Abstract
Animal models of Parkinson’s disease (PD) are important for understanding the mechanisms of the disease and can contribute to developing and validating novel therapeutics. Ideally, these models should replicate the cardinal features of PD, such as progressive neurodegeneration of catecholaminergic neurons and motor defects. Many current PD models emphasize pathological forms of α-synuclein, based on findings that autosomal dominant mutations in α-synuclein and duplications/triplications of the SNCA gene cause PD. In addition, Lewy bodies and Lewy neurites, primarily composed of α-synuclein, represent the predominant pathological characteristics of PD. These inclusions have defined features, such as insolubility in non-ionic detergent, hyperphosphorylation, proteinase K sensitivity, a filamentous appearance by electron microscopy, and β-sheet structure. Furthermore, it has become clear that Lewy bodies and Lewy neurites are found throughout the peripheral and central nervous system, and could account not only for motor symptoms, but also for non-motor symptoms of the disease. The goal of this review is to describe two new α-synuclein-based models: the recombinant adeno-associated viral vector-α-synuclein model and the α-synuclein fibril model. An advantage of both models is that they do not require extensive crossbreeding of rodents transgenic for α-synuclein with other rodents transgenic for genes of interest to study the impact of such genes on PD-related pathology and phenotypes. In addition, abnormal α-synuclein can be expressed in brain regions relevant for disease. Here, we discuss the features of each model, how each model has contributed thus far to our understanding of PD, and the advantages and potential caveats of each model.
Keywords: AAV, fibril, Lewy bodies, Lewy neurites, Parkinson’s disease, α-synuclein
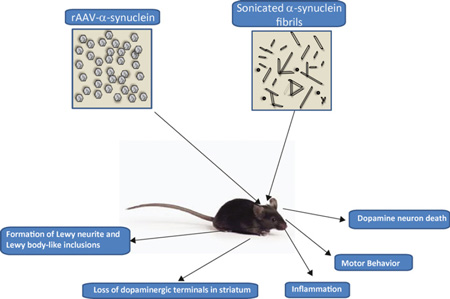
Graphical Abstract
This review describes two α-synuclein-based rodent models of Parkinson’s disease: the rAAV-α-synuclein model and the α-synuclein fibril model. The key features of these models are described, and the extent to which they recapitulate features of PD, such as α-synuclein inclusion formation, loss of dopaminergic synapses in the striatum, motor defects, inflammation, and dopamine neuron death.
Animal models of Parkinson’s disease (PD) are critical for understanding the underlying disease mechanisms and for identifying and developing potential therapeutics that could eventually be tested in clinical trials (Fischer et al. 2016). An ideal model would replicate most, if not all, of the behavioral and pathologic characteristics of PD. For example, PD is a progressive disease with symptoms becoming more severe over time. These include motor symptoms, such as bradykinesia, resting tremor, rigidity, and non-motor symptoms, such as autonomic dysfunction, anosmia, sleep disturbances, cognitive dysfunction, anxiety, and depression. One of the primary pathologic characteristics is a progressive loss of catecholeminergic neurons of the substantia nigra pars compacta (SNpc) and locus coeruleus (Sulzer and Surmeier 2013). Lewy bodies and Lewy neurites are the other pathologic hallmarks of PD, of which α-synuclein is the primary constituent. The Lewy inclusions are not simply ‘aggregates’ of the protein, but have defined features. They appear as 10–15 nm filaments with electron microscopy, are insoluble in anionic detergents, are proteinase K sensitive, and have post-translational modifications, such as phosphorylation on serine-129, nitration, oxidation, and ubiquitination (Kuzuhara et al. 1988; Baba et al. 1998; Goedert et al. 1998; Spillantini et al. 1998; Giasson et al. 2000; Fujiwara et al. 2002). Morphologically, Lewy bodies in the neuronal soma appear as spherical or reniform (Spillantini et al. 1997; Braak et al. 2003a). Lewy neurites appear as spindle-like threads and neuroaxonal spheroids within axons and dendrites (Dickson et al. 1991; Duda et al. 2000; Braak et al. 2003a). Lewy bodies and Lewy neurites are found throughout the nervous system in PD including, but not limited to, the enteric nervous system, sympathetic ganglion, nerves of the cardiac conduction system, dorsal motor nucleus of the vagus nerve, spinal cord, brainstem, hypothalamus, hippocampus, and cortex (Braak et al. 2003a). Analyses of Lewy neurite and Lewy body pathology in brains from PD patients that range from cases with mild pathology to those with a heavy burden of pathology show that Lewy bodies and Lewy neurites form in a topographically predictable sequence, first appearing in the olfactory bulb and dorsal motor nucleus, followed by the pedunculopontine nucleus, raphe nucleus, and SNpc, and then the diagonal band of broca, hippocampus, and cortex (Braak et al. 2003a). Although the purpose of the Braak staging study was not to correlate symptoms with the appearance of pathology, there is a striking association between symptom development and Lewy body/neurite progression. For example, anosmia, depression, and sleep disorders appear early in PD and may be associated with olfactory bulb and brainstem pathology, motor deficits could reflect basal ganglia pathology, and cognitive symptoms (which arise later in PD) may reflect cortical pathology. Overall, these data suggest that pathology spreads among functionally interconnected neuron networks (Braak et al. 2003b).
Traditional models of PD include neurotoxin models, such as 6-hydroxydopamine or MPTP, to lesion dopamine neurons of the SNpc. These models can successfully predict the efficacy of dopaminergic-based therapeutics to alleviate motor symptoms. However, many compounds that showed neuroprotection in the neurotoxin models have failed in clinical trials (Athauda and Foltynie 2015). The lack of predictive power of the neurotoxin model with respect to neuroprotection has led to a focus on additional models of PD emphasizing pathologic α-synuclein. This is largely based on the discovery of missense mutations of α-synuclein linked to familial forms of PD, and the finding that α-synuclein is the major component of Lewy bodies (Polymeropoulos et al. 1997; Spillantini et al. 1997; Kruger et al. 1998). Subsequent to these landmark studies, SNCA gene duplications/triplications and gene polymorphisms in the SNCA promoter region have been shown to cause PD (Singleton et al. 2003). These and missense mutations reduce the age of onset of the disease. Furthermore, genome-wide association studies consistently identify SNCA as a risk factor for PD (Pankratz et al. 2009; Satake et al. 2009; Simon-Sanchez et al. 2009; Edwards et al. 2010; Nalls et al. 2014). Several different transgenic lines of mice over-expressing either human full length or disease-associated mutant α-synuclein under different promoters have been developed as PD models. Overall, the transgenic α-synuclein mice can, to a variable extent, model some of the neuropathologic and behavioral phenotypes similar to PD. However, loss of neurons in the SNpc or locus coeruleus, brain regions relevant for PD, does not occur for most of the transgenic models, and the emergence of pathology and phenotypes is often coincident with cell death. The advantages and limitations of these transgenic lines have been reviewed extensively elsewhere (see (Magen and Chesselet 2010; Chesselet et al. 2008; Chesselet 2008). The remainder of the review will focus on newer α-synuclein models of PD, the recombinant adeno-associated viral vector (rAAV-α-synuclein) and α-synuclein fibril models.
rAAV-α-synuclein increases expression of α-synuclein in the SNpc
The first studies utilizing rAAV vectors showed that dopaminergic neurons in the SNpc can be transduced and express wild-type or mutant α-synuclein for long periods of time (Kirik et al. 2002; Klein et al. 2002). Table 1 lists the studies utilizing rAAV-α-synuclein. Expression of the human wild-type α-synuclein or the PD-associated mutant, A53T-α-synuclein leads to progressive loss of dopaminergic neurons in the SNpc, and loss of dopamine terminals in the striatum. This study also showed significant defects in motor behavior, but a critical threshold of 40–50% of neuron loss must be achieved for such impairments to manifest. Although rAAV2 is the serotype that has been most extensively studied, because the production and purification methods were developed earlier than for the other serotypes, over the past several years new AAV serotypes have been discovered and implemented in this model, such as rAAV2/1, rAAV2/5, rAAV2/6, rAAV2/7, and rAAV2/8, which show increased transduction and spread of α-synuclein expression (McFarland et al. 2009b; Koprich et al. 2010; Lundblad et al. 2012). The vast majority of studies utilize the rAAV-α-synuclein in rats, but the model has been adopted in mice which provide more options for availability of knockout and transgenic mice to test whether certain genes protect and enhance neurodegeneration. However, the phenotypes in the mice are variable and further characterization of rAAV-α-synuclein in mice is recommended (St Martin et al. 2007; Theodore et al. 2008; Ulusoy et al. 2012; Harms et al. 2013; Oliveras-Salva et al. 2013; Song et al. 2015).
Table 1.
Studies using rAAV-synuclein and main findings related to PD phenotypes
| References | Serotype | purification | Promoter | Species/ strain |
cDNA | Titer (gp/mL)* | Time after injection |
Increase in α-synuclein Expression in SNpc by immunoblot |
Loss of Dopaminergic neurons |
Reduction in Dopaminergic innervation in striatum |
Pathology | Behavior |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kirik et al. (2002) | 2 | lodixanol gradient followed by heparin sulfate affinity column |
CMV/CBA | Rat/SD | Human WT α-synuclein Human A53T α-synuclein GFP |
8.2 × 1011 1.4 × 1012 1.4 × 1011 |
3 weeks 8 weeks 27 weeks 3 weeks 8 weeks 27 weeks |
N.D. | 20% (*) 60% (*) 40% (*) (WT and A53T pooled) 20% (N.S.) 10% (N.S.) 10% (N.S.) |
15% (NS) 50% (*) 20% (*) |
Punctate α-synuclein |
No overall significant differences in apomorphine induced rotation, or paw reaching tests, but the 25% of rats that had 60–80% lesion, there were significant defects in behavior |
| Klein et al. (2002) | 2 | lodixanol gradient followed by heparin sulfate affinity column |
CMV/CBA | Rat/SD | Human A30P α-synuclein GFP |
1.4 × 1013 1.4 × 1013 |
52 weeks | N.D. | 53% | N.D. | Punctate α-synuclein |
No changes in rotarod or amphetamine- induced rotation behavior |
| Yamada et al. (2004) | 2 | Centrifugation | CMV | Rat/SD | Human WT α-synuclein GFP |
4.2 × 1011 5.6 × 1011 |
13 weeks | 50%(*) 5% |
N.D. | p129 α-synuclein | Slight but N.S. increase in apomorphine induced turning |
|
| Gorbatyuk et al. (2008, 2010) | 5 | lodixanol gradient followed by heparin sulfate affinity column |
CMV/CBA | Rat/SD | Human WT α-synuclein Human 129A α-synuclein Human 129D α-synuclein GFP |
0.9 × 1013 1 × 1013 1.7 × 1013 |
4 weeks 8 weeks 26 weeks 4 weeks 8 weeks 26 weeks 4 weeks 8 weeks 26 weeks 4 weeks 8 weeks 26 weeks |
4× 4× 4× |
20% (N.S.) 40% (N.S.) 60% (*) 70% (*) 70% (*) No change No change 10% (N.S.) 10% (N.S.) 10% (N.S.) |
No change Reduced levels No change |
Both punctate α-synuclein and diffuse staining Mostly diffuse α-synuclein Punctate α-synuclein |
|
| Chung et al. (2009) | 2 | heparin sulfate affinity column |
Synapsin | Rat/SD | Human A53T α-synuclein GFP |
1.8 × 1012 1.5 × 1012 |
17 weeks 24 weeks 17 weeks 24 weeks |
N.D | 32% (*) 56.3 (*) No change No change |
Activated microglia; Increased cytokines |
||
| Azeredo Da Silveira et al. (2009) | 6 | heparin sulfate affinity column |
CMV | Rat/SD | Human WT α-synuclein Human S129A α-synuclein Human S129D α-synuclein Human A30P α-synuclein Human A30P-S129A α-synuclein Human A30P-S129D α-synuclein |
1.7 × 1011TU/mL TU:4.4 × 107 TU:1.4 × 107 1.2 × 1011TU/mL TU:4.4 × 107 TU:1.4 × 107 1.7 × 1011TU/mL TU:1.2 × 1011 TU:4.4 × 107 0.9 × 1011TU/mL TU:4.4 × 107 TU:1.4 × 107 1.9 × 1011TU/mL TU:4.4 × 107 TU:1.4 × 107 1.4 × 1011TU/mL TU:4.4 × 107 TU:1.4 × 107 |
8 weeks 8 weeks 8 weeks 8 weeks 8 weeks 8 weeks |
2 × 2 × 2× |
20% (N.S.) 10% (N.S.) 10% (N.S) 70%(*) 45%(*) 35% (*) 10% (N.S.) 7% (N.S.) No Change 20%(N.S.) 15%(N.S.) 10%(N.S.) 70% (*) 25%(*) 30%(*) No change No change No change |
5%(*) 15%(*) No Change 3%(N.S.) 15%(N.S) No change |
Significant increase in ThioS and PK resistant species; by EM amorphous aggregates associated with lysosome and phagosome-like organelles occasional filaments |
|
| McFarland et al. (2009a, 2009b) | 8 | lodixanol gradient | CBA WPRE |
Rat/SD | Human WT α-synuclein Human S129A α-synuclein Human S129D α-synuclein GFP |
1.4 × 1013 7.7 × 1012 1.4 × 1013 1.16 × 1014 |
6 weeks | 1.24–2 × 1.78 × 1.19× |
20%(*) 30%(*) 27%(*) |
20% (NS) 30% (NS) 20% (NS) |
α-synuclein aggregates in a subset of cells |
|
| Sanchez-Guajardo (2010) | 5 | lodixanol gradient followed by ion exchange |
CBA | Rat/SD | Human WT α-synuclein GFP |
6.2 × 1013 6.7 × 1013 7.9 × 1012 |
4 weeks 8 weeks 15 weeks |
2 groups: 1 no change, 1 groups with 50% |
TH fiber loss in both groups |
In group with TH fiber loss, no death, increase in microglia and MHCII at 4 weeks In group with 50% loss of TH+neurons, increase in microglia and MNCII at 8 weeks |
||
| Ulusoy et al. (2010a, 2010b) | 5 | lodixanol gradient followed by ion exchange |
CMV/CBA | Rat/SD | Human WT α-synuclein Human α-synucleinΔ110 Human WT α-synuclein + Human α-synucleinΔ110 GFP |
6.2 × 1013 9.2 × 1013 2.7 × 1013 |
3 weeks 8 weeks 15 weeks 3 weeks 8 weeks 15 weeks 3 weeks 8 weeks 15 weeks |
N.D. | ~10% (NS) ~10% (NS) ~10% (NS) ~10% (NS) ~10% (NS) ~10% (NS) ~10% (NS) ~20% (NS) ~40% (*) |
No change ~20% (NS) ~25% (NS) ~10% (NS) ~10% (NS) ~40% (*) ~10% (NS) ~30% (*) ~40% (*) |
Small somal accumulations; pS129-synuclein Small somal accumulation Small somal and neuritic accumulation- more abundant than WT or synuclein Δ110 alone; pS129- synuclein |
No change in apomorphine or amphetamine- induced rotations No change in apomorphine or amphetamine- induced rotations Increase in apomorphine or amphetamine- induced rotations only in rats with >40% lesion (overall effect N.S.) |
| Koprich et al. (2010) | 1/2 chimera | heparin sulfate affinity column |
CMV/CBA +WPRE |
Rat/SD | Human A53T α-synuclein GFP |
5 × 1012 | 3 weeks | N.D. | 52% (*) 24% (*) |
60%(*) No change |
PK resistant α-synuclein |
N.D. |
| Koprich et al. (2011) | 1/2 chimera | heparin sulfate affinity column |
CBA/CMV +WPRE |
Rat/SD | Human A53T α-synuclein GFP |
1.7 × 1012 5.1 × 1011 1.7 × 1012 5.1 × 1011 |
3 weeks 6 weeks 3 weeks 6 weeks 3 weeks 6 weeks |
N.D. | No change 28%(*) No change No change No change No change |
7%(*) 25%(*) 13%(*) No change |
57% forelimb asymmetry 57% forelimb asymmetry 39% forelimb asymmetry No change No change No change |
|
| Decressac et al. (2011) | 6 | lodixanol gradient followed by ion exchange |
CMV/CBA | Rat/SD | Human WT α-synuclein GFP |
3.7 × 1013 2.5 × 1012 |
8 weeks | N.D. | 60% (*) | 53% (*) | N.D. | Significant increase in amphetamine- induced rotation |
| Decressac et al. (2012a, 2012b, 2012c) | 6 | lodixanol gradient followed by ion exchange |
Synapsin 1 | Rat/SD | Human WT α-synuclein |
3.7 × 1012 3.1 × 108/3 µL was injected = 1.1 × 1013gp/mL |
8 weeks | 33% TH and VMAT | Defects in stepping test only at 3 weeks Significant defects in performance in stepping test, cylinder test and rotation test at 8 weeks |
|||
| Decressac et al. (2012a, 2012b, 2012c) | 6 | lodixanol gradient followed by ion exchange |
Synapsin 1 + WPRE enhancer sequence CMV/CBA -WPRE enhancer sequence |
Rat/SD | Human WT α-synuclein Human WT α-synuclein GFP |
3.1 × 108/3 µL was injected= 1.1 × 1013gp/mL 3.1 × 108/3 µL was injected= 1.1 × 1013gp/mL |
10 days 3 weeks 5 weeks 8 weeks 16 weeks 10 d 3 weeks 5 weeks 8 weeks 16 weeks 10 d 3 weeks 5 weeks 8 weeks 16 weeks |
N.D. | 10%(NS) 42%(*) 60%(*) 80%(*) 75%(*) 10%(NS) 15%(NS) No change No change 10%(NS) 10%(NS) 10%(NS) |
30%(*) 80%(*) 80%(*) 10%(NS) 10%(NS) No change No change No change No change No change |
pS129-α-synuclein |
Significant defects in cylinder and amphetamine- induced rotation tests at 8 weeks and 16 weeks No defects in cylinder and amphetamine- induced rotation No defects in cylinder and amphetamine- induce d rotation |
| Decressac et al. (2013) | 6 | lodixanol gradient followed by ion exchange |
Synapsin I | Rat/SD | Human WT α-synuclein GFP |
2.1 × 1012 3.8 × 1011 2.1 × 1012 3.8 × 1011 |
8 weeks | N.D. | 80%(*) 30% (NS) |
80%(*) 30% (NS) |
For high titer: significant reduction in left paw use, and adjusting steps and increase in amphetamine induced rotations |
|
| Taschenberger et al. (2012) | 5 | Synapsin I WPRE |
Rat/Wistar | Human WT A- synuclein Human A30P α-synuclein Human A56P α-synuclein Human A56P/A76P α-synuclein GFP |
Injected 2 µL of 1.2 × 108 transducing units for all constructs |
2 weeks 4 weeks 8 weeks 14 weeks 2 weeks 4 weeks 8 weeks 14 weeks 2 weeks 4 weeks 8 weeks 14 weeks 2 weeks 4 weeks 8 weeks 14 weeks 14 weeks |
43% (*) 48% (*) 56% (*) 69% (*) ~20% (NS) 27% (*) 31% (*) 39% (*) ~20% (NS) 48% (*) 54% (*) 42% (*) ~20% (NS 50% (*) 47% (*) 46% (*) 5.6%(NS) |
95% (*) 60% (*) 70% (*) 30% (NS) |
PK resistant α-synuclein PK resistant α-synuclein Significantly less PK resistant α-synuclein Significantly less PK resistant α-synuclein No PK resistant α-synuclein |
|||
| Gaugler et al. (2012) | 6 | Heparin Column | PGK WPRE |
Rat/SD | Human WT α-synuclein Human A30P α-synuclein Human WT α-synuclein/WPRE Human A53T α-synuclein/WPRE |
8 × 1010 TU/mL (4.1 × 1013 vg/mL) 1.0 × 1011TU/mL (1.7 × 1014 vg/mL) 1.3 × 1010 TU/mL (5.8 × 1013 vg/mL 6.4 × 109 TU/mL (1.3 × 1014 vg/mL |
16 Weeks | 25%(*) No change 60%(*) 60%(*) |
30%(*) No change 50%(*) 50%(*) |
2.8 × 107 TUs of AAV-pgk- α-synuclein WT produced significant reduction in spontaneous behavior; 1.4 × 107 and 2.8 × 107 TU of AAV-pgk- α-synuclein WT produced significant increases in apomorphine- induced rotation but not amphetamine- induced rotation For viruses made with WPRE: 1.25 × 107 TU of AAV-pgk- α-synuclein WT and AAV-pgk-A53T- α-synuclein WT produced reductions in spontaneous activity and increases in apomorphine induced rotations |
||
| Gombash (2013) | 5 | lodixanol gradient followed by ion exchange |
CMV/CBA WPRE |
Rat/SD | Human WT α-synuclein GFP |
1.0 × 1014 5.9 × 1013 1.0 × 1013 2.2 × 1012 1.2 × 1013 |
4 weeks 8 weeks 12 weeks 4 weeks 8 weeks 12 weeks 4 weeks 8 weeks 12 weeks 4 weeks 8 weeks 12 weeks 12 weeks |
70%(*) 90%(*) 50%(*) 35% (NS) 60%(*) 5%(NS) 15% (NS) |
40%(*) 40%(*) |
No significant defect in cylinder test, adjusting steps test or bilateral tactile stimulation Defect s in ultrasonic vocalizations Decrease in forelimb in cylinder and adjusting steps tests; No significant defect in cylinder test, adjusting steps test or bilateral tactile stimulation |
||
| Daher et al. (2014, 2015) | 1 | Affinity column | CBA WPRE |
Rat/Long Evans/SD | Human α-synuclein GFP |
5 × 1012 5 × 1012 |
4 weeks 8 weeks 12 weeks |
2× | 25% (*) 25% (*) 25% (*) 10% (NS) |
Increase in nitrosylated α-synuclein |
||
| Davies et al. 2014; | 2 | heparin sulfate affinity column |
Synapsin | Rat/SD | Human A53T α-synuclein GFP |
2 × 1012 | 19 weeks | N.D. | 34% (*) | N.D. | Increase in higher molecular weight α-synuclein by western blot |
N.D. |
| Bourdenx et al. (2015) | 9 | lodixanol gradient | CMV-IE WPRE |
Rat/SD/Wistar/ Lewis Mouse/C57BI/6/ senescence- accelerated mouse- prone 8 (SAMP8) and SAMP1 |
Human A53T α-synuclein |
In rats: 72 h 1 week 4 weeks 8 weeks 16 weeks In mice: 20 weeks |
In rats: ~30% ~50% ~60% ~70% ~80% ~25% in C57BI/6 only |
In rats: No change ~30% ~75% ~90% ~90% ~30% in all mouse strains |
Increase in p- α-synuclein |
Decrease in spontaneous activity from 8 weeks on Decrease in number of adjusting steps from 4 weeks onward; altered gait timing and inter- limb coordination reduced control over limb trajectory and velocity; Impairments in horizontal ladder task |
||
| Cauda et al. (2015) | 6 | lodixanol gradient followed by ion exchange |
CMV/CBA | Rat/SD | Human WT α-synuclein GFP |
7.7 × 1014 2.3 × 1011 genome copies in 3 uL injected |
8 weeks | 43%(*) | 30%(*) | Significant impairment in ledged beam walking test |
||
| Daniel et al. (2015) | 6 | Heparin column | PGK | Rat/SD | Human WT α-synuclein |
1.42 × 1010 TU/mL | 14 weeks | 2× | 50%(*) | 30%(*) | Increase in p- α-synuclein; Induction of reactive astrocytosis and increase in microglia |
Significant increase in amphetamine- induced rotations |
| Rocha (2015) | 2 | heparin sulfate affinity column |
Synapsin | Rat/SD | Human A53T α-synuclein GFP |
2.0 × 1012 | 24 weeks | 50%(*) | ||||
| Salganik et al. (2015) | 5 | lodixanol gradient followed by heparin sulfate affinity |
Rat/F344 2 months 24 months |
Human A53T α-synuclein |
9.7 × 1012 |
6 weeks 12 weeks 6 weeks 12 weeks |
2 × |
20% (NS) 60%(*) 70%(*) 85%(*) |
Starting at 3 month, significant increase in amphetamine- induced rotations |
|||
| St. Martin (2007) | 2 | lodixanol gradient followed by heparin sulfate affinity column |
CMV/CBA WPRE |
Mice/C57BL/6 | Human WT α-synuclein GFP |
8.9 × 1010 1.7 × 1011 |
4 weeks 12 weeks 24 weeks 4 weeks 12 weeks 24 weeks |
N.D. | No change 9%(NS) 25%(*) 9% 7% No change |
N.D. | N.D. | N.D. |
| Theodore et al. (2008) | 2 | heparin sulfate affinity column |
CMV/CBA WPRE |
Mice/C57BL/6 | Human WT α-synuclein GFP |
3.5 × 1012 5.3 × 1012 |
2 weeks 4 weeks 12 weeks |
No Change | Microglial activation; infiltration of B and T lymphocytes; increased expression of proinflammatory cytokines |
|||
| Ulusoy et al. (2012) | 2,5,6 | lodixanol gradient followed by ion exchange |
Synapsin 1 WPRE |
Harlan C57BL/6 | Human WT α-synuclein GFP |
0.7 × 1012 1.5 × 1012 |
8 weeks | 30–35%(*) | Increase in p- α-synuclein |
|||
| Oliveras-Salva et al. (2013) | 7 | lodixanol step gradient |
Synapsin 1 WPRE |
Mice/C57BL/6 | Human WT α-synuclein Human A53T α-synuclein GFP |
8 × 1011 4 × 1011 2.6 × 1011 4 × 1011 8 × 1011 |
4 weeks 8 weeks 4 weeks 8 weeks 4 weeks 8 weeks 4 weeks 8 weeks 4 weeks |
~30 × ~40 × |
57%(*) 82%(*) 45%(*) 50%(*) 19%(*) 23%(*) 51%(*) 59%(*) No change |
73(*) 86(*) 56(*) 86(*) 17(*) 56(*) 56(*) 91(*) |
Urea soluble α-synuclein; p129 α-synuclein Urea soluble α-synuclein; p129 α-synuclein |
forelimb asymmetry at 12 weeks only with intermediate titer only time spent on rotarod at 14 weeks (intermediate and high titer) distance in open field at 15 weeks (intermediate and high titer) |
| Song (2015) | 1 | Chloroform and NaCI |
CMV | C57BL/6 | Human WT α-synuclein GFP |
3 × 1011 | 4 weeks 8 weeks 12 weeks |
20%(NS) 34%(*) 50%(*) |
10%(NS) 25%(*) 45%(*) |
Significant reduction in open field test at 8 and 12 weeks; Increased time to descend pole in pole test, and reduced score in swim test |
To produce the virus, typically HEK293 cells are transfected with 2–3 plasmids: a transfer plasmid encoding human α-synuclein flanked by rAAV2 inverted terminal repeats which are necessary for replication and packaging; a plasmid which encodes the replication proteins from rAAV2 and capsid proteins a from a particular serotype (1, 2, 5, 7, or 8); and a plasmid that encodes helper factors necessary for a productive infection (also see (Ulusoy et al. 2008) for review). The helper function and capsid genes can be combined into a single plasmid. Since the studies cited in this review all utilize the rAAV2 plasmid and inverted terminal repeats, we will refer only to the capsid serotype, e.g., rAAV2 refers to rAAV2 plasmid, capsid serotype 2, rAAV5, refers to rAAV2 plasmid, capsid serotype 5. After virus production, it is purified, typically by centrifugation through an iodixanol gradient followed by an ion exchange chromatography. Next, the virus is concentrated, and the viral titer, expressed as genome particles per milliliter (gp/mL), is determined using DNA dot blot or real-time PCR, although it is recommended that functional titer units can also be determined (see Caveats and considerations below). The authors also direct the readers.
How the rAAV-α-synuclein model recapitulates features of PD
One of the greatest advantages of the rAAV-α-synuclein model is that α-synuclein can be expressed in dopaminergic neurons of the SNpc, a cell population particularly vulnerable in PD. Expression can be induced in non-transgenic rats and mice, or transgenic or knockout animals to analyze the impact of any gene of interest on the pathologic and behavioral phenotypes, without the need for extensive breeding of transgenic rodents (Ulusoy et al. 2010b; Van der Perren et al. 2015).
In general, rAAV-α-synuclein expression leads to a progressive loss of dopaminergic neurons in the SNpc, and loss of terminals in the striatum, similar to PD brains, although the extent of neurodegeneration in the rAAV-α-synuclein model is variable (Table 1). Some studies show that rAAV-α-synuclein expression in the SNpc causes defects in behavioral tests, such as increased amphetamineor apomorphine-induced rotations, increased forepaw asymmetry in cylinder tests, and decreased number of steps in the adjusting steps test (Kirik et al. 2002; Ulusoy et al. 2010a; Decressac et al. 2011, 2012b,c, 2013; Koprich et al. 2011; Gaugler et al. 2012; Gombash et al. 2013; Oliveras-Salva et al. 2013; Bourdenx et al. 2015; Caudal et al. 2015; Daniel et al. 2015; Salganik et al. 2015; Song et al. 2015). It is important to note that rAAV- α-synuclein is typically unilaterally injected because tests of asymmetric motor behavior can be easily quantified. These behavioral tests rely on asymmetry in motor behavior produced by unilateral losses of dopamine neuron function. For example, in the rotation behavior tests, amphetamine or apomorphine is administered, which either increase levels of synaptic dopamine or activates postsynaptic dopamine receptors, respectively. Animals will turn toward the side with dysfunctional dopamine neurons. The cylinder test and stepping test also measure asymmetry in use of forelimbs.
What has the rAAV-α-synuclein model taught us about PD?
rAAV-α-synuclein studies highlight that models of PD must take pathological α-synuclein into account. For example, earlier studies using neurotoxin models of PD suggested that glial-derived neurotrophic factor (GDNF) family ligands may be neuroprotective, but several clinical trials of these ligands have failed to show significant benefits (Olanow et al. 2015). One possible explanation for the discrepancy could be that the toxin models did not sufficiently take into account the contribution of pathologic α-synuclein. For example, α-synuclein inclusions could interfere with neurotrophic receptor signaling or trafficking (Chung et al. 2009; Volpicelli-Daley et al. 2014a). Indeed, subsequent studies in viral-based α-synuclein models suggest that this model is more effective at predicting response in human disease, as GDNF failed to prevent dopaminergic neuron death (Lo Bianco et al. 2004; Decressac et al. 2011). Further, this work provided mechanistic insight; over-expression of α-synuclein prevented GDNF-induced activation of Nurr1 and consequently prevented the intended effects on expression of tyrosine hydroxylase (TH), aromatic l-amino acid decarboxylase, and other molecules involved in dopamine metabolism. Interestingly, exogenous over-expression of Nurr1 does protect against α-synuclein-induced toxicity, and thus direct activation of transcription factors involved in dopamine neuron survival may be a more promising strategy than GDNF ligands (Decressac et al. 2012a).
The progressive time course of the α-synuclein models has provided interesting data suggesting that synaptic abnormalities in the striatum precede neurodegeneration. In α-synuclein transgenic mice that over-express human α-synuclein under the Thy-1 promoter, extracellular dopamine is elevated at 6 months of age, long before reductions in striatal tissue dopamine content and TH levels which occur at 14 months (Lam et al. 2011). The rAAV-α-synuclein model provides a more accessible means of studying dopamine synaptic dysfunction; as early as 10 days after injection of rAAV6-α-synuclein into the SNpc, there is a significant reduction in dopamine reuptake rates in the striatum, suggesting impairments in the dopamine transporter (Lundblad et al. 2012), followed by a reduction in evoked dopamine release at 3 weeks. All of these changes occur before the onset of dopaminergic cell death and suggest that synaptic and axonal defects contribute to the development of PD symptoms well before neuron death ensues. A recent study suggests that the dopamine transporter and α-synuclein interact at the cell surface, and abnormal α-synuclein may influence the activity of the dopamine transporter, causing these early changes in dopaminergic activity (Butler et al. 2015). The rapid early loss of striatal dopamine function seen in the rAAV-α-synuclein model replicates the pattern seen in human disease. Indeed, 1 year after diagnosis, there is about a 50% loss of dopaminergic terminals in the dorsal putamen of PD patients, and by 4–5 years after diagnosis, 90% of dopaminergic terminals are gone (Kordower et al. 2013).
In addition to help understanding the etiology of PD, the rAAV-α-synuclein model has also helped to elucidate the contribution of genes associated with PD to degeneration of dopamine neurons. For example, heterozygous loss of function mutations in the gene encoding glucosecerebrosidase (GBA) can increase the risk of PD (Tayebi et al. 2003). Co-injection of rAAV2-GBA with rAAV2-A53T-α-synuclein protected against dopamine neuron loss in the SNpc (Rocha et al. 2015). In addition to GBA, autosomal recessive loss of function mutations in the gene encoding Parkin, an E3 ubiquitin ligase, are associated with juvenile parkinsonism (Kitada et al. 1998). Viral-induced co-expression of Parkin and α-synuclein in the substantia nigra reduces the extent of neuron loss produced by expression of α-synuclein alone (Lo Bianco et al. 2004; Yamada et al. 2005). However, in the absence of parkin in mice does not exacerbate α-synuclein over-expression-induced neuron death (Van Rompuy et al. 2015). Thus, the relationship between α-synuclein in parkin requires further investigation.
In addition to GBA and Parkin, other proteins implicated in degradative pathways have been revealed as putative therapeutic targets using the rAAV-α-synuclein. Nedd4, a ubiquitin ligase, targets α-synuclein for degradation in the endosome/lysosome pathway (Chung et al. 2013; Tardiff et al. 2013; Davies et al. 2014). Expression of Nedd4 in the SNpc protects against neurodegeneration caused by rAAV-α-synuclein expression and reduces accumulation of α-synuclein (Davies et al. 2014). NAB2, a potent activator of Nedd4, also rescues α-synuclein toxicity in yeast and induced pluripotent stem cell models, further supporting increased Nedd4 activity as a potential therapeutic target (Chung et al. 2013; Tardiff et al. 2013). Another modulator of degradative pathways is transcription factor EB (TFEB) which responds to lysosomal disruption or nutrient starvation by translocating from lysosomes to the nucleus where it increases expression of genes involved in autophagosome and lysosome biogenesis (Settembre et al. 2012). Expression of TFEB in the SNpc reduces levels of high molecular weight aggregates of α-synuclein produced by rAAV-α-synculein expression and protects against neurodegeneration (Decressac et al. 2013). Expression of lysosomal-associated membrane protein 2a, which facilitates chaperone-mediated autophagy, in the SNpc, protects against α-synuclein-induced neuron loss, suggesting that enhancing α-synuclein degradation may be a therapeutic strategy (Xilouri et al. 2013). In addition, autosomal recessive mutations in another protein, ATP13A2, cause PD. ATP13A2 localizes to lysosomes and facilitates transport of cations across membranes. The expression of wild-type ATP13A2, however, did not protect against rAAV6-α-synuclein-induced degeneration, although it reduced toxicity in yeast, Caenorhabditis elegans and cell culture models of PD (Daniel et al. 2015). Thus, these data highlight the need to test potential therapeutic targets in valid preclinical models.
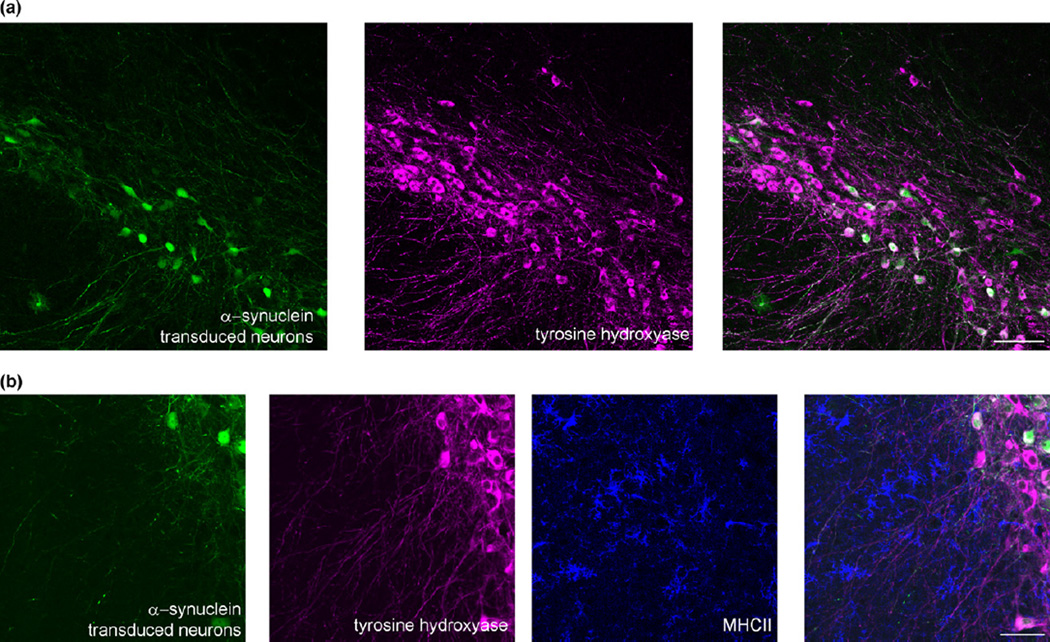
Neuroinflammation is one of the most robust phenotypes found by rAAV-α-synuclein over-expression (either wild-type or A53T), and replicates many features seen in human PD (Fig. 1) (Theodore et al. 2008; Chung et al. 2009; Cao et al. 2010; Sanchez-Guajardo et al. 2010; Harms et al. 2013; Daher et al. 2014, 2015; Allen Reish and Standaert 2015; Daniel et al. 2015). These changes include early activation of microglia, increased pro-inflammatory cytokine expression, increased adaptive and innate immune responses, and infiltration of lymphocytes before cell loss (Allen Reish and Standaert 2015). This inflammatory induction is caused by over-expression of α-synuclein, and not the virus itself, because control mice injected with rAAV-green fluorescent protein (GFP) do not show neuroinflammatory responses. The increase in inflammation likely contributes to neurodegeneration. Further supporting a role for inflammation in the progression of PD, are the findings from genome-wide association studies that polymorphisms in the HLA-DR locus, a component of the human major histocompatibility complex II, is associated with late-onset PD (Hamza et al. 2010). Mice completely lacking the major histocompatibility complex II gene show attenuated inflammatory responses to rAAV2-α-synuclein and protection against cell loss (Harms et al. 2013). Similarly, absence of the fractalkine receptor, which localizes to microglia, monocytes dendritic cells, and natural killer cells, reduces rAAV2-α-synuclein-induced inflammation (Thome et al. 2015). Thus, targeting signaling in the inflammatory pathway may provide a therapeutic target that can prevent the progression for PD.
Fig. 1.
Representative images of a mouse substantia nigra pars compacta transduced with rAAV2-CBΑ-synuclein-IRES-EGFP-WPRE. (a) The eGFP shows neurons transduced with rAAV2-α-synuclein and the magenta image shows tyrosine hydroxylase-positive neurons. Scale bar = 100 µm. B) The eGFP shows neurons transduced with rAAV2-α-synuclein, the magenta image shows tyrosine hydroxylase-positive neurons, and the blue shows major histocompatibility complex II (MHCII)-positive activated microglia near α-synuclein expressing neurons. Unpublished data. Scale bar = 50 µm.
The rAAV2-α-synuclein model has helped to demonstrate the therapeutic potential of small molecule neuroprotective agents, especially inhibitors of leucine-rich repeat kinase 2 (LRRK2). Autosomal dominant mutations in LRRK2 are the most common genetic causes of PD (West 2015). The most prevalent LRRK2 mutation, G2019S, up-regulates its kinase activity (West et al. 2005). The complete absence of the LRRK2 gene protects against dopamine neuron cell loss induced by rAAV1-α-synuclein over-xpression by inhibiting neuroinflammatory responses (Daher et al. 2014). In contrast, rats over-expressing the G2019S–LRRK2 mutation show enhanced rAAV1-α-synuclein-induced dopamine neuron cell loss and inflammation. Administered by oral gavage over the course of 4 weeks, PF-06447475, a potent, selective LRRK2 inhibitor, prevents neurodegeneration and neuroinflammation (Daher et al. 2015). Identifying more effective LRRK2 inhibitors is a very intense area of development and novel inhibitors will be also tested in the future. Overall, the rAAV-α-synuclein model has provided critical preclinical data supporting the potential efficacy of LRRK2 inhibitors for the treatment of PD.
Caveats and considerations
Some of the most important considerations for the rAAV-α-synuclein model are the promoter, inclusion of the woodchuck promoter response element, the serotype, how the virus is purified, and how the titer is determined. High quality, pure, and standardized viruses are critical, also see (Fischer et al. 2016; Ulusoy et al. 2008). The most common promoters to direct expression of α-synuclein include the hybrid cytomegalovirus, chicken b-actin (CBA), phosphoglycerate kinase (PGK), and human synapsin I promoters. Inclusion of a woodchuck hepatitis post-transcriptional regulatory element improves expression levels (Decressac et al. 2012c). The capsid serotype of the rAAV is also an important consideration. The AAV1, AAV5, and AAV8 serotypes show increased transduction efficiency in the SNpc compared to the AAV2 (McFarland et al. 2009a). Recently, the rAAV7 serotype was reported to show high levels of α-synuclein expression in the mouse SNpc and produced a robust loss of dopaminergic neurons (Oliveras-Salva et al. 2013).
The method of purification of the virus also varies across studies. Purification of rAAV is critical for obtaining a high yield of infectious rAAV and to remove contaminants, such as residual cellular proteins from the crude lysate and endotoxin that could produce non-specific neuron death. In most laboratories, centrifugation through an iodixonal gradient is the first step of purification, followed by a heparin sulfate affinity column chromatography for AAV2, or cation exchange for other serotypes. Determination of titer is also critical. Most studies use DNA dot blot or qPCR of viral genomic DNA to determine genome particles/mL. However, this method does not determine the efficiency of transduction and even a high viral titer could contain empty capsid and thus not produce efficient transduction. To determine the functional titer unit (TU), HEK293 cells can be transduced with the virus and the number of eGFP or α-synculein expressing cells can be determined by flow cytometry or another method. Before embarking on studies that could take several months and use many animals, it is recommended the functional titer unit also be determined (e.g., see (Daniel et al. 2015; Azeredo da Silveira et al. 2009).
The amount of protein produced after transduction with rAAV-α-synuclein may vary. Typically, there is an approximately 2–4 fold increase in α-synuclein levels compared to endogenous protein, levels similar to what might be caused by α-synuclein triplication in PD patients (Gorbatyuk et al. 2008, 2010; Azeredo da Silveira et al. 2009; McFarland et al. 2009a; Daher et al. 2014, 2015; Daniel et al. 2015; Salganik et al. 2015). However, a recent study reported up to 30-fold increases in α-synuclein, However, a recent study reported up to 30-fold increases in α-synuclein. While this level of expression produces robust phenotypes, it may not accurately reflect what occurs in PD (Oliveras-Salva et al. 2013). More studies that quantify α-synuclein expression biochemically to determine levels of expression in this model are needed.
The species, strain, and ages of rodents in which the rAAV-α-synuclein is injected is also critical. The strain of mice is also likely to be important; rAAV9-α-synuclein was reported to produce robust dopamine neuron degeneration in the SNpc of C57Bl/6 mice but not other mouse strains (Bourdenx et al. 2015). This finding emphasizes that any study comparing knockout mice or transgenic mice to study the role of any gene of interest in rAAV-α-synuclein-mediated phenotypes should use congenic mouse lines on the same background, such as C57BL/6J. In addition, injection of rAAV5-α-synuclein into 24-month-old rats produced substantially more robust and rapid neuron death compared to rats injected at 2 months old (Salganik et al. 2015). α-synuclein levels increase with age (Chu and Kordower 2007), suggesting that the ability to clear α-synuclein may partly account for this effect, although it is also possible that the production of α-synuclein by AAV vectors may differ in aged animals.
Yet another technical consideration is that injections into the SNpc are technically difficult, especially in the mouse brain, and precision is necessary to maintain animal numbers at a minimum and produce significant data. Another caveat of the rAAV-α-synuclein model is that it does not seem to entirely recapitulate the features of Lewy bodies and Lewy neurites found in PD brains. rAAV-α-synuclein expression increases abundance of α-synuclein phosphorylated at Ser129 (Yamada et al. 2004; Koprich et al. 2010; Ulusoy et al. 2010a; Decressac et al. 2012c; Oliveras-Salva et al. 2013; Bourdenx et al. 2015; Daniel et al. 2015) and nitrosylated α-synuclein (Daher et al. 2014, 2015), both markers of abnormal α-synuclein. It also produces proteinase K (Azeredo da Silveira et al. 2009; Taschenberger et al. 2012) and urea-resistant aggregates (Oliveras-Salva et al. 2013). One outstanding study showed that α-synuclein aggregates are thioflavin S positive and thus have an amyloid conformation. The ultrastructure examination showed mostly amorphous aggregates and only a few 10–15 nm filaments that are characteristic of α-synuclein fibrils in PD brains (Azeredo da Silveira et al. 2009). While α-synuclein does appear to be localized to puncta in transduced neurons overexpressing α-synuclein, these aggregates do not have the morphological characteristics of Lewy bodies and Lewy neurites. Controls are another important consideration with the rAAV-α-synuclein model. Vectors expressing GFP are used most often, but, GFP alone can cause some toxicity. Also, expression of a protein that is expressed in the mammalian nervous system but does not aggregate would be a useful control. However, a general consensus has not been reached as the best control for α-synuclein over-expression (Ulusoy et al. 2010a).
Pre-formed fibrils of α-synuclein induce formation of Lewy body and Lewy neurite-like α-synuclein inclusions
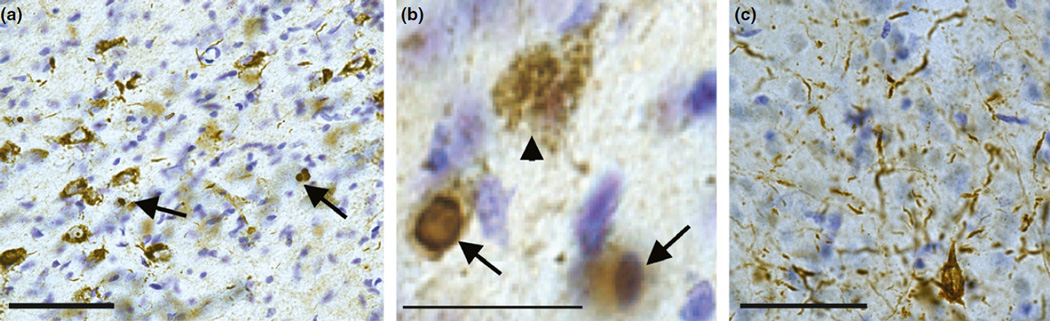
Sonicated fibrils made from recombinant α-synuclein, when added to primary cultured neurons or injected into the striatum or other brain areas, can produce robust formation of inclusions that resemble Lewy bodies and Lewy neurites found in diseased brains. Thus, this model allows investigation of the impact of misfolded α-synuclein on the neuronal function and consequent phenotypes, and determination of whether preventing inclusion formation can reverse these phenotypes. The fibrils are taken up by cells, likely by micropinocytosis (Holmes et al. 2013), gain entry to the cytoplasm by an as yet unknown mechanism and induce normal endogenous α-synuclein to adopt a conformation such that it forms inclusions that grow and propagate throughout the neurons. These inclusions have many features of those found in PD brains: they are filamentous by electron microscopy, hyperphosphorylated, ubiquitinated, insoluble in anionic detergent, and morphologically resemble the threadlike and neuroaxonal spheroid Lewy neurites, and the dense spherical Lewy bodies (Fig. 2) (Volpicelli-Daley et al. 2011, 2014b). One of the most surprising findings is that fibrilinduced inclusions can be formed from endogenous α-synuclein (Volpicelli-Daley et al. 2011; Luk et al. 2012). This is important because endogenous α-synuclein is primarily a presynaptic protein (Maroteaux et al. 1988). Indeed, we found that inclusions form first in axons and then spread to the soma and dendrites (Volpicelli-Daley et al. 2011), as suggested from pathologic studies of PD brains (Braak et al. 2003a). Over-expression of α-synuclein causes it to be highly expressed in the soma and dendrites and throughout the cytosol where it is not typically found. Thus, this model allows us to more closely replicate the physiological processes that likely occur in the brain. Over time, the inclusions cause defects in neuronal excitability and connectivity, and eventually led to neuron death. Importantly, addition of the fibrils to neurons from α-synuclein knockout mice did not display phospho-α-synuclein inclusions, defects in neuronal function or neuron death. In addition, soluble, monomeric, disordered α-synuclein does not cause inclusion formation (Volpicelli-Daley et al. 2011, 2014b). Thus, corruption of endogenous α-synuclein into abnormal conformations is critical for propagation of the pathology and defects in neuronal function. It is critical to point out, however, that the species of α-synuclein that induces the physiological defects is currently unknown; oligomers of α-synuclein could be responsible or amyloidogenic fibrils. Indeed, it has been suggested that sequestration of α-synuclein into the amyloid inclusions may be protective.
Fig. 2.
Injections of fibrils into the substantia nigra pars compacta induces the formation of pS129-α-synuclein inclusions with morphologies similar to Lewy bodies and Lewy neurites found in PD. Twenty micrograms of fibrils were injected into the substantia nigra pars compacta in 12-week-old male rats. After 4 weeks, animals were perfused and immunohistochemistry was performed for pS129-α-synuclein by DAB staining with Nissl counter-stain. (a) Representative image of the SNpc showing inclusions in the soma and neurites Scale bar is 30 µm). (b) Higher magnification of (a) showing the typical morphologies of pS129-α-synuclein inclusions in the SNpc neurons. The inclusions ranged from puncta in the soma to dense, spherical inclusions (c) Abundant Lewy-neurite-like inclusions in the amygdala. Unpublished data.
Similar to rAAV-α-synuclein models, inducing inclusion formation with protein fibrils is advantageous because transgenic over-expression of human α-synuclein is not required; researchers can thus understand the impact of any gene of interest on aggregate formation utilizing knockout mice or transgenic mice without having to crossbreed in to transgenic α-synuclein lines. Also, inclusion formation occurs in neurons from hippocampus, cortex, and midbrain and other brain regions (Volpicelli-Daley et al. 2011, 2014b; Luk et al. 2012; Dryanovski et al. 2013; Masuda-Suzukake et al. 2013, 2014; Aime et al. 2015; Paumier et al. 2015), and thus the impact of these aggregates on diverse neuronal populations can be examined.
Another advantage of the fibril model is that inclusions resembling bona fide Lewy bodies and Lewy neurites can be produced in primary neurons in culture. Over-expression of α-synuclein in vitro, whether by viral-mediated vectors or transient transfections, does not produce inclusions in primary neurons with features, such as those found in PD brains, and similarly, neurons plated from transgenic mice over-expressing mutant or wild-type α-synuclein do not show pathology. Thus, the fibril-induced inclusion model in primary neurons opens up a wide avenue for experiments examining the cell biological mechanisms by which α-synuclein inclusions cause neuronal dysfunction. For example, experiments using live-cell imaging fluorescent microscopy and physiology can now be performed. Finally, the primary neuronal model also allows screening of putative therapeutics without the blood brain barrier.
How the model in which exogenous fibrils induce endogenous α-synuclein to form inclusions recapitulates features of PD
Sonicated fibrils have been injected into the striatum, SNpc, entorhinal cortex, and even the muscle of mice and rats (Luk et al. 2012; Masuda-Suzukake et al. 2013, 2014; Paumier et al. 2015). Regardless of the injection site, α-synuclein inclusions form at the injection site and appear in brain areas distant from the site of injection. For example, unilateral injections of fibrils into the mouse and rat striatum produces inclusions ipsilateral to the injection site in the striatum, SNpc, amygdala, thalamus, and frontal and insular cortex (Luk et al. 2012; Masuda-Suzukake et al. 2013; Paumier et al. 2015). The inclusions in these brain regions are apparent by 4 weeks after injections. By 8–24 weeks after injections, inclusions also develop in the contralateral cortex, and striatum, but inclusions in the SNpc remain ipsilateral to the injection. These inclusions are phosphorylated on Ser129, and colocalize with ubiquitin and p62. In mice, 24 weeks following injection, there is about a 30% loss of TH-positive neurons in the nigra (Luk et al. 2012). In rats, 8 weeks following injection, there is about a 30% loss of TH-positive neurons and an 80% loss of TH-positive terminals in the striatum (Paumier et al. 2015). Injections into two sites of the striatum, to increase the affected area, produces slightly more robust results with about 41% loss of TH-positive neurons (Paumier et al. 2015). In the rat brain, the cell and terminal loss occurs bilaterally even when fibrils were only injected on one side of the striatum and α-synuclein inclusions only appeared on the injected side (Paumier et al. 2015). Interestingly, 4 weeks after fibril injections in rats, there is about a 50% decrease in TH staining in the striatum, consistent with rAAV-α-synuclein producing dopamine dysregulation in the striatum at early time points. With respect to motor defects, although only 30% loss of TH-positive neurons in the SNpc was achieved in mice, they show robust defects in a wire hang test and rotarod test. In rats, there were no significant effects in the amphetamine-induced rotation or cylinder tasks, but there were significant defects in an ultrasonic vocalization test.
Inclusion formation from the same neurons can also be visualized over time in live animals using multiphoton laser scanning microscopy. In transgenic mice expressing human synuclein-GFP (Rockenstein et al. 2005) injected with fibrils in the primary sensory cortex, formation of α-synuclein-GFP inclusions could be visualized in live mice up to 13 months after injection (Osterberg et al. 2015). Fluorescence recovery after photobleaching experiments showed that recruitment of α-synuclein into inclusions reduced its mobility. Thus, this method allows visualization and quantification of the dynamics of inclusion formation without having to perfuse the animal and perform immunohistochemistry. In addition, this technique allows the same neurons to be imaged over extensive periods of time. A powerful extension of technique is to determine how potential therapeutics targeted at preventing inclusion formation alters these dynamics.
What has the fibril model taught us about PD?
Because the fibril model can produce inclusion formation in primary neurons, this has opened up opportunities for cell biological studies examining the mechanisms by which inclusions impact neuronal function. For example, 4 days after fibril-induced inclusions have begun to develop in axons, there are major defects in neuronal synchronization (Volpicelli-Daley et al. 2011). The impairments do not occur in neurons from α-synuclein knockout mice treated with fibrils, demonstrating that the corruption of endogenous α-synuclein is responsible for the reduced neuronal synchronization. The defects occur well before any neuron death, suggesting that neuronal dysfunction emerges in response to abnormal α-synuclein very early before neurodegeneration begins. This is important in light of the development of novel techniques showing the presence of α-synuclein aggregates at a very early stage in brains of patients with PD and dementia with Lewy bodies (Kramer and Schulz-Schaeffer 2007; Roberts et al. 2015).
In addition, defects in axonal transport have been implicated in the development of PD. First, rAAV2-A53T-α-synuclein expression in the substantia nigra causes reduced striatal expression of synaptic vesicle markers and members of the kinesin family of molecular motors involved in anterograde transport (Chung et al. 2009). These changes occur at time points before neuronal death, again suggesting axonal defects precede neuron death. Because fibrils can induce formation of α-synuclein inclusions from endogenously expressed α-synuclein in primary neurons in culture, this allows live-cell imaging studies with the spatial and temporal resolution for the visualization and quantification of organelle transport. Formation of α-synuclein inclusions at early time points, before neuron death, reduces velocities and mobilities of Rab7-positive late endosomes, TrkB neurotrophin receptors, and LC3-positive autophagosomes (Daher et al. 2014). However, axonal transport of mitochondria and synaptic vesicle precursors remain normal. In addition, electron microscopy analyses showed that α-synuclein inclusions do not fill the axonal cytoplasm or grossly disrupt the microtubule tracts. This suggests that the α-synuclein inclusions do not impair axonal transport by occluding the axon nor do the inclusions cause a generalized disruption of transport proteins, such as kinesin or dynein. It is likely that the α-synuclein inclusions selectively affect a subset of adaptor proteins found in endosome/autophagosome compartments. Furthermore, this selective disruption of axonal transport of signaling endosomes in axons harboring inclusions may account for the failure of neurotrophic factors to prevent neurodegeneration (Marks et al. 2010; Lewis and Standaert 2011).
The role of mitochondrial dysfunction in PD has been a long-standing subject of debate. The results of a recent study suggest that mitochondrial stress is downstream of inclusion formation (Dryanovski et al. 2013). When neurons were incubated with fibrils for several days, α-synuclein inclusions were found in the soma and dendrites, and there was evidence of increased mitochondrial oxidant stress, particularly in the dendrites. The mitochondrial stress was not present in control neurons and was blocked by treatment with an NADPH oxidase inhibitor. This study also suggests that increased lysosomal NADPH oxidase activity could have contributed to the source of mitochondrial oxidant stress. Indeed, many gene products implicated in PD cause lysosomal defects and thus increased reactive oxygen species and mitochondrial damage may be downstream from damage to other organelles, such as endosomes and lysosomes, which in turn impact mitochondria.
Another recent study using the fibril-induced inclusion formation model shows a role for the proapoptotic protein, tribbles pseudokinase 3 (Trib3), in dopamine neuron degeneration (Aime et al. 2015). Trib3 is a scaffold for signaling pathways and expression is induced by metabolic stress. This study showed that Trib3 expression was increased in cell culture neuronal models treated with either fibrils or the toxins, 6-hydroxydopamine or MPP+, which have been used in traditional models of PD to lesion dopaminergic neurons. Trib3 expression was also increased in the SNpc of PD patients. Increased expression of Trib3-induced cell death of cultured midbrain dopaminergic neurons, and shRNA knockdown of Trib3 was protective. The authors further showed that Trib3 interacts with Parkin and increased levels of Trib3 reduced Parkin levels, which could cause diminished neuronal survival.
A recent study using fibrils demonstrates that immunotherapy may successfully prevent the progress of the disease (Tran et al. 2014). In this study, antibodies against pathologic α-synuclein were used in an effort to prevent the spread and toxicity of α-synuclein. In mice that received intrastriatal injections of fibrils, intraperitoneal injections of monoclonal antibodies reduced fibril-induced pathological α-synuclein injections and rescued the dopaminergic loss in the SNpc, and improved the motor behavior defects. Thus, these experiments using the fibril model demonstrate the promising therapeutic potential of immunotherapy (Tran et al. 2014).
Caveats and considerations
Similar to the rAAV-α-synuclein model, there are some considerations for the fibril model to work. The first consideration is whether to use fibrils from human or mouse α-synuclein. Even though mouse and human α-synuclein share 95% identity, their efficiency in inducing inclusions formation appears to differ. In primary neurons, both mouse and human fibrils seed formation of inclusions. In non-transgenic mice or rats, mouse fibrils produce robust pathology and about 30–40% TH-positive neuron loss (Luk et al. 2012; Paumier et al. 2015). Although one study showed that human fibrils produced phospho-α-synuclein inclusions in non-transgenic mice (Masuda-Suzukake et al. 2013), another study using human fibrils in rats did not produce abundant phospho-α-synuclein inclusions and did not cause significant loss of TH-positive neurons (Peelaerts et al. 2015). However, the combination of rAAV7-induced expression of human α-synuclein and human fibrils produced robust pathology, with about 50% TH-positive neuron loss and defects in forepaw use as measured by the cylinder test (Peelaerts et al. 2015).
Another consideration is the injection site. Currently, most studies have injected the fibrils into the striatum. The striatum has a large area and the fibrils do not efficiently spread through the tissue. Even with two-site injections into the striatum, only a 41% loss of TH-positive neurons in the SNpc was produced (Paumier et al. 2015), which does not meet the critical threshold for behavioral phenotypes. It is possible that injecting the fibrils directly into the SNpc which has a much smaller volume may produce more robust neuron loss. But, as with the rAAV-α-synuclein model, injections into the SNpc are technically challenging. Further, because the fibrils do not spread to the same extent as virus, precision in the surgeries is even more critical.
When making fibrils, it is important to start with a concentration of α-synuclein monomer of about 5 mg/mL (350 µM), as concentrations less than this take much longer to fibrillize. Also, monomeric α-synuclein with modifications, such as a His tag for purification, does not fibrillize as efficiently. We have found that ordering lyophilized α-synuclein from commercially available sources does not result in efficient fibril formation. In general, purification of α-synuclein from Escherichia coli requires boiling the protein (α-synuclein remains soluble after boiling), followed by gel affinity chromatography, and then by anion exchange chromatography. After purification, it is critical to ensure to assess potential contamination of the α-synuclein preparation with endotoxin. Once fibrils are formed, it is crucial to characterize them with sedimentation assays and thioflavin T binding (Volpicelli-Daley et al. 2014b). Typically, at least half of the α-synuclein should be in the pellet after a 100 000 g spin for the model to work efficiently. Storage conditions are also critical. The fibrils will disaggregate at 4°C (Bousset et al. 2013) and with repeated freeze/thaws. In addition, the fibrils, which range in size from 1 µm to 200 nm, need to be sonicated with a probe tip sonicator such that they break in to small fragments (on average 50 nm by transmission electron microscopy) before adding them to neurons or injecting them into the mice. Typically, bath sonicators are not sufficient to break the fibrils into small fragments. It is recommended to check for efficient sonication using transmission electron microscopy when adopting this protocol in the lab for the first time.
Monomeric α-synuclein is an important control for these experiments. Because α-synuclein forms fibrils and oligomers so quickly, it is important to keep the monomer on ice, to spin at high speed (100 000 g) at 4°C and take only the supernatant before adding to neurons or injecting into rodent brains. As with the sonicated fibrils, it is recommended to examine the monomer preparation using transmission electron microscopy to ensure absence of oligomers or short fibrils. Although we have found that monomer does not induce inclusion formation (Volpicelli-Daley et al. 2011, 2014b), it is important to show that monomer alone is not causing effects independent of inclusion formation. Additionally, performing experiments using neurons from α-synuclein knockout mice is recommended to determine if phenotypes are produced from entrainment of endogenous α-synuclein and not solely from the exogenously added fibrils.
Finally, rAAV-α-synuclein studies have tested viral titers and species and strains of rodents for the most efficient and robust phenotypes. It is recommended that similar experiments be performed with the fibril models, such as testing different concentrations of fibrils, different injections sites, and comparing different species and strains of rodents. In addition, as with the rAAV-α-synuclein model, it will be of great interest if injections of fibrils into aged rodents, which may have higher levels of α-synuclein, produces more robust results. For example, the in vivo study that first demonstrated the fibril model (Luk et al. 2012) used C57BL6/C3H mice which have a mutation in the toll-like receptor 4 (TLR4), a receptor responsible for activating the innate immune system, and it is possible that this genotype may influence the spread of pathologic α-synuclein in this strain of mouse.
One of the drawbacks of the fibril model is the lack of knowledge about the spread of pathologic α-synuclein and the exact mechanism by which the fibrils induce endogenous α-synuclein to misfold. Additionally, although fibril-induced inclusion formation is a novel system to study pathology and deficits caused by PD, the model initiates pathology and does not allow determination of how α-synuclein converts from a monomeric, normal conformation to a beta-sheet, pathogenic conformation. Recent studies suggest that, normally, α-synuclein forms higher order alpha-helical oligomers that can bind to membranes and are resistant to form pathologic conformations (Burre et al. 2014; Dettmer et al. 2015a,b). Cellular stressors and PD-linked mutations in α-synuclein impair the ability of α-synuclein to adopt its normal conformation and increase levels of unfolded monomer that can go on to form beta-sheets, protofibrils, and fibrils. A recent study used a novel technique microelectron diffraction to examine the three-dimensional structure of ‘invisible crystals’ of the non-amyloid component of α-synuclein (NAC domain, residues 68–78) which is the domain responsible for association into fibrils (Rodriguez et al. 2015). This study also examined the structure of a domain immediately upstream of the NAC domain, called the preNAC domain (residues 47–56) and showed that it can adopt a beta-sheet conformation that can form protofibrils. The data from the paper suggest that the PD-associated A53T-α-synuclein mutation might enhance the interaction of the NAC domain and preNAC domain in fibrils, increasing its propensity to form fibrils. Thus, emerging studies are making progress in understanding how α-synuclein converts to a pathological conformation (Volpicelli-Daley and Standaert 2016).
Future directions
The rAAV-α-synuclein model has made progress toward understanding how genes implicated in PD accelerate or protect against α-synuclein-induced pathology. The rAAV-α-synuclein model has also been important in revealing that the neuroinflammation plays a major role in the neurodegenerative process. It will be of great interest if these findings can be replicated using the fibril model. If phenotypes can be replicated using two PD models, it provides even greater support for the validity of a therapeutic target.
Furthermore, several questions remain unsolved. For example, why do the different α-synuclein mutations manifest with distinct disease phenotypes (Kasten and Klein 2013)? Why do certain subpopulations of neurons appear to be more susceptible to forming α-synuclein inclusions (Taguchi et al. 2014)? How is abnormal α-synuclein released by neurons and transmitted to neighboring neurons? Also, what are the factors that initiate conversion of normal α-synuclein expressed in the neuron to abnormal, b-sheet oligomers and amyloid fibrils? Are oligomers or amyloid fibrils (or a combination of both) the toxic species? Why do approximately 10% of people have abundant Lewy bodies and Lewy neurites with no symptoms of disease? The nature of the toxic conformer of α-synuclein also remains controversial and it has been suggested by some that oligomers are toxic, while mature Lewy bodies are inert or even protective. Finally, what are the mechanisms by which other genes implicated in PD, such as GBA, LRRK2, ATP13A2, VPS35, and tau, impact formation of pathologic α-synuclein. These questions may be answered with the use of the rAAV-α-synuclein and fibril models or may require the development of novel models.
Overall, the goal of developing models of PD is to understand how the disease develops and how to slow or halt the progression of the disease. The fibril model recapitulates many features of PD: neuron loss, inclusions with biochemical and morphologic features similar to Lewy bodies and Lewy neurites, spread of pathology throughout the brain and defects in motor behaviors. Similar to rAAV-α-synuclein studies, future studies using fibrils composed of α-synuclein with mutations linked to human disease, such as A53T, A30P, E46K, H50Q, and G51D, could help determine how these mutations influence the development of disease. These mutations manifest with distinct clinical phenotypes and it will be interesting to determine if the extent they produce distinct profiles with respect to toxicity and spread throughout the brain. Both models have been employed primarily to study dopaminergic neurons in the substantia nigra, but they may also be adaptable to study the extranigral pathology thought to be responsible for nonmotor defects, such as cognitive decline and depression. For example, injection of rAAV5-α-synuclein into the dopaminergic ventral tegmental area and the cholinergic medial septum/diagonal band of Broca produced pathology and defect in motor and learning tasks without overt cell death (Hall et al. 2013). In addition, injection of fibrils in the striatum produces robust pathology in the striatum, SNpc, amygdala, and cortex (Luk et al. 2012). Direct injections of fibrils into the hippocampus can produce pathology in this brain region as well. Overall, the fibril model or combined AAV-α-synuclein and fibril models recapitulate many of the features found in PD, and thus provide the best models to date for this disease and offer the most promise of discovering promising therapeutics.
Acknowledgments
This work was funded by awards from the American Parkinson Disease Association and Michael J Fox Foundation to LVD and NIH P20 award NS0925230 to DGS.
All experiments were conducted in compliance with the ARRIVE guidelines.
Abbreviations used
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- 6-OHDA
6-hydroxydopamine
- GDNF
glial-derived neurotrophic factor
- ITRs
inverted terminal repeats
- LRRK2
leucine-rich repeat kinase
- PD
Parkinson’s disease
- rAAV
recombinant adeno-associated viral vector
- SNpc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
- WPRE
woodchuck promoter response element
- WT
wild-type
Footnotes
conflict of interest disclosure
The authors have no conflict of interest to declare.
References
- Aime P, Sun X, Zareen N, et al. Trib3 Is elevated in Parkinson’s disease and mediates death in Parkinson’s disease models. J. Neurosci. 2015;35:10731–10749. doi: 10.1523/JNEUROSCI.0614-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen Reish HE, Standaert DG. Role of alpha-synuclein in inducing innate and adaptive immunity in Parkinson disease. J. Parkinsons Dis. 2015;5:1–19. doi: 10.3233/JPD-140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athauda D, Foltynie T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat. Rev. Neurol. 2015;11:25–40. doi: 10.1038/nrneurol.2014.226. [DOI] [PubMed] [Google Scholar]
- Azeredo da Silveira S, Schneider BL, Cifuentes-Diaz C, Sage D, Abbas-Terki T, Iwatsubo T, Unser M, Aebischer P. Phosphorylation does not prompt, nor prevent, the formation of alpha-synuclein toxic species in a rat model of Parkinson’s disease. Hum. Mol. Genet. 2009;18:872–887. doi: 10.1093/hmg/ddn417. [DOI] [PubMed] [Google Scholar]
- Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am. J. Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- Bourdenx M, Dovero S, Engeln M, et al. Lack of additive role of ageing in nigrostriatal neurodegeneration triggered by alpha-synuclein overexpression. Acta Neuropathol. Commun. 2015;3:46. doi: 10.1186/s40478-015-0222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousset L, Pieri L, Ruiz-Arlandis G, et al. Structural and functional characterization of two alpha-synuclein strains. Nat. Commun. 2013;4:2575. doi: 10.1038/ncomms3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003a;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural. Transm. 2003b;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- Burre J, Sharma M, Sudhof TC. alpha-synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc. Natl Acad. Sci. USA. 2014;111:E4274–E4283. doi: 10.1073/pnas.1416598111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler B, Saha K, Rana T, Becker JP, Sambo D, Davari P, Goodwin JS, Khoshbouei H. Dopamine Transporter Activity Is Modulated by alpha-synuclein. J. Biol. Chem. 2015;290:29542–29554. doi: 10.1074/jbc.M115.691592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Theodore S, Standaert DG. Fcgamma receptors are required for NF-kappaB signaling, microglial activation and dopaminergic neurodegeneration in an AAV-synuclein mouse model of Parkinson’s disease. Mol. Neurodegener. 2010;5:42. doi: 10.1186/1750-1326-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudal D, Alvarsson A, Bjorklund A, Svenningsson P. Depressive-like phenotype induced by AAV-mediated overexpression of human alpha-synuclein in midbrain dopaminergic neurons. Exp. Neurol. 2015;273:243–252. doi: 10.1016/j.expneurol.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Chesselet MF. In vivo alpha-synuclein overexpression in rodents: a useful model of Parkinson’s disease? Exp. Neurol. 2008;209:22–27. doi: 10.1016/j.expneurol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesselet MF, Fleming S, Mortazavi F, Meurers B. Strengths and limitations of genetic mouse models of Parkinson’s disease. Parkinsonism Relat. Disord. 2008;14(Suppl 2):S84–S87. doi: 10.1016/j.parkreldis.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Kordower JH. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: is this the target for Parkinson’s disease? Neurobiol. Dis. 2007;25:134–149. doi: 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Chung CY, Koprich JB, Siddiqi H, Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J. Neurosci. 2009;29:3365–3373. doi: 10.1523/JNEUROSCI.5427-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Khurana V, Auluck PK, et al. Identification and rescue of alpha-synuclein toxicity in Parkinson patient-derived neurons. Science. 2013;342:983–987. doi: 10.1126/science.1245296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher JP, Volpicelli-Daley LA, Blackburn JP, Moehle MS, West AB. Abrogation of alpha-synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proc. Natl Acad. Sci. USA. 2014;111:9289–9294. doi: 10.1073/pnas.1403215111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher JP, Abdelmotilib HA, Hu X, et al. Leucine-rich Repeat Kinase 2 (LRRK2) Pharmacological Inhibition Abates alpha-synuclein Gene-induced Neurodegeneration. J. Biol. Chem. 2015;290:19433–19444. doi: 10.1074/jbc.M115.660001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel G, Musso A, Tsika E, Fiser A, Glauser L, Pletnikova O, Schneider BL, Moore DJ. alpha-synuclein-induced dopaminergic neurodegeneration in a rat model of Parkinson’s disease occurs independent of ATP13A2 (PARK9) Neurobiol. Dis. 2015;73:229–243. doi: 10.1016/j.nbd.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Davies SE, Hallett PJ, Moens T, et al. Enhanced ubiquitin-dependent degradation by Nedd4 protects against alpha-synuclein accumulation and toxicity in animal models of Parkinson’s disease. Neurobiol. Dis. 2014;64:79–87. doi: 10.1016/j.nbd.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decressac M, Ulusoy A, Mattsson B, Georgievska B, Romero-Ramos M, Kirik D, Bjorklund A. GDNF fails to exert neuroprotection in a rat alpha-synuclein model of Parkinson’s disease. Brain. 2011;134:2302–2311. doi: 10.1093/brain/awr149. [DOI] [PubMed] [Google Scholar]
- Decressac M, Kadkhodaei B, Mattsson B, Laguna A, Perlmann T, Bjorklund A. alpha-synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Sci. Transl. Med. 2012a;4:163ra156. doi: 10.1126/scitranslmed.3004676. [DOI] [PubMed] [Google Scholar]
- Decressac M, Mattsson B, Bjorklund A. Comparison of the behavioural and histological characteristics of the 6-OHDA and alpha-synuclein rat models of Parkinson’s disease. Exp. Neurol. 2012b;235:306–315. doi: 10.1016/j.expneurol.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Decressac M, Mattsson B, Lundblad M, Weikop P, Bjorklund A. Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of alpha-synuclein in midbrain dopamine neurons. Neurobiol. Dis. 2012c;45:939–953. doi: 10.1016/j.nbd.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Bjorklund A. TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein toxicity. Proc. Natl Acad. Sci. USA. 2013;110:E1817–E1826. doi: 10.1073/pnas.1305623110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer U, Newman AJ, Soldner F, et al. Parkinson-causing alpha-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation. Nat. Commun. 2015a;6:7314. doi: 10.1038/ncomms8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer U, Selkoe D, Bartels T. New insights into cellular alpha-synuclein homeostasis in health and disease. Curr. Opin. Neurobiol. 2015b;36:15–22. doi: 10.1016/j.conb.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Ruan D, Crystal H, Mark MH, Davies P, Kress Y, Yen SH. Hippocampal degeneration differentiates diffuse Lewy body disease (DLBD) from Alzheimer’s disease: light and electron microscopic immunocytochemistry of CA2–3 neurites specific to DLBD. Neurology. 1991;41:1402–1409. doi: 10.1212/wnl.41.9.1402. [DOI] [PubMed] [Google Scholar]
- Dryanovski DI, Guzman JN, Xie Z, Galteri DJ, Volpicelli-Daley LA, Lee VM, Miller RJ, Schumacker PT, Surmeier DJ. Calcium entry and alpha-synuclein inclusions elevate dendritic mitochondrial oxidant stress in dopaminergic neurons. J. Neurosci. 2013;33:10154–10164. doi: 10.1523/JNEUROSCI.5311-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda JE, Lee VM, Trojanowski JQ. Neuropathology of synuclein aggregates. J. Neurosci. Res. 2000;61:121–127. doi: 10.1002/1097-4547(20000715)61:2<121::AID-JNR1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Edwards TL, Scott WK, Almonte C, et al. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann. Hum. Genet. 2010;74:97–109. doi: 10.1111/j.1469-1809.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer DL, Gombash SE, Kemp CJ, Manfredsson FP, Polinski NK, Duffy MF, Sortwell CE. Viral Vector-Based Modeling of Neurodegenerative Disorders: Parkinson’s Disease. Methods Mol. Biol. 2016;1382:367–382. doi: 10.1007/978-1-4939-3271-9_26. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. alpha-synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- Gaugler MN, Genc O, Bobela W, et al. Nigrostriatal overabundance of alpha-synuclein leads to decreased vesicle density and deficits in dopamine release that correlate with reduced motor activity. Acta Neuropathol. 2012;123:653–669. doi: 10.1007/s00401-012-0963-y. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Davies SW. Filamentous nerve cell inclusions in neurodegenerative diseases. Curr. Opin. Neurobiol. 1998;8:619–632. doi: 10.1016/s0959-4388(98)80090-1. [DOI] [PubMed] [Google Scholar]
- Gombash SE, Manfredsson FP, Kemp CJ, et al. Morphological and behavioral impact of AAV2/5-mediated overexpression of human wildtype alpha-synuclein in the rat nigrostriatal system. PLoS ONE. 2013;8:e81426. doi: 10.1371/journal.pone.0081426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk OS, Li S, Sullivan LF, Chen W, Kondrikova G, Manfredsson FP, Mandel RJ, Muzyczka N. The phosphorylation state of Ser-129 in human alpha-synuclein determines neurodegeneration in a rat model of Parkinson disease. Proc. Natl Acad. Sci. USA. 2008;105:763–768. doi: 10.1073/pnas.0711053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk OS, Li S, Nguyen FN, et al. alpha-synuclein expression in rat substantia nigra suppresses phospholipase D2 toxicity and nigral neurodegeneration. Mol. Ther. 2010;18:1758–1768. doi: 10.1038/mt.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Jewett M, Landeck N, Nilsson N, Schagerlof U, Leanza G, Kirik D. Characterization of cognitive deficits in rats overexpressing human alpha-synuclein in the ventral tegmental area and medial septum using recombinant adeno-associated viral vectors. PLoS ONE. 2013;8:e64844. doi: 10.1371/journal.pone.0064844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza TH, Zabetian CP, Tenesa A, et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat. Genet. 2010;42:781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Cao S, Rowse AL, et al. MHCII is required for alpha-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J. Neurosci. 2013;33:9592–9600. doi: 10.1523/JNEUROSCI.5610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes BB, DeVos SL, Kfoury N, et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl Acad. Sci. USA. 2013;110:E3138–E3147. doi: 10.1073/pnas.1301440110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten M, Klein C. The many faces of alpha-synuclein mutations. Mov. Disord. 2013;28:697–701. doi: 10.1002/mds.25499. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, Mandel RJ, Bjorklund A. Parkinsonlike neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J. Neurosci. 2002;22:2780–2791. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Klein RL, King MA, Hamby ME, Meyer EM. Dopaminergic cell loss induced by human A30P alpha-synuclein gene transfer to the rat substantia nigra. Hum. Gene Ther. 2002;13:605–612. doi: 10.1089/10430340252837206. [DOI] [PubMed] [Google Scholar]
- Koprich JB, Johnston TH, Reyes MG, Sun X, Brotchie JM. Expression of human A53T alpha-synuclein in the rat substantia nigra using a novel AAV1/2 vector produces a rapidly evolving pathology with protein aggregation, dystrophic neurite architecture and nigrostriatal degeneration with potential to model the pathology of Parkinson’s disease. Mol. Neurodegener. 2010;5:43. doi: 10.1186/1750-1326-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprich JB, Johnston TH, Huot P, Reyes MG, Espinosa M, Brotchie JM. Progressive neurodegeneration or endogenous compensation in an animal model of Parkinson’s disease produced by decreasing doses of alpha-synuclein. PLoS ONE. 2011;6:e17698. doi: 10.1371/journal.pone.0017698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain. 2013;136:2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer ML, Schulz-Schaeffer WJ. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J. Neurosci. 2007;27:1405–1410. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kuzuhara S, Mori H, Izumiyama N, Yoshimura M, Ihara Y. Lewy bodies are ubiquitinated. A light and electron microscopic immunocytochemical study. Acta Neuropathol. 1988;75:345–353. doi: 10.1007/BF00687787. [DOI] [PubMed] [Google Scholar]
- Lam HA, Wu N, Cely I, et al. Elevated tonic extracellular dopamine concentration and altered dopamine modulation of synaptic activity precede dopamine loss in the striatum of mice overexpressing human alpha-synuclein. J. Neurosci. Res. 2011;89:1091–1102. doi: 10.1002/jnr.22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TB, Standaert DG. Parkinson’s disease, primates, and gene therapy: vive la difference? Mov. Disord. 2011;26:2–3. doi: 10.1002/mds.23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Bianco C, Schneider BL, Bauer M, Sajadi A, Brice A, Iwatsubo T, Aebischer P. Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an alpha-synuclein rat model of Parkinson’s disease. Proc. Natl Acad. Sci. USA. 2004;101:17510–17515. doi: 10.1073/pnas.0405313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad M, Decressac M, Mattsson B, Bjorklund A. Impaired neurotransmission caused by overexpression of alpha-synuclein in nigral dopamine neurons. Proc. Natl Acad. Sci. USA. 2012;109:3213–3219. doi: 10.1073/pnas.1200575109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magen I, Chesselet MF. Genetic mouse models of Parkinson’s disease The state of the art. Prog. Brain Res. 2010;184:53–87. doi: 10.1016/S0079-6123(10)84004-X. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, Bartus RT, Siffert J, et al. Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DM, Hasegawa M. Prion-like spreading of pathological alpha-synuclein in brain. Brain. 2013;136:1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Suzukake M, Nonaka T, Hosokawa M, Kubo M, Shimozawa A, Akiyama H, Hasegawa M. Pathological alpha-synuclein propagates through neural networks. Acta Neuropathol. Commun. 2014;2:88. doi: 10.1186/s40478-014-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland NR, Fan Z, Xu K, Schwarzschild MA, Feany MB, Hyman BT, McLean PJ. alpha-synuclein S129 phosphorylation mutants do not alter nigrostriatal toxicity in a rat model of Parkinson disease. J. Neuropathol. Exp. Neurol. 2009a;68:515–524. doi: 10.1097/NEN.0b013e3181a24b53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland NR, Lee JS, Hyman BT, McLean PJ. Comparison of transduction efficiency of recombinant AAV serotypes 1, 2, 5, and 8 in the rat nigrostriatal system. J. Neurochem. 2009b;109:838–845. doi: 10.1111/j.1471-4159.2009.06010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls MA, Pankratz N, Lill CM, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 2014;46:989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, Bartus RT, Volpicelli-Daley LA, Kordower JH. Trophic factors for Parkinson’s disease: to live or let die. Mov. Disord. 2015;30:1715–1724. doi: 10.1002/mds.26426. [DOI] [PubMed] [Google Scholar]
- Oliveras-Salva M, Van der Perren A, Casadei N, Stroobants S, Nuber S, D’Hooge R, Van den Haute C, Baekelandt V. rAAV2/7 vector-mediated overexpression of alpha-synuclein in mouse substantia nigra induces protein aggregation and progressive dose-dependent neurodegeneration. Mol. Neurodegener. 2013;8:44. doi: 10.1186/1750-1326-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterberg VR, Spinelli KJ, Weston LJ, Luk KC, Woltjer RL, Unni VK. Progressive aggregation of alpha-synuclein and selective degeneration of lewy inclusion-bearing neurons in a mouse model of parkinsonism. Cell Rep. 2015;10:1252–1260. doi: 10.1016/j.celrep.2015.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratz N, Wilk JB, Latourelle JC, et al. Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum. Genet. 2009;124:593–605. doi: 10.1007/s00439-008-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumier KL, Luk KC, Manfredsson FP, et al. Intrastriatal injection of pre-formed mouse alpha-synuclein fibrils into rats triggers alpha-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiol. Dis. 2015;82:185–199. doi: 10.1016/j.nbd.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, Baekelandt V. alpha-synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522:340–344. doi: 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Roberts RF, Wade-Martins R, Alegre-Abarrategui J. Direct visualization of alpha-synuclein oligomers reveals previously undetected pathology in Parkinson’s disease brain. Brain. 2015;138:1642–1657. doi: 10.1093/brain/awv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha EM, Smith GA, Park E, et al. Glucocerebrosidase gene therapy prevents alpha-synucleinopathy of midbrain dopamine neurons. Neurobiol. Dis. 2015;82:495–503. doi: 10.1016/j.nbd.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Schwach G, Ingolic E, et al. Lysosomal pathology associated with alpha-synuclein accumulation in transgenic models using an eGFP fusion protein. J. Neurosci. Res. 2005;80:247–259. doi: 10.1002/jnr.20446. [DOI] [PubMed] [Google Scholar]
- Rodriguez JA, Ivanova MI, Sawaya MR, et al. Structure of the toxic core of alpha-synuclein from invisible crystals. Nature. 2015;525:486–490. doi: 10.1038/nature15368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salganik M, Sergeyev VG, Shinde V, Meyers CA, Gorbatyuk MS, Lin JH, Zolotukhin S, Gorbatyuk OS. The loss of glucose-regulated protein 78 (GRP78) during normal aging or from siRNA knockdown augments human alpha-synuclein (alpha-syn) toxicity to rat nigral neurons. Neurobiol. Aging. 2015;36:2213–2223. doi: 10.1016/j.neurobiolaging.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS ONE. 2010;5:e8784. doi: 10.1371/journal.pone.0008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake W, Nakabayashi Y, Mizuta I, et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat. Genet. 2009;41:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- Settembre C, Zoncu R, Medina DL, et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012;31:1095–1108. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Sanchez J, Schulte C, Bras JM, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat. Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, et al. alpha-synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Song LK, Ma KL, Yuan YH, Mu Z, Song XY, Niu F, Han N, Chen NH. Targeted overexpression of alpha-synuclein by rAAV2/1 vectors induces progressive nigrostriatal degeneration and increases vulnerability to MPTP in mouse. PLoS ONE. 2015;10:e0131281. doi: 10.1371/journal.pone.0131281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl Acad. Sci. USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Martin JL, Klucken J, Outeiro TF, et al. Dopaminergic neuron loss and up-regulation of chaperone protein mRNA induced by targeted over-expression of alpha-synuclein in mouse substantia nigra. J. Neurochem. 2007;100:1449–1457. doi: 10.1111/j.1471-4159.2006.04310.x. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Surmeier DJ. Neuronal vulnerability, pathogenesis, and Parkinson’s disease. Mov. Disord. 2013;28:715–724. doi: 10.1002/mds.25187. [DOI] [PubMed] [Google Scholar]
- Taguchi K, Watanabe Y, Tsujimura A, Tatebe H, Miyata S, Tokuda T, Mizuno T, Tanaka M. Differential expression of alpha-synuclein in hippocampal neurons. PLoS ONE. 2014;9:e89327. doi: 10.1371/journal.pone.0089327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardiff DF, Jui NT, Khurana V, et al. Yeast reveal a “druggable” Rsp5/Nedd4 network that ameliorates alpha-synuclein toxicity in neurons. Science. 2013;342:979–983. doi: 10.1126/science.1245321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger G, Garrido M, Tereshchenko Y, Bahr M, Zweckstetter M, Kugler S. Aggregation of alphaSynuclein promotes progressive in vivo neurotoxicity in adult rat dopaminergic neurons. Acta Neuropathol. 2012;123:671–683. doi: 10.1007/s00401-011-0926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayebi N, Walker J, Stubblefield B, et al. Gaucher disease with parkinsonian manifestations: does glucocerebrosidase deficiency contribute to a vulnerability to parkinsonism? Mol. Genet. Metab. 2003;79:104–109. doi: 10.1016/s1096-7192(03)00071-4. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cao S, McLean PJ, Standaert DG. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J. Neuropathol. Exp. Neurol. 2008;67:1149–1158. doi: 10.1097/NEN.0b013e31818e5e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome AD, Standaert DG, Harms AS. Fractalkine signaling regulates the inflammatory response in an alpha-synuclein model of Parkinson disease. PLoS ONE. 2015;10:e0140566. doi: 10.1371/journal.pone.0140566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran HT, Chung CH, Iba M, Zhang B, Trojanowski JQ, Luk KC, Lee VM. alpha-synuclein immunotherapy blocks uptake and templated propagation of misfolded alpha-synuclein and neurodegeneration. Cell Rep. 2014;7:2054–2065. doi: 10.1016/j.celrep.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulusoy A, Bjorklund T, Hermening S, Kirik D. In vivo gene delivery for development of mammalian models for Parkinson’s disease. Exp. Neurol. 2008;209:89–100. doi: 10.1016/j.expneurol.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Ulusoy A, Decressac M, Kirik D, Bjorklund A. Viral vector-mediated overexpression of alpha-synuclein as a progressive model of Parkinson’s disease. Prog. Brain Res. 2010a;184:89–111. doi: 10.1016/S0079-6123(10)84005-1. [DOI] [PubMed] [Google Scholar]
- Ulusoy A, Decressac M, Kirik D, Bjöorklund A. Viral vector-mediated overexpression of α-synuclein as a progressive model of Parkinson’s disease. Prog Brain Res. 2010b;184:89–111. doi: 10.1016/S0079-6123(10)84005-1. [DOI] [PubMed] [Google Scholar]
- Ulusoy A, Bjorklund T, Buck K, Kirik D. Dysregulated dopamine storage increases the vulnerability to alpha-synuclein in nigral neurons. Neurobiol. Dis. 2012;47:367–377. doi: 10.1016/j.nbd.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Van der Perren A, Van den Haute C, Baekelandt V. Viral vector-based models of Parkinson’s disease. Curr. Top Behav. Neurosci. 2015;22:271–301. doi: 10.1007/7854_2014_310. [DOI] [PubMed] [Google Scholar]
- Van Rompuy AS, Oliveras-Salva M, Van der Perren A, Corti O, Van den Haute C, Baekelandt V. Nigral overexpression of alpha-synuclein in the absence of parkin enhances alpha-synuclein phosphorylation but does not modulate dopaminergic neurodegeneration. Mol. Neurodegener. 2015;10:23. doi: 10.1186/s13024-015-0017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Standaert DG. Invisible killers. Mov. Disord. 2016;31:44. doi: 10.1002/mds.26465. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Gamble KL, Schultheiss CE, Riddle DM, West AB, Lee VM. Formation of alpha-synuclein Lewy neurite-like aggregates in axons impedes the transport of distinct endosomes. Mol. Biol. Cell. 2014a;25:4010–4023. doi: 10.1091/mbc.E14-02-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Lee VM. Addition of exogenous alpha-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous alpha-synuclein to Lewy body and Lewy neurite-like aggregates. Nat. Protoc. 2014b;9:2135–2146. doi: 10.1038/nprot.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AB. Ten years and counting: moving leucine-rich repeat kinase 2 inhibitors to the clinic. Mov. Disord. 2015;30:180–189. doi: 10.1002/mds.26075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc. Natl Acad. Sci. USA. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xilouri M, Brekk OR, Landeck N, Pitychoutis PM, Papasilekas T, Papadopoulou-Daifoti Z, Kirik D, Stefanis L. Boosting chaperone-mediated autophagy in vivo mitigates alpha-synuclein-induced neurodegeneration. Brain. 2013;136:2130–2146. doi: 10.1093/brain/awt131. [DOI] [PubMed] [Google Scholar]
- Yamada M, Iwatsubo T, Mizuno Y, Mochizuki H. Overexpression of alpha-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of alpha-synuclein and activation of caspase-9: resemblance to pathogenetic changes in Parkinson’s disease. J. Neurochem. 2004;91:451–461. doi: 10.1111/j.1471-4159.2004.02728.x. [DOI] [PubMed] [Google Scholar]
- Yamada M, Mizuno Y, Mochizuki H. Parkin gene therapy for alpha-synucleinopathy: a rat model of Parkinson’s disease. Hum. Gene Ther. 2005;16:262–270. doi: 10.1089/hum.2005.16.262. [DOI] [PubMed] [Google Scholar]