal crystalloids, which have been associated with higher risk of PCa [34,35]. Higher ejaculatory frequency may be linked to lowering of psychological tension and central sympathetic nervous system suppression, which could dampen the stimulation of prostate epithelial cell division [36]. Given the lack of modifiable risk factors identified for PCa to date, the specific biological mechanisms underlying these associations are worthy of further investigation.
5. Conclusions
This large prospective study provides the strongest evidence to date of a beneficial role of ejaculation in prevention of PCa, a disease for which relatively little is understood about etiology generally and knowledge of modifiable risk factors is particularly scant. The results are robust to adjustment for many dietary, lifestyle, and screening behaviors, but additional work on the underlying biological mechanisms should be undertaken to corroborate these findings given the potential for residual confounding. More frequent ejaculation in the absence of risky sexual behaviors could represent an important means of reducing the profound medical costs and physical and psychological side effects of unnecessary diagnosis and treatment of low-risk tumors, even though it appears to be less strongly associated with aggressive disease.
Supplementary Material
Acknowledgments
We are grateful to the participants and staff of the Health Professionals Follow-up Study for their valuable contributions. In addition, we would like to thank the following state cancer registries for their cooperation and assistance: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY. JRR and KMW assume full responsibility for analyses and interpretation of these data. Finally, we are indebted to David Havelick for his enthusiastic support of this project.
Financial disclosures: Jennifer R. Rider certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: The Health Professionals Follow-Up Study is supported in part by grants UM1 CA167552, P01 CA055075, P01 CA133891, and P01 CA141298. Jennifer R. Rider, Kathryn M. Wilson, and Lorelei A. Mucci are supported by Prostate Cancer Foundation Young Investigator Awards. The sponsors played no role in the study.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.eururo.2016.03.027.
Footnotes
Author contributions: Jennifer R. Rider had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Rider, Mucci, Giovannucci.
Acquisition of data: Rider, Wilson, Mucci, Giovannucci.
Analysis and interpretation of data: Rider, Wilson, Sinnott, Mucci, Giovannucci.
Drafting of the manuscript: Rider, Wilson.
Critical revision of the manuscript for important intellectual content: Rider, Wilson, Sinnott, Kelly, Mucci, Giovannucci.
Statistical analysis: Rider, Wilson, Sinnott, Kelly.
Obtaining funding: Giovannucci.
Administrative, technical, or material support: None.
Supervision: Mucci, Giovannucci.
Other: None.
References
- 1.Soerjomataram I, Lortet-Tieulent J, Parkin DM, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380:1840–1850. doi: 10.1016/S0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- 2.Gardner JR, Livingston PM, Fraser SF. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol. 2014;32:335–346. doi: 10.1200/JCO.2013.49.5523. [DOI] [PubMed] [Google Scholar]
- 3.Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the Health Professionals Follow-up Study. J Clin Oncol. 2011;29:726–732. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richman EL, Kenfield SA, Stampfer MJ, Paciorek A, Carroll PR, Chan JM. Physical activity after diagnosis and risk of prostate cancer progression: data from the cancer of the prostate strategic urologic research endeavor. Cancer Res. 2011;71:3889–3895. doi: 10.1158/0008-5472.CAN-10-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson KM, Giovannucci EL, Mucci LA. Lifestyle and dietary factors 1 in the prevention of lethal prostate cancer. Asian J Androl. 2012;14:365–374. doi: 10.1038/aja.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Discacciati A, Wolk A. Lifestyle and dietary factors in prostate cancer prevention. Recent Results Cancer Res. 2014;202:27–37. doi: 10.1007/978-3-642-45195-9_3. [DOI] [PubMed] [Google Scholar]
- 7.Isaacs JT. Prostatic structure and function in relation to the etiology of prostatic cancer. Prostate. 1983;4:351–366. doi: 10.1002/pros.2990040405. [DOI] [PubMed] [Google Scholar]
- 8.Leitzmann MF, Platz EA, Stampfer MJ, Willett WC, Giovannucci E. Ejaculation frequency and subsequent risk of prostate cancer. JAMA. 2004;291:1578–1586. doi: 10.1001/jama.291.13.1578. [DOI] [PubMed] [Google Scholar]
- 9.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the Health Professionals Follow-up Study. Int J Cancer. 2007;121:1571–1578. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. Clinical practice guidelines in oncology: prostate cancer. Fort Washington, PA: NCCN; 2016. [Google Scholar]
- 11.Greenland S, Finkle WD. A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol. 1995;142:1255–1264. doi: 10.1093/oxfordjournals.aje.a117592. [DOI] [PubMed] [Google Scholar]
- 12.Popiolek M, Rider JR, Andren O, et al. Natural history of early, localized prostate cancer: a final report from three decades of follow-up. Eur Urol. 2013;63:428–435. doi: 10.1016/j.eururo.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 14.Jahn JL, Giovannucci EL, Stampfer MJ. The high prevalence of undiagnosed prostate cancer at autopsy: implications for epidemiology and treatment of prostate cancer in the prostate-specific antigen era. Int J Cancer. 2015;137:2795–2802. doi: 10.1002/ijc.29408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 16.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 2014 [Google Scholar]
- 17.Fenton KA, Johnson AM, McManus S, Erens B. Measuring sexual behaviour: methodological challenges in survey research. Sex Transm Infect. 2001;77:84–92. doi: 10.1136/sti.77.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tourangeau R, Yan T. Sensitive questions in surveys. Psychol Bull. 2007;133:859–883. doi: 10.1037/0033-2909.133.5.859. [DOI] [PubMed] [Google Scholar]
- 19.Sarma AV, McLaughlin JC, Wallner LP, et al. Sexual behavior, sexually transmitted diseases and prostatitis: the risk of prostate cancer in black men. J Urol. 2006;176:1108–1113. doi: 10.1016/j.juro.2006.04.075. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez L, Galan Y, Jimenez R, et al. Sexual behaviour, history of sexually transmitted diseases, and the risk of prostate cancer: a case-control study in Cuba. Int J Epidemiol. 2005;34:193–197. doi: 10.1093/ije/dyh332. [DOI] [PubMed] [Google Scholar]
- 21.Hayes RB, Pottern LM, Strickler H, et al. Sexual behaviour, STDs and risks for prostate cancer. Br J Cancer. 2000;82:718–725. doi: 10.1054/bjoc.1999.0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dennis LK, Dawson DV. Meta-analysis of measures of sexual activity and prostate cancer. Epidemiology. 2002;13:72–79. doi: 10.1097/00001648-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Honda GD, Bernstein L, Ross RK, Greenland S, Gerkins V, Henderson BE. Vasectomy, cigarette smoking, and age at first sexual intercourse as risk factors for prostate cancer in middle-aged men. Br J Cancer. 1988;57:326–331. doi: 10.1038/bjc.1988.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spence AR, Rousseau MC, Parent ME. Sexual partners, sexually transmitted infections, and prostate cancer risk. Cancer Epidemiol. 2014;38:700–707. doi: 10.1016/j.canep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Rotkin ID. Studies in the epidemiology of prostatic cancer: expanded sampling. Cancer Treat Rep. 1977;61:173–180. [PubMed] [Google Scholar]
- 26.Ahmadi H, Allameh F, Baradaran N, et al. Circulating sex hormones play no role in the association between sexual activity and the risk of prostate cancer. J Sex Med. 2011;8:905–913. doi: 10.1111/j.1743-6109.2010.02115.x. [DOI] [PubMed] [Google Scholar]
- 27.Giles GG, Severi G, English DR, et al. Sexual factors and prostate cancer. BJU Int. 2003;92:211–216. doi: 10.1046/j.1464-410x.2003.04319.x. [DOI] [PubMed] [Google Scholar]
- 28.Hayes RB, de Jong FH, Raatgever J, et al. Physical characteristics and factors related to sexual development and behaviour and the risk for prostatic cancer. Eur J Cancer Prev. 1992;1:239–245. doi: 10.1097/00008469-199204000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Rosenblatt KA, Wicklund KG, Stanford JL. Sexual factors and the risk of prostate cancer. Am J Epidemiol. 2001;153:1152–1158. doi: 10.1093/aje/153.12.1152. [DOI] [PubMed] [Google Scholar]
- 30.Oishi K, Okada K, Yoshida O, et al. A case-control study of prostatic cancer in Kyoto, Japan: sexual risk factors. Prostate. 1990;17:269–279. doi: 10.1002/pros.2990170403. [DOI] [PubMed] [Google Scholar]
- 31.Saitz TR, Serefoglu EC, Trost LW, Thomas R, Hellstrom WJ. The pre-treatment prevalence and types of sexual dysfunction among patients diagnosed with prostate cancer. Andrology. 2013;1:859–863. doi: 10.1111/j.2047-2927.2013.00137.x. [DOI] [PubMed] [Google Scholar]
- 32.Choo R, Long J, Gray R, Morton G, Gardner S, Danjoux C. Prospective survey of sexual function among patients with clinically localized prostate cancer referred for definitive radiotherapy and the impact of radiotherapy on sexual function. Support Care Cancer. 2010;18:715–722. doi: 10.1007/s00520-009-0675-6. [DOI] [PubMed] [Google Scholar]
- 33.Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol Cancer. 2006;5:17. doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svatek RS, Karam JA, Rogers TE, Shulman MJ, Margulis V, Benaim EA. Intraluminal crystalloids are highly associated with prostatic adenocarcinoma on concurrent biopsy specimens. Prostate Cancer Prostatic Dis. 2007;10:279–282. doi: 10.1038/sj.pcan.4500954. [DOI] [PubMed] [Google Scholar]
- 35.Del Rosario AD, Bui HX, Abdulla M, Ross JS. Sulfur-rich prostatic intraluminal crystalloids: a surgical pathologic and electron probe X-ray microanalytic study. Hum Pathol. 1993;24:1159–1167. doi: 10.1016/0046-8177(93)90210-8. [DOI] [PubMed] [Google Scholar]
- 36.Newman HF, Reiss H, Northup JD. Physical basis of emission, ejaculation, and orgasm in the male. Urology. 1982;19:341–350. doi: 10.1016/0090-4295(82)90186-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.