Abstract
Alzheimer's disease (AD) is a gradually debilitating disease that leads to dementia. The molecular mechanisms underlying AD are still not clear, and at present no reliable biomarkers are available for the early diagnosis. In the last several years, together with oxidative stress and neuroinflammation, altered cholesterol metabolism in the brain has become increasingly implicated in AD progression. A significant body of evidence indicates that oxidized cholesterol, in the form of oxysterols, is one of the main triggers of AD. The oxysterols potentially most closely involved in the pathogenesis of AD are 24-hydroxycholesterol and 27-hydroxycholesterol, respectively deriving from cholesterol oxidation by the enzymes CYP46A1 and CYP27A1. However, the possible involvement of oxysterols resulting from cholesterol autooxidation, including 7-ketocholesterol and 7β-hydroxycholesterol, is now emerging. In a systematic analysis of oxysterols in post-mortem human AD brains, classified by the Braak staging system of neurofibrillary pathology, alongside the two oxysterols of enzymatic origin, a variety of oxysterols deriving from cholesterol autoxidation were identified; these included 7-ketocholesterol, 7α-hydroxycholesterol, 4β-hydroxycholesterol, 5α,6α-epoxycholesterol, and 5β,6β-epoxycholesterol. Their levels were quantified and compared across the disease stages. Some inflammatory mediators, and the proteolytic enzyme matrix metalloprotease-9, were also found to be enhanced in the brains, depending on disease progression. This highlights the pathogenic association between the trends of inflammatory molecules and oxysterol levels during the evolution of AD. Conversely, sirtuin 1, an enzyme that regulates several pathways involved in the anti-inflammatory response, was reduced markedly with the progression of AD, supporting the hypothesis that the loss of sirtuin 1 might play a key role in AD. Taken together, these results strongly support the association between changes in oxysterol levels and AD progression.
Abbreviations: α-epoxy, 5α,6α-epoxycholesterol; β-epoxy, 5β,6β-epoxycholesterol; 24-OH, 24-hydroxycholesterol; 25-OH, 25-hydroxycholesterol; 27-OH, 27-hydroxycholesterol; 4β-OH, 4β-hydroxycholesterol; 7β-OH, 7β-hydroxycholesterol; 7α-OH, 7α-hydroxycholesterol; 7-K, 7-ketocholesterol; 7-OH-4-C, 7α-hydroxy-3-oxo-4-cholestenoic acid; Aβ, amyloid β; AD, Alzheimer's disease; BBB, blood brain barrier; COX-2, cyclooxigenase 2; CSF, cerebrospinal fluid; IL, interleukin; LXR, liver X receptor; MCI, mild cognitive impairment; MCP-1, monocyte chemotactic protein 1; MMP-9, matrix metalloprotease 9; NFT, neurofibrillary tangles; PBS, phosphate buffered saline; p-tau, hyperphosphorylated tau; ROS, reactive oxygen species; SIRT-1, sirtuin 1; SPE, solid phase extraction
Keywords: Alzheimer's disease, Oxysterols, Sirtuin-1, Inflammation, Cholesterol metabolism
Highlights
-
•
Changes in brain oxysterol levels may influence AD progression.
-
•
Oxysterol accumulation in the brain may amplify neuroinflammation.
-
•
SIRT-1 loss during AD progression may favor neuroinflammation.
-
•
Oxysterols and SIRT-1 might be useful markers for early AD diagnosis.
1. Introduction
Alzheimer's disease (AD) is a slowly progressive neurodegenerative disorder that leads to dementia. It is characterized by intracellular neurofibrillary tangles (NFT) made of hyperphosphorylated tau (p-tau) protein and extracellular amyloid beta (Aβ) plaques [1]. Although experimental evidence confirms that Aβ accumulation precedes and drives NFT formation, thereby facilitating the development of tau pathology [2], [3], intraneuronal lesions consisting of abnormal tau protein are seen to develop from the onset of AD until the end-phase of the underlying pathological process [4]. Recent evidence indicates that the AD process begins in nuclei of the lower brainstem, which send diffuse projections to a variety of vulnerable brain sites and, in particular, to the cerebral cortex [4], [5], [6], [7], [8].
At present, no reliable biomarkers for early diagnosis of AD are available; only a provisional diagnosis can be made, and that generally not until the final phase of the disease. Definitive diagnosis requires post-mortem evaluation of NFT and senile plaques in the brain [9], [10]. Thanks to the development of sensitive immunocytochemical methods, it is now possible to reliably detect intraneuronal neurofibrillary location and the severity of changes with disease progression. The Braak staging system for intraneuronal lesions differentiates among initial, intermediate, and late phases of the disease process, in both non-symptomatic and symptomatic AD patients; six AD stages (I–VI) have been identified (transentorhinal stages I–II: clinically silent cases; limbic stages Ill–IV: incipient AD; neocortical stages V-VI: fully developed AD) [11].
The molecular mechanisms underlying the disease are still not clear, and remain controversial. However, in recent years, it has become increasingly clear that altered cholesterol metabolism in the brain is involved in AD development, and hypercholesterolemia is considered a potential risk factor [12], [13], [14], [15]. Thanks to consistent research evidence, it is now believed that cholesterol oxidized products, known as oxysterols, are the link connecting altered cholesterol metabolism and hypercholesterolemia to this neurodegenerative disease [14].
Cholesterol is produced in situ in the brain, where its content exceeds that of any other organ. In order to prevent cholesterol accumulation in the brain, it can be oxidized by enzymes of the cytochrome p450 family, forming oxysterols. In particular, cholesterol is primarily converted by CYP46A1 into 24-hydroxycholesterol (24-OH), also called cerebrosterol, which, unlike its parent compound, is able to cross the blood brain barrier (BBB) and reaches the peripheral circulation. Another oxysterol present in the brain is 27-hydroxycholesterol (27-OH), produced by CYP27A1 and then transformed into 7α-hydroxy-3-oxo-4-cholestenoic acid (7-OH-4-C) by the enzyme CYP7B; 7-OH-4-C crosses the BBB, and reaches the liver where it is eliminated [16]. However, most 27-OH flows in the opposite direction, from the circulation into the brain. In addition to those oxysterols, others deriving from cholesterol autoxidation have been identified post-mortem in human AD brains, including 7-ketocholesterol (7-K), 7α-hydroxycholesterol (7α-OH), 4β-hydroxycholesterol (4β-OH), 5α,6α-epoxycholesterol (α-epoxy), and 5β,6β-epoxycholesterol (β-epoxy); the trend of their concentrations during disease stages has been examined [17].
In the normal aging brain, oxysterol homeostasis is closely controlled by pathways regulating both cholesterol biosynthesis and efflux, and oxysterol enzymatic formation. Moreover, several routes by which cholesterol metabolites may be exported from the brain or imported into it have been demonstrated [18], [19]. However, toxic amounts of oxysterols can accumulate in the brain, in particular due to the increased flux of these sterol molecules from the peripheral circulation into the brain; this is caused by increased permeability of the BBB, owing to its impairment [20]. Aging may lead to partial disruption of BBB integrity, but the barrier's function can also be significantly affected in neurodegenerative diseases, including AD. Among the causes of this impairment, hypercholesterolemia associated to oxidative stress can damage the BBB [21]; furthermore, BBB integrity and function can be partially damaged by oxysterols themselves [22]. Oxysterols that accumulate in the brain certainly play a fundamental role in AD development, by enhancing oxidative stress and inflammation, as well as Aβ formation and accumulation, with subsequent neuron death [14].
It has been widely reported that increased oxidative stress in the brain during AD intensifies neurodegeneration, by favoring generation of reactive oxygen species (ROS), lipid peroxidation, and oxysterol formation [23], [24]. Apart from oxidative stress, AD pathology is also associated with neuroinflammation [14], [25], [26]. AD development is characterized by the enhancement of many inflammatory mediators in the brain, including several cytokines and proteolytic enzymes, such as matrix metalloprotease 9 (MMP-9), produced by microglia, by astrocytes, and even by neurons [25], [26]. Up-regulation of the enzyme cyclooxigenase 2 (COX-2) has also been observed during inflammation, mainly in neurons but also in reactive microglia [27], [28]. Moreover, during AD progression, a marked reduction of sirtuin-1 (SIRT-1) has been demonstrated. SIRT-1 is a deacetylase that regulates several cellular pathways, involved not only in the anti-inflammatory response but also in other neuroprotective mechanisms through which it promotes neuron survival, by deacetylating a broad spectrum of molecules [29]. In this connection, it has been hypothesized that the loss of SIRT-1 protein in the brain contributes to the pathogenesis of AD [30].
The study elucidates the pathogenic association between changes in the concentration of several oxysterols present in AD brains and the progression of the disease, as well as the association between oxysterol levels and neuroinflammation. In this connection, a systematic analysis of oxysterols at different Braak stages of the AD brain was carried out. Several oxysterols of both enzymatic and non-enzymatic origin, some never previously identified in the brain, were found and quantified, and the profile of their amounts was found to clearly differ depending on the severity of the disease. Moreover, taking into account the different Braak stages, a new possible association between inflammatory molecules and oxysterol levels trend throughout the evolution of this disease emerges. Lastly, the results support the hypothesis that the loss of SIRT-1 might play a key role in AD progression.
2. Materials and methods
2.1. Histological and immunohistochemical analysis
In the brains included in the present study, routine neuropathological examination excluded relevant lesions such as tumors, significant vascular disease/stroke, or inflammation. Tissue from the frontal and occipital cortex of thirteen AD patients and four control subjects were obtained from the Carlo Besta Institute of Neurology (Milan, Italy).
Specimens were assessed histologically by hematoxylin and eosin, cresyl violet for Nissl substance, Heidenhein-Woelcke for myelin and thioflavine S for amyloid as well as by immunohistochemistry using antibodies against Aβ (4G8 1:4000, Signet Laboratories, Dedham, MA) after formic acid pre-treatment for 30 min, and against p-tau (AT8 1:300, Innogenetics, Ghent, Belgium). The immunoreaction was visualized using the EnVision Plus/Horseradish Peroxidase system (DakoCytomation) and 3-3’-diaminobenzidine as chromogen.
2.2. Oxysterol quantification
Brain tissues were washed with phosphate-buffered saline (PBS) to remove contaminating blood and stored at −80 °C before being analyzed separately. Oxysterols were measured by isotope dilution mass spectrometry, essentially as described elsewhere [31] with the exception of the solid phase extraction (SPE) step, which was repeated twice to eliminate cholesterol. Analyses were run on an Agilent 6890N gas chromatograph equipped with a 7683 series automatic liquid sampler, and interfaced with an Agilent 5973 mass spectrometer (Agilent Technologies; Palo Alto, CA). Separation was achieved on a 30 m capillary column (HP-5MS 30×0.25 mm ID, 0.25 µm thickness). The mass spectrometer was set to the selected ion monitoring mode; the molecules were monitored with ions at mass/charge ratio (m/z): 463 m/z for [2H7]-27-hydroxycholesterol, [2H7]-24-hydroxycholesterol, [2H7]-7α-hydroxycholesterol, [2H7]-7β-hydroxycholesterol, [2H7]-4α-hydroxycholesterol, and [2H7]-4β-hydroxycholesterol; 456 m/z for [2H0]-27-hydroxycholesterol, [2H0]-24-hydroxycholesterol, [2H0]-7α-hydroxycholesterol, [2H0]-7β-hydroxycholesterol, [2H0]-4α-hydroxycholesterol, and [2H0]-4β-hydroxycholesterol; 481 m/z for [2H7]-5α,6α-epoxycholesterol, and [2H7]-5β,6β-epoxycholesterol; 474 m/z for [2H0]-5α,6α-epoxycholesterol, and [2H0]-5β,6β-epoxycholesterol; 137 m/z for [2H6]-25-hydroxycholesterol, and 131 m/z for [2H0]-25-hydroxycholesterol; 479 m/z for [2H7]-7-ketocholesterol, and [2H0]-7-ketocholesterol (Avanti Polar Lipids). Quantification of oxysterols was by the internal standard ratio method.
2.3. RNA extraction and cDNA synthesis
Total RNA was extracted using TRIzol Reagent (Applied Biosystems, Monza, Italy) following the manufacturer's instructions. RNA was dissolved in RNase-free water fortified with RNase inhibitors (RNase SUPERase-In; Ambion, Austin, TX, USA). The amount and purity (A260/A280 ratio) of the extracted RNA were assessed spectrophotometrically. cDNA was synthesized by reverse transcription from 2 μg RNA with a commercial kit and random primers (High-Capacity cDNA Reverse Transcription Kit; Applied Biosystems) following the manufacturer's instructions.
2.4. Real time RT-PCR
Singleplex real-time RT-PCR was performed on 30 ng of cDNA using TaqMan Gene Expression Assay kits prepared for human SIRT-1, COX-2, interleukin-1α (IL-1α), IL-6, IL-8, monocyte chemotactic protein 1 (MCP-1), MMP-9, and β2-microglobulin, TaqMan Fast Universal PCR Master Mix, and 7500 Fast Real-Time PCR System (Applied Biosystems). Negative controls did not include cDNA. The oligonucleotide sequences are not revealed by the manufacturer because of proprietary interests. The cycling parameters were as follows: 20 s at 95 °C for AmpErase UNG activation, 3 s at 95 °C for AmpliTaq Gold DNA polymerase activation, 40 cycles of 3 s at 95 °C (melting), and 30 s at 60 °C (annealing⁄extension). The fractional cycle number (Ct) at which fluorescence passes the threshold in the amplification plot of fluorescence signal versus cycle number was determined for each gene considered. The results were then normalized to the expression of β2-microglobulin, as housekeeping gene. Relative quantification of target gene expression was achieved with a mathematical method [32].
3. Results
3.1. Neuropathological characterization of AD and control brains by immunohistochemistry
In the four control brains (age at death: 60–71 years) there was no evidence of Aβ or tau pathology. Significant AD pathology (Aβ deposits and neurofibrillary changes) has been observed in the other brains, which were classified based on the Braak staging system of neurofibrillary pathology [33]. Five brains were at stages I or II (age at death: 72–86 years), and eight were at stages IV to VI (age at death: 68–82 years) (Fig. 1).
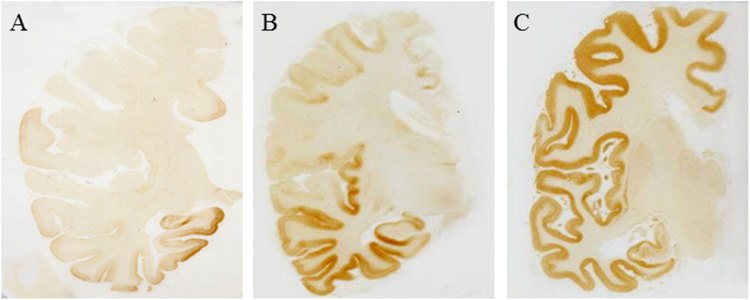
Fig. 1.
Distribution of neurofibrillary changes in AD brain specimens. Immunohistochemistry with monoclonal antibody AT8 to p-tau (immunoreactivity corresponds to brown reaction product) revealed severe involvement of all regions of the cerebral cortex by neurofibrillary changes (C, Braak stage VI), that can spare the primary motor and sensory areas (B, Braak stage IV) or involve selectively the mesial temporal areas (A, Braak stage II).
In the brains at Braak stages I–II of neurofibrillary changes, Aβ deposits were moderate to numerous, with a clear preponderance of preamyloid deposits (i.e. immunoreactive for Aβ but lacking the tinctorial properties of amyloid as yellow fluorescence after thioflavine S staining). In the brains at Braak stages IV to VI of neurofibrillary changes, the large majority of Aβ deposits were under the form of senile plaques (fluorescent after thioflavine S staining).
3.2. Oxysterol levels in the frontal and occipital cortex of AD brains during AD progression
The study comprised autopsy specimens from the frontal and occipital cortex of AD brains, classified as early or late AD based on the Braak staging system of neurofibrillary pathology (early AD: stages I and II; late AD: stages IV–VI). In the control brains, the presence of senile plaques and tau pathology was excluded. Oxysterols were identified and quantified in brain samples by isotope dilution gas chromatography/mass spectrometry, to determine their levels in normal and AD brains. In particular, levels of oxysterols in early and late AD brains were determined, to highlight any changes in those levels during disease progression.
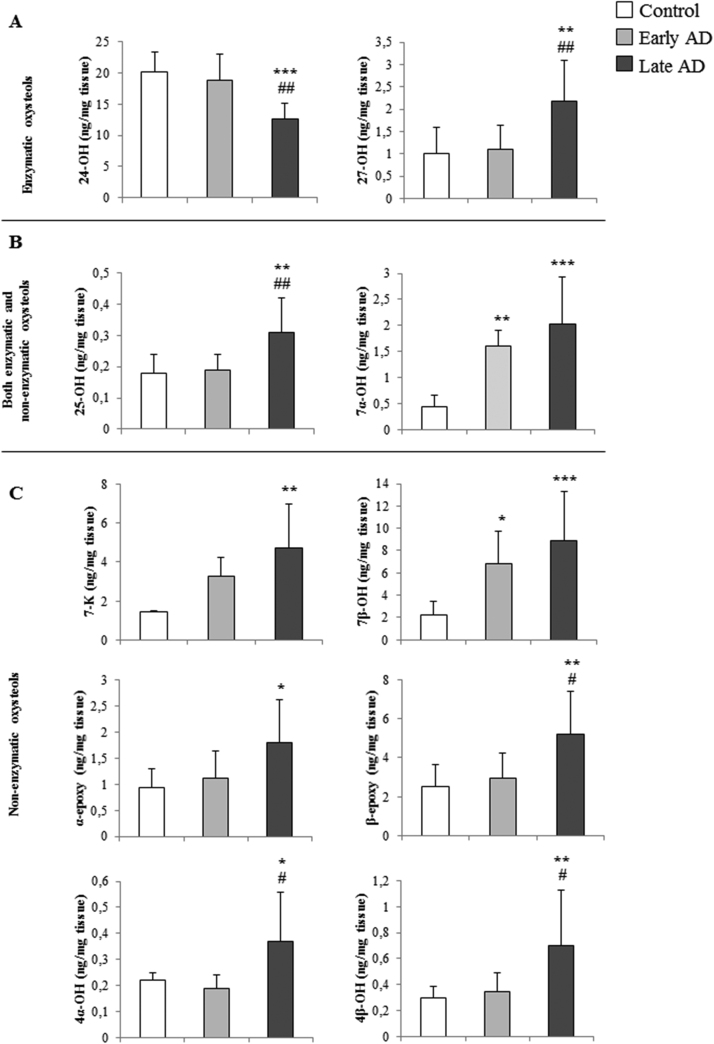
Of interest were data concerning the levels of ten oxysterols recovered from the cerebral frontal and occipital cortex, two of enzymatic origin (24-OH and 27-OH) (Fig. 2A), six of non-enzymatic origin (7-K, 4α-OH, 4β-OH, 7β-OH, α-epoxy and β-epoxy) (Fig. 2C), and two of both enzymatic and non-enzymatic origin (25-OH and 7α-OH) (Fig. 2B). Interestingly, it emerged that levels of all oxysterols except 24-OH increased during disease progression, this increment becoming markedly significant in late AD, compared to control brains and even, in some cases, compared to early AD brains. Levels of 7α-OH and 7β-OH were already elevated in the early stages of AD (Fig. 2). Concerning 24-OH, in early AD its level was similar to control brains, whereas it was significantly decreased at advanced disease stages (late AD), compared to controls and early AD brains (Fig. 2A).
Fig. 2.
Quantification of oxysterols present in autopsy samples of frontal and occipital cortex from AD brains. Oxysterol levels were quantified by isotope dilution mass spectrometry in ex vivo samples of AD brains classified by the Braak staging system. The figure gives the levels of: (A) two oxysterols of enzymatic origin, 24-hydroxycholesterol (24-OH) and 27-hydroxycholesterol (27-OH); (B) the oxysterols produced both enzymatically and non-enzymatically, 25-hydroxycholesterol (25-OH) and 7α-hydroxycholesterol (7α-OH); (C) the oxysterols of non-enzymatic origin, 7-ketocholesterol (7-K), 7β-hydroxycholesterol (7β-OH), 5α,6α-epoxycholesterol (α-epoxy) and 5β,6β-epoxycholesterol (β-epoxy), 4α-hydroxycholesterol (4α-OH) and 4β-hydroxycholesterol (4β-OH). Early AD (Braak stages I, II); late AD (Braak stages IV–VI). Control brain specimens: n=4; early AD specimens: n=5; late AD specimens: n=8. Brain tissues from the frontal and occipital cortex were analyzed separately. *P<0.05,**P<0.01, and ***P<0.001 vs controls; #P<0.05 and ##P<0.01 vs early AD.
Of note, as Fig. 2 shows, an examination of oxysterol levels in early and late AD brains clearly shows that they change significantly in the cerebral cortex with AD progression. Conversely, when all data regarding AD brains were grouped together (total AD) without considering the disease stage of the donor, the increase in levels of certain oxysterols (e.g. 25-OH, 27-OH, 4β-OH, α-epoxy, and β-epoxy) was no longer significant compared to control brains (Table 1). The reduction of 24-OH level was less significant, but still considerable.
Table 1.
Identification and quantification of oxysterols in the frontal and occipital cortex of AD brains (Total AD) by isotope dilution gas chromatography/mass spectrometry.
| Control (ng/mg tissue) | Total AD (ng/mg tissue) | ||
|---|---|---|---|
| Enzymatic oxysterols | 24-hydroxycholesterol | 20.13±3.15 | 14.86±4.41* |
| 27-hydroxycholesterol | 1.01±0.59 | 1.64±0.91 | |
| Both enzymatic and non enzymatic oxysterols | 25-hydroxycholesterol | 0.18±0.06 | 0.25±0.10 |
| 7α-hydroxycholesterol | 0.44±0.23 | 1.79±0.66*** | |
| Non enzymatic oxysterols | 7-ketocholesterol | 1.46±0.06 | 4.10±1.90** |
| 7β-hydroxycholesterol | 2.27±1.14 | 7.86±3.70*** | |
| α-epoxycholesterol | 0.94±0.36 | 1.39±0.73 | |
| β-epoxycholesterol | 2.50±1.14 | 4.36±2.18 | |
| 4α-hydroxycholesterol | 0.22±0.03 | 0.30±0.18* | |
| 4β-hydroxycholesterol | 0.30±0.09 | 0.52±0.36 | |
AD, Alzheimer’s disease. Control brain specimens: n=4; Total AD (Braak stages I to VI) specimens: n=13.
* P<0.05; ** P<0.01; *** P<0.001 vs controls.
These results suggest that subdivision of specimens by disease stage may be of fundamental importance to clarify which events or molecules might contribute to neurodegeneration. Considering the brains of AD patients regardless of disease stage could flatten the results, and fail to reveal some important details, which only appear at a given time in the development of AD.
3.3. CYP46A1 and CYP27A1 expression levels in AD brain samples
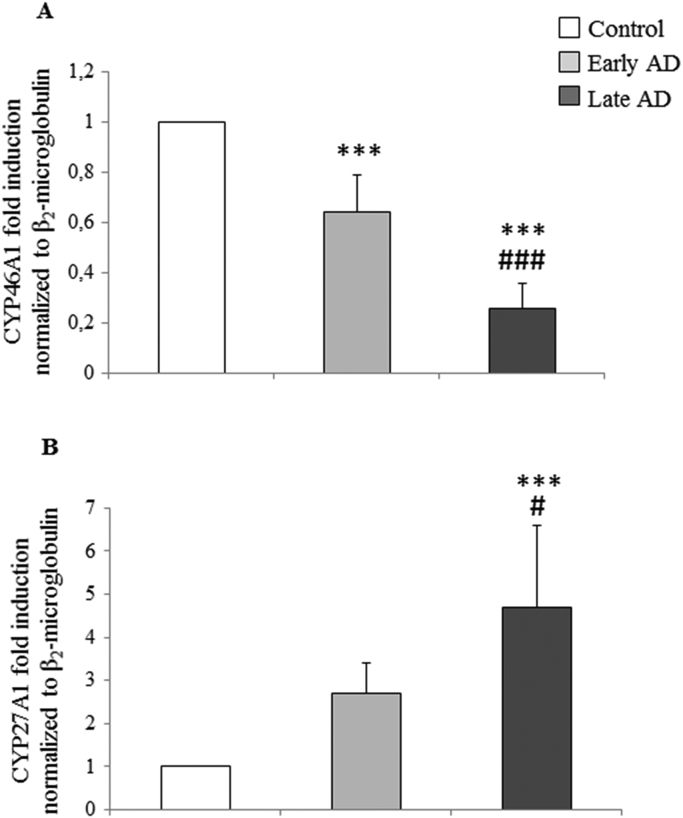
Expression of the enzymes responsible for converting cholesterol into the most widely studied oxysterols in the brain, 24-OH and 27-OH, i.e. CYP46A1 and CYP27A1 respectively, was quantified in the frontal and occipital cortex of ex vivo samples from normal and AD brains. The AD brain specimens were subdivided by disease stage into early and late AD.
As shown in Fig. 3, CYP46A1 mRNA levels, measured by real time RT-PCR, were significantly decreased, in both early-stage AD (about −40% vs control) and, more markedly, in late-stage AD (about −80% vs control). Conversely, CYP27A1 mRNA levels were increased in both early-stage (about 2.5-fold) and late-stage (about 4.5-fold) AD brains, compared to controls, but this increase was only statistically significant in late-stage AD. The results concerning CYP46A1 and CYP27A1 expression levels (Fig. 3) respectively reflect those for 24-OH and for 27-OH levels in AD brains (Fig. 2A): the loss of 24-hydroxylase leads to the reduction of 24-OH levels, and the increase of 27-hydroxylase leads to the accumulation of 27-OH.
Fig. 3.
Measurement of the expression levels of CYP46A1 and CYP27A1 in AD brain specimens. Gene expression was quantified by real-time RT-PCR in specimens at different stages of AD. Brain specimens of healthy subjects were taken as controls. Data, normalized to β2-microglobulin, are expressed as mean values±SD. Early AD (Braak stages I, II); late AD (Braak stages IV to VI). Control brain specimens: n=4; early AD specimens: n=5; late AD specimens: n=8. Brain tissues from the frontal and occipital cortex were analyzed separately. ***P<0.001vs controls; #P<0.05 and ###P<0.001 vs early AD.
3.4. Expression of some mediators involved in AD neuroinflammation
To investigate a correlation between oxysterol content and inflammatory response in AD brains, the expression of some pro-inflammatory molecules in the cerebral frontal and occipital cortex of AD patients was measured. Again brain specimens were divided into two groups (early and late AD) by disease severity.
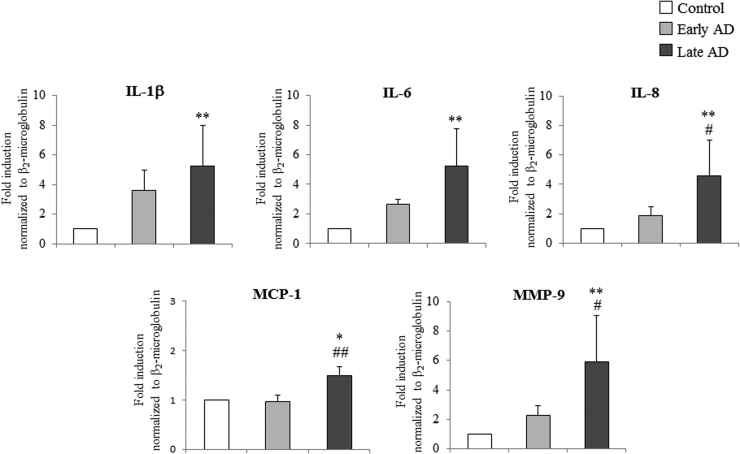
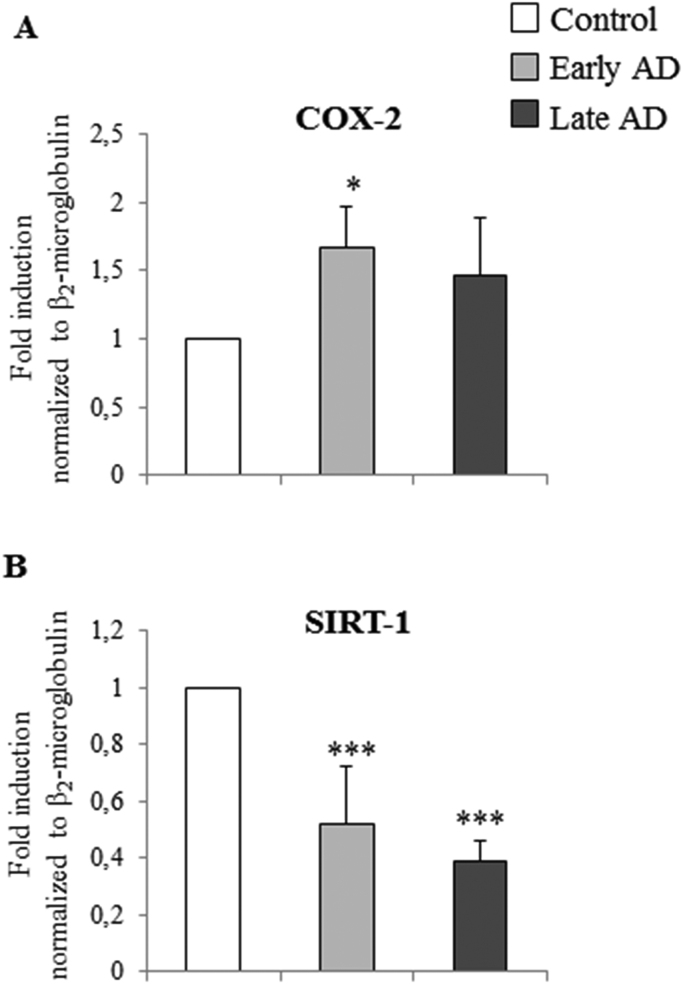
As Fig. 4 shows, mRNA levels of IL-1β, IL-6, and IL-8, and of MMP-9, were markedly increased (about 5-fold vs controls) in late AD brains, while MCP-1 expression was less up-regulated, but still significant in late AD samples. Concerning the enzyme COX-2, which is involved in the inflammatory response, its overexpression in AD brains clearly emerged, with a significant increment already in early AD (about 1.7-fold induction vs controls) (Fig. 5A).
Fig. 4.
Measurement of the expression levels of some inflammatory molecules in AD brain specimens. Gene expression of IL-1β, IL-6, IL-8, MCP-1 and MMP-9 was quantified by real-time RT-PCR in specimens at different stages of AD. Brain specimens of healthy subjects were taken as controls. Data, normalized to β2-microglobulin, are expressed as mean values±SD. Early AD (Braak stages I, II); late AD (Braak stages IV to VI). Control brain specimens: n=4; early AD specimens: n=5; late AD specimens: n=8. Brain tissues from the frontal and occipital cortex were analyzed separately. *P<0.05 and **P<0.01 vs controls; #P<0.05 and ##P<0.01 vs early AD.
Fig. 5.
Measurement of the expression levels of COX-2 and SIRT-1 in AD brain specimens. Gene expression of COX-2 and SIRT-1 was quantified by real-time RT-PCR in specimens at different stages of AD. Brain specimens of healthy subjects were taken as controls. Data, normalized to β2-microglobulin, are expressed as mean values±SD. Early AD (Braak stages I, II); late AD (Braak stages IV–VI). Control brain specimens: n=4; early AD specimens: n=5; late AD specimens: n=8. Brain tissues from the frontal and occipital cortex were analyzed separately. *P<0.05 and ***P<0.001 vs controls.
Since SIRT-1 is involved in many inflammatory disorders, to confirm its role during AD progression, SIRT-1 mRNA levels were also evaluated in the same autopsy samples of AD brains. Real-time RT-PCR revealed a statistically-significant decrease in SIRT-1 expression in both early (about −50%) and late (about −60%) AD compared to controls (Fig. 5B). These results suggest that the reduction of SIRT-1 levels during the progression of AD favors and amplifies neuroinflammation and, consequently, neurodegeneration.
Of note, because of the consistent association between changes in oxysterol levels (Fig. 2) and increased expression levels of inflammatory molecules (Figs. 4 and 5A) in the AD brain, oxysterols might be considered as new markers of both AD progression and AD neuroinflammation.
4. Discussion
The histopathological hallmarks of AD are senile plaques made up of Aβ peptide, and NFT consisting of hyperphosphorylated tau protein [1]. Aβ and p-tau have long been considered as the primary cause of AD, but alternative proposals have recently been made, suggesting that oxidative stress, inflammation, and dysregulated lipid homeostasis within the diseased brain may be the key points leading to Aβ and p-tau formation and accumulation [14], [34]. However, the sequence of events occurring during AD progression is not yet understood in detail, and the molecular mechanisms remain to be fully elucidated. Therapeutic strategies to counteract AD are thus still unsatisfactory.
The lack of specific markers for early diagnosis has stimulated researchers to look for new molecules whose levels change in the course of the disease. In this connection, oxysterols, cholesterol oxidation products, might be new targets for monitoring AD progression: significant changes in the concentrations of several oxysterols have been observed in post-mortem AD brains compared to healthy subjects. These changes in the brain might reflect changes in oxysterol levels in the cerebrospinal fluid (CSF) and peripheral circulation [14].
Increased oxysterol concentrations in the brain may promote cellular damage, causing neuron dysfunction and degeneration, and could contribute to neuroinflammation and amyloidogenesis [14]. These compounds can also contribute to excitotoxic brain injury [35]. In 2009, Hascalovici and collaborators identified 7α-OH, 4β-OH, α- and β-epoxy, and 7-K in post-mortem human AD brains, and their levels were higher at the mild cognitive impairment (MCI) disease stage [17]. Recently, Ahonen and collaborators reported that the two main oxysterols found in mouse AD brain samples, in relatively high concentrations, were 24-OH (1.0–1.6×105 ng/g) and 27-OH (90–210 ng/g). Moreover, consistent levels of 25-OH, 7α-OH, 7β-OH and 7-K were also detected [36]. Increased levels of 24-OH, 7-K, and β-epoxy have also been detected in the areas of the rat hippocampus undergoing neuroinflammation after kainite-induced excitotoxic injury [37], [38].
To support the idea that changes in oxysterol content of the brain might reflect changes in their levels in the peripheral circulation, and in the light of the fact that oxysterols can cross the BBB, the flux of more than twenty cholesterol metabolites between brain and circulation was recently analyzed in twenty AD patients. Changes in concentrations, between jugular and forearm veins, of eighteen oxysterols, five cholestenoic acids and three cholenoic acids were measured. Among these cholesterol metabolites, 24-OH, 7-OH-4-C, 7β-OH, 7-K and also 6-oxo-5α-hydroxycholesterol were exported from the brain; of them, that exported in the largest quantities was 24-OH. Conversely, 27-OH was imported into the brain [19]. Of note, 27-OH is one of the major oxysterols in the blood circulation, and its high levels have been related to hypercholesterolemia and oxidative stress [39]. Two other cholesterol metabolites, 7α,25-dihydroxycholest-4-en-3-one and 7α,(25 R)26-hydroxycholest-4-en-3-one, are reported to be exported from the brain [18]. Oxysterols and cholestenoic acids have also been identified and quantified in mouse CSF, and the findings compared with concentrations of the same metabolites found in the plasma, in order to clarify cholesterol metabolism. Concentrations of oxysterols were lower in the CSF than in the plasma, but 7α,24-dihydroxycholesterol and 7α,24-dihydroxycholest-4-en-3-one, both of enzymatic origin, were only identified in the CSF [40].
This paper presents, for the first time, a systematic analysis of oxysterols in the brains of patients with different stages of AD development. Brain samples obtained at autopsy from individuals affected by AD were examined by immunohistochemistry for the two pathological hallmarks of this disease, extracellular amyloid deposits and intraneuronal NFT. It was observed that the trends in levels of 24-OH and 27-OH, the two oxysterols of enzymatic origin, were opposed during the course of the disease: in the early stages of AD, levels of 24-OH and 27-OH were, respectively, slightly below and slightly above those of controls. Of note, at the later stages of AD, 24-OH levels were markedly decreased (about −40%) compared to control brains, while 27-OH levels were approximately double those of controls (Fig. 2A); both these changes were statistically significant compared to levels of the two oxysterols in early stages, stressing that changes in their concentrations play an important role in the end-phase of the disease. The same trend as 27-OH was observed for 25-OH, β-epoxy, 4α-OH, and 4β-OH, oxysterols mainly deriving from cholesterol autoxidation by ROS during oxidative stress (Fig. 2B and 2C). Concerning the other oxysterols, 7α-OH, 7β-OH, 7-K and α-epoxy, the levels increased progressively from the earliest stages of the disease, peaking in the late stages, in most cases the difference being significant compared to controls (Fig. 2B and 2C).
If the levels of the various oxysterols at early and at late AD stages are considered separately (Fig. 2), all changes in concentrations are evident and statistically significant; this is particular so at the later disease stages. In contrast, analyzing all AD brain specimens together, some differences in oxysterol levels are no longer significant versus controls (Table 1). In particular, concerning the oxysterols generally considered to be potentially involved in AD, 24-OH and 27-OH, it was observed that the reduction in the former was much less significant versus controls, while the increase in the latter was no more significant versus controls (Table 1).
These results highlight the importance of discriminating among the different stages of progression of the disease when performing any analysis, in order to clarify at what stage biochemical or molecular changes occur; this is necessary in order to develop targeted therapeutic strategies for use at the appropriate time, so as to prevent or reduce neuronal damage.
The same analytical approach was applied to measuring expression levels of the cholesterol hydroxylases CYP46A1 and CYP27A1, respectively responsible for the formation of 24-OH and of 27-OH. It was found that CYP46A1 expression dramatically decreased during AD progression. In early AD brains it was already significantly reduced, by about 40%, but in late AD brains it was reduced by as much as 80% (Fig. 3A). Conversely, CYP27A1 expression was markedly increased, in particular in the later phases of AD (Fig. 3B). These results are in agreement with the trend in 24-OH and 27-OH levels, quantified at early and late stages in AD brains. The significant decrement of both CYP46A1 and 24-OH in the late stages of AD reflects a selective loss of neuronal cells expressing the 24-hydroxylase. As demonstrated by Brown 3rd and collaborators, CYP46A1 is expressed in neurons and astrocytes in the normal brain, but during AD progression the levels of 24-OH decrease markedly because of the neuronal damage [41]. However, it has been observed that, in the damaged area, and especially around senile plaques, there is an ectopic induction of CYP46A1 in the astrocytes, leading to some 24-OH production, but without compensating for the decrease of that oxysterol [41], [42]. Conversely, various reasons might explain the marked increment of CYP27A1 and 27-OH in the end-phases of AD. Immunocytochemical studies have shown that, in the normal brain, CYP27A1 is expressed in the neurons, astrocytes and oligodendrocytes. In AD brains, the number of neurons expressing 27-hydroxylases decreases and, as a consequence of neuron loss, expression of CYP27A1 may also be reduced; however, 27-OH levels remain elevated because CYP27A1 is expressed in astrocytes and oligodendrocytes, leading to in situ generation of 27-OH [41]. Accumulation of 27-OH in the brain is also due to the increased flux of this oxysterol across the BBB, either because of hypercholesterolemia associated to oxidative stress [21], or of damaged BBB integrity [20]. An alternative explanation for the accumulation of 27-OH is the reduced activity of CYP7B, the neuronal enzyme responsible for 27-OH metabolism; this reduction arises from the reduced CYP7B expression in the brain of AD patients, because of neuron loss [43].
From these considerations, it is clear that the balance between 24-OH and 27-OH levels is important, and that an increased ratio of 27-OH to 24-OH in AD brains is consistent with AD pathogenesis. Thus it is likely that reduced levels of 24-OH may accelerate disease progression, and that the increased levels of 27-OH may be insufficient to compensate for this: the shift in balance between the two oxysterols might lead to increased generation and accumulation of Aβ and NFT with consequent neurodegeneration [16], [21], [44].
Besides changes in oxysterol levels, it has also become evident that inflammation and oxidative stress are self-reinforcing during AD progression: inflammation can be triggered or enhanced by oxidative stress, through activation of the redox sensitive transcription factor NF-κB, and vice versa [14], [23].
The brain regions that are vulnerable to neurodegeneration, as in the case of AD, exhibit increased oxidative damage, in terms of DNA oxidation, protein oxidation, and lipid peroxidation, together with depletion of antioxidant enzymes; these occur before the appearance of senile plaques and NFT [45], [46], [47].
Elevated inflammatory response, characterized by the release of cytokines, adhesion molecules, proteolytic enzymes and other inflammatory mediators, has been detected in regions of the brain of AD patients, both post-mortem and in vivo, and in animal models of AD [26], [48], [49], [50]. Interestingly, in recent years genetic variations in inflammation related genes have been investigated in AD. Several genetic studies have reported significant associations between different polymorphisms of genes encoding for pro- and anti-inflammatory molecules and AD [51], [52]. It has also been observed that inflammatory changes are a relatively early pathogenic events in AD pathology, and that they precede the late stages, characterized by tau-related pathology [53]. However, the precise role played by pro-inflammatory molecules in AD remains controversial. In the early stages of AD, neuroinflammation appears to be beneficial in slowing AD progression, whereas in the later stages, chronic inflammation is detrimental to neurons [54].
This study provides evidence that the expression levels of various inflammatory mediators are markedly increased in AD brain samples. In particular, IL-1β, IL-6, and IL-8, MCP-1 and MMP-9 mRNA levels were all significantly up-regulated in brain specimens from the frontal and occipital cortex of late-stage AD (Fig. 4). These inflammatory molecules are produced by glia cells and neurons. Interleukins are important mediators for glia cell recruitment and activation, as is leukocyte infiltration around the areas of neuroinflammation; interleukins can also initiate the further production of signaling molecules and stimulate production of other inflammatory mediators [25], [26]. Expression of MMP-9, a major MMP that has been identified in neuroinflammation, is regulated, among others factors, by cytokines [55].
Furthermore, it is increasingly apparent that the COX-2-dependent pathway is associated with neuroinflammation and neurodegeneration. Indeed, COX-2 expression and activity is up-regulated during neuroinflammation, with subsequent production of prostaglandins, and its overexpression is mainly observed in damaged neurons, which can be promoted by cytokine release [28], [56], [57], [58]. Further, leukocyte migration across a damaged BBB is also influenced by COX-2 production, supporting the idea that increased peripheral inflammation is capable of propagating sustained and damaging neuroinflammation [59]. In this connection, among the lipid mediators produced by COX-2, prostaglandin E2 activates NF-κB, which, in turn, may stimulate leukocyte recruitment into the inflamed brain by inducing MCP-1 transcription [60], [61]. The present study investigated the implication of COX-2, the key player in the neuroinflammatory response. A statistically significant increase (about 1.5-fold vs controls) of COX-2 expression levels was observed in the early stage of the disease (Fig. 5A). The data are in agreement with other studies on patients at different stages of AD pathology, which show increased neuronal expression of COX-2 in the neocortex of patients with early Braak stage AD [62]. Moreover, as previously demonstrated in vitro in human neuroblastoma SH-SY5Y cells, the oxysterols mainly involved in AD are capable of synthesizing COX-2 in response to inflammatory mediator release [63]. The results reported here thus confirm the involvement of oxysterols in neuroinflammation.
Another molecule involved in the pathology of AD is SIRT-1, a deacetylase predominantly localized in nuclei, which takes part in several crucial neuroprotective pathways [64]. It has been demonstrated to regulate the anti-inflammatory response by activating the nuclear receptor liver X receptor (LXR) via deacetylation of Lys432 [65], or by inhibiting NF-κB through deacetylation of the Lys310 residue of RelA/p65 [66]. Overexpression of SIRT-1 or its activation by resveratrol have also been shown to be neuroprotective by markedly reducing NF-κB signaling stimulated by Aβ, in microglial cells [67]. Another mechanism by which SIRT-1 exerts its neuroprotective effect concerns its ability to reduce the pathological accumulation of Aβ both in vitro and in vivo. In particular, SIRT-1 increases α-secretase production and activity in mouse primary neurons [68]. Moreover, SIRT-1 overexpression, mediated by its agonist resveratrol, leads to the reduction of oligomerized Aβ peptides and of oxidative stress [69]. SIRT-1 also inhibits the tau-related AD phenotype: it directly deacetylates tau protein at multiple residues, making the protein more susceptible to proteasomal degradation. SIRT-1 deficiency leads to hyperacetylation of tau, with the consequent accumulation of p-tau [70]. Taking into account these findings, it may be assumed that the decreased levels of SIRT-1 during AD progression could accelerate neurodegeneration by: i) activating NF-κB in microglia, thus promoting neuroinflammation; ii) inhibiting the expression of α-secretase, leading to Aβ production; iii) favoring tau accumulation by inhibiting its clearance. Therefore, therapeutic up-regulation of SIRT-1 may provide opportunities for the amelioration of AD neuropathology and related conditions by protecting against neuronal loss.
The significant decrease in SIRT-1 levels in autopsy samples from AD patients has already been reported, but its crucial role during the different phases of AD progression has not been elucidated. Julien and collaborators, after having analyzed various cerebral regions in autopsy specimens, provided the first evidence of a down-regulation of SIRT-1 in the cerebral cortex of patients with MCI or AD. They reported that SIRT-1 decreases in parallel with the accumulation of tau protein, and that the greater loss of SIRT-1 protein and mRNA levels is probably a relatively late event [29].
In contrast, the present study found a highly significant SIRT-1 decrease already in early AD specimens, becoming even greater in late AD (Fig. 5B). These data are in agreement with a study by Lutz and collaborators, which found a loss of SIRT-1 during AD progression by the Braak staging system of neurofibrillary degeneration. Alongside the decrease in SIRT-1 expression, which is inversely correlated with tau and amyloid protein accumulation, they also observed that the loss of SIRT-1 is dependent on the neuronal population, after analyzing different brain subregions [71].
Concerning the involvement of COX-2 and SIRT-1 in neuroinflammation, it is clear from the present data that SIRT-1 expression is inversely proportional to the expression of COX-2 (Fig. 5). It may thus be suggested that the loss of SIRT-1 during AD progression sustains neuroinflammation and neuronal damage. In support of this hypothesis, it has been demonstrated that docosahexaenoic acid, a representative omega-3 polyunsaturated fatty acid, protects against obesity-associated inflammation by suppressing insulin-induced expression of COX-2, through up-regulation of SIRT-1, in human colon epithelial cells [72].
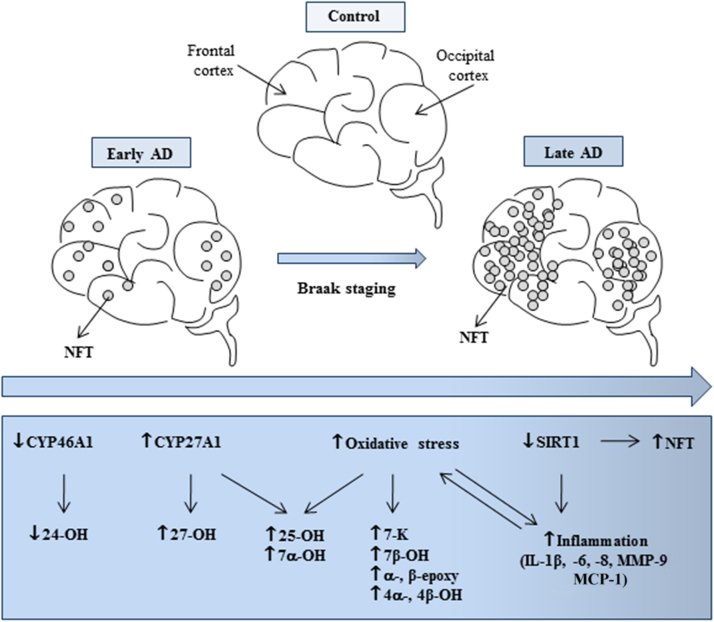
In summary, this study provides important insights into the contribution of oxysterols, as well as that of SIRT-1 and other inflammatory mediators, to the occurrence and development of neurodegenerative changes in AD (Fig. 6). Further, because the concentration of certain oxysterols in CSF and the peripheral circulation reflects the number of metabolically-active neurons in the brain [14] and, thus, the progression of AD, their levels in these biological fluids might be associated to the degree of neuronal damage; this might provide a means of identifying early or late phases of the disease. Another promising serum protein marker for early AD could be SIRT-1 [73]. For this reason, oxysterols and SIRT-1 might represent novel predictive biomarkers for the early diagnosis of AD.
Fig. 6.
A hypothetical scheme for the involvement of oxysterols in the different stages of AD progression.
5. Conclusions
Further studies will be needed to identify all the molecular pathways that are modulated or altered by oxysterols at different stages of AD, in order to find targeted therapeutic strategies that may have different effects on distinct vulnerable brain areas, to prevent or to treat this disabling disease.
Acknowledgments
The authors thank the CRT Foundation (Turin) and the University of Turin (Italy).
References
- 1.Querfurth H.W., LaFerla F.M. Alzheimer’s disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Gotz J., Chen F., van Dorpe J., Nitsch R.M. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 3.Lewis J., Dickson D.W., Lin W.L., Chisholm L., Corral A., Jones G., Yen S.H., Sahara N., Skipper L., Yager D., Eckman C., Hardy J., Hutton M., McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 4.Braak H., Thal D.R., Ghebremedhin E., Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 5.Braak H., Del Tredici K. Alzheimer's pathogenesis: is there neuron-to-neuron propagation? Acta Neuropathol. 2011;121:589–595. doi: 10.1007/s00401-011-0825-z. [DOI] [PubMed] [Google Scholar]
- 6.Braak H., Del Tredici K. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain. 2015;138:2814–2833. doi: 10.1093/brain/awv236. [DOI] [PubMed] [Google Scholar]
- 7.Parvizi J., Van Hoesen G.W., Damasio A. The selective vulnerability of brainstem nuclei to Alzheimer's disease. Ann. Neurol. 2001;49:53–66. doi: 10.1002/1531-8249(200101)49:1<53::aid-ana30>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 8.Simic G., Stanic G., Mladinov M., Jovanov-Milosevic N., Kostovic I., Hof P.R. Does Alzheimer's disease begin in the brainstem. Neuropathol. Appl. Neurobiol. 2009;35:532–554. doi: 10.1111/j.1365-2990.2009.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyman B.T., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Carrillo M.C., Dickson D.W., Duyckaerts C., Frosch M.P., Masliah E., Mirra S.S., Nelson P.T., Schneider J.A., Thal D.R., Thies B., Trojanowski J.Q., Vinters H.V., Montine T.J. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montine T.J., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Dickson D.W., Duyckaerts C., Frosch M.P., Masliah E., Mirra S.S., Nelson P.T., Schneider J.A., Thal D.R., Trojanowski J.Q., Vinters H.V., Hyman B.T. National Institute on Aging–Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braak H., Alafuzoff I., Arzberger T., Kretzschmar H., Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y.L., Wang L.M., Chen Y., Gao J.Y., Marshall C., Cai Z.Y., Hu G., Xiao M. Changes in astrocyte functional markers and β-amyloid metabolism-related proteins in the early stages of hypercholesterolemia. Neuroscience. 2016;316:178–191. doi: 10.1016/j.neuroscience.2015.12.039. [DOI] [PubMed] [Google Scholar]
- 13.Dias H.K., Brown C.L., Polidori M.C., Lip G.Y., Griffiths H.R. LDL-lipids from patients with hypercholesterolaemia and Alzheimer’s disease are inflammatory to microvascular endothelial cells: mitigation by statin intervention. Clin. Sci. 2015;129:1195–1206. doi: 10.1042/CS20150351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamba P., Testa G., Gargiulo S., Staurenghi E., Poli G., Leonarduzzi G. Oxidized cholesterol as the driving force behind the development of Alzheimer’s disease. Front Aging Neurosci. 2015;7:119. doi: 10.3389/fnagi.2015.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue-Shan Z., Juan P., Qi W., Zhong R., Li-Hong P., Zhi-Han T., Zhi-Sheng J., Gui-Xue W., Lu-Shan L. Imbalanced cholesterol metabolism in Alzheimer’s disease. Clin. Chim. Acta. 2016;456:107–114. doi: 10.1016/j.cca.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 16.Björkhem I., Cedazo-Minguez A., Leoni V., Meaney S. Oxysterols and neurodegenerative diseases. Mol. Asp. Med. 2009;30:171–179. doi: 10.1016/j.mam.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Hascalovici J.R., Vaya J., Khatib S., Holcroft C.A., Zukor H., Song W., Arvanitakis Z., Bennett D.A., Schipper H.M. Brain sterol dysregulation in sporadic AD and MCI: relationship to heme oxygenase-1. J. Neurochem. 2009;110:1241–1253. doi: 10.1111/j.1471-4159.2009.06213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crick P.J., William Bentley T., Abdel-Khalik J., Matthews I., Clayton P.T., Morris A.A., Bigger B.W., Zerbinati C., Tritapepe L., Iuliano L., Wang Y., Griffiths W.J. Quantitative charge-tags for sterol and oxysterol analysis. Clin. Chem. 2015;61:400–411. doi: 10.1373/clinchem.2014.231332. [DOI] [PubMed] [Google Scholar]
- 19.Iuliano L., Crick P.J., Zerbinati C., Tritapepe L., Abdel-Khalik J., Poirot M., Wang Y., Griffiths W.J. Cholesterol metabolites exported from human brain. Steroids. 2015;99:189–193. doi: 10.1016/j.steroids.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leoni V., Masterman T., Patel P., Meaney S., Diczfalusy U., Björkhem I. Side chain oxidized oxysterols in cerebrospinal fluid and the integrity of blood-brain and blood-cerebrospinal fluid barriers. J. Lipid Res. 2003;44:793–799. doi: 10.1194/jlr.M200434-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Björkhem I. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J. Intern. Med. 2006;260:493–508. doi: 10.1111/j.1365-2796.2006.01725.x. [DOI] [PubMed] [Google Scholar]
- 22.Dias I.H., Polidori M.C., Griffiths H.R. Hypercholesterolaemia-induced oxidative stress at the blood-brain barrier. Biochem. Soc. Trans. 2014;42:1001–1005. doi: 10.1042/BST20140164. [DOI] [PubMed] [Google Scholar]
- 23.Leszek J., Barreto G.E., Gąsiorowski K., Koutsouraki E., Ávila-Rodrigues M., Aliev G. Inflammatory mechanisms and oxidative stress as key factors responsible for progression of neurodegeneration: role of Brain Innate Immune System. CNS Neurol. Disord. Drug Targets. 2016;15:329–336. doi: 10.2174/1871527315666160202125914. [DOI] [PubMed] [Google Scholar]
- 24.Venkateshappa C., Harish G., Mahadevan A., Srinivas Bharath M.M., Shankar S.K. Elevated oxidative stress and decreased antioxidant function in the human hippocampus and frontal cortex with increasing age: implications for neurodegeneration in Alzheimer’s disease. Neurochem Res. 2012;37:1601–1614. doi: 10.1007/s11064-012-0755-8. [DOI] [PubMed] [Google Scholar]
- 25.Heneka M.T., O’Banion M.K., Terwel D., Kummer M.P. Neuroinflammatory processes in Alzheimer’s disease. J. Neural Transm. (Vienna) 2010;117:919–947. doi: 10.1007/s00702-010-0438-z. [DOI] [PubMed] [Google Scholar]
- 26.Lyman M., Lloyd D.G., Ji X., Vizcaychipi M.P., Ma D. Neuroinflammation: the role and consequences. Neurosci. Res. 2014;79:1–12. doi: 10.1016/j.neures.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Consilvio C., Vincent A.M. Feldman EL. Neuroinflammation, COX-2, and ALS--a dual role? Exp. Neurol. 2004;187:1–10. doi: 10.1016/j.expneurol.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Yasojima K., Schwab C., McGeer E.G., McGeer P.L. Distribution of cyclooxygenase-1 and cyclooxygenase-2 mRNAs and proteins in human brain and peripheral organs. Brain Res. 1999;830:226–236. doi: 10.1016/s0006-8993(99)01389-x. [DOI] [PubMed] [Google Scholar]
- 29.Julien C., Tremblay C., Emond V., Lebbadi M., Salem N., Jr, Bennett D.A., Calon F. Sirtuin 1 decrease parallels the accumulation of tau in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2009;68:48–58. doi: 10.1097/NEN.0b013e3181922348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonda D.J., Lee H.G., Camins A., Pallàs M., Casadesus G., Smith M.A., Zhu X. The sirtuin pathway in ageing and Alzheimer disease: mechanistic and therapeutic considerations. Lancet Neurol. 2011;10:275–279. doi: 10.1016/S1474-4422(11)70013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iuliano L., Micheletta F., Natoli S., Ginanni Corradini S., Iappelli M., Elisei W., Giovannelli L., Violi F., Diczfalusy U. Measurement of oxysterols and alpha-tocopherol in plasma and tissue samples as indices of oxidant stress status. Anal. Biochem. 2003;312:217–223. doi: 10.1016/s0003-2697(02)00467-0. [DOI] [PubMed] [Google Scholar]
- 32.Livak J.K., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 34.Petrov A.M., Kasimov M.R., Zefirov A.L. Brain cholesterol metabolism and its defects: linkage to neurodegenerative diseases and synaptic dysfunction. Acta Nat. 2016;8:58–73. [PMC free article] [PubMed] [Google Scholar]
- 35.Ma M.T., Zhang J., Farooqui A.A., Chen P., Ong W.Y. Effects of cholesterol oxidation products on exocytosis. Neurosci. Lett. 2010;476:36–41. doi: 10.1016/j.neulet.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 36.Ahonen L., Maire F.B., Savolainen M., Kopra J., Vreeken R.J., Hankemeier T., Myöhänen T., Kylli P., Kostiainen R. Analysis of oxysterols and vitamin D metabolites in mouse brain and cell line samples by ultra-high-performance liquid chromatography-atmospheric pressure photoionization-mass spectrometry. J. Chromatogr. A. 2014;1364:214–222. doi: 10.1016/j.chroma.2014.08.088. [DOI] [PubMed] [Google Scholar]
- 37.He X., Jenner A.M., Ong W.Y., Farooqui A.A., Patel S.C. Lovastatin modulates increased cholesterol and oxysterol levels and has a neuroprotective effect on rat hippocampal neurons after kainate injury. J. Neuropathol. Exp. Neurol. 2006;65:652–663. doi: 10.1097/01.jnen.0000225906.82428.69. [DOI] [PubMed] [Google Scholar]
- 38.Kim J.H., Jittiwat J., Ong W.Y., Farooqui A.A., Jenner A.M. Changes in cholesterol biosynthetic and transport pathways after excitotoxicity. J. Neurochem. 2010;112:34–41. doi: 10.1111/j.1471-4159.2009.06449.x. [DOI] [PubMed] [Google Scholar]
- 39.Brown A.J., Jessup W. Oxysterols: sources, cellular storage and metabolism, and new insights into their roles in cholesterol homeostasis. Mol. Asp. Med. 2009;30:111–122. doi: 10.1016/j.mam.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Crick P.J., Beckers L., Baes M., Van Veldhoven P.P., Wang Y., Griffiths W.J. The oxysterol and cholestenoic acid profile of mouse cerebrospinal fluid. Steroids. 2015;99:172–177. doi: 10.1016/j.steroids.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown J., 3rd, Theisler C., Silberman S., Magnuson D., Gottardi-Littell N., Lee J.M., Yager D., Crowley J., Sambamurti K., Rahman M.M., Reiss A.B., Eckman C.B., Wolozin B. Differential expression of cholesterol hydroxylases in Alzheimer’s disease. J. Biol. Chem. 2004;279:34674–34681. doi: 10.1074/jbc.M402324200. [DOI] [PubMed] [Google Scholar]
- 42.Bogdanovic N., Bretillon L., Lund E.G., Diczfalusy U., Lannfelt L., Winblad B., Russell D.W., Björkhem I. On the turnover of brain cholesterol in patients with Alzheimer’s disease. Abnormal induction of the cholesterol-catabolic enzyme CYP46 in glial cells. Neurosci. Lett. 2001;314:45–48. doi: 10.1016/s0304-3940(01)02277-7. [DOI] [PubMed] [Google Scholar]
- 43.Yau J.L., Rasmuson S., Andrew R., Graham M., Noble J., Olsson T., Fuchs E., Lathe R., Seckl J.R. Dehydroepiandrosterone 7-hydroxylase CYP7B: predominant expression in primate hippocampus and reduced expression in Alzheimer’s disease. Neuroscience. 2003;121:307–314. doi: 10.1016/s0306-4522(03)00438-x. [DOI] [PubMed] [Google Scholar]
- 44.Glöckner F., Meske V., Lütjohann D., Ohm T.G. Dietary cholesterol and its effect on tau protein: a study in apolipoprotein E-deficient and P301L human tau mice. J. Neuropathol. Exp. Neurol. 2011;70:292–301. doi: 10.1097/NEN.0b013e318212f185. [DOI] [PubMed] [Google Scholar]
- 45.Resende R., Moreira P.I., Proença T., Deshpande A., Busciglio J., Pereira C., Oliveira C.R. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic. Biol. Med. 2008;44:2051–2057. doi: 10.1016/j.freeradbiomed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 46.Sultana R., Perluigi M., Butterfield D.A. Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic. Biol. Med. 2013;62:157–169. doi: 10.1016/j.freeradbiomed.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thanan R., Oikawa S., Hiraku Y., Ohnishi S., Ma N., Pinlaor S., Yongvanit P., Kawanishi S., Murata M. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int. J. Mol. Sci. 2014;16:193–217. doi: 10.3390/ijms16010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cagnin A., Brooks D.J., Kennedy A.M., Gunn R.N., Myers R., Turkheimer F.E., Jones T., Banati R.B. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358:461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- 49.Hayes A., Thaker U., Iwatsubo T., Pickering-Brown S.M., Mann D.M. Pathological relationships between microglial cell activity and tau and amyloid beta protein in patients with Alzheimer’s disease. Neurosci. Lett. 2002;331:171–174. doi: 10.1016/s0304-3940(02)00888-1. [DOI] [PubMed] [Google Scholar]
- 50.Johnston H., Boutin H., Allan S.M. Assessing the contribution of inflammation in models of Alzheimer’s disease. Biochem. Soc. Trans. 2011;39:886–890. doi: 10.1042/BST0390886. [DOI] [PubMed] [Google Scholar]
- 51.Flex A., Giovannini S., Biscetti F., Liperoti R., Spalletta G., Straface G., Landi F., Angelini F., Caltagirone C., Ghirlanda G., Bernabei R. Effect of proinflammatory gene polymorphisms on the risk of Alzheimer’s disease. Neurodegener. Dis. 2014;13:230–236. doi: 10.1159/000353395. [DOI] [PubMed] [Google Scholar]
- 52.Vasto S., Candore G., Listì F., Balistreri C.R., Colonna-Romano G., Malavolta M., Lio D., Nuzzo D., Mocchegiani E., Di Bona D., Caruso C. Inflammation, genes and zinc in Alzheimer’s disease. Brain Res. Rev. 2008;58:96–105. doi: 10.1016/j.brainresrev.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Eikelenboom P., van Exel E., Hoozemans J.J., Veerhuis R., Rozemuller A.J., van Gool W.A. Neuroinflammation - an early event in both the history and pathogenesis of Alzheimer’s disease. Neurodegener. Dis. 2010;7:38–41. doi: 10.1159/000283480. [DOI] [PubMed] [Google Scholar]
- 54.Sodhi R.K., Singh N. Liver X receptors: emerging therapeutic targets for Alzheimer’s disease. Pharm. Res. 2003;72:45–51. doi: 10.1016/j.phrs.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Candelario-Jalil E., Yang Y., Rosenberg G.A. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 57.Hein A.M., O’Banion M.K. Neuroinflammation and memory: the role of prostaglandins. Mol. Neurobiol. 2009;40:15–32. doi: 10.1007/s12035-009-8066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sil S., Ghosh T. Cox-2 plays a vital role in the impaired anxiety like behavior in colchicine induced rat model of Alzheimer disease. Behav. Neurol. 2016;2016:1501527. doi: 10.1155/2016/1501527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fiala M., Liu Q.N., Sayre J., Pop V., Brahmandam V., Graves M.C., Vinters H.V. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood-brain barrier. Eur. J. Clin. Investig. 2002;32:360–371. doi: 10.1046/j.1365-2362.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- 60.Choi S.H., Aid S., Choi U., Bosetti F. Cyclooxygenases-1 and -2 differentially modulate leukocyte recruitment into the inflamed brain. Pharm. J. 2010;10:448–457. doi: 10.1038/tpj.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poligone B., Baldwin A.S. Positive and negative regulation of NF-kappaB by COX-2: roles of different prostaglandins. J. Biol. Chem. 2001;276:38658–38664. doi: 10.1074/jbc.M106599200. [DOI] [PubMed] [Google Scholar]
- 62.Hoozemans J.J., Veerhuis R., Rozemuller J.M., Eikelenboom P. Neuroinflammation and regeneration in the early stages of Alzheimer’s disease pathology. Int. J. Dev. Neurosci. 2006;24:157–165. doi: 10.1016/j.ijdevneu.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Testa G., Gamba P., Badilli U., Gargiulo S., Maina M., Guina T., Calfapietra S., Biasi F., Cavalli R., Poli G., Leonarduzzi G. Loading into nanoparticles improves quercetin’s efficacy in preventing neuroinflammation induced by oxysterols. PLoS One. 2014;9:e96795. doi: 10.1371/journal.pone.0096795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herskovits A.Z., Guarente L. Sirtuin deacetylases in neurodegenerative diseases of aging. Cell Res. 2013;23:746–758. doi: 10.1038/cr.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X., Zhang S., Blander G., Tse J.G., Krieger M., Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol. Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 66.Yeung F., Hoberg J.E., Ramsey C.S., Keller M.D., Jones D.R., Frye R.A., Mayo M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen J., Zhou Y., Mueller-Steiner S., Chen L.F., Kwon H., Yi S., Mucke L., Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J. Biol. Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 68.Qin W., Yang T., Ho L., Zhao Z., Wang J., Chen L., Zhao W., Thiyagarajan M., MacGrogan D., Rodgers J.T., Puigserver P., Sadoshima J., Deng H., Pedrini S., Gandy S., Sauve A.A., Pasinetti G.M. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J. Biol. Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- 69.Albani D., Polito L., Batelli S., De Mauro S., Fracasso C., Martelli G., Colombo L., Manzoni C., Salmona M., Caccia S., Negro A., Forloni G. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide. J. Neurochem. 2009;110:1445–1456. doi: 10.1111/j.1471-4159.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- 70.Min S.W., Cho S.H., Zhou Y., Schroeder S., Haroutunian V., Seeley W.W., Huang E.J., Shen Y., Masliah E., Mukherjee C., Meyers D., Cole P.A., Ott M., Gan L. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67:953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lutz M.I., Milenkovic I., Regelsberger G., Kovacs G.G. Distinct patterns of sirtuin expression during progression of Alzheimer’s disease. Neuromol. Med. 2014;16:405–414. doi: 10.1007/s12017-014-8288-8. [DOI] [PubMed] [Google Scholar]
- 72.Song N.Y., Na H.K., Baek J.H., Surh Y.J. Docosahexaenoic acid inhibits insulin-induced activation of sterol regulatory-element binding protein 1 and cyclooxygenase-2 expression through upregulation of SIRT1 in human colon epithelial cells. Biochem. Pharm. 2014;92:142–148. doi: 10.1016/j.bcp.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 73.Kumar R., Chaterjee P., Sharma P.K., Singh A.K., Gupta A., Gill K., Tripathi M., Dey A.B., Dey S. Sirtuin1: a promising serum protein marker for early detection of Alzheimer’s disease. PLoS One. 2013;8:e61560. doi: 10.1371/journal.pone.0061560. [DOI] [PMC free article] [PubMed] [Google Scholar]