Abstract
The obligate intracellular parasite Toxoplasma gondii exploits cells of the immune system to disseminate. Upon infection, parasitized dendritic cells (DCs) and microglia exhibit a hypermigratory phenotype in vitro that has been associated with enhancing parasite dissemination in vivo in mice. One unresolved question is how parasites commandeer parasitized cells to achieve systemic dissemination by a ‘Trojan horse’ mechanism. By chromatography and mass spectrometry analyses, we identified an orthologue of the 14-3-3 protein family, T. gondii 14-3-3 (Tg14-3-3), as mediator of DC hypermotility. We demonstrate that parasite-derived polypeptide fractions enriched for Tg14-3-3 or recombinant Tg14-3-3 are sufficient to induce the hypermotile phenotype when introduced by protein transfection into murine DCs, human DCs or microglia. Further, gene transfer of Tg14-3-3 by lentiviral transduction induced hypermotility in primary human DCs. In parasites expressing Tg14-3-3 in a ligand regulatable fashion, over-expression of Tg14-3-3 was correlated with induction of hypermotility in parasitized DCs. Localization studies in infected DCs identified Tg14-3-3 within the parasitophorous vacuolar space and a rapid recruitment of host cell 14-3-3 to the parasitophorous vacuole membrane. The present work identifies a determinant role for Tg14-3-3 in the induction of the migratory activation of immune cells by T. gondii. Collectively, the findings reveal Tg14-3-3 as a novel target for an intracellular pathogen that acts by hijacking the host cell’s migratory properties to disseminate.
Introduction
Toxoplasma gondii is a widespread obligate intracellular parasite capable of infecting humans and virtually all other warm-blooded vertebrates (Tenter et al., 2000). It is estimated to infect more than one-third of the global human population. T. gondii infection is normally efficiently controlled by a functional immune system, but in immunocompromised individuals, such as individuals with AIDS, toxoplasmosis remains a leading cause of focal central nervous system disease. While it is well established that T. gondii is capable of crossing biological barriers such as the blood-brain, blood-ocular and maternal-fetal barriers (Barragan et al., 2008), the precise mechanisms as to how the parasite achieves passage and wide dissemination remain elusive.
To replicate, T. gondii actively invades host cells, including immune cells, and dendritic cells (DCs) are one of the first innate immune cells to encounter T. gondii after the parasite crosses the intestinal epithelium (Denkers et al., 2012, Hunter et al., 2012). Being an early source of IL-12 driving protective Th1 responses, ablation of DCs results in failure to mount protective immunity and lethal outcome in murine toxoplasmosis (Liu et al., 2006, Mashayekhi et al., 2011). Besides the determinant roles that DCs play in controlling the infection, the invasive T. gondii tachyzoites employ successful mechanisms to subvert the migratory machinery of DCs, and promote their rapid and widespread dissemination throughout the host by a ‘Trojan horse’ mechanism (Courret et al., 2006, Lambert et al., 2006, Fuks et al., 2012). Upon active invasion by T. gondii, DCs and primary microglia rapidly undergo dramatic morphological changes accompanied by an induction of a hypermotile phenotype, promoted by the activation of GABAergic signaling pathways in infected DCs (Dellacasa-Lindberg et al., 2011, Fuks et al., 2012, Weidner et al., 2013, Kanatani et al., 2015). Hypermigration of parasitized DCs has been linked to enhanced dissemination and parasitic loads in mice for apicomplexan infections with T. gondii and Neospora caninum (Lambert et al., 2006, Lambert et al., 2009, Collantes-Fernandez et al., 2012). However, the parasitic effector molecules that are responsible for the onset of the hypermigratory phenotype have not been elucidated.
14-3-3 proteins are a family of ubiquitously expressed proteins of all eukaryotic cells and, as dimerized adapter proteins, they are involved in the regulation of diverse cellular processes including cytoskeleton organization, cell migration, apoptosis and cell cycle control (Sluchanko et al., 2010). Further, human isoforms of 14-3-3 promote cell migration and cancer metastasis through cytoskeletal remodeling (Somanath et al., 2009, Freeman et al., 2011). 14-3-3 sequences have been identified in several apicomplexan parasites, e.g. in Plasmodiae, T. gondii, N. caninum and Cryptosporidiae (Siles-Lucas Mdel et al., 2003). The precise roles of the 14-3-3 proteins in the Apicomplexa, and specifically of T. gondii 14-3-3 (Tg14-3-3), however, remain chiefly unknown (Assossou et al., 2003, Assossou et al., 2004, Lorestani et al., 2012).
Herein, we identify a T. gondii effector molecule responsible for the initiation of the migratory activation elicited in DCs shortly after parasite invasion. We show that Tg14-3-3 is sufficient to induce a hypermigratory phenotype in DCs.
Results
Identification of T. gondii-derived polypeptides associated with hypermigration of DCs
Previous work has demonstrated that DCs exhibit a dramatic hypermigratory phenotype within minutes after active invasion by T. gondii tachyzoites (Lambert et al., 2006, Weidner et al., 2013). As DC hypermotility was dependent on intracellular parasite localization and secretory organelle discharge, but not parasite attachment to the cell surface (Weidner et al., 2013), we hypothesized that parasite-derived effector molecules mediated the hypermigratory phenotype. We therefore fractionated parasite lysates by ion exchange chromatography (Fig. 1A), performed protein transfection on DCs with the eluted fractions and assessed DC migration. Importantly, the T. gondii lysate and eluted polypeptide fraction (active fraction - AF), alike live T. gondii tachyzoites, induced a significantly enhanced transmigration of DCs across transwell filters (Fig 1B). Similarly, in motility assays, the AF induced significantly increased velocities in transfected DCs, in sharp contrast to the flow through (FT) fraction (Fig. 1C). In the absence of the transfection reagent, the AF induced non-significant effects on DC migration (Fig. S1A), indicating that intracellular delivery was necessary for induction of motility. Altogether, these data demonstrate that parasite-derived polypeptide fractions delivered into the cytosol can induce a hypermigratory phenotype in DCs, similar to T. gondii infection.
Fig. 1. Purification of a migration-inducing fraction in total T. gondii tachyzoite lysates.
A. Ion exchange chromatography of total tachyzoite lysates as described in experimental procedures. Two fractions were collected designated Active Fraction (AF) and Flow Through (FT). Coomassie Blue-stained SDS-PAGE gel of total T. gondii lysate (PRU-RFP), AF and FT, at equal total protein concentrations is provided as inset.
B. Transmigration of murine DCs following protein transfection with T. gondii lysates (2 μg), AF (2 μg) and FT (2 μg) fractions into murine DCs. ‘Transfection control’ indicates transfection reagent only and ‘T. gondii’ indicates challenge with freshly egressed T. gondii tachyzoites. Histograms show the fold change in transmigration frequency (median ± SD) relative to the transfection control (normalized to 1) from five independent experiments and over three separate fractionation events. Asterisks indicate significant differences (*: p < 0,05; **: p < 0,005; non-significant (ns): p > 0,05, Student’s t-test).
C. Motility plots of DCs transfected with the indicated protein fractions, challenged with live T. gondii tachyzoites or treated with the transfection reagent alone. Plots depict cell track analyses compiled from three independent experiments. Median velocity ± SEM are provided.
D. Western blot analysis of total fibroblast (HFF) lysates, total murine dendritic cell (DC) lysates, T. gondii lysates, AF and FT using pan-14-3-3 antibody.
E. Velocity analysis of DCs transfected with indicated protein fractions, ± preincubation with the R18 peptide, by a motility assay. Data represent compiled analysis from three independent experiments (median ± SEM). Asterisks indicate significant differences (**: p < 0,001; ns: p > 0,05, Student’s t-test).
Next, we attempted to identify the putative polypeptides that induced DC hypermotility. Separation of the protein fractions by SDS-PAGE (Fig. 1A), followed by mass spectrometry comparison analyses identified a polypeptide band (≈35 kDa MW,) present in the AF, but absent in the non-active FT fraction. The polypeptide was identified as T. gondii 14-3-3 (Tg14-3-3, TGME49_263090) by MALDI-TOF (Fig. S3A). Based on the known roles of 14-3-3 in cell motility and the presence of Tg14-3-3 in parasite-derived secretory fractions (Zhou et al., 2005), we chose Tg14-3-3 as a rational candidate and tested its hypothetical implication in host cell hypermotility. Western blotting analysis corroborated an enrichment of Tg14-3-3 in AF and an absence of detectable signal in FT fractions (Fig. 1D). Finally, to confirm a role for Tg14-3-3 in the induction of DC hypermotility, we took advantage of a competitive inhibitor of 14-3-3 proteins (R18) (Wang et al., 1999). Molecular modeling of Tg14-3-3, based on the previously determined crystal structure of the human 14-3-3 zeta form in complex with R18 (Yang et al., 2006), indicated that the inhibiting R18 peptide can readily take a similar position in the positively charged cleft of Tg14-3-3 (Fig. S2A) Indeed, pre-incubation of the AF with R18 abolished hypermotility in transfected DCs without affecting the FT fractions (Fig. 1E). Taken together, these data demonstrate that delivery of parasite-derived polypeptide fractions containing Tg14-3-3 into the host cell cytosol is associated with the induction of a hypermigratory phenotype in DCs.
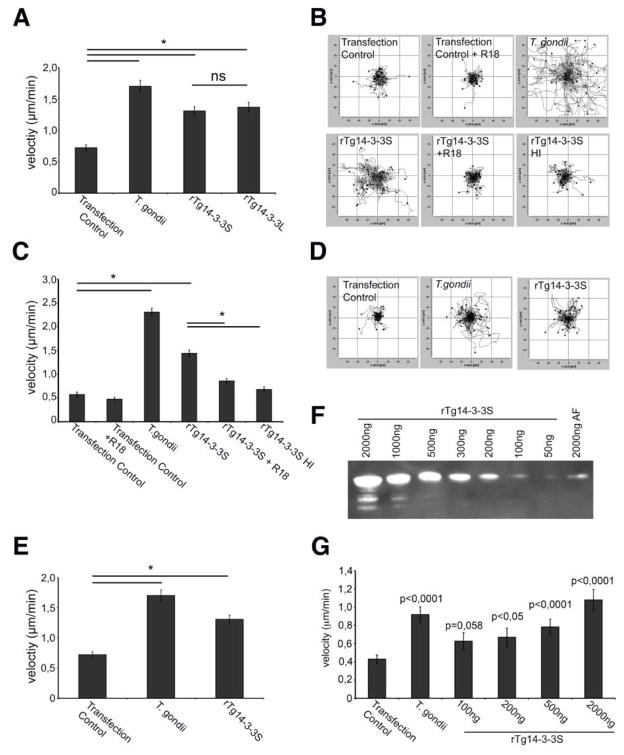
Recombinant Tg14-3-3 induces hypermotility in DCs and microglia
Natively purified polypeptide fractions have the disadvantage of being composite. To assess the role of Tg14-3-3 in the absence of co-eluting T. gondii proteins, we generated recombinant Tg14-3-3 (rTg14-3-3) protein. First, because analyses of the AF obtained by ion exchange chromatography fractionation of T. gondii lysates identified Tg14-3-3 (TGME49_263090) by MALDI-TOF (Fig. S3A), we characterized Tg14-3-3 present in the AF. Recombinant proteins of the full length annotated sequence (rTg14-3-3L) and the amino acid sequence with highest homology with other 14-3-3 were expressed (rTg14-3-3S) (Fig. S2B, Fig. S3B, C and D). Western blot analyses detected that native Tg14-3-3 in the AF and rTg14-3-3S migrated identically (Fig. S3E). Further, endogenous tagged Tg14-3-3 migrated accordingly (Fig. S3F). Altogether, we conclude that rTg14-3-3S corresponds to the native Tg14-3-3 retrieved in AF.
Importantly, protein transfection of rTg14-3-3 into murine DCs resulted in a significant increase in DC velocity (Fig. 2 A, B and C), that was absent in the protein transfection control with recombinant GFP (Fig S1B). We observed no measureable differences between the two recombinant proteins (rTg14-3-3L, rTg14-3-3S) in the ability to induce a hypermotile phenotype when transfected into DCs (Fig. 2A). Because we lacked evidence that a native variant of Tg14-3-3 corresponding to rTg14-3-3L was expressed, we chose to focus our efforts on characterizing the effects of Tg14-3-3S, the isoform that is most similar to other eukaryotic forms of 14-3-3. Pre-incubation of rTg14-3-3S with the inhibitor R18 prior to transfection significantly reduced velocity and, heat-inactivated rTg14-3-3S failed to induce hypermotility in DCs (Fig. 2B and C). Corroborating our findings in DCs, murine microglia cells (BV2) transfected with rTg14-3-3S exhibited hypermotility, reaching velocities similar to those induced by live T. gondii infection (Fig. 2D and E). A titration of rTg14-3-3S revealed presence of native Tg14-3-3 in the range of 100–200 ng/2 μg AF of Toxoplasma lysate (Fig. 2F) and transfection of rTg14-3-3S within this range or higher range induced DC hypermotility dose-dependently (Fig. 2G).
Fig. 2. Recombinant T. gondii 14-3-3 (rTg14-3-3) induce hypermotility in DCs and microglia.
A. Murine DCs were challenged with freshly egressed tachyzoites (PRU-RFP, T. gondii), transfected with either rTg14-3-3S or rTg14-3-3L or mock transfected with only transfection reagent (transfection control) as indicated under experimental procedures. Data represent the velocity of 50 cells (median ± SEM) from three independent experiments. Asterisks indicate significant differences (* p < 0,001; ns: p > 0,05, Student’s t-test).
B. Motility plots of murine DCs were challenged with freshly egressed tachyzoites (T. gondii), transfected with rTg14-3-3S or mock transfected with only transfection reagent (transfection control). rTg14-3-3S was also pre-incubated with R18 or heat-inactivated (HI) prior to transfection. For each condition, the plots depict cell track analyses (μm) of 50 single cells during 60 min.
C. Velocity analysis of cells under the same conditions as (B). Data represent compiled analysis (median ± SEM) from three independent experiments. Asterisks indicate significant differences (* p < 0,001; ns: p > 0,05, Student’s t-test).
D. Motility plots of microglia (BV2) challenged with freshly egressed tachyzoites (T. gondii), transfected with rTg14-3-3S or mock transfected (transfection control). The velocity of 50 individual cells was measured during 60 min.
E. Velocity analysis of cells under the same conditions as (D). Data represent compiled analysis (median ± SEM) from two independent experiments (* p < 0,001; ns: p > 0,05, Student’s t-test).
F. Titration of rTg14-3-3S to compare the amount of native 14-3-3 in 2μg of AF by Western blotting using a pan-14-3-3Ab as indicated in experimental procedures.
G. Murine DCs were challenged with freshly egressed tachyzoites (PRU-RFP, T. gondii), mock transfected, or transfected with the indicated concentration of rTg14-3-3S as indicated under experimental procedures. Data represent the velocity of 50 cells (median ± SEM) from two independent experiments. P values (related to the transfection control) are provided using the Student’s t-test.
Lentiviral over-expression of Tg14-3-3 is sufficient to induce hypermotility in DCs
Next, we sought out to determine the impact of Tg14-3-3 (31 kDa) on human monocyte-derived DCs (MoDCs) using a lentiviral expression system. Transduced MoDCs were identified by their ZsGreen expression (Fig. S4), and therefore could be specifically tracked. A significant elevation of migratory velocity was observed in MoDCs transduced with Tg14-3-3, but not in mock-transduced MoDCs. Importantly, Tg14-3-3-transduced MoDCs exhibited hypermotility similar to T. gondii-infected MoDCs (Fig. 3A and B), while motility was not elevated in MoDCs expressing only ZsGreen protein. Altogether, these data are in line with the results obtained with rTg14-3-3 and corroborate that Tg14-3-3, alone, is sufficient to induce a hypermotile state in MoDCs.
Fig. 3. Lentiviral over-expression of Tg14-3-3 is sufficient to induce hypermotility in human DCs.
A. Human monocyte-derived dendritic cells (MoDCs) were transduced with a lentiviral vector expressing Tg14-3-3 (Tg14-3-3S) or the empty vector. Plots depict the motility plots (μm) of 50 single cells during 60 min from one representative donor that were non-infected, non-treated (NI, NT), challenged with freshly egressed tachyzoites (T. gondii), treated only with the cationic polymer (polybrene), transduced with pLVX (empty vector) or transduced with pLVX14-3-3 (Tg14-3-3).
B. Velocity analysis of cells under the same conditions as (D). Data represent compiled analysis from three healthy donors (median ± SEM). Asterisks indicate significant differences (* p < 0,001; ns: p > 0,05, Student’s t-test).
In an effort to study the effects of a loss of Tg14-3-3, we attempted to delete the 14-3-3 ORF in the parasite by applying two parallel CRISPR/Cas9-based approaches (Shen et al., 2014, Sidik et al., 2014). Multiple independent transfection experiments resulted in non-viable parasites and indicated that the deletion of the 14-3-3 ORF is likely not tolerated by the parasite (Fig. S5).
Endogenous over-expression of Tg14-3-3 in T. gondii tachyzoites mediates enhanced migration of infected DCs
Since the localization of 14-3-3 proteins presumably determines their effector functions, we sought to determine the impact of endogenous over-expression of Tg14-3-3 by intracellular tachyzoites on host cell migration. A T. gondii line over-expressing YFP-tagged Tg14-3-3 in a ligand regulatable fashion (RH-DDYFP-Tg14-3-3) (Lorestani et al., 2012) allowed visualization of YFP-Tg14-3-3 expression by fluorescence microscopy (Fig. 4A). In the absence of the induction by the ligand Shield1, RH-DDYFP-Tg14-3-3 expressed undetectable levels of YFP-Tg14-3-3 while native Tg14-3-3 expression was maintained (Fig. 4B, Fig. S6A). Importantly, induced expression of YFP-Tg14-3-3 was accompanied by a significant increase in velocity (Fig 4C and D) and in transmigration frequency of MoDCs (Fig 4E), with non-significant effects by Shield1 on motility (Fig. S6B). These data demonstrate that endogenous over-expression of Tg14-3-3 results in a migratory activation of MoDCs. Next, we analyzed the localization of YFP-Tg14-3-3 by TEM and immunogold labeling using an antibody directed against YFP. Consistent with previous reports (Assossou et al., 2003, Lorestani et al., 2012), an intraparasitic cytosolic localization of YFP-Tg14-3-3 was detected (Fig. 4F and G). Importantly, an extraparasitic localization of YFP-Tg14-3-3 was consistently observed in the perivacuolar space beneath the parasitophorous vacuole membrane (PVM) (Fig. 4F and G, Fig. S7). Taken together, these data support our recombinant protein and transduction data, that Tg14-3-3 is sufficient to induce a hypermotile phenotype in MoDCs, and that a portion of the protein localizes to the perivacuolar space.
Fig. 4. Endogenous, inducible over-expression of Tg14-3-3 potentiates the hypermotility phenotype in DCs.
A. YFP-expression in replicating vacuoles in HFFs was assessed by titrating Shield1 in the culture medium (250nM, 500nM and 1μM). 1μM was used throughout the experiments. Scale bar – 50μm.
B. YFP-tagged-14-3-3 in tachyzoite lysates could be detected by Western blotting with an anti-GFP antibody that cross-reacts with the YFP epitope.
C. MoDCs were challenged with RH-DDYFP-Tg14-3-3 for 2 h and then Shield1 was added to the culture medium for the final 2 h of incubation after which a motility assay was performed. The histograms show the frequency of the accumulated distances (μm) related to the total cell population, recorded during 60 min. Red lines indicate median migrated distance. The trajectory of individual cells is provided as a motility plot (inset) for 50 infected MoDCs from one healthy donor.
D. Velocity analysis of cells under the same conditions as (C). Data represent a compiled analysis (median ± SEM) from three healthy donors. Asterisks indicate significant differences as compared to the non-infected control (* p < 0,001, ** p < 0,0001, Student’s t-test).
E. MoDCs were challenged with freshly egressed tachyzoites (± Shield1) for 4–6 h. Data are representative of the average transmigration rate (± SD) from three healthy donors. Asterisks indicate significant differences as compared to the non-infected control (* p < 0,05, ** p < 0,005, Student’s t-test).
F. Immunogold labeling of MoDCs infected with RH-DDYFP-Tg14-3-3 tachyzoites (+ Shield1) at MOI1 for 24 h, using an anti-GFP antibody followed by incubation with a secondary antibody conjugated to 12 nm colloidal gold. Inset is taken at 60k magnification. Gold particles within the tachyzoites (tachy) are circled in red, while the gold particles within the perivacuolar space (PS) are indicated with arrows.
G. Average number of gold particles detected inside the tachyzoites (tachy) and within the perivacuolar space (PS) (± SEM) compiled from 15 separate fields from two healthy donors, performed under same conditions as in (F). The negative control is MoDCs challenged with freshly egressed PRU-RFP expressing tachyzoites. Asterisks indicate significant differences as compared to the negative control (** p < 0,001, * p < 0,05, Dunnett’s test).
Host cell 14-3-3 is rapidly recruited to the parasitophorous vacuole (PV) of T. gondii
To determine localization of host 14-3-3 following T. gondii infection, MoDCs were challenged with T. gondii, followed by immunofluorescence staining of the PVM-marker GRA7 and 14-3-3 using separate 14-3-3 antibodies. The 14-3-3 staining signal consistently formed a distinct ring around the PVM that co-localized, at least in part, with GRA7 vacuolar staining (Fig. 5A and B, Fig. S8). Recruitment of host 14-3-3 to the PVM was rapid, with a distinct pattern being visible 6 h after T. gondii invasion and further reinforced by 24 h (Fig. 5C). Host 14-3-3 signal localized to the PVM and not to the surface of T. gondii, as indicated by absence of co-localisation with SAG1 staining (Fig. 5C). TEM immunogold staining confirmed the concentration and localization of host 14-3-3 to the perivacuolar space beneath the PVM (Fig. 5D).
Fig. 5. Host 14-3-3 is rapidly recruited to the parasitophorous vacuole (PV) of T. gondii.
A. MoDC were challenged with freshly egressed GFP-expressing T. gondii tachyzoites (PTGluc, MOI1) for 24 h and stained with pan-14-3-3 antibody (red) and GRA7 antibody (blue).
B. MoDCs were challenged with PTGluc tachyzoites (MOI1) for 24 h and stained with pan-14-3-3 antibody (red) and GRA7 antibody (blue). Fluorescence intensity (FI) profile plots at different emission wavelengths are provided, corresponding to the signals of 14-3-3 (red) and GRA7 (blue). Scale bar: 10μm.
C. Time course analysis of recruitment of 14-3-3 to the PV. MoDCs were challenged with PTGluc tachyzoites (MOI 2) for 6 h (upper panel) or MOI 1 for 24 h (lower panel). Cells were fixed and stained with pan-14-3-3 antibody (red) and SAG1 antibody (blue).
D. Immunogold labeling of MoDCs infected with RH-DDYFP-Tg14-3-3 tachyzoites (+ Shield 1) at MOI1 for 24 h, using a pan-14-3-3 antibody followed by incubation with a secondary antibody conjugated to 12 nm colloidal gold. Inset is taken at 60k magnification.
Discussion
The tachyzoite stage of T. gondii plays an important role in pathogenesis during acute and reactivated toxoplasmosis. DCs and microglia have been implicated in the dissemination of the parasite by a ‘Trojan horse’ mechanism. Thus, our studies on the migratory characteristics of parasitized DCs and microglia are directly relevant to dissemination-related acute pathology and to reactivation of chronic infection. In this context, our studies identify, for the first time, a parasite-derived molecule that mediates host cell hypermotility.
Our findings establish that parasite-derived Tg14-3-3 is sufficient to induce a hypermigratory phenotype in parasitized DCs and microglia. We demonstrate this by separate approaches. First, native tachyzoite-derived fractions enriched for Tg14-3-3 and recombinant Tg14-3-3 induced hypermigration in a blocking-peptide (R18) inhibitable fashion. Second, gene transfer of Tg14-3-3 by lentiviral transduction induced hypermotility in primary DCs. Third, over-expression of Tg14-3-3 in tachyzoites was correlated with enhanced induction of hypermotility in parasitized DCs.
Seven genes encode 14-3-3 in most mammals, including humans, while protists have a least one (Fu et al., 2000, Freeman et al., 2011). In T. gondii, one gene codes for Tg14-3-3 (TGME49_263090). Eukaryotes can tolerate the loss of a single 14-3-3 gene if multiple genes are expressed, however deletion of all 14-3-3 genes, as experimentally determined in yeast, results in death (Cognetti et al., 2002). Similarly, two separate CRISP/cas-9-based approaches targeting the Tg14-3-3 gene yielded non-viable parasites.
Tg14-3-3 was previously identified in the parasitophorous vacuole (PV) and in excreted/secreted fractions of T. gondii tachyzoites (Assossou et al., 2004, Zhou et al., 2005). Tg14-3-3 was not detected in the host cell cytosol by the current approach. To address this, methods with higher sensitivity would likely be needed. Alternatively, it is plausible that Tg14-3-3 does not traffic to the host cell cytosol However, its localization to the PV advocates for a role as effector molecule in the infected cell. In this context, the observed strong recruitment of host cell 14-3-3 around the replicating parasitic vacuole is intriguing. Also, the onset of the hypermigratory phenotype after recombinant protein transfection shown here indicate rapid and direct effector functions by Tg14-3-3 on host cell motility and correspond well with the onset of hypermigration minutes after parasite invasion (Weidner et al., 2013). Additional Tg14-3-3 functions are also possible. For example, 14-3-3 proteins participate in transcriptional regulation (Tzivion et al., 2011) and T. gondii is known to manipulate the host cell transcriptome via secreted effector molecules (Hakimi et al., 2015).
The finding that transfection of recombinant Tg14-3-3 is sufficient to induce DC hypermotility indicates that the counterparts for effector functions ought to reside among host cell molecules, rather than among parasite-derived molecules, and does not exclude an additional implication of parasite-derived molecules. One means by which 14-3-3 regulates cellular processes is by modulating protein localization (Muslin et al., 2000, Dougherty et al., 2004). One possible mechanism may include the observed recruitment of host cell 14-3-3 around the replicating parasitic vacuole. Hypothetically, the regulatory functions of host cell 14-3-3 in cell motility may be up- or downmodulated as a consequence of its sequestration to the PV. We speculate that the functions of Tg14-3-3 and host 14-3-3 in cell migration may be linked and mediated by their direct physical interaction or vicinity. 14-3-3 proteins form homo- and/or hetero-dimer interactions and future research needs to test whether host- and parasite-14-3-3 interactions occur under physiological conditions. Interestingly, GABAergic signaling pathways in DCs are implicated in the hypermigratory phenotype (Fuks et al., 2012) and recent reports have implicated 14-3-3 molecules as regulators of GABA receptor signaling in neurological models (Benke et al., 2012, Qian et al., 2012). Thus, this implies a putative hypothetical link to GABA receptor regulation and future investigations will be important to establish the molecular interplay of Tg14-3-3 with putative host counterparts. Additionaly, among different strains of T. gondii and N. caninum significant differences exist in the induction of the hypermigratory phenotype in vitro and in vivo (Lambert et al., 2009, Collantes-Fernandez et al., 2012). For example, T gondii type II strains consistently induce a more pronounced hypermigratory phenotype compared to type I strains. Future research needs to determine whether the genotype-related differences are explained by differential expression levels and/or differences in protein trafficking of Tg14-3-3 (Hammoudi et al., 2015).
Our findings are well in line with the implications of 14-3-3 in the pathogenesis of disease. 14-3-3 proteins regulate the actin cytoskeleton remodeling and cell migration of eukaryotic cells and enhanced expression of 14-3-3 promotes metastasis and invasivity of various cancer types (Mhawech, 2005, Freeman et al., 2011). In Plasmodium berghei infection, Pb14-3-3 participates in the remodeling of the erythrocytic cytoskeleton (Lalle et al., 2011) and fungal 14-3-3 mediates adhesive interactions to epithelial cells (da Silva Jde et al., 2013). In helminthic infections, parasite 14-3-3 orthologues have also proven to be immunodominant (Siles-Lucas et al., 1998, Yang et al., 2015). Similarly, reactivity to Tg14-3-3 was found in individuals with acute toxoplasmosis (Assossou et al., 2004) and Tg14-3-3 could thus play a role in the immune control of the infection. Also, the activation of host 14-3-3 could be implicated in immune control mechanisms, as host 14-3-3 has been associated with phagocytosis and microbial resistance (Ulvila et al., 2011) and autoantibodies to host 14-3-3 correlate with protection against severe malaria (Duarte et al., 2012).
Here, we add novel insight into the contribution of 14-3-3 molecules to the spectrum of disease. Our findings reveal that a 14-3-3 orthologue is utilized by the apicomplexan parasite T. gondii to hijack host cell migration and thereby promote dissemination. The results highlight novel molecular perspectives in the manipulation of host cells by microorganisms, with an impact on pathogenesis, and in alternative pathways that can regulate leukocyte migration.
Experimental procedures
Parasites
T. gondii tachyzoites of the RFP-expressing PRU-RFP (Pepper et al., 2008), GFP-expressing PTGluc and RH-LDMluc (Hitziger et al., 2005) or RH-14-3-3-Myc3x (Lorestani et al., 2012) were kept on a 2-day passage cycle in human foreskin fibroblast (HFF) monolayers. Cells were cultivated in Dulbecco’s modified Eagle’s medium (DMEM, GE Healthcare Life Sciences) with 10% fetal bovine serum (FBS), gentamicin (20 μg/ml, Life Technologies), L-glutamine (2 mM, Life Technologies) and HEPES (0.01 M, Life Technologies), referred to as complete medium (CM).
Generation of RH-DD-YFP-14-3-3 parasites
Parasites conditionally expressing 14-3-3 (TGME49_263090) fused to a destabilization domain (DD) (Herm-Gotz et al., 2007) and YFP were generated by cloning PCR amplifying the 14-3-3 coding sequence using 063090-AvrII-F (cagcctaggATGGCGGAGGAAATCAAGAATC) and 063090-EcoRV-R (gcggatatcTTACTGATCAGCTTGTTCTGC) primers (Lorestani et al., 2012) via AvrII and EcoRV cloning sites in the following plasmid: (PmeI)ptub-(BglII)-DD-YFP-(AvrII)-YFP(EcoRV)/sagCAT. RHΔHX parasites were transfected with 25 μg of plasmid DNA and a stable transgenic parasites line was selected under 20 μM chloramphenicol. A clonal line was obtained by limiting dilution. One μm of Shield1 (Clontech Laboratories) was added to the culture medium to induce expression and replenished after 12 h.
Murine DCs, human DCs and microglia
Mouse bone marrow-derived dendritic cells (DCs) were purified from the bone marrow of C57BL/6 mice and typified as previously described (Fuks et al., 2012). Briefly, cells from bone marrow of 6–10 week old C57BL/6 mice were cultivated in RPMI 1640 with 10% fetal bovine serum (FBS), gentamicin (20 μg/ml), glutamine (2 mM) and HEPES (0.01 M) (all reagents from Life Technologies), and supplemented with 10 ng/ml recombinant mouse GM-CSF (Peprotech). Medium was replenished on days 2 and 4. Loosely adherent cells were harvested on day 6. The Regional Animal Research Ethical Board, Stockholm, Sweden, approved all protocols involving animals following proceedings described in EU legislation.
To generate human monocyte-derived DC (MoDCs), buffy coats obtained from healthy blood donors at the Karolinska University Hospital Blood Center were treated with 1 ml RosetteSep (StemCell Technologies) per 15 ml of buffy coat, followed by centrifugation on Lymphoprep™ (StemCell Technologies) gradients. The population, defined as monocytes, exhibited CD14+ (Dakocytomation) and < 1% CD3+/19+ (BD Biosciences) as evaluated by flow cytometry (FACSCalibur, BD Biosciences). MoDCs were generated as described previously (Lambert et al., 2006). Briefly, purified cells were cultured in six-well tissue culture plates (5×106 cells per well) for 7 d in CM supplemented with 100 ng/ml GM-CSF (Peprotech) and 12.5 ng/ml IL-4 (Peprotech). After 72 h, the medium was changed and cytokines replenished. MoDCs were defined by the expression of CD1a, CD11b, CD14 (DakoCytomation), CD80, CD83, CD86, HLA-DR, CD11a and CD18 (BD Biosciences). The Regional Ethics Committee, Stockholm, Sweden, approved protocols involving human cells. All donors received written and oral information upon donation of blood. Written consent was obtained for utilization of white blood cells for research purposes.
BV2 immortalized murine microglia were cultured in CM (Blasi et al., 1990).
Fractionation of T. gondii lysates, ion exchange chromatography and mass spectrometry
Approximately 3×108 tachyzoites were purified from HFF monolayers by centrifugation and filtration through 3 μm filters (Osmonics Inc), and subjected to French Press lysis. The tachyzoite lysis was then centrifuged at 2000 × g for 10 min to pellet intact tachyzoites and insoluble proteins. The supernatant was collected and brought to 5 ml with Buffer 1 (PBS, pH adjusted to 7.0) and loaded on a 1 ml HiTrap MonoQ HP column (GE Healthcare) equilibrated with Buffer 1. Bound proteins were eluted by increasing the salt concentration to 600 mM KCl and collected as the Active Fraction (AF). Unbound proteins were also collected and designated as Flow Through (FT). Proteins from total T. gondii lysates, AF, and the FT were separated by reducing SDS-page and stained with Coomassie Brilliant Blue G-250. Individual bands were excised from the gel, reduced, alkylated with iodoacetamide and digested with trypsin (Promega modified porcine trypsin). Tryptic peptides were extracted and analysed with a MALDI-TOF instrument (Bruker Utraflex tof/tof). Spectra were subtracted for background signals and the remaining signals was used for search in the NCBInr database and ToxoDB’s ME49 strain reference genome, without restriction, using the Mascot search engine (MatrixScience.com) allowing one missed cleavage and oxidation of methionines.
T. gondii excretory/secretory antigen (ESA) was prepared as previously described (Zhou et al., 2005).
Recombinant T. gondii 14-3-3 (rTg14-3-3)
The Tg14-3-3 coding sequences were synthesized (GeneArt, LifeTechnologies) and expressed through a pRSET-A expression vector (LifeTechnologies) with a N-Terminal His6-tag in T7express competent cells (NEB) at 37°C for 4h in the presence of 0.4 mM IPTG. The protein was purified as previously described (Nurmi et al., 2006). Briefly rTg14-3-3 was isolated through a NiNTA agarose column (GE Healthcare) from the clarified lysate (PBS, 0.05% Triton-X100, 10 mM Imidazol, 1 mM PMSF, 2 mM MgCl2, 50 μg/ml DNAse I). The eluted protein was further purified using HiTrap MonoQ chromatography (GE Healthcare) in 20 mM Tris pH 7.0, 50 mM NaCl, 2 mM DTT, with linear NaCl gradient elution. Final purification was performed on a Superdex 200 10/300 size-exclusion column (GE Healthcare) in PBS, 2 mM DTT and 2 mM EDTA. The His-tag was removed by digestion with 0.2 U of Enterokinase (LifeTechnologies) per 40 μg 14-3-3 and incubating according to the manufacturer’s instruction followed by a pass over NiNTA agarose and gel filtration chromatography.
Protein Transfection
Transfection of mixed protein fractions from total tachyzoite lysates or purified recombinant Tg14-3-3 was performed as instructed by the manufacturer (SAINT-PhD™, Synvolux Therapeutics). Briefly, 1×105 DCs or microglia were incubated with 2μg of protein, diluted in HBS and mixed with 20μl of SAINT-PhD™ reagent in serum-free medium. Transfection was performed at 37°C, 5% CO2 for 4 h. As a technical control, recombinant β-galactosidase (β-gal) was transfected into the cells and the cytosolic β-gal activity was determined by a colorimetric assay.
Western blotting
Purified T. gondii lysates or DCs were resuspended in Laemmli sample buffer with protease inhibitors (Thermo Scientific), sonicated and boiled. Proteins were separated by 4–20% SDS-PAGE (Life Technologies), and blotted onto PVDF membrane (Millipore), followed by Western blotting with indicated antibodies (pan-14-3-3 (Santa Cruz), GFP (Abcam), GRA7 mAb, SAG1 mAb), and detected using HRP-conjugated species-specific secondary antibodies (Life Technologies). Proteins were revealed by enhanced chemiluminescence in a BioRad ChemiDoc™ XRS+ (GE Healthcare). For the rTg14-3-3 titration experiments, the recombinant protein and the AF were quantified using the BCA protein assay kit (ThermoFisher). Two-color Westerns were performed with rabbit anti-GFP (Abcam) and mouse anti-14-3-3 (Santa Cruz). For detection, goat-anti rabbit IRDye 800CW (LI-COR) and goat anti-mouse IRDye 680RD (LI-COR) were used and imaging was performed on the Odyssey Fc Dual-Mode Imaging System (LI-COR).
Production of lentivirus and transduction
Lentivirus production was done using lipofectamine transfection. Briefly, pLVX-IRES-ZsGreen (Clontech) expressing Tg14-3-3 gene, or the empty vector were co-transfected with pΔ8.91 packaging vector and pCMV-VSVg envelope vector into HEK293FT cells and the resulting supernatant was harvested after 60 h (Burns et al., 1993, Stewart et al., 2003). The primers used to amplify Tg14-3-3 for insertion into pLVX-IRES-ZsGreen were F: 5′-TAAGCAGAATTCATGGCGGAGGAAATCAAGAATC -3′ and R: 5′-TAAGCAGGATCCATGGCGGAGGAAATCAAG -3′. Recovered lentiviral particles were centrifuged to eliminate cell debris, filtered through 0.45 μm cellulose acetate filters, concentrated by ultracentrifugation and resuspended in 100 μl phosphate-buffered saline (PBS). Titers were determined by infecting HEK293FT cells with serial dilutions of concentrated lentivirus and quantified by flow cytometry (FACSVerse flow cytometer, Becton Dickinson). DCs were transduced twice (MOI 3, days 3 and 5). ZsGreen-expression was verified by epifluorescence microscopy or flow cytometry.
Approach to knock out Tg14-3-3 in RHΔKU80 parasites
To delete the 14-3-3 ORF from the genome of RHΔKU80 Toxoplasma parasites, a CRISPR/Cas9-based approach was followed (Shen et al., 2014, Sidik et al., 2014). Two guide RNAs were designed as previously reported (Sidik et al., 2014) targeting the 5′ and 3′ region of the 14-3-3 ORF (Fig. S5). Guide RNAs were cloned in the pU6 FLAG-Cas9 expression plasmid (Sidik et al., 2014) and a total of 40 μg of each plasmid DNA alone or jointly were transfected together with a PCR product harboring a 5′-dhfr-HXGPRT-3′dhfr cassette flanked by 35 bp homologous regions to the respective region at the 5′ and 3′ ORF of 14-3-3 targeted by CRISPR/Cas9 restriction. Transfected parasites were selected with 25 μg/ml mycophenolic acid and 50 μg/ml xanthine (Donald et al., 1996). Three independent transfection experiments were carried out.
Transfection parameters for 14-3-3 3xMyc parasites (Lorestani et al., 2012) were the same as stated above and parasites were analyzed 36 h after transfection for 14-3-3 3xMyc expression by IFA, using Myc-antibody (1:50, Santa Cruz Biotech) and FLAG-antibody (1:500, Sigma) to stain for FLAG-tagged Cas9. The nuclei were stained with DAPI.
Transmigration and motility assays
For the motility assay, DCs were challenged with freshly egressed tachyzoites or treated as indicated, as previously described (Weidner et al., 2013). Bovine collagen I (0.75 mg/ml, Invitrogen) was added to the cells and then loaded onto a labtech chamber slide (Nalge Nunc International) where the medium chambers were removed, but the gaskets were left intact. The slide was spun at 1000 rpm for 5 s to concentrate the cells to the bottom of the slide. The cells were imaged every min for 45–60 min on a Zeiss AxioImager microscope, equipped with an AxioCam MRm camera and AxioVision software (Zeiss). Motility patterns were compiled using ImageJ (image stabilizer software and manual tracking plugins). In the transmigration assay, infection of cells and quantification of migrated cells were conducted as previously described (Lambert et al., 2006). Briefly, DCs were plated at a density of 1×106 cells/well and incubated with freshly egressed T. gondii tachyzoites (MOI3) or treated as indicated, for 4h at 37°C and 5% CO2. DCs were then transferred into transwell filters (8μm pore size; BD biosciences) and incubated for 16h at 37°C and 5% CO2. Migrated DCs were quantified in a hemocytometer.
Immunofluorescence
DCs were plated on poly-L-lysine coated coverslips and incubated with freshly egressed T. gondii tachyzoites (MOI 3, 6 h or ON). Cells were fixed in 4% paraformaldehyde for 20 min and permeabilized with 0.1 % Triton X-100 in PBS with 100 mM glycine for 20 min. Blocking was done in 5% FBS in PBS for 1 h and incubated with primary antibodies: pan 14-3-3 (H-8) sc-1657 (Santa Cruz Biotechnology), pan 14-3-3 (Rabbit) #8312 (Cell Signaling Technologies), pan 14-3-3 (K-19) sc-629 (Santa Cruz Biotechnology), mAb DG52 to the surface protein SAG1 and polyclonal rabbit Ab to the dense granule protein 7 – GRA7 (R6; Statens Bakteriologiska Laboratorium, Solna, Sweden) for 2 h at RT or ON at 4°C. After washing with PBS, cells were incubated with secondary antibodies for 1 h. Coverslips were mounted and imaged by confocal microscopy. Images were acquired on a Zeiss LSM780 confocal microscope equipped with a GaAsP detector using Zen black 2011 software (Zeiss). Digital images were processed an analyzed using Photoshop CS6 software (Adobe). Color balance, brightness, and contrast settings were manipulated to generate final images. All changes were applied equally to entire image.
Immunogold electron microscopy
5×106 DCs were challenged with PRU-RFP or RH-DDYFP-Tg14-3-3 (+Shield1) for 24h (MOI 1). Cells were fixed in 4% PFA, 0.1% glutaraldehyde (GA) in 0.1 M phosphate buffer for 60 min. Cells were washed, collected and cryosectioned as previously described (Goldenberg et al., 2007). Sections were cut at 90 nm thickness and picked up in a 1:1 mixture of 2% methylcellulose and 2,5M sucrose, placed on formvar-coated nickel grids (3mm diameter) and stored at 4°C. For immunolabelling, grids were washed as previously described (Goldenberg et al., 2007) and blocked in blocking buffer containing 5% skim milk powder, 1% BSA-c (Aurion). Rabbit anti-GFP (Abcam), was added to the grid at 2.5 μg/ml in 5% skim milk powder, 0.1% BSA-c (dilution buffer) for 1.5 h. Following PBS washes, the samples were blocked again. The secondary antibody conjugated to 12 nm colloidal gold (Jackson ImmunoResearch) was diluted 1 in 20 and incubated with the samples for 1 hour. After PBS washes, the cells were fixed with 2% GA, washed with PBS, dH2O, and 1.8% methylcellulose, 0.2% uranyl acetate in dH2O on ice. Grids were examined using a Hitachi 7500 transmission electron microscope operating at an accelerating voltage of 80 kV. Images were obtained using an Olympus SIS Megaview II digital camera and iTEM software
Supplementary Material
Fig. S1. Migratory characteristics of DCs after protein transfection
A. Velocity analysis of DCs challenged with AF (2 μg) in absence or presence of protein transfection reagent. Data represent compiled analysis from two independent experiments (median ± SEM). Statistics performed using the Student’s t-test; n.s: p > 0,05.
B. Velocity analysis of DCs challenged with recombinant GFP (rGFP). Data represent compiled analysis from two independent experiments (median ± SEM). Statistics performed using the Student’s t-test.
Fig. S2. Amino acid sequence analyses and predicted tertiary structure of Tg14-3-3.
A. A molecular model of Tg14-3-3 was generated based on the crystal structure of human 14-3-3ζ in complex with parts of the R18 peptide (PDB ID: 1A38) (left panel). A visualization of the estimated surface charge potentials (blue = positive, red = negative) reveals the large degree of conservation between the human 14-3-3 structure (left panel) and Tg14-3-3 model (middle panel). Overlay (right panel) of the two with human 14-3-3ζ in light gray, R18 in pink and Tg14-3-3 in dark grey demonstrates the large overall structural conservation including the potential R18 binding site in the Tg14-3-3 structure. All amino acids within 4Å of the R18 peptide were analyzed and found to be conserved between Tg14-3-3 and human 14-3-3 (see alignment, B).
B. Alignment of the primary amino acid sequence of Tg14-3-3 with Neospora caninum 14-3-3, human and mouse 14-3-3ε illustrates strong conservation. The amino acids that make up the 14-3-3 binding site are indicated with a red asterisk. Secondary structure elements are shown based on the X-ray structure of human 14-3-3ε (PDB-ID: 2BR9).
Fig. S3. Characterization of Tg14-3-3 expressed by T. gondii
A. Peptides identified by MALDI-TOF in the AF. The full annotated amino acid sequence of the Tg14-3-3 gene (TGME49_263090) is shown. Identified peptides are indicated in red, resulting in > 60% coverage of the peptide sequence.. Underlined sequence indicates N-terminal sequence of Tg14-3-3 that is absent in annotated 14-3-3 sequences for other species (Fig. S2B). Arrow indicates the position of the methionine used to express the rTg14-3-3S form.
B. Schematic representation of recombinant proteins expressed. The full amino acid sequence (rTg14-3-3L) as annotated or the amino acid sequence with highest homology to other 14-3-3 sequences (rTg14-3-3S) were recombinantly expressed (see Fig. S2B) as indicated in experimental procedures. For each part of the sequence, the percentage of coverage by MALDI-TOF analyses of the AF/lysate is indicated.
C. The purity and aggregation status of the purified recombinant 14-3-3 assessed by SDS-PAGE. Recombinant protein with His-tag was generated and His-tag was cleaved as indicated under experimental procedures. Note that rTg14-3-3S has a predicted MW of 31 kDa and runs at ≈ 35 kDa by SDS-PAGE.
D. Gel filtration chromatography revealing the final proteins to be highly pure and free of aggregation.
E. Western blot shows migration of the two recombinant Tg14-3-3 proteins (rTg14-3-3L, rTg14-3-3S) and native Tg14-3-3.
F. Western blot showing endogenous c-myc3x-tagged Tg14-3-3, running as a single band at about 39 kDa (C-myc3x represents about 4 kDa).
Fig. S4. Lentiviral transduction of MoDCs was assessed by microscopy.
A, B. Micrographs show respectively non-infected, non-transduced (NI, NT) MoDCs and Tg14-3-3- ZsGreen-transduced MoDCs generated as indicated under experimental procedures. Overlayed image of ZsGreen and DIC is provided. Scale bar – 100 μm.
Fig. S5. CRISP/Cas9-based approach to knock out Tg14-3-3.
A. Schematic representation of the applied knockout strategy. Two pU6 Cas9 plasmids encoding guide RNAs binding either the 5′ or 3′ end of the 14-3-3 ORF were jointly transfected in RHΔKU80 parasites. A HXGPRT expression cassette with flanking 35 bp homologous regions (red) was offered to repair the double strand break.
B. To verify specificity of guide RNAs, pU6 Cas9 plasmids encoding 14-3-3 5′ and 3′ gRNAs were transfected in a cell line expression endogenously 3xMyc (red) tagged 14-3-3.
C. 14-3-3 3xMyc parasites transfected with different pU6-Cas9 plasmids were analyzed by IFA 36 h after transfection. Expression of mock gRNA (Sidik et al., 2014) does not interfere with 14-3-3 3xMyc (red) level. Transfection of either 14-3-3 5′, 3′ alone or a combination of both gRNAs abrogates 14-3-3 3xMyc expression. Transfected parasites were identified by FLAG-Cas9 (green) expression. Nuclei were stained with DAPI. Scale bar 10 μm.
Fig. S6. Expression of DDYFP-Tg14-3-3 and effects of Shield1 on DC motility
A. Expression of DDYFP-Tg14-3-3. DDYFP-Tg14-3-3 expressing tachyzoites were incubated in presence or absence of Shield1 (1μM) for 24 h while replicating in HFF monolayers. Purified T. gondii lysates were immunoblotted against anti-GFP antibody (cross-reacts with YFP), shown in green and anti-14-3-3 antibody shown in red.
B. Effects of Shield1 on DC motility. MoDCs were challenged with freshly egressed T. gondii (RH-LDMluc) or incubated in complete medium (non-infected) for 4 h. Where indicated, Shield1 was added to the culture medium for the final 2 h of incubation, after which a motility assay was performed as indicated under experimental procedures. Data represent the velocity analysis (median median ± SEM) from 2 healthy donors. n.s. indicates non-significant differences (P > 0,05; Mann Whitney U test).
Fig. S7. Localization of YFP-Tg14-3-3 by TEM and immunogold labeling.
Immunogold labeling of MoDCs infected with RH-DDYFP-Tg14-3-3 (+ Shield1) at MOI1 for 24 h, using an anti-GFP antibody followed by incubation with a secondary antibody conjugated to 12 nm colloidal gold as further described in experimental procedures. Four separate micrographs from two independent preparations are provided. Gold particles within the tachyzoites are circled in red, while the gold particles within the perivacuolar space are indicated with arrows.
Fig. S8. Characterization of recruitement of host cell 14-3-3 to the PV with separate antibodies.
A. MoDCs were challenged with freshly egressed RFP-expressing T. gondii tachyzoites (PRU-RFP, MOI 1) for 24 h, stained and imaged by epifluorescence microscopy as indicated under experimental procedures. The pan-14-3-3 antibody (green) was produced by immunizing animals with a synthetic peptide corresponding to residues surrounding Met223 of human 14-3-3γ protein (Pan 14-3-3 (Rabbit) #8312, Cell Signaling Technologies).
B. Procedure as in (A) using a pan 14-3-3 antibody that was raised against a peptide mapping at the N-terminus of 14-3-3 β of human origin N-terminus of 14-3-3 β of human origin (Pan 14-3-3 (K-19) sc-629; Santa Cruz Biotechnology). Scale bar – 10μm
Acknowledgments
We thank Robert Temkin for TEM, Polya Rosin, Fabrice Neihers and Tatiana Sandalova for critical input and advice. This work was supported by grants from the Swedish Research Council (to AB) and a fellowship from the Mats Sundin Foundation (to JMW), MOP-68992 grant from Canadian Institutes of Health Research (to REH), National Institutes of Health grants AI081924 and AI110690 (to MJG) and by the Deutsche Forschungs-gemeinschaft (to KE).
Footnotes
The authors declare that no conflicts of interest exist.
References
- Assossou O, Besson F, Rouault JP, Persat F, Brisson C, Duret L, et al. Subcellular localization of 14-3-3 proteins in Toxoplasma gondii tachyzoites and evidence for a lipid raft-associated form. FEMS microbiology letters. 2003;224:161–168. doi: 10.1016/S0378-1097(03)00479-8. [DOI] [PubMed] [Google Scholar]
- Assossou O, Besson F, Rouault JP, Persat F, Ferrandiz J, Mayencon M, et al. Characterization of an excreted/secreted antigen form of 14-3-3 protein in Toxoplasma gondii tachyzoites. FEMS microbiology letters. 2004;234:19–25. doi: 10.1016/j.femsle.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Barragan A, Hitziger N. Transepithelial migration by Toxoplasma. Sub-cellular biochemistry. 2008;47:198–207. doi: 10.1007/978-0-387-78267-6_16. [DOI] [PubMed] [Google Scholar]
- Benke D, Zeilhofer HU. Divorce of obligatory partners in pain: disruption of GABA(B) receptor heterodimers in neuralgia. The EMBO journal. 2012;31:3234–3236. doi: 10.1038/emboj.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. Journal of neuroimmunology. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognetti D, Davis D, Sturtevant J. The Candida albicans 14-3-3 gene, BMH1, is essential for growth. Yeast. 2002;19:55–67. doi: 10.1002/yea.804. [DOI] [PubMed] [Google Scholar]
- Collantes-Fernandez E, Arrighi RB, Alvarez-Garcia G, Weidner JM, Regidor-Cerrillo J, Boothroyd JC, et al. Infected dendritic cells facilitate systemic dissemination and transplacental passage of the obligate intracellular parasite Neospora caninum in mice. PloS one. 2012;7:e32123. doi: 10.1371/journal.pone.0032123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courret N, Darche S, Sonigo P, Milon G, Buzoni-Gatel D, Tardieux I. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2006;107:309–316. doi: 10.1182/blood-2005-02-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de da Silva JF, de Oliveira HC, Marcos CM, da Silva RA, da Costa TA, Calich VL, et al. Paracoccidoides brasiliensis 30 kDa adhesin: identification as a 14-3-3 protein, cloning and subcellular localization in infection models. PloS one. 2013;8:e62533. doi: 10.1371/journal.pone.0062533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellacasa-Lindberg I, Fuks JM, Arrighi RB, Lambert H, Wallin RP, Chambers BJ, Barragan A. Migratory activation of primary cortical microglia upon infection with Toxoplasma gondii. Infection and immunity. 2011;79:3046–3052. doi: 10.1128/IAI.01042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkers EY, Schneider AG, Cohen SB, Butcher BA. Phagocyte responses to protozoan infection and how Toxoplasma gondii meets the challenge. PLoS pathogens. 2012;8:e1002794. doi: 10.1371/journal.ppat.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald RGK, Carter D, Ullman B, Roos DS. Insertional tagging, cloning, and expression of the Toxoplasma gondii hypoxanthine-xanthine-guanine phosphoribosyltransferase gene. Journal of Biological Chemistry. 1996;271:14010–14019. doi: 10.1074/jbc.271.24.14010. [DOI] [PubMed] [Google Scholar]
- Dougherty MK, Morrison DK. Unlocking the code of 14-3-3. Journal of cell science. 2004;117:1875–1884. doi: 10.1242/jcs.01171. [DOI] [PubMed] [Google Scholar]
- Duarte J, Herbert F, Guiyedi V, Franetich JF, Roland J, Cazenave PA, et al. High levels of immunoglobulin E autoantibody to 14-3-3 epsilon protein correlate with protection against severe Plasmodium falciparum malaria. The Journal of infectious diseases. 2012;206:1781–1789. doi: 10.1093/infdis/jis595. [DOI] [PubMed] [Google Scholar]
- Freeman AK, Morrison DK. 14-3-3 Proteins: diverse functions in cell proliferation and cancer progression. Seminars in cell & developmental biology. 2011;22:681–687. doi: 10.1016/j.semcdb.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Subramanian RR, Masters SC. 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol. 2000;40:617–647. doi: 10.1146/annurev.pharmtox.40.1.617. [DOI] [PubMed] [Google Scholar]
- Fuks JM, Arrighi RB, Weidner JM, Kumar Mendu S, Jin Z, Wallin RP, et al. GABAergic signaling is linked to a hypermigratory phenotype in dendritic cells infected by Toxoplasma gondii. PLoS pathogens. 2012;8:e1003051. doi: 10.1371/journal.ppat.1003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg NM, Grinstein S, Silverman M. Golgi-bound Rab34 is a novel member of the secretory pathway. Mol Biol Cell. 2007;18:4762–4771. doi: 10.1091/mbc.E06-11-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimi MA, Bougdour A. Toxoplasma’s ways of manipulating the host transcriptome via secreted effectors. Current opinion in microbiology. 2015;26:24–31. doi: 10.1016/j.mib.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Hammoudi PM, Jacot D, Mueller C, Di Cristina M, Dogga SK, Marq JB, et al. Fundamental Roles of the Golgi-Associated Toxoplasma Aspartyl Protease, ASP5, at the Host-Parasite Interface. PLoS pathogens. 2015;11:e1005211. doi: 10.1371/journal.ppat.1005211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herm-Gotz A, Agop-Nersesian C, Munter S, Grimley JS, Wandless TJ, Frischknecht F, Meissner M. Rapid control of protein level in the apicomplexan Toxoplasma gondii. Nature methods. 2007;4:1003–1005. doi: 10.1038/nmeth1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitziger N, Dellacasa I, Albiger B, Barragan A. Dissemination of Toxoplasma gondii to immunoprivileged organs and role of Toll/interleukin-1 receptor signalling for host resistance assessed by in vivo bioluminescence imaging. Cellular microbiology. 2005;7:837–848. doi: 10.1111/j.1462-5822.2005.00517.x. [DOI] [PubMed] [Google Scholar]
- Hunter CA, Sibley LD. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nature reviews. 2012;10:766–778. doi: 10.1038/nrmicro2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatani S, Uhlen P, Barragan A. Infection by Toxoplasma gondii Induces Amoeboid-Like Migration of Dendritic Cells in a Three-Dimensional Collagen Matrix. PloS one. 2015;10:e0139104. doi: 10.1371/journal.pone.0139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalle M, Curra C, Ciccarone F, Pace T, Cecchetti S, Fantozzi L, et al. Dematin, a component of the erythrocyte membrane skeleton, is internalized by the malaria parasite and associates with Plasmodium 14-3-3. J Biol Chem. 2011;286:1227–1236. doi: 10.1074/jbc.M110.194613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert H, Hitziger N, Dellacasa I, Svensson M, Barragan A. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cellular microbiology. 2006;8:1611–1623. doi: 10.1111/j.1462-5822.2006.00735.x. [DOI] [PubMed] [Google Scholar]
- Lambert H, Vutova PP, Adams WC, Lore K, Barragan A. The Toxoplasma gondii-shuttling function of dendritic cells is linked to the parasite genotype. Infection and immunity. 2009;77:1679–1688. doi: 10.1128/IAI.01289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Fan YT, Dias A, Esper L, Corn RA, Bafica A, et al. Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. J Immunol. 2006;177:31–35. doi: 10.4049/jimmunol.177.1.31. [DOI] [PubMed] [Google Scholar]
- Lorestani A, Ivey FD, Thirugnanam S, Busby MA, Marth GT, Cheeseman IM, Gubbels MJ. Targeted proteomic dissection of Toxoplasma cytoskeleton sub-compartments using MORN1. Cytoskeleton (Hoboken) 2012;69:1069–1085. doi: 10.1002/cm.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS, et al. CD8alpha(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity. 2011;35:249–259. doi: 10.1016/j.immuni.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhawech P. 14-3-3 proteins--an update. Cell research. 2005;15:228–236. doi: 10.1038/sj.cr.7290291. [DOI] [PubMed] [Google Scholar]
- Muslin AJ, Xing H. 14-3-3 proteins: regulation of subcellular localization by molecular interference. Cell Signal. 2000;12:703–709. doi: 10.1016/s0898-6568(00)00131-5. [DOI] [PubMed] [Google Scholar]
- Nurmi SM, Gahmberg CG, Fagerholm SC. 14-3-3 proteins bind both filamin and alphaLbeta2 integrin in activated T cells. Ann N Y Acad Sci. 2006;1090:318–325. doi: 10.1196/annals.1378.035. [DOI] [PubMed] [Google Scholar]
- Pepper M, Dzierszinski F, Wilson E, Tait E, Fang Q, Yarovinsky F, et al. Plasmacytoid dendritic cells are activated by Toxoplasma gondii to present antigen and produce cytokines. J Immunol. 2008;180:6229–6236. doi: 10.4049/jimmunol.180.9.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z, Micorescu M, Yakhnitsa V, Barmack NH. Climbing fiber activity reduces 14-3-3-theta regulated GABA(A) receptor phosphorylation in cerebellar Purkinje cells. Neuroscience. 2012;201:34–45. doi: 10.1016/j.neuroscience.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Brown KM, Lee TD, Sibley LD. Efficient gene disruption in diverse strains of Toxoplasma gondii using CRISPR/CAS9. MBio. 2014;5:e01114–01114. doi: 10.1128/mBio.01114-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidik SM, Hackett CG, Tran F, Westwood NJ, Lourido S. Efficient genome engineering of Toxoplasma gondii using CRISPR/Cas9. PloS one. 2014;9:e100450. doi: 10.1371/journal.pone.0100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siles-Lucas M, Felleisen RS, Hemphill A, Wilson W, Gottstein B. Stage-specific expression of the 14-3-3 gene in Echinococcus multilocularis. Molecular and biochemical parasitology. 1998;91:281–293. doi: 10.1016/s0166-6851(97)00208-9. [DOI] [PubMed] [Google Scholar]
- del Siles-Lucas MM, Gottstein B. The 14-3-3 protein: a key molecule in parasites as in other organisms. Trends in parasitology. 2003;19:575–581. doi: 10.1016/j.pt.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Sluchanko NN, Gusev NB. 14-3-3 proteins and regulation of cytoskeleton. Biochemistry Biokhimiia. 2010;75:1528–1546. doi: 10.1134/s0006297910130031. [DOI] [PubMed] [Google Scholar]
- Somanath PR, Byzova TV. 14-3-3beta-Rac1-p21 activated kinase signaling regulates Akt1-mediated cytoskeletal organization, lamellipodia formation and fibronectin matrix assembly. Journal of cellular physiology. 2009;218:394–404. doi: 10.1002/jcp.21612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS, et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9:493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzivion G, Dobson M, Ramakrishnan G. FoxO transcription factors; Regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta. 2011;1813:1938–1945. doi: 10.1016/j.bbamcr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Ulvila J, Vanha-aho LM, Kleino A, Vaha-Makila M, Vuoksio M, Eskelinen S, et al. Cofilin regulator 14-3-3zeta is an evolutionarily conserved protein required for phagocytosis and microbial resistance. Journal of leukocyte biology. 2011;89:649–659. doi: 10.1189/jlb.0410195. [DOI] [PubMed] [Google Scholar]
- Wang B, Yang H, Liu YC, Jelinek T, Zhang L, Ruoslahti E, Fu H. Isolation of high-affinity peptide antagonists of 14-3-3 proteins by phage display. Biochemistry. 1999;38:12499–12504. doi: 10.1021/bi991353h. [DOI] [PubMed] [Google Scholar]
- Weidner JM, Kanatani S, Hernandez-Castaneda MA, Fuks JM, Rethi B, Wallin RP, Barragan A. Rapid cytoskeleton remodelling in dendritic cells following invasion by Toxoplasma gondii coincides with the onset of a hypermigratory phenotype. Cellular microbiology. 2013;15:1735–1752. doi: 10.1111/cmi.12145. [DOI] [PubMed] [Google Scholar]
- Yang J, Pan W, Sun X, Zhao X, Yuan G, Sun Q, et al. Immunoproteomic profile of Trichinella spiralis adult worm proteins recognized by early infection sera. Parasites & vectors. 2015;8:20. doi: 10.1186/s13071-015-0641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Lee WH, Sobott F, Papagrigoriou E, Robinson CV, Grossmann JG, et al. Structural basis for protein-protein interactions in the 14-3-3 protein family. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17237–17242. doi: 10.1073/pnas.0605779103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XW, Kafsack BF, Cole RN, Beckett P, Shen RF, Carruthers VB. The opportunistic pathogen Toxoplasma gondii deploys a diverse legion of invasion and survival proteins. The Journal of biological chemistry. 2005;280:34233–34244. doi: 10.1074/jbc.M504160200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Migratory characteristics of DCs after protein transfection
A. Velocity analysis of DCs challenged with AF (2 μg) in absence or presence of protein transfection reagent. Data represent compiled analysis from two independent experiments (median ± SEM). Statistics performed using the Student’s t-test; n.s: p > 0,05.
B. Velocity analysis of DCs challenged with recombinant GFP (rGFP). Data represent compiled analysis from two independent experiments (median ± SEM). Statistics performed using the Student’s t-test.
Fig. S2. Amino acid sequence analyses and predicted tertiary structure of Tg14-3-3.
A. A molecular model of Tg14-3-3 was generated based on the crystal structure of human 14-3-3ζ in complex with parts of the R18 peptide (PDB ID: 1A38) (left panel). A visualization of the estimated surface charge potentials (blue = positive, red = negative) reveals the large degree of conservation between the human 14-3-3 structure (left panel) and Tg14-3-3 model (middle panel). Overlay (right panel) of the two with human 14-3-3ζ in light gray, R18 in pink and Tg14-3-3 in dark grey demonstrates the large overall structural conservation including the potential R18 binding site in the Tg14-3-3 structure. All amino acids within 4Å of the R18 peptide were analyzed and found to be conserved between Tg14-3-3 and human 14-3-3 (see alignment, B).
B. Alignment of the primary amino acid sequence of Tg14-3-3 with Neospora caninum 14-3-3, human and mouse 14-3-3ε illustrates strong conservation. The amino acids that make up the 14-3-3 binding site are indicated with a red asterisk. Secondary structure elements are shown based on the X-ray structure of human 14-3-3ε (PDB-ID: 2BR9).
Fig. S3. Characterization of Tg14-3-3 expressed by T. gondii
A. Peptides identified by MALDI-TOF in the AF. The full annotated amino acid sequence of the Tg14-3-3 gene (TGME49_263090) is shown. Identified peptides are indicated in red, resulting in > 60% coverage of the peptide sequence.. Underlined sequence indicates N-terminal sequence of Tg14-3-3 that is absent in annotated 14-3-3 sequences for other species (Fig. S2B). Arrow indicates the position of the methionine used to express the rTg14-3-3S form.
B. Schematic representation of recombinant proteins expressed. The full amino acid sequence (rTg14-3-3L) as annotated or the amino acid sequence with highest homology to other 14-3-3 sequences (rTg14-3-3S) were recombinantly expressed (see Fig. S2B) as indicated in experimental procedures. For each part of the sequence, the percentage of coverage by MALDI-TOF analyses of the AF/lysate is indicated.
C. The purity and aggregation status of the purified recombinant 14-3-3 assessed by SDS-PAGE. Recombinant protein with His-tag was generated and His-tag was cleaved as indicated under experimental procedures. Note that rTg14-3-3S has a predicted MW of 31 kDa and runs at ≈ 35 kDa by SDS-PAGE.
D. Gel filtration chromatography revealing the final proteins to be highly pure and free of aggregation.
E. Western blot shows migration of the two recombinant Tg14-3-3 proteins (rTg14-3-3L, rTg14-3-3S) and native Tg14-3-3.
F. Western blot showing endogenous c-myc3x-tagged Tg14-3-3, running as a single band at about 39 kDa (C-myc3x represents about 4 kDa).
Fig. S4. Lentiviral transduction of MoDCs was assessed by microscopy.
A, B. Micrographs show respectively non-infected, non-transduced (NI, NT) MoDCs and Tg14-3-3- ZsGreen-transduced MoDCs generated as indicated under experimental procedures. Overlayed image of ZsGreen and DIC is provided. Scale bar – 100 μm.
Fig. S5. CRISP/Cas9-based approach to knock out Tg14-3-3.
A. Schematic representation of the applied knockout strategy. Two pU6 Cas9 plasmids encoding guide RNAs binding either the 5′ or 3′ end of the 14-3-3 ORF were jointly transfected in RHΔKU80 parasites. A HXGPRT expression cassette with flanking 35 bp homologous regions (red) was offered to repair the double strand break.
B. To verify specificity of guide RNAs, pU6 Cas9 plasmids encoding 14-3-3 5′ and 3′ gRNAs were transfected in a cell line expression endogenously 3xMyc (red) tagged 14-3-3.
C. 14-3-3 3xMyc parasites transfected with different pU6-Cas9 plasmids were analyzed by IFA 36 h after transfection. Expression of mock gRNA (Sidik et al., 2014) does not interfere with 14-3-3 3xMyc (red) level. Transfection of either 14-3-3 5′, 3′ alone or a combination of both gRNAs abrogates 14-3-3 3xMyc expression. Transfected parasites were identified by FLAG-Cas9 (green) expression. Nuclei were stained with DAPI. Scale bar 10 μm.
Fig. S6. Expression of DDYFP-Tg14-3-3 and effects of Shield1 on DC motility
A. Expression of DDYFP-Tg14-3-3. DDYFP-Tg14-3-3 expressing tachyzoites were incubated in presence or absence of Shield1 (1μM) for 24 h while replicating in HFF monolayers. Purified T. gondii lysates were immunoblotted against anti-GFP antibody (cross-reacts with YFP), shown in green and anti-14-3-3 antibody shown in red.
B. Effects of Shield1 on DC motility. MoDCs were challenged with freshly egressed T. gondii (RH-LDMluc) or incubated in complete medium (non-infected) for 4 h. Where indicated, Shield1 was added to the culture medium for the final 2 h of incubation, after which a motility assay was performed as indicated under experimental procedures. Data represent the velocity analysis (median median ± SEM) from 2 healthy donors. n.s. indicates non-significant differences (P > 0,05; Mann Whitney U test).
Fig. S7. Localization of YFP-Tg14-3-3 by TEM and immunogold labeling.
Immunogold labeling of MoDCs infected with RH-DDYFP-Tg14-3-3 (+ Shield1) at MOI1 for 24 h, using an anti-GFP antibody followed by incubation with a secondary antibody conjugated to 12 nm colloidal gold as further described in experimental procedures. Four separate micrographs from two independent preparations are provided. Gold particles within the tachyzoites are circled in red, while the gold particles within the perivacuolar space are indicated with arrows.
Fig. S8. Characterization of recruitement of host cell 14-3-3 to the PV with separate antibodies.
A. MoDCs were challenged with freshly egressed RFP-expressing T. gondii tachyzoites (PRU-RFP, MOI 1) for 24 h, stained and imaged by epifluorescence microscopy as indicated under experimental procedures. The pan-14-3-3 antibody (green) was produced by immunizing animals with a synthetic peptide corresponding to residues surrounding Met223 of human 14-3-3γ protein (Pan 14-3-3 (Rabbit) #8312, Cell Signaling Technologies).
B. Procedure as in (A) using a pan 14-3-3 antibody that was raised against a peptide mapping at the N-terminus of 14-3-3 β of human origin N-terminus of 14-3-3 β of human origin (Pan 14-3-3 (K-19) sc-629; Santa Cruz Biotechnology). Scale bar – 10μm