Abstract
Objectives
To develop data-driven criteria for clinically inactive disease on and off therapy for juvenile dermatomyositis (JDM).
Methods
The PRINTO database contains 275 patients with active JDM evaluated prospectively up to 24 months. Thirty-eight patients off therapy at 24 months were defined as clinically inactive and included in the reference group. These were compared with a random sample of 76 patients who had active disease at study baseline. Individual measures of muscle strength/endurance, muscle enzymes, physician's and parent's global disease activity/damage evaluations, inactive disease criteria derived from literature, and other ad hoc criteria, were evaluated for sensitivity, specificity, and Cohen's kappa agreement.
Results
The individual measures that best characterised inactive disease (sensitivity and specificity >0.8 and Cohen's K>0.8) were manual muscle testing (MMT)≥78, physician global assessment of muscle activity=0, physician global assessment of overall disease activity (PhyGloVAS) ≤0.2, the Childhood Myositis Assessment Scale (CMAS) ≥48, the Disease Activity Score (DAS) ≤3 and the Myositis Disease Activity Assessment Visual Analogue Scale (MYOACT) ≤0.2. The best combination of variables to classify a patient as being in a status of inactive disease on or off therapy is at least 3 out of 4 of the following criteria: creatine kinase ≤150, CMAS≥48, MMT≥78, and PhyGloVAS≤0.2. After 24 months, 30/31 (96.8%) patients were inactive off therapy and 69/145 (47.6%) were inactive on-therapy.
Conclusion
PRINTO established data-driven criteria, with clearly evidence-based cut off values, to identify JDM patients with clinically inactive disease. These criteria can be used in clinical trials, in research and in clinical practice.
Keywords: juvenile dermatomyositis, core set measures, inactive disease, disease activity, Dermatomyositis, Outcomes research
Juvenile Dermatomyositis (JDM) is a systemic autoimmune vasculopathy that primarily involves skin and muscles, and is characterised by proximal muscle weakness and typical rashes, including Gottron's papules and heliotrope rash. While mortality has been greatly reduced to 2-3%,1 morbidity has increased, with 70-80% of the juvenile patients having cumulative damage after a mean of 8 years.2-5
With the advent of new therapies and treatment strategies for JDM,6 inactive disease has become a realistic therapeutic target in clinical practice.3, 5 However, no formal criteria clinically inactive disease for JDM are in place. Differences in previously-used criteria have been noted between studies, and most of the criteria include generic concepts without clearly defined cut-off values or operational definitions.2, 7-10
The aim of this project was to develop data-driven criteria of clinically inactive disease by analysing a large international prospective cohort of JDM. The overall goal was to propose criteria which could have practical applicability in the current clinical practice, research and in future clinical trials.
PATIENTS AND METHODS
Database content
Centres of the Paediatric Rheumatology International Trials Organisation (PRINTO)11 prospectively collected data on clinical, laboratory and therapeutic modalities in consecutive patients who had probable/definite JDM;12, 13 were younger than 18 years; and were in an active phase of their disease, defined as either the need to start corticosteroid therapy or a new immunosuppressive medication or to have a major dose increase. Written or verbal informed consent/assent was obtained as per local requirements.
The database contains the 6 PRINTO JDM core set measures14 assessed longitudinally (0, 6, 12 and 24 months): 1) physician's global assessment of the patient's overall disease activity on a 10-cm visual analogue scale (MD-GLOVAS);15 2) muscle strength/endurance through the Childhood Myositis Assessment Scale (CMAS),16-18 or the Manual Muscle Testing of 8 muscle groups (MMT);19 3) global disease activity assessment through the Disease Activity Score (DAS);20 4) functional ability through the Childhood Health Assessment Questionnaire (C-HAQ);21-24 5) parent's global assessment of the overall patient's well-being on a 10-cm VAS (Par GLOVAS);15, 21, 22 6) health-related quality of life through the physical summary score (PhS) of the Child Health Questionnaire (CHQ).22, 25 Additional measures were the Myositis Disease Activity Assessment (MDAA) which combines the Myositis Disease Activity Assessment Visual Analogue Scale (MYOACT) including the physician evaluation of extra muscular activity (MD-ExtraMuscVAS) and muscle activity (MD-MuscVAS) and the Myositis Intention to Treat Activity Index (MITAX);26 the psychosocial summary score (PsS) of the CHQ;22, 25 serum muscle enzymes: creatine kinase (CPK), lactate dehydrogenase (LDH), aldolase, aspartate aminotransferase (AST), alanine aminotransferase (ALT),27-31 whose results were standardized as previously described;32 and the Myositis Damage Index (MDI).4, 26, 33 Scoring and content of all the instruments have been previously reported.14
Study design and inclusion criteria
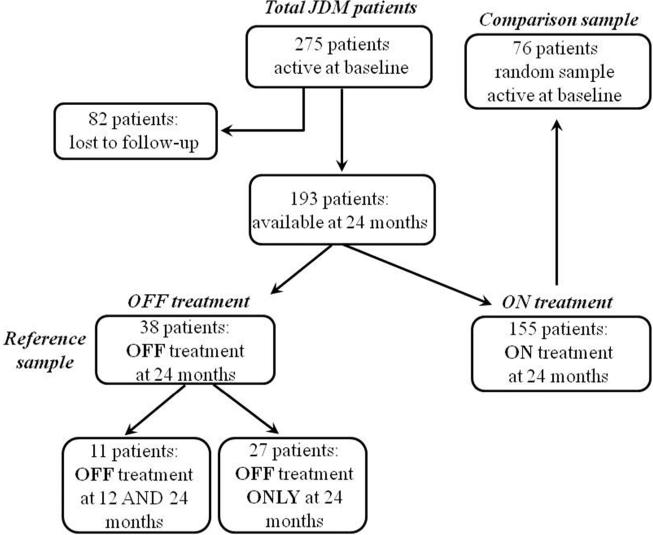
To identify the features that were suitable as candidates criteria for clinically inactive disease, we followed the classification approach. The purpose was to separate patients with inactive disease (reference sample) from patients with active disease (comparison sample), with high sensitivity and specificity. As shown in Figure 1, the reference sample was represented by patients off therapy with an inactive disease status by definition at 24 months; in addition patients were declared stable or improved with respect to the previous visit by the physician and/or the parents. In order to avoid selection biases the comparison sample was represented by the baseline data (patient active by study inclusion criteria) of a computer generated random list of patients who were still on therapy after 24 months of follow-up. Similarly to the work done in juvenile idiopathic arthritis (JIA), we defined clinically inactive disease as a single point in time status with clinically and biologically quiescent disease that can be on/off therapy. When this criteria is met for at least 6 or 12 continuous months, this status is called clinical remission on- or off therapy, respectively.34, 35
Figure 1.
Flow chart of the study patients.
Validation process
Four steps were used for the analysis.
In Step 1 (cut-off selection) the reference sample off therapy at 24 months was described. The hypothesis was that the descriptive values for each variable considered would be representative of inactive disease status and able to discriminate inactive from active patients. Based on the descriptive statistics (mean±standard deviation [SD], minimum, 20th percentile, median, 80th percentile and maximum) of each variable, we then selected candidate cut-offs that could properly describe a patient as clinically inactive off therapy. The strategy was as follows: for those variables where the lowest value correlated with inactive disease (e.g. muscle enzymes), we chose the median, 20th percentile or the minimum values. In contrast, those variables in which higher values correlate with disease inactivity (e.g. CMAS, MMT), the median, 80th percentile or the maximum value was considered to be the best cut-off value. Additional candidate cut-offs were also derived from the literature.2, 7, 8, 10
In Step 2 (accuracy measures), we evaluated the ability (accuracy) of the cut-offs for each variable to discriminate the reference sample from the comparison sample by calculating the sensitivity/specificity, and Cohen's kappa36. The kappa statistic according to Landis & Koch37 was categorised as follows: 0.01-0.2=slight; 0.21-0.4=fair; 0.41-0.6=moderate; 0.61-0.8=substantial; 0.81-1=almost perfect agreement. In this step we expected that only a few, if any, of the active patients in the comparison group would have values consistent with cut-offs selected as representative of the reference sample.
In Step 3 (inactive disease criteria testing) we tested 19 candidate criteria (combination of individual measures and related cut-offs) of inactive disease derived from the literature3, 9, 38-44 and an additional 35 criteria that, based on the results of Step 1 and 2, were deemed clinically reasonable by the Steering Committee of the project (DL, AP, NR, AR, AM, PM, EP), based on the results of Step 1 and 2, by calculating their sensitivity/specificity, and Cohen's kappa36. Moreover in this step we expected that only a few, if any, of the patients in the comparison sample would fit inactive disease criteria, while most, if not all, of the patients in the reference sample would.
Finally, in Step 4 (criteria confirmation), we applied the top criteria selected in Step 3 to both the reference sample off therapy and the remaining patients still on therapy at 24 months (Figure 1). In this analysis, among the patients still on therapy at 24 months, we identified the subgroup of patients who met the criteria of inactive disease on medication, and differentiated them from those patients who did not meet the criteria of inactive disease and who were therefore considered to have still active JDM despite 24 months of therapy. Our hypothesis was that, if our top criteria selected in Step 3 allowed us to correctly identify the group with inactive disease on therapy at 24 months, the individual activity in this group should be quite similar to the reference sample.
Data were entered into an Access XP database and analyzed with Excel XP (Microsoft), XLSTAT 6.1.9 Addinsoft, Statistica 6.0 (StatSoft, Inc), and Stata 7.0 (Stata Corporation).
RESULTS
Demographic characteristics
A total of 275 active JDM patients were retrieved from the PRINTO database (Figure 1); 168 (61%) were females with a median age at disease onset of 7.2 years (interquartile range (IQR) 4.3-10.2), and a median disease duration of 0.6 years (IQR 0.2-2.1).14 There were no differences in baseline demographic, clinical and laboratory characteristics between the 193 (70.2%) patients with 2 years follow-up and the remaining 82 lost to follow-up. These 193 patients were divided into 2 groups: 38 (20%) were off therapy (reference group) and 155 (80%) were still on therapy. From these 155 patients, we randomly extracted 76 patients and used their baseline data (when the patients were active by inclusion criteria) as a comparison sample in a 1:2 ratio. There were no statistically significant differences in baseline demographic, clinical and laboratory characteristics between the reference and the comparison sample.
Step 1. Clinically inactive disease cut-off selection
Table 1 shows the descriptive statistics related to disease activity/damage characteristics of the 38 patients off therapy included in the reference sample. For those variables, where the lowest value correlated with normality, we chose as cut-off the median, the 20th percentile or the minimum (e.g. 0 for C-HAQ) while for those measures in which higher values signified inactivity (e.g. 52 for CMAS) we selected the median, the 80th percentile or the maximum. In addition we also considered literature derived cut-offs.
Table 1.
Descriptive statistics of disease activity measures in the 38 patients (reference sample) off therapy at 24 months after entry into study. Candidate cut-off values for inactive disease are indicated in parenthesis.
| Measures | Mean ± SD | 20th percentile | Median | 80th percentile |
|---|---|---|---|---|
| Muscle strength/activity | ||||
| CMAS (range: 0-52) ↓ | 50.1±6.1 | 50 | 52 | 52 |
| MMT (range: 0–80) ↓ | 78.4±8.7 | 80 | 80 | 80 |
| DAS (range: 0–20) ↑ | 1.7±1.9 | 0 | 1.0 | 3 |
| MYOACT (range: 0-10) ↑ | 0.1±0.1 | 0 | 0 | 0.1 |
| MITAX (range: 0-63) ↑ | 1.2±2.6 | 0 | 0 | 1.0 |
| Muscle enzymes | ||||
| CPK (n.v.: 0-150 u/l) ↑ | 122±221 | 43.5 | 69.4 | 133.2 |
| LDH (n.v.: 50-150 u/l) ↑ | 120±82 | 67.2 | 97.2 | 164 |
| AST/SGOT (n.v.: 0-35 u /l) ↑ | 21.3±9.2 | 14.7 | 21.3 | 28.3 |
| ALT/SGPT (n.v.: 0-35 u/l) ↑ | 14.7±9.4 | 7.0 | 13.1 | 21.6 |
| Aldolase (n.v.: 0-6 u/l) ↑ | 3.7±2.3 | 1.7 | 3.3 | 5.4 |
| Physician's evaluations | ||||
| MD-GLOVAS (0–10-cm scale) ↑ | 0.1±0.2 | 0 | 0 | 0 |
| MD-ExtraMuscVAS (0-10 cm scale) ↑ | 0±0.2 | 0 | 0 | 0 |
| MD-MuscVAS MD-MuscVAS (0-10 cm scale) ↑ | 0±0.1 | 0 | 0 | 0 |
| Parent's evaluations | ||||
| Par GLOVAS (0-10 cm scale) ↑ | 0.3±1 | 0 | 0 | 0.3 |
| PainVAS (0-10 cm scale) ↑ | 0.3±1 | 0 | 0 | 0.2 |
| C-HAQ (range 0–3) ↑ | 0.1±0.4 | 0 | 0 | 0.1 |
| CHQ PhS (nv: 40-60) ↓ | 52.7±4.6 | 52.4 | 53.3 | 54.7 |
| CHQ PsS (nv: 40-60) ↓ | 57.4±5.8 | 54.9 | 57.9 | 62.2 |
| Damage measures | ||||
| Phy Global Damage (0-10 cm scale VAS) ↑ | 0.4±1.5 | 0 | 0 | 0.3 |
| MDI se (0-10 cm scale) ↑ | 0.1±0.2 | 0 | 0 | 0.1 |
| MDI chi (range: 0-35) ↑ | 0.6±0.8 | 0 | 0 | 1.0 |
| MDI ex (range: 0-16) ↑ | 0.3±0.6 | 0 | 0 | 1.0 |
↑ indicates that a higher score for that variable denotes worse disease activity; ↓ indicates that a lower score denotes worse disease activity; VAS=visual analogue scale, u/l=units/litre;
CMAS=Childhood Myositis Assessment Scale; MMT=Manual Muscle Testing DAS=Disease Activity Score; MYOACT=Myositis Disease Activity Assessment; MITAX=Myositis Intent-to-Treat Activity Index A-E version; CPK= Creatine kinase; LDH=Lactate dehydrogenase; AST=Aspartate aminotransferase; ALT=Alanine aminotransferase; ALD=Aldolase; n.v.=normal values; MD-GLOVAS=Physician's global assessment of patient's overall disease activity; MD-ExtraMuscVAS= Physician's global assessment of extra-skeletal disease activity; MD-MuscVAS MD-MuscVAS=Physician's global assessment of muscle activity; Par GloVAS=Parent's global assessment of patient's overall well-being; PainVAS=Parent's global assessment of child's pain; C-HAQ= Childhood Health Assessment Questionnaire; CHQ PhS=CHQ physical summary score; CHQ PsS=CHQ psychosocial summary score; MDI se severity of damage score; MDI chi=extent of damage score in children; MDI ex=extended damage score.
Step 2. Inactive disease cut-offs values accuracy
Table 2 reports the frequency of patients observed with a particular cut-off as well as the results of accuracy measurements for the reference and the comparison samples. The frequency of patients fitting a particular cut-off was very high for the reference sample and conversely very low for the comparison sample. Exceptions were represented by a low frequency of patients with active JDM but normal serum muscle enzymes, no pain and normal quality of life (CHQ PhS and PsS).
Table 2.
The frequency of patients who met the selected cut-off value in the reference group (off therapy at 24 months) versus the comparison sample (baseline data of a random sample of patients selected from all patients on therapy at 24 months). The cut-off points were selected at step 2 (see Table 1) or derived from literature. The last 3 columns report accuracy measures (sensitivity, specificity and Cohen's k).
| Measures | Reference sample Off therapy patients N=38 | Comparison sample On therapy N=76 | Sensitivity | Specificity | Cohen's k |
|---|---|---|---|---|---|
| Muscle strength/activity | |||||
| CMAS=522 | 23/36 | 1/75 | 0.64 | 0.99 | 0.68 |
| CMAS ≥ 487 | 32/36 | 3/75 | 0.89 | 0.96 | 0.86 |
| MMT=802 | 32/36 | 0/76 | 0.89 | 1.00 | 0.92 |
| MMT ≥ 787 | 34/36 | 2/76 | 0.94 | 0.97 | 0.92 |
| DAS=02 | 15/37 | 0/76 | 0.41 | 1.00 | 0.48 |
| DAS ≤ 38 | 30/37 | 2/76 | 0.81 | 0.97 | 0.81 |
| MYOACT=02 | 20/33 | 0/75 | 0.61 | 1.00 | 0.68 |
| MYOACT ≤ 0.22 | 30/33 | 3/75 | 0.91 | 0.96 | 0.87 |
| MYTAX=02 | 19/31 | 0/75 | 0.61 | 1.00 | 0.69 |
| Muscle enzymes | |||||
| CPK ≤ 150 | 24/28 | 25/75 | 0.86 | 0.67 | 0.42 |
| LDH ≤ 150 | 23/31 | 9/73 | 0.74 | 0.88 | 0.61 |
| CPK ≤ 150 or LDH ≤ 150 | 23/32 | 7/75 | 0.72 | 0.91 | 0.64 |
| Aldolase ≤ 6.0 | 13/14 | 7/42 | 0.93 | 0.83 | 0.67 |
| AST ≤ 35 | 29/31 | 17/67 | 0.94 | 0.75 | 0.60 |
| ALT ≤ 35 | 31/31 | 38/73 | 1.00 | 0.48 | 0.35 |
| AST ≤ 35 or ALT ≤ 35 | 29/31 | 21/74 | 0.94 | 0.72 | 0.55 |
| Physician's evaluations | |||||
| MD-GLOVAS=02 | 27/33 | 1/76 | 0.82 | 0.99 | 0.84 |
| MD-GLOVAS ≤ 0.22 | 28/33 | 1/76 | 0.85 | 0.99 | 0.86 |
| MD-ExtraMuscVAS=0 | 29/33 | 13/76 | 0.88 | 0.83 | 0.66 |
| MD-MuscVAS=0 | 30/33 | 2/76 | 0.91 | 0.97 | 0.89 |
| Parent's evaluations | |||||
| Par GloVAS=02 | 20/29 | 4/75 | 0.69 | 0.95 | 0.67 |
| Par GloVAS ≤ 0.22 | 23/29 | 5/75 | 0.79 | 0.93 | 0.73 |
| Pain VAS=02 | 22/29 | 15/75 | 0.76 | 0.80 | 0.52 |
| Pain VAS ≤ 0.22 | 24/29 | 15/75 | 0.83 | 0.80 | 0.57 |
| C-HAQ=02, 10 | 23/29 | 6/76 | 0.79 | 0.92 | 0.71 |
| CHQ PhS ≥ 402 | 26/27 | 21/65 | 0.96 | 0.68 | 0.53 |
| CHQ PsS ≥ 402 | 27/27 | 46/65 | 1.00 | 0.29 | 0.20 |
| Damage measures | |||||
| Global Damage=02 | 24/35 | 22/73 | 0.69 | 0.70 | 0.36 |
| MDI se=02 | 20/35 | 20/74 | 0.57 | 0.73 | 0.29 |
| MDI chi=02 | 18/34 | 26/75 | 0.53 | 0.65 | 0.17 |
| MDI ex=02 | 25/34 | 43/75 | 0.74 | 0.43 | 0.13 |
VAS=visual analogue scale, u/l=units/litre;
CMAS=Childhood Myositis Assessment Scale; MMT=Manual Muscle Testing DAS=Disease Activity Score; MYOACT=Myositis Disease Activity Assessment; MITAX=Myositis Intent-to-Treat Activity Index A-E version; CPK= Creatine kinase; LDH=Lactate dehydrogenase; AST=Aspartate aminotransferase; ALT=Alanine aminotransferase; ALD=Aldolase; =MD-GLOVAS= Physician's global assessment of patient's overall disease activity; MD-ExtraMuscVAS= Physician's global assessment of extra-skeletal disease activity; MD-MuscVAS MD-MuscVAS=Physician's global assessment of muscle activity; Par GloVAS=Parent's global assessment of patient's overall well-being; PainVAS=Parent's global assessment of child's pain; C-HAQ= Childhood Health Assessment Questionnaire; CHQ PhS=CHQ physical summary score; CHQ PsS=CHQ psychosocial summary score; MDI se severity of damage score; MDI chi=extent of damage score in children; MDI ex=extended damage score.
For muscle strength/activity measures those variables with an almost perfect agreement, included MMT=802 or ≥ 78,7 CMAS≥487, MYOACT≤0.22 and DAS≤ 3 in decreasing order of k.8 Similarly the MD-GLOVAS≤0.2 or=0 and the physician global assessment of muscle activity (MD-MuscVAS)=0, showed a Cohen's k>0.8.
A substantial agreement was observed for the C-HAQ=0, MYOACT=0, the Par GLOVAS (≤ 0.2 or =0), the MD-ExtraMuscVAS, and for some muscle enzyme levels (normal aldolase, LDH, or combination of normal CPK and LDH). For all the other variables, the cut-off values analysed held an agreement which was slight to moderate. Of note, when the cut-off was set to the lowest (e.g. DAS=0) or maximum values (e.g. CMAS=52) the sensitivity and accuracy measurements were lower.
Step 3. Inactive disease criteria testing
Table 3 reports the frequency of patients who fit the top inactive disease criteria tested and their accuracy measurements. The top criteria (C1-C6) required the presence of 3/4 measures fitting the related cut-offs. They all required the presence of normal muscle enzymes (CPK, aldolase or LDH), as well as muscle strength (MMT) or endurance (CMAS), and a low MD-GLOVAS. Since inactive disease criteria from the literature consistently asked for normal muscle enzymes, the Steering Committee retained them despite their non-optimal performance in Step 2. For example the inactive criterion 1 requires the combination of at least 3/4 measures being normal according to specific cut-offs: CPK≤150 or CMAS≥48 or MMT≥78 or MD-GLOVAS ≤0.2. Criteria 1, 2, 4 and 6 are similar with the only difference represented by the muscle enzymes considered (CPK or aldolase or LDH, or the combination of LDH/ALT). Criterion 3 required the use of the MYOACT≤0.2 instead of the MD-GLOVAS, while criterion 5 required an MMT=80 instead of an MMT≥78. The 19 criteria derived from the literature consistently showed lower accuracy measures (fair to moderate agreement).
Table 3.
Frequency of patients who fit the criteria for inactive disease in the reference sample (off therapy at 24 months) versus the comparison sample (baseline data of a random sample of patients selected from all patients on therapy at 24 months). Criteria were formulated from step 2 (Tables 1 and 2) or derived from literature3, 9, 38-44. The last 3 columns report accuracy measures (sensitivity, specificity and Cohen's k). Definitions are sorted by decreasing values of Cohen's k and numbered as criteria 1 (C1),C2 etc.
| Inactive disease criteria | Reference sample Off therapy patients N=38 | Comparison sample Active patients N=76 | Sensitivity | Specificity | Cohen's k |
|---|---|---|---|---|---|
| Top criteria derived by the Study Steering Committee | |||||
| C1. 3 of 4: (CPK≤150; CMAS≥48; MMT≥78; MD-GLOVAS ≤0.2) | 30/31 | 0/76 | 0.97 | 1.0 | 0.98 |
| C2. 3 of 4: (Aldolase≤6; CMAS≥48; MMT≥78; MD-GLOVAS ≤0.2) | 29/30 | 0/75 | 0.97 | 1.0 | 0.98 |
| C3. 3 of 4: (CPK≤150; CMAS≥48; MMT≥78; MYOACT ≤0.2) | 31/33 | 0/76 | 0.94 | 1.0 | 0.96 |
| C4. 3 of 4: (LDH≤150; CMAS≥48; MMT≥78; MD-GLOVAS ≤0.2) | 31/33 | 0/76 | 0.94 | 1.0 | 0.96 |
| C5. 3 of 4: (CPK≤150; CMAS≥48; MMT=80; MD-GLOVAS ≤0.2) | 30/32 | 0/76 | 0.94 | 1.0 | 0.95 |
| C6. 3 of 4: (LDH≤150 AND ALT≤35; CMAS≥48; MMT≥78; MD-GLOVAS ≤0.2) | 30/32 | 0/75 | 0.94 | 1.0 | 0.95 |
| Top criteria derived from literature | |||||
| C7. MMT=80 AND (CPK, LDH, AST, ALT)=normal AND MITAX=03 | 18/31 | 0/76 | 0.58 | 1.0 | 0.66 |
| C8. MMT=80 AND CPK=Normal AND MITAX=0 38 | 14/29 | 0/76 | 0.48 | 1.0 | 0.57 |
| C9. MD-GLOVAS =0 AND MMT=80 AND (CPK, LDH, AST, ALT)=normal AND MITAX=0 42 | 11/30 | 0/76 | 0.37 | 1.0 | 0.45 |
| C10. MMT=80 AND Enzymes normal AND MITAX=043 | 11/31 | 0/76 | 0.35 | 1.0 | 0.44 |
| C11. MITAX=0 AND MMT=80 AND CMAS=52 AND Enzymes=normal9 | 9/32 | 0/76 | 0.28 | 1.0 | 0.36 |
| C12. MMT=80 AND (CPH or LDH normal) AND Skin DAS=041 | 9/34 | 0/76 | 0.26 | 1.0 | 0.33 |
| C13. Skin DAS=0 AND MMT=80 AND Enzymes=normal44 | 9/34 | 0/76 | 0.26 | 1.0 | 0.33 |
| C14. Skin DAS=0 AND MMT=80 AND Enzymes normal AND MITAX=039, 40 | 7/34 | 0/76 | 0.21 | 1.0 | 0.26 |
C=criteria; CPK=creatine kinase; CMAS=Childhood Myositis Assessment Scale; MMT=Manual Muscle Testing; MD-GLOVAS=Physician's global assessment of patient's overall disease activity; MYOACT=Myositis Disease Activity Assessment; LDH=Lactate dehydrogenase; ALT=Alanine aminotransferase; AST=Aspartate aminotransferase; MITAX=Myositis Intent-to-Treat Activity Index A-E version;
None of the patients in the comparison sample group fitted any of the definitions of inactive disease reported in Table 3. Of note, when all criteria were applied to the subgroup of 11 patients who were off therapy at 12 and 24 months (and therefore in clinical remission off therapy for 12 months) their accuracy measures improved with a sensitivity/specificity/k=1.
For the subsequent analysis, since the top 2 criteria (C1 and 2) had overlapping accuracy performance, with the only difference being that v 1 requires normal CPK and criterion 2 normal aldolase, it was decided to choose criterion 1 since CPK is more universally used. In this dataset, for example, the frequency of patients with CPK available was 269/275 patients (97.8%) versus 145/275 patients (52.7%) with aldolase.
Step 4 Inactive disease criteria confirmation
When we applied the top inactive disease criterion 1 (C1) (3/4 measures among CPK≤150 or CMAS≥48 or MMT≥78 or MD-GLOVAS ≤0.2), there were 30/31 (96.8%) who were inactive off therapy at month 24 (reference sample) and 69/145 (47.6%) who were inactive on-therapy at month 24. Table 4 shows the comparison of disease activity/damage measures in these 2 groups of patients who met the top inactive disease criteria C1 at month 24. There were no statistically significant differences in the measures examined with the exception of MMT, MD-GLOVAS, and CHQ PsS which were slightly worse in the group of patients still on-therapy at month 24.
Table 4.
Comparison of patients who met the top inactive criteria (3/4 measure among CPK≤150 or CMAS≥48 or MMT≥78 or MD-GLOVAS ≤0.2) between the group of patients off therapy at 24 months (reference sample) and the group of patients on treatment at 24 months.
| Patients who met the best inactive disease criteria | Reference sample OFF therapy Evaluable: N=31/38 (inactive=30/31; 96.8%) Median (1st- 3rd q) |
ON treatment Evaluable: N=145/155 (inactive=69/145; 47.6%) Median (1st- 3rd q) |
P° |
|---|---|---|---|
| Muscle strength/activity | |||
| CMAS (range 0-52) ↓ | 52 (52-52) | 52 (51-52) | 0.18 |
| MMT (range 0–80) ↓ | 80 (80-80) | 80 (79-80) | 0.016 |
| DAS (range 0–20) ↑ | 1 (0-3) | 1 (0-4.5) | 0.26 |
| MYOACT (range 0-10) ↑ | 0 (0-0.02) | 0 (0-0.07) | 0.28 |
| MITAX (range 0-63) ↑ | 0 (0-1) | 0 (0-1) | 0.13 |
| Muscle enzymes | |||
| CPK (n.v.: 0-150 u/l) ↑ | 71.9 (49.7-130.9) | 72.1 (41.8-121.7) | 0.67 |
| LDH (n.v.: 50-150 u/l) ↑ | 115.4 (77.3-163.7) | 118.4 (77.6-153) | 0.83 |
| Aldolase n.v.: (0-6 u/l) ↑ | 3.4 (2.6-5.3) | 3.6 (2.6-5.2) | 0.99 |
| AST (n.v.: 0-35 u /l) ↑ | 21.7 (14.7-28.3) | 18.5 (13.1-24.9) | 0.22 |
| ALT (n.v.: 0-35 u/l) ↑ | 13.1 (7.0-21.6) | 11.7 (7-17.5) | 0.41 |
| Physician's evaluation | |||
| MD-GLOVAS (0–10-cm scale) ↑ | 0 (0-0) | 0 (0-0.2) | 0.006 |
| MD-ExtraMuscVAS (0-10 cm scale) ↑ | 0 (0-0) | 0 (0-0) | 0.17 |
| MD-MuscVAS (0-10 cm scale) ↑ | 0 (0-0) | 0 (0-0) | 0.11 |
| Parent's evaluations | |||
| Par GloVAS (0-10 cm scale) ↑ | 0 (0-0) | 0 (0-0.2) | 0.11 |
| PainVAS (0-10 cm scale) ↑ | 0 (0-0) | 0 (0-0.1) | 0.41 |
| C-HAQ (range 0-3) ↑ | 0 (0-0.06) | 0 (0-0.12) | 0.82 |
| CHQ PhS (normal values: 40-60) ↓ | 53.2 (52.6-54.3) | 53.2 (51.7-55.4) | 0.61 |
| CHQ PsS (normal values: 40-60) ↓ | 58.7 (56.3-62.2) | 52.8 (47.7-58.1) | 0.001 |
| Damage measures | |||
| Global Damage (0-10 cm scale) ↑ | 0 (0-0.1) | 0 (0-0.3) | 0.13 |
| MDI se (0-10 cm scale) ↑ | 0 (0-0.05) | 0 (0-0.07) | 0.55 |
| MDI chi (range 0–35) ↑ | 0 (0-1) | 0 (0-1) | 0.51 |
| MDI ex (range 0 -16) ↑ | 0 (0-0.5) | 0 (0-0) | 0.80 |
Evaluable = number of patients in the group who have available measurements needed for calculating the criteria. P°: values refer to the Mann-Whitney U test.
↑ indicates that a higher score for that variable denotes worse disease activity; ↓ indicates that a lower score denotes worse disease activity; VAS=visual analogue scale, u/l=units/litre;
CMAS=Childhood Myositis Assessment Scale; MMT=Manual Muscle Testing DAS=Disease Activity Score; MYOACT=Myositis Disease Activity Assessment; MITAX=Myositis Intent-to-Treat Activity Index A-E version; CPK= Creatine kinase; LDH=Lactate dehydrogenase; AST=Aspartate aminotransferase; ALT=Alanine aminotransferase; ALD=Aldolase; n.v.=normal values; MD-GLOVAS=Physician's global assessment of patient's overall disease activity; MD-ExtraMuscVAS=Physician's global assessment of extra-skeletal disease activity; MD-MuscVAS MD-MuscVAS=Physician's global assessment of muscle activity; Par GloVAS=Parent's global assessment of patient's overall well-being; PainVAS=Parent's global assessment of child's pain; C-HAQ=Childhood Health Assessment Questionnaire; CHQ PhS=CHQ physical summary score; CHQ PsS=CHQ psychosocial summary score; MDI se severity of damage score; MDI chi=extent of damage score in children; MDI ex=extended damage sc.
DISCUSSION
Using a data-driven based approach, PRINTO identified a definition to classify JDM patients as clinically inactive on/off therapy if at least 3 out of 4 measures meet the proposed inactivity cut-offs: CPK≤150, CMAS≥48, MMT≥78, and PhyGloVAS≤0.2.
The PRINTO database contains JDM patients with high disease activity at baseline, short disease duration, and followed for 24 months.6, 14, 45 This time frame was chosen with the rational to have a sufficiently long follow-up to induce inactivity. Indeed after 24 months we identified that 20% of the patients were off therapy. When the disease activity status of this reference sample was analysed, we found that measures related to muscle strength/endurance, disease activity, muscle enzymes and patient's reported outcome had values that were very close to normal. For example the mean MD-GLOVAS was 0.1±0.2 on a scale of 0-10 cm. The fact that this measure was not exactly equal to 0 can be interpreted either as the physician's aversion to consider extremely low level of disease activity (close but not overlapping with 0) in order to discontinue therapies, or as the inherent measurement error of the VAS scale in which a line close to 0 means in reality exactly 0, as observed also in other diseases such as JIA.46 In future studies this measurement error could be avoided with the use of more precise tools such as the 21 circle VAS.47 Similarly the mean values for muscle strength/endurance were close but not overlapping with the extreme range of the scales, while for muscle enzymes all descriptive measures where below the upper range of normal values. The myositis literature has numerous definitions of inactive disease which, however, in many cases are not evidence-based or lack the operational cut-off value in order for a particular variable to be called normal.3, 9, 38-44 In order to overcome these problems, our rational was to select the best cut-off values for each variable to be able to differentiate active from inactive patients. The variables which showed the best accuracy (Cohen's Kappa 0.8-1.0) were muscle strength/endurance and some disease activity tools and the MD-GLOVAS, while a substantial agreement (Cohen's Kappa 0.61-0.8) was observed for parent's reported outcome and muscle enzymes. Of note, most of these measures are part of the PRINTO JDM14 and IMACS48 core set measures for the evaluation of response to therapy. This demonstrates that the core set measures are probably the key measures to evaluate the disease status of the patient for each phase of the disease.
One of the challenges in JDM, as well as in other rheumatic diseases, is the lack of gold standard for the evaluation of the disease status of a patient. By selecting variables for inactivity derived from literature3, 9, 38-44 and those based on our Step 2 process, our analytical process allowed us to develop multiple combinations of inactive disease criteria and rank these in the order of their best accuracy to characterise an inactive patient. The results showed that all the definitions derived from the literature had only a fair to moderate agreement. The best performing definition of inactive disease, with an almost perfect agreement, was related to a definition set up by the steering committee. As in the literature, we elected to retain muscle enzymes in the definition, despite their individual accuracy in terms of agreement was substantial but not almost perfect. The top definitions selected all have some common characteristics, such as a minimum number of individual measures to be observed as normal (in general 3 out of 4). All the top combinations require the presence of normal muscle enzyme(s), muscle strength/endurance or a MD-GLOVAS close to normal. The similarity of the top definitions, and the partial overlap with the variables that are currently used to evaluate response to therapy can be interpreted as a measure of convergent validity of the process.14, 48 Theoretically a patient could fit the definition of inactive disease with abnormal muscle enzymes, abnormal muscle strength/endurance, or a MD-GLOVAS close to normal. However, it is unlikely that a physician will give a score ≤0.2 in a child with abnormal muscle enzymes or abnormal muscle strength/endurance unless it was due to damage. It has been reported that patients with longstanding disease and substantial damage cannot achieve normal MMT or CMAS score,9 but patients in the PRINTO dataset had short disease duration and very minimal damage at baseline.
In the final analytical step we applied the top definition to the remaining part of the sample still on therapy after 24 months of therapy, and we found that almost 50% of patients fit the definition of inactive disease on therapy, with only minimal clinical and laboratory differences between the group inactive off and on therapy. Similar to other serious paediatric rheumatic diseases, medication reduction can be a challenge in JDM, with physicians hesitant to discontinue medications even when disease is inactive. In comparison to adult myositis, paediatric patients tend to receive corticosteroids and immunosuppressive medications for longer periods with 80% of JDM patients still receiving medications more than 2 years after the diagnosis, despite 50% of the patients meeting the proposed inactive disease criteria.6, 14
Limitations of our study included that the dataset did not have a physician rating of inactive disease, it could not evaluate the predictive ability of the definition to predict subsequent flares. , that we were able to identify in this data set only 11 patients who were in clinical remission off therapy for 12 continuous months, that 30% of the patients were lost to follow-up, and that the criteria will need to be validated in future prospective intervention study.34, 35 The strength of our criteria lies in the fact that it has been tested in a large number of patients from many countries.
In conclusion, using a data-driven approach PRINTO established criteria for clinically inactive disease in JDM, with evidence-based cut-offs for muscle strength/endurance, muscle enzymes and physical global evaluation of disease activity. These criteria can be used in clinical trials, in research and in clinical practice.
ACKNOWLEDGMENTS
Supported by a grant from the European Union (contract no. QLG1-CT-2000-00514), by IRCCS G. Gaslini, Genoa, Italy.
Dr Dragana Lazarevic was the recipient of the European League Against Rheumatism (EULAR) Scientific Training Bursaries. Dr Paivi M. Miettunen attended the PRINTO headquarter at Gaslini Hospital in Genoa (Italy) as part of her visiting professorship sabbatical. Dr. Lisa Rider was supported by the intramural research program of the National Institute of Environmental Health Sciences, National Institutes of Health.
We also need to thank all members of PRINTO who participated as investigators in the study and whose enthusiastic effort made this work possible.
Footnotes
Competing Interest
None declared.
Contributor statement
- conception and design, or analysis and interpretation of data
- drafting the article or revising it critically for important intellectual content
- final approval of the version to be published.
REFERENCES
- 1.Huber A, Feldman BM. Long-term outcomes in juvenile dermatomyositis: how did we get here and where are we going? Curr Rheumatol Rep. 2005;7:441–6. doi: 10.1007/s11926-005-0048-1. [DOI] [PubMed] [Google Scholar]
- 2.Ravelli A, Trail L, Ferrari C, et al. Long-term outcome and prognostic factors of juvenile dermatomyositis: A multinational, multicenter study of 490 patients. Arthritis Care Res (Hoboken ) 2010;62:63–72. doi: 10.1002/acr.20015. [DOI] [PubMed] [Google Scholar]
- 3.Kim S, El-Hallak M, Dedeoglu F, et al. Complete and sustained remission of juvenile dermatomyositis resulting from aggressive treatment. Arthritis Rheum. 2009;60:1825–30. doi: 10.1002/art.24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rider LG, Lachenbruch PA, Monroe JB, et al. Damage extent and predictors in adult and juvenile dermatomyositis and polymyositis as determined with the myositis damage index. Arthritis Rheum. 2009;60:3425–35. doi: 10.1002/art.24904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldman BM, Rider LG, Reed AM, Pachman LM. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet. 2008;371:2201–12. doi: 10.1016/S0140-6736(08)60955-1. [DOI] [PubMed] [Google Scholar]
- 6.Hasija R, Pistorio A, Ravelli A, et al. Approaches for the treatment of new onset and flared juvenile dermatomyositis: An international multicenter PRINTO study. Arthritis Rheum. 2011 doi: 10.1002/art.30475. [DOI] [PubMed] [Google Scholar]
- 7.Sanner H, Kirkhus E, Merckoll E, et al. Long-term muscular outcome and predisposing and prognostic factors in juvenile dermatomyositis: A case-control study. Arthritis Care Res (Hoboken ) 2010;62:1103–11. doi: 10.1002/acr.20203. [DOI] [PubMed] [Google Scholar]
- 8.Sanner H, Gran JT, Sjaastad I, Flato B. Cumulative organ damage and prognostic factors in juvenile dermatomyositis: a cross-sectional study median 16.8 years after symptom onset. Rheumatology (Oxford) 2009;48:1541–7. doi: 10.1093/rheumatology/kep302. [DOI] [PubMed] [Google Scholar]
- 9.Oddis CV, Rider LG, Reed AM, et al. International consensus guidelines for trials of therapies in the idiopathic inflammatory myopathies. Arthritis Rheum. 2005;52:2607–15. doi: 10.1002/art.21291. [DOI] [PubMed] [Google Scholar]
- 10.Huber AM, Lang B, LeBlanc CMA, et al. Medium- and long-term functional outcomes in a multicenter cohort of children with juvenile dermatomyositis. Arthritis Rheum. 2000;43:541–9. doi: 10.1002/1529-0131(200003)43:3<541::AID-ANR9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 11.Ruperto N, Martini A, for the Paediatric Rheumatology International Trials Organisation (PRINTO) Networking in pediatrics: the example of the Pediatric Rheumatology International Trials Organisation (PRINTO). Arch Dis Child. 2011;96:596–601. doi: 10.1136/adc.2010.188946. [DOI] [PubMed] [Google Scholar]
- 12.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 13.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403–7. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 14.Ruperto N, Ravelli A, Pistorio A, et al. The provisional Pediatric Rheumatology International Trial Organization/American College of Rheumatology/European League Against Rheumatism disease activity core set for the evaluation of response to therapy in juvenile dermatomyositis: a prospective validation study. Arthritis Rheum. 2008;59:4–13. doi: 10.1002/art.23248. [DOI] [PubMed] [Google Scholar]
- 15.Rider LG, Feldman BM, Perez MD, et al. Development of validated disease activity and damage indices for the juvenile idiophatic inflammatory myopathies. I. Physician, parent, and patients global assessments. Arthritis Rheum. 1997;40:1976–83. doi: 10.1002/art.1780401109. [DOI] [PubMed] [Google Scholar]
- 16.Lovell DJ, Lindsley CB, Rennebohm RM, et al. Development of validated disease activity and damage indices for the juvenile idiopathic inflammatory myopathies - II. The Childhood Myositis Assessment Scale (CMAS): a quantitative tool for the evaluation of muscle function. Arthritis Rheum. 1999;42:2213–9. doi: 10.1002/1529-0131(199910)42:10<2213::AID-ANR25>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Rennebohm RM, Jones K, Huber AM, et al. Normal scores for nine maneuvers of the childhood myositis assessment scale. Arthritis Rheum Arthritis Care Res. 2004;51:365–70. doi: 10.1002/art.20397. [DOI] [PubMed] [Google Scholar]
- 18.Huber AM, Feldman BM, Rennebohm RM, et al. Validation and clinical significance of the childhood myositis assessment scale for assessment of muscle function in the juvenile idiopathic inflammatory myopathies. Arthritis Rheum. 2004;50:1595–603. doi: 10.1002/art.20179. [DOI] [PubMed] [Google Scholar]
- 19.Rider LG, Koziol D, Giannini EH, et al. Validation of manual muscle testing and a subset of eight muscles for adult and juvenile idiopathic inflammatory myopathies. Arthritis Care Res (Hoboken ) 2010;62:465–72. doi: 10.1002/acr.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bode RK, Klein-Gitelman MS, Miller ML, Lechman TS, Pachman LM. Disease activity score for children with juvenile dermatomyositis: Reliability and validity evidence. Arthritis Rheum Arthritis Care Res. 2003;49:7–15. doi: 10.1002/art.10924. [DOI] [PubMed] [Google Scholar]
- 21.Singh G, Athreya BH, Fries JF, Goldsmith DP. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis Rheum. 1994;37:1761–9. doi: 10.1002/art.1780371209. [DOI] [PubMed] [Google Scholar]
- 22.Ruperto N, Ravelli A, Pistorio A, et al. Cross-cultural adaptation and psychometric evaluation of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ) in 32 countries. Review of the general methodology. Clin Exp Rheumatol. 2001;19:S1–S9. [PubMed] [Google Scholar]
- 23.Huber AM, Hicks JE, Lachenbruch PA, et al. Validation of the Childhood Health Assessment Questionnaire in the Juvenile Idiopathic Myopathies. J Rheumatol. 2001;28:1106–11. [PubMed] [Google Scholar]
- 24.Apaz MT, Saad-Magalhaes C, Pistorio A, et al. Health-related quality of life of patients with juvenile dermatomyositis: results from the Pediatric Rheumatology International Trials Organisation multinational quality of life cohort study. Arthritis Rheum. 2009;61:509–17. doi: 10.1002/art.24343. [DOI] [PubMed] [Google Scholar]
- 25.Landgraf JM, Abetz L, Ware JE. The CHQ User's Manual. (First Edition) 1996 [Google Scholar]
- 26.Isenberg DA, Allen E, Farewell V, et al. International consensus outcome measures for patients with idiopathic inflammatory myopathies. Development and initial validation of myositis activity and damage indices in patients with adult onset disease. Rheumatology. 2004;43:49–54. doi: 10.1093/rheumatology/keg427. [DOI] [PubMed] [Google Scholar]
- 27.Guzman J, Petty RE, Malleson PN. Monitoring disease activity in juvenile dermatomyositis: the role of Von Willebrand factor and muscle enzymes. J Rheumatol. 1994;21:739–43. [PubMed] [Google Scholar]
- 28.Rider LG, Miller FW. Laboratory evaluation of the inflammatory myopathies. Clin Diag Lab Immunol. 1995;2:9. doi: 10.1128/cdli.2.1.1-9.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rider LG, Prasad K, Feldman BM, et al. Relationships among laboratory tests and global disease activity assessments in juvenile dermatomyositis. Arthritis Rheum. 1996;39(suppl.):S191. [Google Scholar]
- 30.Rider LG. Assessment of disease activity and its sequelae in children and adults with myositis. Curr Opin Rheumatol. 1996;8:495–506. doi: 10.1097/00002281-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Ramanan AV, Feldman BM. The role of muscle enzymes in JDM. Pediatr Rheumatol Online J. 2005;3:1–4. [Google Scholar]
- 32.Ruperto N, Ravelli A, Cuttica R, et al. The Pediatric Rheumatology International Trials Organization criteria for the evaluation of response to therapy in juvenile systemic lupus erythematosus: Prospective validation of the disease activity core set. Arthritis Rheum. 2005;52:2854–64. doi: 10.1002/art.21230. [DOI] [PubMed] [Google Scholar]
- 33.Sultan SM, Allen E, Cooper RG, et al. Interrater reliability and aspects of validity of the myositis damage index. Ann Rheum Dis. 2011;70:1272–6. doi: 10.1136/ard.2010.142117. [DOI] [PubMed] [Google Scholar]
- 34.Wallace CA, Ruperto N, Giannini E, et al. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31:2290–4. [PubMed] [Google Scholar]
- 35.Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken ) 2011;63:929–36. doi: 10.1002/acr.20497. [DOI] [PubMed] [Google Scholar]
- 36.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 1977 [Google Scholar]
- 37.Landis JR, Koch GC. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 38.Naji P, Shahram F, Nadji A, Davatchi F. Effect of early treatment in polymyositis and dermatomyositis. Neurol India. 2010;58:58–61. doi: 10.4103/0028-3886.60398. [DOI] [PubMed] [Google Scholar]
- 39.Na SJ, Kim SM, Sunwoo IN, Choi YC. Clinical characteristics and outcomes of juvenile and adult dermatomyositis. J Korean Med Sci. 2009;24:715–21. doi: 10.3346/jkms.2009.24.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stringer E, Singh-Grewal D, Feldman BM. Predicting the course of juvenile dermatomyositis: significance of early clinical and laboratory features. Arthritis Rheum. 2008;58:3585–92. doi: 10.1002/art.23960. [DOI] [PubMed] [Google Scholar]
- 41.Vancsa A, Ponyi A, Constantin T, Zeher M, Danko K. Pregnancy outcome in idiopathic inflammatory myopathy. Rheumatol Int. 2007;27:435–9. doi: 10.1007/s00296-006-0239-8. [DOI] [PubMed] [Google Scholar]
- 42.Mielnik P, Wiesik-Szewczyk E, Olesinska M, Chwalinska-Sadowska H, Zabek J. Clinical features and prognosis of patients with idiopathic inflammatory myopathies and anti-Jo-1 antibodies. Autoimmunity. 2006;39:243–7. doi: 10.1080/08916930600623767. [DOI] [PubMed] [Google Scholar]
- 43.Chowdhary V, Wakhlu A, Agarwal A, Misra R. Outcome in juvenile dermatomyositis. Indian Pediatr. 2002;39:931–5. [PubMed] [Google Scholar]
- 44.Fisler RE, Liang MG, Fuhlbrigge RC, Yalcindag A, Sundel RP. Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis. J Am Acad Dermatol. 2002;47:505–11. doi: 10.1067/mjd.2002.122196. [DOI] [PubMed] [Google Scholar]
- 45.Ruperto N, Pistorio A, Ravelli A, et al. The pediatric rheumatology international trials organization provisional criteria for the evaluation of response to therapy in juvenile dermatomyositis. Arthritis Care Res. 2010;62:1533–41. doi: 10.1002/acr.20280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foell D, Wulffraat N, Wedderburn LR, et al. Methotrexate withdrawal at 6 vs 12 months in juvenile idiopathic arthritis in remission: a randomized clinical trial. JAMA. 2010;303:1266–73. doi: 10.1001/jama.2010.375. [DOI] [PubMed] [Google Scholar]
- 47.Filocamo G, Davi S, Pistorio A, et al. Evaluation of 21-numbered circle and 10-centimeter horizontal line visual analog scales for physician and parent subjective ratings in juvenile idiopathic arthritis. J Rheumatol. 2010;37:1534–41. doi: 10.3899/jrheum.091474. [DOI] [PubMed] [Google Scholar]
- 48.Rider LG, Giannini EH, Brunner HI, et al. International consensus on preliminary definitions of improvement in adult and juvenile myositis. Arthritis Rheum. 2004;50:2281–90. doi: 10.1002/art.20349. [DOI] [PubMed] [Google Scholar]