Abstract
Isolated hepatocytes from young (4–6 mo) and old (24–26 mo) F344 rats were exposed to increasing concentrations of menadione, a vitamin K derivative and redox cycling agent, to determine whether the age-related decline in Nrf2-mediated detoxification defenses resulted in heightened susceptibility to xenobiotic insult. An LC50 for each age group was established, which showed that aging resulted in a nearly 2-fold increase in susceptibility to menadione (LC50 for young: 405 μM; LC50 for old: 275 μM). Examination of the known Nrf2-regulated pathways associated with menadione detoxification revealed, surprisingly, that NAD(P)H: quinone oxido-reductase 1 (NQO1) protein levels and activity were induced 9-fold and 4-fold with age, respectively (p=0.0019 and p=0.018; N=3), but glutathione peroxidase 4 (GPX4) declined by 70% (p=0.0043; N=3). These results indicate toxicity may stem from vulnerability to lipid peroxidation instead of inadequate reduction of menadione semi-quinone. Lipid peroxidation was 2-fold higher, and GSH declined by a 3-fold greater margin in old versus young rat cells given 300 µM menadione (p<0.05 and p≤0.01 respectively; N=3). We therefore provided 400 µM N-acetyl-cysteine (NAC) to hepatocytes from old rats before menadione exposure to alleviate limits in cysteine substrate availability for GSH synthesis during challenge. NAC pretreatment resulted in a >2-fold reduction in cell death, suggesting that the age-related increase in menadione susceptibility likely stems from attenuated GSH-dependent defenses. This data identifies cellular targets for intervention in order to limit age-related toxicological insults to menadione and potentially other redox cycling compounds.
Abbreviations: ARE, Antioxidant Response Element; BSO, Buthionine-S,R-Sulfoximine; CPR, Cytochrome P450 reductase; DCPIP, Dichlorophenolindophenol; GPX4, Glutathione Peroxidase 4; LDHA, Lactate dehydrogenase A; LOO, Lipid peroxide; MDA, Malonyldialdehyde; NQO1, NAD(P)H:quinone oxido-reductase 1; NQO2, NAD(P)H:quinone oxido-reductase 2; Nrf2, Nuclear factor (erythroid-derived 2)-like 2; RIPA, Radioimmunoprecipitation assay; TRE, TPA-Response Element
Chemical compounds studies in this article: BSO (PubChem CID: 119565), DMF (PubChem CID: 6228), NAC (PubChem CID: 12035), Menadione (PubChem CID: 4055)
Keywords: Glutathione, Menadione, Redox-cycling, Detoxification Capacity, Aging
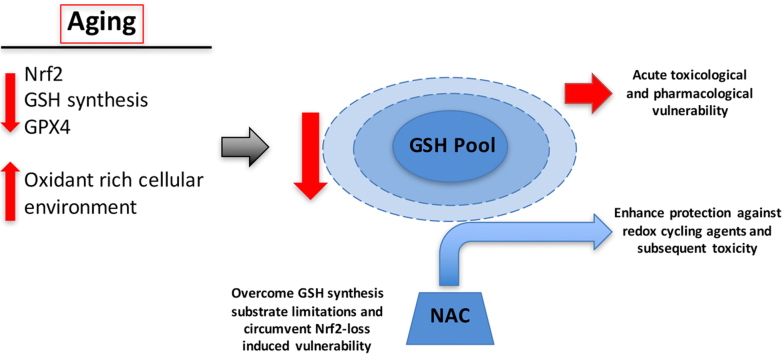
Graphical abstract
Highlights
-
•
Menadione toxicity is nearly two-fold higher with age by LC50 in F344 hepatocytes.
-
•
Glutathione (GSH) loss during menadione treatment is accelerated with age.
-
•
Age-related loss of GPX4 protein correlates with increased menadione-induced MDA.
-
•
NAC maintained GSH mitigates age-related susceptibility to redox cycling menadione.
1. Introduction
A major hallmark of aging, and a key driver for the onset of age-related pathophysiologies across multiple species, including primates, is the disruption of cellular redox homeostatic mechanisms that protect against a variety of environmental, oxidative, pathological, and toxicological insults [1], [2], [3], [4], [5], [6]. Nrf2-dependent phase II detoxification mechanisms in particular, tend to decline with age [7], [8], [9], [10], [11], [12]. The age-related decrease in these detoxification pathways and ensuing increase of reactive oxygen/nitrogen species (ROS/RNS) is well established and is causally linked to various pathologies such as cardiovascular and neurodegenerative diseases, cancer and diabetes [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]. The mechanisms associated with this loss are poorly understood; however, we have found that hepatic Nrf2 protein synthesis declines with aging and that phase II detoxification gene expression is limited [7], [23], [24]. However, despite the age-related decline in basal expression of Nrf2 regulated detoxification enzymes, it remains unknown whether this loss magnifies the toxicological exposure effect of ROS/RNS and xenobiotics detoxified through these pathways. Of particular interest to this work are the age-associated changes to resilience against acute exposure to a redox cycling challenge.
Redox cycling compounds are prooxidant catalysts, which facilitate the transfer of electrons onto oxygen to produce reactive oxygen species (ROS) [25], [26]. These compounds are highly abundant as substituents in xenobiotic compounds (e.g. redox active metals and pesticides) [27], [28], [29], [30], [31], redox active pharmacophores (e.g. anesthetics) [32], and especially, their use in pharmaco chemotherapeutic drugs (e.g. menadione, anthracycline, adriamycin, and doxorubicin) [33], [34], [35], [36], [37]. A decreased capacity to detoxify redox cycling agents in the liver could potentially increase vulnerability to xenobiotic exposures, as well as limiting some medical treatment options such as antibiotics and anti-cancer chemotherapeutics [38], [39], [40], [41]. This is of particular importance as cancer incidence increases exponentially with age [42]. Thus, it is important to determine whether there is an age-related decline in detoxification of redox cycling compounds and if so, which of these types of drugs, toxins, or environmental xenobiotics have a heightened toxicity profile.
In order to test our hypothesis that there is an age-related decline in resilience to redox cycling compounds in the liver, we employed an acute menadione challenge. Menadione, a derivative of vitamin K and a redox cycling agent, and its mechanism of action is well characterized. Herein, we show that hepatocytes from aged rats are acutely more susceptible to menadione insult. Moreover, while certain detoxification enzymes involved in menadione metabolism actually increase with age, the observed age-associated vulnerability to menadione appears to stem from a marked attenuation of Nrf2-regulated GSH-dependent detoxification pathways.
2. Materials and methods
2.1. Reagents
NAC (Cat# 616-91-1), menadione (Cat# 58-27-5), NADPH (Cat# 2646-71-1), dichlorophenolindophenol (DCPIP; Cat# 620-45-1), and protease inhibitor cocktail (Cat# P8340) were ordered from Sigma-Aldrich (St. Louis, MO). Collagenase type IV was purchased from Worthington Biochemical Corporation (Lakewood, NJ). PVDF transfer membrane was purchased from Millipore (Billerica, MA). Dicumarol (Cat# 66-76-2) was ordered from Calbiochem (Darmstadt, Germany).
2.2. Animals
Both young and old male F344 rats were from the National Institute on Aging animal colonies. The rats were housed in the Linus Pauling Institute animal facility and allowed to acclimatize for at least 1 week prior to any experimentation. Animals were maintained on a 12 h light cycle (7 a.m.–7 p.m.) and fed standard chow ad libitum. All animal work was approved and in accordance to IACUC guidelines (Assurance Number: A3229-01). The AAALAC-accredited Laboratory Animal Resources Center (LARC) provided management and veterinary care.
2.3. Hepatocyte isolation
Hepatocyte isolation was performed as described previously [43]. Briefly, after animal sacrifice via AALAC-approved protocols, the liver was perfused via a cannula in the portal vein with Hank's balanced salt solution, pH 7.4. Following removal of blood, liver cells were disassociated using collagenase solution (1 mg/mL). The resultant cell suspension was filtered through sterile gauze to remove connective tissue and debris. Parenchymal cells were isolated using gravity filtration and washed three times with Krebs–Henseleit solution, pH 7.4. Cells were resuspended in Kreb-Henseleit solution and placed in a round bottom flask and rotated at room temperature for 1 h before cell count and viability were assessed using trypan blue exclusion.
2.4. Cell and tissue lysates
For whole cell lysates, suspended cells were harvested by centrifugation at 100×g, washed in Krebs-Henseleit solution, pH 7.4, and sonicated in lysis buffer (50 mM Tris, pH 7.5, containing 1% NP-40 (v/v), 100 mM NaCl, 2 mM EDTA, 2 mM sodium ortho-vanadate) with added protease inhibitors. For tissue, lysates were obtained as previously described by Siegel et al.. [44]. Briefly, tissue was homogenized using a dounce homogenizer in RIPA buffer with a volume to weight ratio of 5:1. The homogenate was sonicated 3 times and centrifuged for 15 min at 10,000×g centrifugation (4 °C) before supernatants were collected for assays. For the NQO1 assay, supernatants from tissue lysates were subjected to an additional ultracentrifugation step (30,000×g for 1 h at 4 °C). Protein concentrations of samples were determined either by the Bradford Assay (Cat# 500-0006, BioRad) or Pierce 660 nm assay (Cat# 22660, Thermo Scientific).
2.5. Assessment of menadione toxicity
Hepatocytes were diluted to 4×106 cells/mL using Kreb-Henseleit solution, pH 7.4, and rotated on a MACSMIX (Miltenyi Biotec) rotator placed in a cell culture incubator (5% CO2 at 37° C) to maintain the cells in suspension. Hepatocytes were treated with increasing concentrations of menadione (0, 100, 200, 300, 400, 500, and 600 μM) for 2 h before being assayed for viability using trypan blue exclusion. Menadione was solubilized in dimethylformamide (DMF). DMF was also used as the vehicle control and total DMF in treated cell suspensions was 0.05% by volume for all treatment experiments.
2.6. NQO1 activity assay
NQO1 activity of samples was assayed as described previously by Siegel et al. [44]. Briefly, tissues from young and old animals were prepared as described above before being assayed for the NAD(P)H-dependent reduction of DCPIP by NQO1 in the presence and absence of dicumarol (a reversible NQO1-specific inhibitor). DCPIP reduction was assayed using a DU800 spectrophotometer at 600 nm over 1 min, and NQO1 activity was measured as the dicumarol inhibitable portion of the reduction. Final concentrations of reagents in reaction solution were 0.2 mM NAD(P)H and 40 μM DCPIP with and without 20 μM dicumarol.
2.7. Malondialdehyde quantification
Measurement of the lipid peroxidation product, malonyldialdehyde (MDA), was performed as previously described by Wong et al. [45] and modified by Sommerburg et al. [46]. Briefly, 200 µL of suspended cells (4×106 cells/mL) was mixed with 750 µL of 440 mM phosphoric acid, 250 µL of 42 mM thiobarbituric acid (TBA), and 300 µL of water prior to being boiled for 1 h. The reaction was quenched by placing samples into an ice bath. Samples then had an equal volume (1.5 mL) of 1 M NaOH added before being centrifuged at 16,000×g for 5 min at 10° C. Malondialdehyde was separated from other metabolites by HPLC using a Luna C18(2)] Phenomenex #00G-4252- E0) column in isocratic mode (25 mM potassium phosphate buffer, pH 6.5/methanol [50:50]as eluents) and detected by fluorescence (excitation, 532 nm; emission, 553 nm). Malondialdehyde was quantified relative to a tetramethoxypropane (TMP) standard curve.
2.8. Immunoblotting
Lysates were prepared as described above, sonicated, and proteins were solubilized for PAGE in Laemmli loading buffer containing SDS. Samples were heat-denatured for 5 min at 100 °C. Normalized amounts of protein (30 μg/lane) were run on SDS-PAGE and transferred to PVDF membranes with a semi-dry blotter. Membranes were blocked in PBS containing 1% Tween-20 with either 5% nonfat dry milk or 3% BSA, incubated with primary antibodies for 2 h at room temperature, washed, and incubated with secondary antibodies for 1 h at room temperature, washed, incubated with chemiluminescence reagents, exposed to film, and developed. Images shown were cropped for size/space considerations. Antibodies made to the following proteins were used: GPX4 (Protein Tech - Cat# 14432-1-AP), NQO1 (Abcam - Cat# ab2346), Cytochrome P450 Reductase (CPR, EC 1.6.2.4; Abcam – Cat# 13513), Lactate Dehydrogenase A (LDHA; Cell Signaling Technology - Cat# 2012) and Actin (Sigma-Aldrich – Cat# A5044). Blots were densitometrically analyzed with ImageJ software from NIH.
2.9. Glutathione analysis
Glutathione (GSH) content of suspended cells was determined according to the methods of Fariss and Reed [47] as modified by Smith et al. [7]. Briefly, suspensions were homogenized in an equal volume of 10% (w/v) perchloric acid (PCA) containing 10 mM EDTA. After deproteinization, 200 µL of the supernatant containing internal standard (γ-glutamyl glutamate) was mixed with 50 µL iodoacetic acid (100 mM dissolved in 0.2 mM m-cresol purple) and the pH was adjusted to 10 by using KOH–KHCO3 buffer (2 M KOH:2.4 M KHCO3). Samples were placed in the dark, at room temperature for 1 h. The resulting S-carboxymethyl derivatives were subjected to further chemical modification by addition of an equal volume of 1-fluoro-2,4-dinitrobenzene (1% v/v in absolute ethanol). The solution was incubated overnight in the dark and at room temperature. Samples (75 µL) were separated by HPLC using a Thermo Scientific (Eugene, OR) APS-2 Hypersil column and detected on a Shimadzu (Kyoto, Japan) SPD-10AVP UV–Vis spectrophotometer with the absorbance set at 365 nm. Quantitation was obtained by integration relative to GSH and GSSG standards.
2.10. Statistical analysis
All statistical analysis was performed using Excel (Microsoft, Inc.) and Prism 6 and 7 (GraphPad Software, Inc.). Error bars represent standard error of the mean. For comparisons between two samples, two-sided Student's t-test was used. Differences between samples that resulted in a p-value of ≤0.05 were considered statistically significant. Statistical analyses for multiple comparisons were evaluated by Student's t-test with the Sidak-Bonferroni post-hoc method.
3. Results
3.1. Acute menadione challenge causes hepatocellular damage and loss of viability
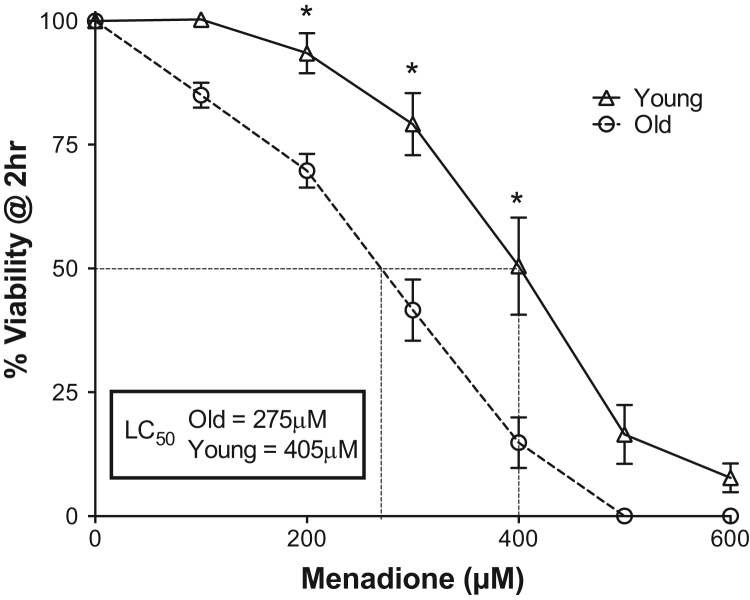
To establish the relative age-associated susceptibility to menadione challenge, aliquots of freshly isolated hepatocytes from young and old rats were incubated with increasing concentrations of menadione, and viability was assessed after 2 h of challenge. Results in Fig. 1 show that regardless of age, increasing concentrations of menadione corresponded with heightened indices of cell damage and loss of viability. For hepatocytes from young rats, the lowest concentration of menadione employed (100 µM) resulted in no changes in toxicity versus vehicle control; in contrast, hepatocytes isolated from old rats lacked any resilience to menadione, demonstrating viability loss at all concentrations tested. The LC50 for menadione was calculated to be 405 µM and 275 µM for young and old rat hepatocytes, respectively (Fig. 1). These results indicate a significantly higher susceptibility to menadione with age (p<0.05, N=3), and suggest that basal cellular defenses to this redox cycling agent are compromised.
Fig. 1.
Acute menadione toxicity is enhanced with age. Isolated hepatocytes from young and old rats were treated with increasing amounts of menadione (0–600 µM). Viability was determined by trypan blue exclusion 2 h after exposure. N=3, *p<0.05..
3.2. Increased vulnerability to menadione with age is not caused by a loss in NQO1 or increase in Cytochrome P450 Reductase (CPR)
Considering the mechanisms associated with its detoxification (Fig. 2), heightened age-dependent vulnerability to menadione may stem from altered activities of one or more antioxidant or phase II detoxification enzymes. As almost all of these enzymes are primarily regulated by Nrf2 [48], [49], [50], [51], [52], [53], [54], we sought to define potential lesions in menadione detoxification, which might serve as the basis for its enhanced toxicity with age.
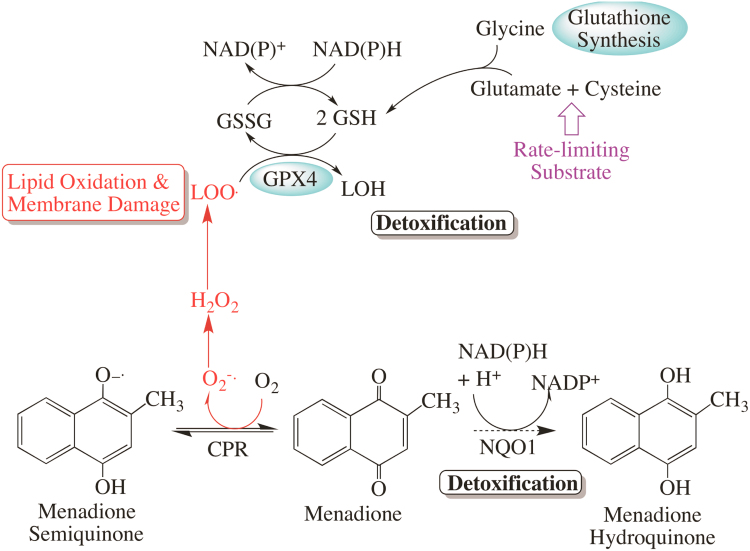
Fig. 2.
Menadione detoxification scheme. Menadione is a potent redox cycling agent which cycles between the quinone and semi-quinone states, producing superoxide, which may eventually lead to oxidative and nitrosative damage. NQO1 detoxifies the menadione quinone by reducing it to the hydroquinone, which is then eliminated. The major resultant oxidative damage from redox cycling is lipid peroxidation (LOO.), which is primarily detoxified by the glutathione-dependent GPX4 enzyme.
CPR converts menadione from the quinone to semiquinone state; therefore, any age-related increase in the reductase would lead to higher levels of redox cycling. However, we found that the CPR protein decreased by ~2-fold in hepatocytes from old animals (Fig. 3A, p=0.0137, N=3). These results suggest that, if anything, there may be less menadione redox cycling with age progression.
Fig. 3.
CPR protein levels decrease while NQO1 protein levels and activity increase with age. CPR protein levels (A), and NQO1 activity (B) and protein levels (C) in liver tissue homogenates from young (Y) and old (O) animals were evaluated. N=3 for A, B and C, *p=0.0137, 0.0185 and 0.0019 respectively.
Because CPR could not account for the elevated toxicity from menadione, we examined the hepatic levels and activity of NQO1, as this enzyme catalyzes the two-electron reduction of menadione to the fully reduced hydroquinone state (Fig. 2). Full reduction limits redox cycling and initiates menadione detoxification and removal from cells. Rather than the hypothesized decline in NQO1 levels with age, we observed that NQO1 protein content and enzyme activity in livers from old rats increased markedly compared to that seen in young animals. As shown in Fig. 3B, NQO1 activity was nearly 4-fold higher with age (p=0.0185, N=3), which is consistent with heightened hepatic protein content of the enzyme (9.3-fold increase, p=0.0019, N=3) in Fig. 3C. While this significant elevation in steady-state NQO1 activity may represent an adaptation toward the heightened pro-oxidant cellular milieu of the aging rat liver, these results nevertheless suggest that NQO1 is not likely a part of the mechanism associated with vulnerability to menadione.
3.3. Age-related decline in hepatic GSH and GPX4 contribute to menadione-mediated cytotoxicity
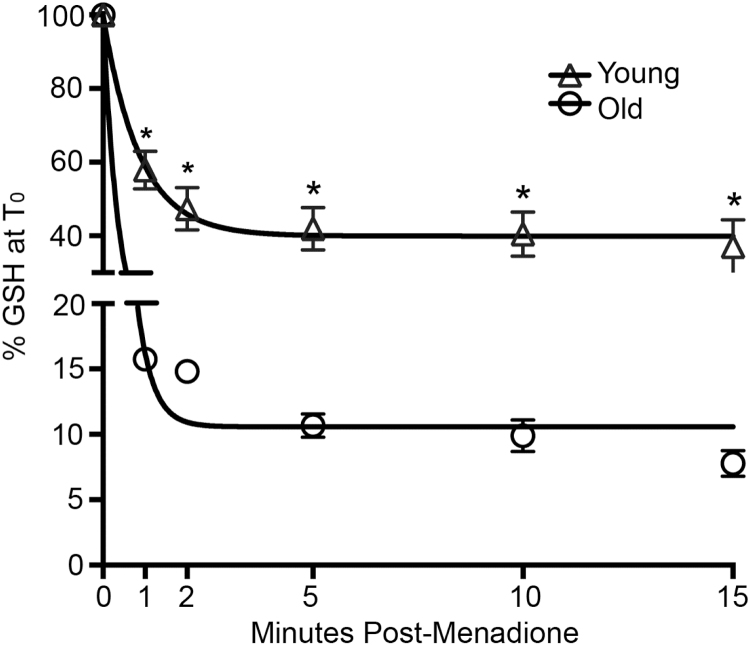
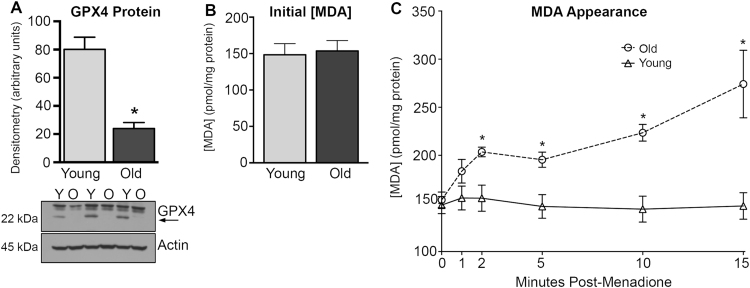
Menadione and the reactive oxygen species initiated by its redox cycling are primarily detoxified by GSH-dependent mechanisms (cf. Fig. 2), which we previously reported to decline significantly with age [7]. In order to determine whether hepatic GSH levels become limiting under menadione insult, hepatocytes from young and old animals were treated with menadione at approximately the LC50 for old rats (300 µM). Results show that menadione rapidly eliminates GSH in both age groups, but it caused a markedly greater loss of GSH hepatocytes from old rats. As shown in Fig. 4, menadione resulted in >80% decline in GSH within one minute in old rat hepatocytes. The menadione-mediated loss of GSH was significantly less severe in cells from young animals (~60% decline). GSH levels at all time points were significantly different between young and old (p≤0.01, N=3). Moreover, GSH levels in the young rat hepatocytes never decreased below a threshold of ~35%. Thus, reserves of hepatic GSH are more rapidly and extensively lost with age, and this precipitous decline in GSH precedes indices of cellular damage and loss of viability.
Fig. 4.
GSH loss during menadione treatment is accelerated with age. Hepatocytes from young and old rats were treated with 300 µM menadione over a 15 min time course and assayed for GSH content. N=3, *p≤0.01.
To further determine age-associated differences in GSH-dependent detoxification of menadione, we examined hepatic concentrations of glutathione peroxidase 4 (GPX4). This enzyme is a primary protectant against phospholipid hydroperoxides, and is also known to play a significant role in protection against redox cycling [55], [56]. Western blot analysis revealed that GPX4 protein levels were significantly lower in old than in young hepatocytes (Fig. 5A; 70%, p=0.0043, N=3), which is consistent with reports of age-related loss in GPX4 levels in multiple tissues, including the liver [57], [58]..
Fig. 5.
GPX4 protein levels are significantly reduced with age which correlates with an increased appearance of menadione-induced MDA. Hepatocytes from young (Y) and old (O) animals were evaluated for GPX4 protein levels by western blot (A). Basal MDA content (B) and MDA accumulation (C) were assayed after menadione treatment. N=3 for A, B and C, *p=0.0043, >0.05, and <0.05 for A, B, and C respectively.
Because GPX4 chiefly protects against lipid peroxidation, we measured hepatic levels of malondialdehyde (MDA), a biomarker of the oxidation of polyunsaturated lipids, through its derivatization with thiobarbituric acid (TBA). Because TBA derivatization may overestimate lipid peroxidation levels, we employed a well-characterized HPLC method to minimize ex vivo artifacts so that relative differences in lipid peroxidation with age and menadione could be discerned. [59] It is interesting to note that, without menadione, no age-associated differences in MDA levels were observed, indicating that basal lipid oxidation and its detoxification are maintained despite the age-related loss of GPX4 (Fig. 5B). However, exposure to menadione at the approximate LC50 for old rat hepatocytes (300 µM), resulted in a rapid appearance of MDA in cells from both age groups (Fig. 5C). Hepatocytes from aged rats were demonstrably more vulnerable to menadione-mediated lipid peroxidation. Initial increases in MDA levels for cells from both young and old animals was observed, followed by a brief stabilization of MDA levels between the 2 and 5 min time points. After this, MDA levels in cells from young animals returned to baseline. In contrast, hepatocytes from old rats displayed a rapid increase in MDA levels after the 2 through 5-min stabilization period. After 15 min, MDA levels were 147.4±13.8 and 274.2±35.1 pmol/mg protein in liver cells from young and old animals, respectively. Moreover, lipid peroxidation levels in hepatocytes from young animals never increased by more than 5%, whereas the extent of lipid oxidation in hepatocytes isolated from old rats were 78% higher and still rising 15 min after menadione challenge. The divergence between young and old is striking and aligns with loss of GPX4, as well as the appearance of cellular damage and loss of viability.
3.4. Age-related menadione toxicity is reversed by GSH precursors
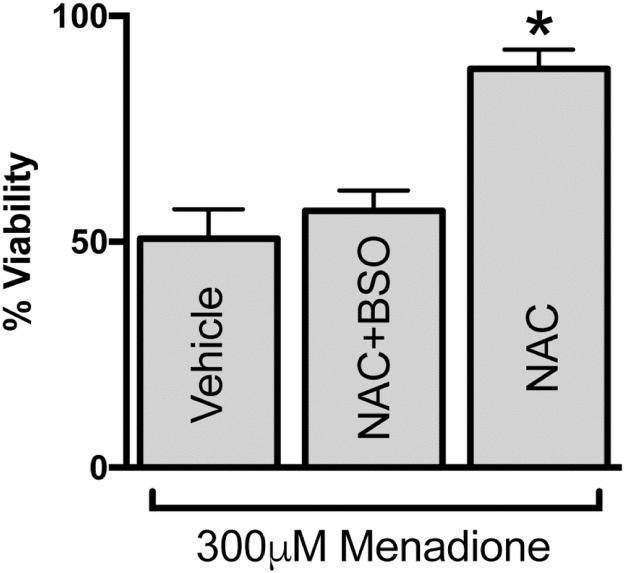
Because GPX4 works at the expense of GSH, and GSH is also lowered with age in our model, we hypothesized that providing cells with NAC should relieve the cysteine substrate limitations for GSH synthesis, and thus increase resistance to menadione insult. A 1 h pretreatment with NAC (400 µM) before menadione exposure significantly increased resistance to menadione in old rat hepatocytes. In fact, our data show that NAC treatment reversed the increased age-associated vulnerability to menadione (Fig. 6, p=0.003, n=5), making this indistinguishable from 300 µM menadione-treated hepatocytes from young animals (Fig. 1). Co-pretreating with buthionine sulfoximine (BSO; 400 µM for 1 h), a specific GSH synthesis inhibitor, eliminated the ability of NAC to rescue hepatocyte viability, which serves as a proof of concept that GSH is the key component.
Fig. 6.
Pretreating cells with NAC prior to menadione exposure improves resistance. Hepatocytes from old animals were pretreated with vehicle (water), 400 µM NAC, or 400 µM NAC & 400 µM BSO for 1 h prior to addition of 300 µM menadione. Viability was determined 2 h later. N=5, *p=0.003 versus vehicle.
4. Discussion
The aging process leads to disparate changes in hepatic detoxification capacity. For example, many phase I enzymes are unaffected by age, while phase II detoxification processes are more variable [60], [61], [62]. Our data exemplifies this with NQO1 levels and activity increasing significantly in aging hepatocytes, but GPX4 appreciably declines. Therefore, while a general age-related loss in basal detoxification capacity is apparent, it is not clear if this decline is at least partly compensatory, or leads to vulnerability to certain classes of xenobiotic compounds. Our present study is important as our data supports the concept that, at least for redox cycling compounds, (e.g. menadione) there is a profound susceptibility with age.
The current study focused on susceptibility to acute menadione insult using freshly isolated hepatocytes from young and old rats. This cell model is appropriate for understanding aging differences in detoxification because these cells retain their aging phenotype with respect to stress resistance. It also allows for a direct assessment of potential age-induced differences in hepatic detoxification capacity at the time of animal sacrifice [43]. Using this hepatocyte model, we previously showed that steady-state Nrf2 levels significantly decline both in hepatocytes and in the aging rat liver [7], [23], [24].
We used menadione in the current study because most of the enzymes involved in its detoxification are Nrf2-dependent. For example, NQO1 maintains menadione in its fully reduced hydroquinone state [25], [26], [63] prior to its further metabolism and removal from the cell (cf. Fig. 2). Additionally, because menadione is a potent redox cycling agent, its mode of acute toxicity results from a cascade of redox cycling, lipid peroxidation, and membrane damage [26], [64], [65], [66], [67]. As such, we chose to examine GSH and GPX4, the principal glutathione peroxidase that is localized to membranes, as the primary players in preventing cell damage from acute menadione exposure [68], [69], [70], [71]. While GPX1 also plays a major role in peroxide detoxification, it is localized to the cytosol and thus is not the initial detoxifier of menadione-induced lipid peroxidation. Thus, xenobiotic disposition and the extent of acute oxidative damage ensuing from menadione exposure are largely dependent on the hepatocellular activity of NQO1, GSH, and GPX4—all of which are Nrf2-regulated mechanisms.
Despite this common tie to Nrf2 associated gene transcription, we found that aging does not uniformly affect all of the enzymatic components involved in menadione detoxification. For example, we observed that hepatic levels of NQO1 and its activity significantly increase with age, while CPR activity is diminished. Overall, these results could be interpreted as an attempt by the hepatocyte to limit levels of the semiquinone species, thereby lowering the rate of redox cycling. However, this protective adaptation against menadione insult in the aging rat liver is counterbalanced by the significant loss of GSH levels and GSH-dependent detoxification enzymes (e.g. GPX4). The reason(s) why certain Nrf2-dependent genes are affected with age while other genes do not change or decline are not presently known. With respect to NQO1, it has been reported that with age, NQO1 levels and/or activity decline [72], increase [73], or remain similar to that seen in young [74]. These results may stem from the varied methods employed to monitor NQO1. We used the reduction of DCPIP as first developed by Ernster et al. [75] and recently optimized by Ross et al. [44]. This method has the benefit of highly purifying the cytosolic fraction through ultracentrifugation and does not assay the reduction of menadione-cytochrome c, which has confounding NQO2 enzymatic activity. Additionally, the NQO1 promoter region contains an embedded TRE (TPA-response element) within the ARE enhancer [76], [77] and demonstrates higher recruitment and binding efficiency for Nrf2 both for basal and induced transcription [78], [79]. These factors may account for the increase we observed in spite of Nrf2 decline. Regardless of the precise mechanism, it is clear that NQO1 is not involved in the age-related enhanced vulnerability to menadione that we observed in this study.
It is equally clear that GSH-dependent defenses are compromised in hepatocytes from aging rats. We and others have previously shown that hepatic GSH declines with age [7], [80], [81], [82], [83], [84]; however, GSH remains at millimolar levels even in very old animals. Thus, it was not known prior to the present study whether the liver is more susceptible to menadione insult that would result from attenuated GSH concentrations. Our results show that menadione causes a rapid loss of GSH in hepatocytes from both young and old rats, but the rate and degree of GSH loss is more extensive in older animals. This lack of capacity to maintain cellular GSH appears to be the most important factor leading to lost resiliency against menadione challenge with age. This concept is buttressed by our results using NAC and NAC+BSO to modulate GSH prior to menadione insult. In particular, NAC, which supplies L-cysteine for GSH synthesis [85], [86], [87], essentially abrogates the increased vulnerability to menadione in aged rat hepatocytes. Taken together, the inability to supply GSH for detoxification, along with diminished GPX4, potentially sets the stage for the aging cell to be susceptible to a variety of toxins, oxidants, and environmental mutagens.
An additional aspect revealed in this study is that GSH levels in young rat hepatocytes never decreased below ~35% of initial levels versus the 90% lost by old. This suggests that when the menadione-mediated oxidation crosses a critical threshold of GSH, cellular toxicity rapidly ensues. Because mitochondria contain a separate GSH pool that approximates this threshold level, it is enticing to suggest that maintenance of mitochondrial GSH is particularly important to resist menadione insult. In this regard, loss of mitochondrial GSH has been demonstrated to cause a decline in mitochondrial membrane potential [66], [88], [89], [90], [91], inducing calcium overload and initiating necro-apoptotic pathways leading to cell death [92], [93], [94], [95], [96]. We are currently investigating the role of mitochondrial GSH in sensitizing the cell toward oxidative insult in aging.
Finally, the results presented in this study identify a specific cellular target to potentially improve detoxification and xenobiotic metabolism. There is a significant clinical history of using NAC to limit toxicity from acute exposure to drugs and toxins [97], [98], [99]. Our data suggest that a similar preventative strategy of NAC administration may be warranted to increase resistance to xenobiotic and drug toxicity in older adults. GSH is a principal detoxicant for environmental xenobiotics, pharmaceuticals, air pollutants, and heavy metals. Providing NAC to increase substrate supply of cysteine may circumvent the age-related decline in GSH synthetic enzymes that attenuation of Nrf2 engenders. Thus, using NAC as a prophylactic instead of an intervention may allow GSH levels to be maintained for detoxification in older adults.
Acknowledgments
This work was supported by NIH Grant P01AT002034 and NIH R012AG17141 and a grant from the Medical Research Foundation of Oregon (Grant number 2014-14-1837). N.O.T. was supported by the NSF's Integrative Graduate Education and Research Traineeship in Aging Research.
References
- 1.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. XThe hallmarks of aging. Cell. 2013;153 doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones D.P. Redox theory of aging. Redox Biol. 2015;5:71–79. doi: 10.1016/j.redox.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Da Silva C.C. Caldeira, Cerqueira F.M., Barbosa L.F., Medeiros M.H.G., Kowaltowski A.J. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell. 2008;7:552–560. doi: 10.1111/j.1474-9726.2008.00407.x. [DOI] [PubMed] [Google Scholar]
- 4.Jones D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006;8:1–16. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 5.Brewer G.J. Epigenetic oxidative redox shift (EORS) theory of aging unifies the free radical and insulin signaling theories. Exp. Gerontol. 2010;45:173–179. doi: 10.1016/j.exger.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paredes J., Jones D., Wilson M., Herndon J. Age-related alterations of plasma glutathione and oxidation of redox potentials in chimpanzee (Pan troglodytes) and rhesus monkey (Macaca mulatta) Age. 2014;36:719–732. doi: 10.1007/s11357-014-9615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suh J.H., Shenvi S.V., Dixon B.M., Liu H., Jaiswal A.K., Liu R.-M., Hagen T.M. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Natl. Acad. Sci. USA. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stohs S.J., Al-Turk W.A., Angle C.R., Heinicke R.J. Glutathione S-transferase activity in liver, lung and intestinal mucosa of aging female mice. Gen. Pharmacol. 1982;13:519–522. doi: 10.1016/0306-3623(82)90028-3. 〈http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7152232〉 [DOI] [PubMed] [Google Scholar]
- 9.Spearman M., Leibman K. Aging selectively alters glutathione S-transferase isozyme concentrations in liver and lung cytosol. Drug Metab. Dispos. 1984;12:661–671. [PubMed] [Google Scholar]
- 10.Carrillo M.C., Nokubo M., Kitani K., Satoh K., Sato K. Age-related alterations of enzyme activities and subunits of hepatic glutathione S-transferases in male and female Fischer-344 rats. Biochim. Biophys. Acta (BBA)/Protein Struct. Mol. 1991;1077:325–331. doi: 10.1016/0167-4838(91)90547-d. [DOI] [PubMed] [Google Scholar]
- 11.Cornet M., Mertens K., Callaerts A., Sonck W., Vercruysse A., Rogiers V. Age- and gender-related changes in the hepatic metabolism of 2-methylpropene and relationship to epoxide metabolizing enzymes. Mech. Ageing Dev. 1994;74:103–115. doi: 10.1016/0047-6374(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 12.Patriarca S., Furfaro A.L., Cosso L., Pesce Maineri E., Balbis E., Domenicotti C., Nitti M., Cottalasso D., Marinari U.M., Pronzato M.A., Traverso N. Heme oxygenase 1 expression in rat liver during ageing and ethanol intoxication. Biogerontology. 2007;8:365–372. doi: 10.1007/s10522-006-9079-x. [DOI] [PubMed] [Google Scholar]
- 13.Ho E., Galougahi K. Karimi, Liu C.-C., Bhindi R., Figtree G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013;1:483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griendling K.K., FitzGerald G.A. Oxidative stress and cardiovascular injury Part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 15.Klaunig J.E., Kamendulis L.M. The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 16.Liu J., Qu W., Kadiiska M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009;238:209–214. doi: 10.1016/j.taap.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maritim A.C., Sanders R.A., Watkins J.B. Diabetes, oxidative stress, and antioxidants: a review. J. Biochem. Mol. Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 18.Rains J.L., Jain S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sekhar R.V., Mckay S.V., Patel S.G., Guthikonda A.P., Reddy V.T., Balasubramanyam A., Jahoor F. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care. 2011;34:162–167. doi: 10.2337/dc10-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh D., LeVault K.R., Barnett A.J., Brewer G.J. A reversible early oxidized redox state that precedes macromolecular ROS damage in aging nontransgenic and 3xTg-AD mouse neurons. J. Neurosci. 2012;32:5821–5832. doi: 10.1523/JNEUROSCI.6192-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh D., Levault K.R., Brewer G.J. Relative importance of redox buffers GSH and NAD(P)H in age-related neurodegeneration and Alzheimer disease-like mouse neurons. Aging Cell. 2014;13:631–640. doi: 10.1111/acel.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshino J., Mills K.F., Yoon M.J., Imai S.I. Nicotinamide mononucleotide, a key NAD + intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shay K.P., Michels A.J., Li W., Kong A.N.T., Hagen T.M. Cap-independent Nrf2 translation is part of a lipoic acid-stimulated detoxification stress response. Biochim. Biophys. Acta – Mol. Cell Res. 2012;1823:1102–1109. doi: 10.1016/j.bbamcr.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith E.J., Shay K.P., Thomas N.O., Butler J.A., Finlay L.F., Hagen T.M. Age-related loss of hepatic Nrf2 protein homeostasis: potential role for heightened expression of miR-146a. Free Radic. Biol. Med. 2015;89:1184–1191. doi: 10.1016/j.freeradbiomed.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lind C., Hochstein P., Ernster L. DT-diaphorase as a quinone reductase: a cellular control device against semiquinone and superoxide radical formation. Arch. Biochem. Biophys. 1982;216:178–185. doi: 10.1016/0003-9861(82)90202-8. [DOI] [PubMed] [Google Scholar]
- 26.Thor H., Smith M.T., Hartzell P., Bellomo G., Jewell S.A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J. Biol. Chem. 1982;257:12419–12425. [PubMed] [Google Scholar]
- 27.Kalinowski D.S., Stefani C., Toyokuni S., Ganz T., Anderson G.J., Subramaniam N.V., Trinder D., Olynyk J.K., Chua A., Jansson P.J., Sahni S., Lane D.J.R., Merlot A.M., Kovacevic Z., Huang M.L.H., Lee C.S., Richardson D.R. Redox cycling metals: pedaling their roles in metabolism and their use in the development of novel therapeutics. Biochim. Biophys. Acta – Mol. Cell Res. 2016;1863:727–748. doi: 10.1016/j.bbamcr.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 28.Jomova K., Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Ercal N., Gurer-Orhan H., Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 30.Bonneh-Barkay D., Langston W.J., Di Monte D.A. Toxicity of redox cycling pesticides in primary mesencephalic cultures. Antioxid. Redox Signal. 2005;7:649–653. doi: 10.1089/ars.2005.7.649. [DOI] [PubMed] [Google Scholar]
- 31.DeLorenzo M.E., Scott G.I., Ross P.E. Toxicity of pesticides to aquatic microorganisms: a review. Environ. Toxicol. Chem. 2001;20:84–98. doi: 10.1897/1551-5028(2001)020<0084:toptam>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Kovacic P., Somanathan R. Mechanism of anesthetic toxicity: metabolism, reactive oxygen species, oxidative stress, and electron transfer. ISRN Anesthesiol. 2011;2011 [Google Scholar]
- 33.Michalska T., Kruk I., Lichszteld K., Kladny J. Hydroxyl radical generation by redox cycling of some chemotherapeutic antibiotics. Toxicol. Environ. Chem. 1996;56:171–176. 〈http://www.scopus.com/inward/record.url?eid=2-s2.0-0030483429&partnerID=40&md5=8c32caa5d8abab42d1a6736b2285fb4e〉 [Google Scholar]
- 34.Sack M., Alili L., Karaman E., Das S., Gupta A., Seal S., Brenneisen P. Combination of conventional chemotherapeutics with redox-active cerium oxide nanoparticles--a novel aspect in cancer therapy. Mol. Cancer Ther. 2014;13:1740–1749. doi: 10.1158/1535-7163.MCT-13-0950. [DOI] [PubMed] [Google Scholar]
- 35.von Montfort C., Alili L., Teuber-Hanselmann S., Brenneisen P. Redox-active cerium oxide nanoparticles protect human dermal fibroblasts from PQ-induced damage. Redox Biol. 2015;4:1–5. doi: 10.1016/j.redox.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wondrak G.T. Redox-directed cancer therapeutics: molecular mechanisms and opportunities. Antioxid. Redox Signal. 2009;11:3013–3069. doi: 10.1089/ars.2009.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zubair H., Khan H.Y., Sohail a, Azim S., Ullah M.F., Ahmad a, Sarkar F.H., Hadi S.M. Redox cycling of endogenous copper by thymoquinone leads to ROS-mediated DNA breakage and consequent cell death: putative anticancer mechanism of antioxidants. Cell Death Dis. 2013;4:e660. doi: 10.1038/cddis.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J.H. Chemotherapy for colorectal cancer in the elderly. World J. Gastroenterol. 2015;21:5158–5166. doi: 10.3748/wjg.v21.i17.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wildiers H. Mastering chemotherapy dose reduction in elderly cancer patients. Eur. J. Cancer. 2007;43:2235–2241. doi: 10.1016/j.ejca.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 40.Wedding U., Honecker F., Bokemeyer C., Pientka L., Höffken K. Tolerance to chemotherapy in elderly patients with cancer. Cancer Control. 2007;14:44–56. doi: 10.1177/107327480701400106. [DOI] [PubMed] [Google Scholar]
- 41.Köhne C.-H., Folprecht G., Goldberg R.M., Mitry E., Rougier P. Chemotherapy in elderly patients with colorectal cancer. Oncologist. 2008;13:390–402. doi: 10.1634/theoncologist.2007-0043. [DOI] [PubMed] [Google Scholar]
- 42.Meza R., Jeon J., Moolgavkar S.H., Luebeck E.G. Age-specific incidence of cancer: phases, transitions, and biological implications. Proc. Natl. Acad. Sci. USA. 2008;105:16284–16289. doi: 10.1073/pnas.0801151105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shenvi, S.V., Dixon, B.M., Shay, K.P., Hagen, T.M., A rat primary hepatocyte culture model for a ging studies Curr. Protoc. Toxicol. 2008. 〈http://dx.doi.org/10.1002/0471140856.tx1407s37〉. [DOI] [PMC free article] [PubMed]
- 44.Siegel D., Kepa J.K., Ross D. Biochemical and genetic analysis of NAD(P)H: quinone oxidoreductase 1 (NQO1) Curr. Protoc. Toxicol. 2007 doi: 10.1002/0471140856.tx0422s32. (Chapter 4, Unit 4.22) [DOI] [PubMed] [Google Scholar]
- 45.Wong S.H.Y., Knight J.A., Hopfer S.M., Zaharia O., Leach C.N., Sunderman F.W. Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin. Chem. 1987;33:214–220. [PubMed] [Google Scholar]
- 46.Sommerburg O., Grune T., Klee S., Ungemach F.R., Siems W.G. Formation of 4-hydroxynonenal and further aldehydic mediators of inflammation during bromotrichlorornethane treatment of rat liver cells. Mediat. Inflamm. 1993;2:27–31. doi: 10.1155/S0962935193000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fariss M.W., Reed D.J. High-performance liquid chromatography of thiols and disulfides: Dinitrophenol derivatives. Methods Enzym. 1987;143:101–109. doi: 10.1016/0076-6879(87)43018-8. [DOI] [PubMed] [Google Scholar]
- 48.Venugopal R., Jaiswal a K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H: quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu H., Itoh K., Yamamoto M., Zweier J.L., Li Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005;579:3029–3036. doi: 10.1016/j.febslet.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 50.Vnukov V.V., Gutsenko O.I., Milutina N.P., Ananyan A.A., Danilenko A.O., Panina S.B., Kornienko I.V. Influence of SkQ1 on expression of Nrf2 transcription factor gene, ARE-controlled genes of antioxidant enzymes and their activity in rat blood leukocytes. Biochemistry. 2015;80:586–591. doi: 10.1134/S0006297915050107. [DOI] [PubMed] [Google Scholar]
- 51.Brigelius-Flohe R. Glutathione peroxidases and redox-regulated transcription factors. Biol. Chem. 2006:1329–1335. doi: 10.1515/BC.2006.166. [DOI] [PubMed] [Google Scholar]
- 52.Wild A., Mulcahy R. Regulation of gamma-glutamylcysteine synthetase subunit gene expression: insights into transcriptional control of antioxidant defenses. Free Radic. Res. 2000;32:281–301. doi: 10.1080/10715760000300291. [DOI] [PubMed] [Google Scholar]
- 53.Chan J.Y., Kwong M. Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic-leucine zipper protein. Biochim. Biophys. Acta Gene Struct. Expr. 2000;1517:19–26. doi: 10.1016/s0167-4781(00)00238-4. [DOI] [PubMed] [Google Scholar]
- 54.Kweon M.H., Park Y. In, Sung H.C., Mukhtar H. The novel antioxidant 3-O-caffeoyl-1-methylquinic acid induces Nrf2-dependent phase II detoxifying genes and alters intracellular glutathione redox. Free Radic. Biol. Med. 2006;40:1349–1361. doi: 10.1016/j.freeradbiomed.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Cao J.Y., Dixon S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016:1–15. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imai H., Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic. Biol. Med. 2003;34:145–169. doi: 10.1016/s0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- 57.Zhang L.P., Maiorino M., Roveri A., Ursini F. Phospholipid hydroperoxide glutathione-peroxidase – specific activity in tissues of rats of different age and comparison with other glutathione peroxidases. Biochim. Biophys. Acta. 1989;1006:140–143. doi: 10.1016/0005-2760(89)90336-6. <Go to ISI>://A1989AY82400022. [DOI] [PubMed] [Google Scholar]
- 58.Glass D., Viñuela A., Davies M.N., Ramasamy A., Parts L., Knowles D., Brown A.A., Hedman A.K., Small K.S., Buil A., Grundberg E., Nica A.C., Di Meglio P., Nestle F.O., Ryten M., Durbin R., McCarthy M.I., Deloukas P., Dermitzakis E.T., Weale M.E., Bataille V., Spector T.D. Gene expression changes with age in skin, adipose tissue, blood and brain. Genome Biol. 2013;14:R75. doi: 10.1186/gb-2013-14-7-r75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Halliwell, B., Gutteridge, J.M.C., 2007. Free Radicals in Biology and Medicine. 〈http://dx.doi.org/10.1016/0891-5849(91)90055-8〉. [DOI] [PubMed]
- 60.Leakey J., Cunny H., Bazare J., Jr., Webb P., Lipscomb J., Slikker W., Jr., Feuers R., Duffy P., Hart R. Effects of aging and caloric restriction on hepatic drug metabolizing enzymes in the fischer 344 rat II: effects on conjugating enzymes. Mech. Ageing Dev. 1989;48:157–166. doi: 10.1016/0047-6374(89)90047-x. [DOI] [PubMed] [Google Scholar]
- 61.Amicarelli F., Di Ilio C., Masciocco L., Bonfigli A., Zarivi O., D’Andrea M.R., Di Giulio C., Miranda M. Aging and detoxifying enzymes responses to hypoxic or hyperoxic treatment. Mech. Ageing Dev. 1997;97:215–226. doi: 10.1016/s0047-6374(97)00061-4. [DOI] [PubMed] [Google Scholar]
- 62.Vyskocilova E., Szotakova B., Skalova L., Bartikova H., Hlavacova J., Bousova I. Age-related changes in hepatic activity and expression of detoxification enzymes in male rats. Biomed. Res. Int. 2013;2013 doi: 10.1155/2013/408573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cadenas E. Antioxidant and prooxidant functions of DT-diaphorase in quinone metabolism. Biochem. Pharmacol. 1995;49:127–140. doi: 10.1016/s0006-2952(94)00333-5. [DOI] [PubMed] [Google Scholar]
- 64.Morrison H., Jernstrom B., Nordenskjold M., Thor H., Orrenius S. Induction of dna damage by menadione (2-methyl-1,4-naphthoquinone) in primary cultures of rat hepatocytes. Biochem. Pharmacol. 1984;33:1763–1769. doi: 10.1016/0006-2952(84)90347-2. [DOI] [PubMed] [Google Scholar]
- 65.Morrison H., Monte D.D.I., Nordenskjold M., Jernstrom B. Induction of cell damage by menadione and benzo(a)-pyrene-3,6-quinone in cultures of adult rat hepatocytes and human fibroblasts. Toxicol. Lett. 1985;28:37–47. doi: 10.1016/0378-4274(85)90007-4. [DOI] [PubMed] [Google Scholar]
- 66.Frei B., Winterhalter K.H., Richter C. Menadione- (2-methyl-1,4-naphthoquinone-) dependent enzymatic redox cycling and calcium release by mitochondria. Biochemistry. 1986;25:4438–4443. doi: 10.1021/bi00363a040. 〈http://www.ncbi.nlm.nih.gov/pubmed/3092856〉 [DOI] [PubMed] [Google Scholar]
- 67.Smith P.F., Alberts D.W., Rush G.F. Menadione-induced oxidative stress in hepatocytes isolated from fed and fasted rats: The role of NADPH-regenerating pathways. Toxicol. Appl. Pharmacol. 1987;89:190–201. doi: 10.1016/0041-008x(87)90040-8. [DOI] [PubMed] [Google Scholar]
- 68.Wefers H., Sies H. Hepatic low-level chemiluminescence during redox cycling of menadione and the menadione-glutathione conjugate: Relation to glutathione and NAD(P)H: quinone reductase (DT-diaphorase) activity. Arch. Biochem. Biophys. 1983;224:568–578. doi: 10.1016/0003-9861(83)90244-8. [DOI] [PubMed] [Google Scholar]
- 69.Ross D., Thor H., Orrenius S., Moldeus P. Interaction of menadione (2-methyl-1,4-naphthoquinone) with glutathione. Chem. Biol. Interact. 1985;55:177–184. doi: 10.1016/s0009-2797(85)80126-5. [DOI] [PubMed] [Google Scholar]
- 70.Chung J.H., Seo D.C., Chung S.H., Lee J.Y., Seung S. a. Metabolism and cytotoxicity of menadione and its metabolite in rat platelets. Toxicol. Appl. Pharmacol. 1997;142:378–385. doi: 10.1006/taap.1996.8048. [DOI] [PubMed] [Google Scholar]
- 71.Chiou T.J., Tzeng W.F. The roles of glutathione and antioxidant enzymes in menadione-induced oxidative stress. Toxicology. 2000;154:75–84. doi: 10.1016/s0300-483x(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 72.Shih P.H., Yen G.C. Differential expressions of antioxidant status in aging rats: the role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology. 2007;8:71–80. doi: 10.1007/s10522-006-9033-y. [DOI] [PubMed] [Google Scholar]
- 73.Hyun D.-H., Emerson S.S., Jo D.-G., Mattson M.P., de Cabo R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc. Natl. Acad. Sci. USA. 2006;103:19908–19912. doi: 10.1073/pnas.0608008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tung B.T., Rodriguez-Bies E., Ballesteros-Simarro M., Motilva V., Navas P., Lopez-Lluch G. Modulation of endogenous antioxidant activity by resveratrol and exercise in mouse liver is age dependent. J. Gerontol. – Ser. A Biol. Sci. Med. Sci. 2014;69:398–409. doi: 10.1093/gerona/glt102. [DOI] [PubMed] [Google Scholar]
- 75.Ernster L. DT diaphorase. Methods Enzymol. 1967;10:309–317. [Google Scholar]
- 76.Li Y. Regulation of human NAD(P)H: Quinone oxidoreductase gene: role of api binding site contained within human antioxidant response element. J. Biol. Chem. 1992;267:15097–15104. [PubMed] [Google Scholar]
- 77.Xie T., Belinsky M., Xu Y., Jaiswal A.K. ARE- and TRE-mediated regulation of gene expression: response to xenobiotics and antioxidants. J. Biol. Chem. 1995;270:6894–6900. doi: 10.1074/jbc.270.12.6894. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen T., Huang H.C., Pickett C.B. Transcriptional regulation of the antioxidant response element. Activation by Nrf2 and repression by MafK. J. Biol. Chem. 2000;275:15466–15473. doi: 10.1074/jbc.M000361200. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen T., Sherratt P.J., Pickett C.B. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 80.Farooqui M.Y., Day W.W., Zamorano D.M. Glutathione and lipid peroxidation in the aging rat. Comp. Biochem. Physiol. B. 1987;88:177–180. doi: 10.1016/0305-0491(87)90097-6. [DOI] [PubMed] [Google Scholar]
- 81.Loguercio C., Taranto D., Vitale L.M., Beneduce F., Del Vecchio C. Blanco, Effect of liver cirrhosis and age on the glutathione concentration in the plasma, erythrocytes, and gastric mucosa of man. Free Radic. Biol. Med. 1996;20:483–488. doi: 10.1016/0891-5849(96)02057-6. [DOI] [PubMed] [Google Scholar]
- 82.Liu R., Choi J. Age-associated decline in gamma-glutamylcysteine synthetase gene expression in rats. Free Radic. Biol. Med. 2000;28:566–574. doi: 10.1016/s0891-5849(99)00269-5. doi:S0891584999002695 [pii] [DOI] [PubMed] [Google Scholar]
- 83.Liu R. Down-regulation of gamma-glutamylcysteine synthetase regulatory subunit gene expression in rat brain tissue during aging. J. Neurosci. Res. 2002;68:344–351. doi: 10.1002/jnr.10217. [DOI] [PubMed] [Google Scholar]
- 84.Erden-Inal M., Sunal E., Kanbak G. Age-related changes in the glutathione redox system. Cell Biochem. Funct. 2002;20:61–66. doi: 10.1002/cbf.937. [DOI] [PubMed] [Google Scholar]
- 85.Miners J.O., Drew R., Birkett D.J. Mechanism of action of paracetamol protective agents in mice in vivo. Biochem. Pharmacol. 1984;33:2995–3000. doi: 10.1016/0006-2952(84)90599-9. [DOI] [PubMed] [Google Scholar]
- 86.Pratt S., Ioannides C. Mechanism of the protective action of n-acetylcysteine and methionine against paracetamol toxicity in the hamster. Arch. Toxicol. 1985;57:173–177. doi: 10.1007/BF00290883. [DOI] [PubMed] [Google Scholar]
- 87.Gouix E., Buisson A., Nieoullon A., Kerkerian-Le Goff L., Tauskela J.S., Blondeau N., Had-Aissouni L. Oxygen glucose deprivation-induced astrocyte dysfunction provokes neuronal death through oxidative stress. Pharmacol. Res. 2014;87:8–17. doi: 10.1016/j.phrs.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 88.Costantini P., Chernyak B.V., Petronilli V., Bernardi P. Modulation of the mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. J. Biol. Chem. 1996;271:6746–6751. doi: 10.1074/jbc.271.12.6746. [DOI] [PubMed] [Google Scholar]
- 89.Halestrap A.P., Woodfield K.Y., Connern C.P. Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J. Biol. Chem. 1997;272:3346–3354. doi: 10.1074/jbc.272.6.3346. [DOI] [PubMed] [Google Scholar]
- 90.Chernyak B.V., Bernardi P. The mitochondrial permeability transition pore is modulated by oxidative agents through both pyridine nucleotides and glutathione at two separate sites. Eur. J. Biochem. 1996;238:623–630. doi: 10.1111/j.1432-1033.1996.0623w.x. [DOI] [PubMed] [Google Scholar]
- 91.Marí M., Morales A., Colell A., García-Ruiz C., Fernández-Checa J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox Signal. 2009;11:2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Green D.R., Reed J.C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. (Review) (59 refs) [DOI] [PubMed] [Google Scholar]
- 93.Fariss M.W., Chan C.B., Patel M., Van Houten B., Orrenius S. Role of mitochondria in toxic oxidative stress. Mol. Interv. 2005;5:94–111. doi: 10.1124/mi.5.2.7. [DOI] [PubMed] [Google Scholar]
- 94.Ott M., Gogvadze V., Orrenius S., Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 95.Nieminen A.-L. Apoptosis and necrosis in health and disease: role of mitochondria. Int. Rev. Cytol. 2003;224:29–55. doi: 10.1016/s0074-7696(05)24002-0. [DOI] [PubMed] [Google Scholar]
- 96.Lemasters J.J., Theruvath T.P., Zhong Z., Nieminen A.-L. Mitochondrial calcium and the permeability transition in cell death. Biochim. Biophys. Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bémeur C., Vaquero J., Desjardins P., Butterworth R.F. N-Acetylcysteine attenuates cerebral complications of non-acetaminophen- induced acute liver failure in mice: antioxidant and anti-inflammatory mechanisms. Metab. Brain Dis. 2010;25:241–249. doi: 10.1007/s11011-010-9201-2. [DOI] [PubMed] [Google Scholar]
- 98.Chun L.J., Tong M.J., Busuttil R.W., Hiatt J.R. Acetaminophen hepatotoxicity and acute liver failure. J. Clin. Gastroenterol. 2009;43:342–349. doi: 10.1097/MCG.0b013e31818a3854. [DOI] [PubMed] [Google Scholar]
- 99.Fontana R.J. Acute liver failure due to drugs. Semin. Liver Dis. 2008;28:175–187. doi: 10.1055/s-2008-1073117. [DOI] [PubMed] [Google Scholar]