Abstract
Aggressive fungal pathogens such as Botrytis and Verticillium spp. cause severe crop losses worldwide. We recently discovered that Botrytis cinerea delivers small RNAs (Bc-sRNAs) into plant cells to silence host immunity genes. Such sRNA effectors are mostly produced by B. cinerea Dicer-like protein 1 (Bc-DCL1) and Bc-DCL2. Here we show that expressing sRNAs that target Bc-DCL1 and Bc-DCL2 in Arabidopsis and tomato silences Bc-DCL genes and attenuates fungal pathogenicity and growth, exemplifying bidirectional cross-kingdom RNAi and sRNA trafficking between plants and fungi. This strategy can be adapted to simultaneously control multiple fungal diseases. We also show that Botrytis can take up external sRNAs and double-stranded RNAs (dsRNAs). Applying sRNAs or dsRNAs that target Botrytis DCL1 and DCL2 genes on the surface of fruits, vegetables, and flowers significantly inhibits gray mold disease. Such pathogen gene-targeting RNAs represent a new generation of environmentally-friendly fungicides.
Small RNAs (sRNAs) are short non-coding regulatory RNAs that are mostly generated by Dicer or Dicer-like (DCL) endoribonucleases from double-stranded RNAs (dsRNAs) or single-stranded RNAs (ssRNAs) with hairpin structures1,2. Mature sRNAs are loaded into Argonaute (AGO) proteins and induce silencing of genes with fully or partially complementary sequences3,4. This phenomenon is called RNA interference (RNAi) and is widely conserved in most eukaryotic organisms. We have recently discovered that Botrytis cinerea, an aggressive plant fungal pathogen, delivers small RNAs (Bc-sRNAs) into host plant cells and utilizes host RNAi machinery to suppress host immunity genes5. These pathogen sRNAs mediate cross-kingdom RNAi in plant hosts and represent a novel class of pathogen effectors that inhibit host immunity for successful infection6,7. Similarly, some animal parasites, such as gastrointestinal nematode Heligmosomoides polygyrus, also deliver sRNAs into mammalian cells and target host genes involved in innate immunity8-12. These studies suggest that sRNA-mediated cross-kingdom RNAi is a conserved virulence mechanism that evolved in both plant and animal eukaryotic pathogens and pests to suppress host immunity. However, it is still not clear how many pathogens and pests have evolved this novel pathogenic mechanism.
While we have demonstrated sRNA trafficking from fungal pathogens to host plants, others have observed that plant transgene-derived artificial sRNAs can induce gene silencing in certain interacting insects13,14, nematodes15,16, fungi17,18, and oomycetes19,20, a phenomenon called host-induced gene silencing (HIGS)21, suggesting that the artificial sRNAs travel from host plants to pathogens and pests. However, bidirectional cross-kingdom RNAi and two-way sRNA trafficking have not been observed between a pathogen and a host, or any two interacting organisms.
Plant diseases caused by eukaryotic pathogens, such as fungi and oomycetes, cause significant crop losses every year. B. cinerea poses a serious threat to almost all vegetables and fruits, as well as many flowers, in their pre- and post-harvest stages by causing gray mold disease22,23. Verticillium dahliae is another economically important fungal pathogen, which causes wilt disease on a wide range of plant species24,25. Current disease control is mainly achieved by fungicide application, which is costly and environmentally hazardous. HIGS has worked effectively against certain fungal and oomycete pathogens, such as Blumeria graminis17,26,27, Puccinia tritici28, Fusarium spp.18,29,30, Phytophthora infestans19,31, and Phytophthora capsici20, but it is restricted to plants with established transformation methods, which is not available to the majority of crop plants. Moreover, genetically modified organisms (GMOs) are still a concern to some consumers. Thus, there is an urgent need to develop effective solutions that are environmentally friendly to control plant diseases caused by pathogens that utilize sRNA effectors, such as B. cinerea.
Results
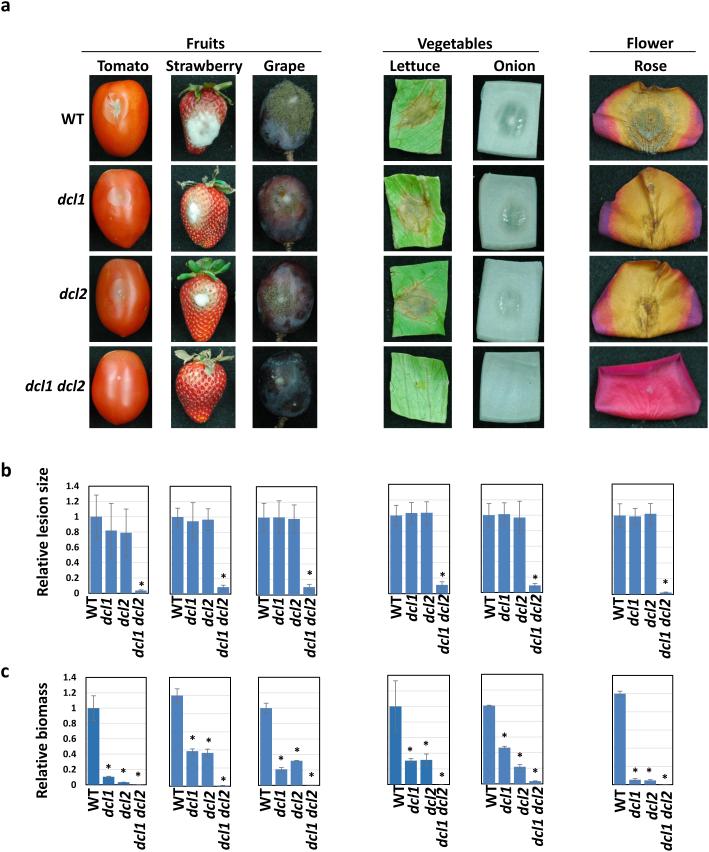
To explore whether cross-kingdom RNAi is bidirectional in B. cinerea-plant interactions and to find a way to protect plants from gray mold disease, we first reasoned that generating HIGS plants producing artificial, traceable sRNAs that target virulence genes in B. cinerea would be a straightforward approach. The B. cinerea genome encodes two DCL genes, Bc-DCL1 and Bc-DCL2 (Bc-DCL1/2). We previously showed that the pathogenicity and growth of B. cinerea were largely compromised when both DCLs were knocked out5. To fully evaluate the contribution of Bc-DCLs to B. cinerea pathogenicity on a wide range of economically important crops, we inoculated various fruits (tomato—Solanum lycopersicum 'Roma', strawberry—Fragaria × ananassa, and grape—Vitis labrusca 'Concord'), vegetables (iceberg lettuce—Lactuca sativa and onion—Allium cepa L.), and flower petals (rose—Rosa hybrida L.) with the dcl1 dcl2 double mutant, dcl1 and dcl2 single mutants and wild-type (WT) strains. Only the dcl1 dcl2 double mutant, but not the dcl1 or dcl2 single mutants, showed much weaker pathogenicity and produced significantly smaller lesions than the WT strain on all the plant samples (Fig. 1a,b, P < 0.01), although all the mutant strains showed reduced growth in planta (Fig. 1c), as well as on cultured media (Supplementary Fig. 1a). More importantly, only the dcl1 dcl2 double mutant, but not the dcl1 or dcl2 single mutants, failed to produce Bc-sRNA effectors5. Thus, elimination of Bc-sRNA effectors contributes more to the reduced virulence seen in dcl1 dcl2 than growth attenuation, highlighting the importance of both Bc-DCLs and sRNA effectors in fungal pathogenicity. We profiled total sRNAs isolated from the dcl1 dcl2 double mutant and the WT strain. In the WT strain, Bc-sRNAs ranged from 20 to 35 nucleotides (nt) in length (Supplementary Fig. 1b), with an enrichment of 24–27-nt species. The abundance of 20–27-nt sRNA species was clearly reduced but not completely eliminated in the dcl1 dcl2 double mutant (Supplemental Fig. 1b), pointing to the existence of DCL-independent sRNA biogenesis pathways in B. cinerea, as reported in Neurospora crassa32,33. Most strikingly, the sRNAs derived from retrotransposons mostly long terminal repeats (LTRs) (ranging from 20–26-nt and peaking at 21–22-nt) were almost completely eliminated in the dcl1 dcl2 double mutant (Supplementary Fig. 1b), which indicates that Bc-DCL1/2 are responsible for generating almost all sRNAs from retrotransposons. The sRNAs from intergenic non-coding (IGN) regions (mainly the 21-, 22- and 24-nt sRNAs) and antisense to open reading frames (ORFs-antisense, mainly the 21-22-nt sRNAs) were also largely reduced in dcl1 dcl2, although the sRNAs from the sense transcripts of ORFs (ORFs-sense) were not changed significantly. This result is consistent with our previous findings that the majority of predicted sRNA effectors are from retrotransposon LTRs5. Bc-DCL1/2 are largely responsible for generating sRNA effectors (Supplementary Table 1) and contribute significantly to B. cinerea pathogenicity. Although it is not known whether HIGS works in B. cinerea, these results show that Bc-DCL1/2 are ideal targets for testing whether HIGS would be an efficient strategy for controlling gray mold disease and whether cross-kingdom RNAi in the opposite direction from the plant host to B. cinerea occurs.
Figure 1. B. cinerea dcl1 dcl2 double mutant, but not the dcl1 or dcl2 single mutant, displays reduced virulence on fruits, vegetables, and flower petals.
(a) B. cinerea dcl1 dcl2 double mutant shows compromised virulence on fruits (tomato, strawberry, and grape), vegetables (lettuce and onion), and flower petals (rose), while B. cinerea dcl1 and dcl2 single mutants showed similar virulence as the WT strain. (b) Relative lesion sizes of the infected plant samples were measured 3 days post inoculation (dpi) for lettuce, onion, and strawberry and 5 dpi for tomato, grape, and rose petal using ImageJ, and error bars indicate the standard deviations (SD) of 10 samples. (c) B. cinerea relative DNA content (relative biomass) was measured by quantitative PCR. Error bars indicate the SD of three technical replicates. Asterisks indicate statistically significant differences (P < 0.01). Similar results were obtained from at least three biological replicates.
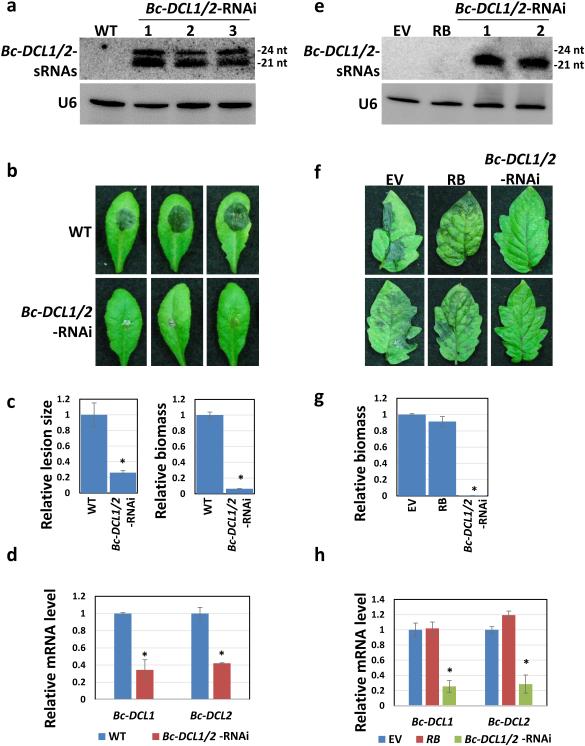
We generated transgenic Bc-DCL1/2-RNAi Arabidopsis plants expressing hairpin RNAs34 containing two segments from non-conserved regions of Bc-DCL1 and Bc-DCL2, respectively. We avoided the conserved functional domains to eliminate any off-target effects on DCL genes in plants or other organisms. These selected Bc-DCL DNA regions have only 3.5% to 4.8% sequence homology to Arabidopsis DCLs (Supplementary Fig. 2a), and indeed, no host DCL genes were silenced (Supplementary Fig. 2b). The hairpin RNA products in the transgenic plants were processed into sRNAs (Bc-DCL1/2-sRNAs) (Fig. 2a). These RNAi plants exhibited much smaller lesions and less fungal growth after B. cinerea infection when compared with the WT plants (Fig. 2b,c). Relative expression of Bc-DCL1 and Bc-DCL2 was clearly suppressed in B. cinerea collected from the transgenic plants compared to those from WT plants (Fig. 2d). These results suggest that RNAi signals against Bc-DCL1/2 produced in plant cells moved into the fungal cells and efficiently silenced Bc-DCL1 and Bc-DCL2, which led to suppression of fungal virulence and growth and inhibition of disease.
Figure 2. Arabidopsis and tomato Bc-DCL1/2-RNAi plants confer enhanced resistance against B. cinerea infection.
(a) Bc-DCL1/2-sRNAs were highly expressed in the Arabidopsis transgenic plants, as detected by Northern blot. (b) Arabidopsis Bc-DCL1/2-RNAi plants show enhanced disease resistance against B. cinerea. Similar results were obtained from three biological replicates. (c) Relative lesion sizes were measured 3 dpi using imageJ, and error bars indicate the SD of 10 samples. B. cinerea relative biomass was measured 3 dpi by quantitative PCR, and error bars indicate the SD of three technical replicates. (d) The expression of Bc-DCL1 and Bc-DCL2 were downregulated in infected Arabidopsis Bc-DCL1/2-RNAi plants, as measured by quantitative RT-PCR. (e) Northern blot analysis reveals the expression levels of Bc-DCL1/2-sRNAs in the tomato Bc-DCL1/2-RNAi plants through virus-induced gene silencing (VIGS). (f) Tomato Bc-DCL1/2-RNAi plants were more resistant to B. cinerea compared to control plants (EV and RB). Similar results were obtained from three biological replicates. (g) B. cinerea relative biomass was measured 3 dpi, and error bars indicate the SD of three technical replicates. (h) Bc-DCL1 and Bc-DCL2 were silenced in infected tomato Bc-DCL1/2-RNAi plants, as measured by quantitative RT-PCR. Asterisks indicate statistically significant differences (P < 0.01).
To determine whether this Bc-DCL1/2-targeting RNAi strategy could also efficiently control gray mold disease in tomato plants, we introduced the same Bc-DCL1/2 fragment into the tobacco rattle virus (TRV) silencing system35, which triggered the expression of Bc-DCL1/2-sRNAs in tomato. Three weeks after agro-infiltration, the tomato plants produced large amounts of Bc-DCL1/2-sRNAs (Fig. 2e) and were infected with B. cinerea using spray inoculation. The tomato leaves expressing Bc-DCL1/2-sRNAs displayed very mild to almost no disease symptoms at 3 dpi (Fig. 2f,g), whereas the controls, which either expressed sRNAs-targeting an potato late blight resistance gene RB that is not present in tomato36 or the TRV2 empty vector (EV), both showed very severe water soaked disease lesions (Fig. 2f,g). The growth of B. cinerea was also significantly reduced on the leaves expressing Bc-DCL1/2-sRNAs compared with the controls, and the expression of Bc-DCL1 and Bc-DCL2 was largely reduced in B. cinerea grown on these leaves as well (Fig. 2h). These data further support that cross-kingdom RNAi is bidirectional in B. cinerea-plant interactions, and fungal DCLs are ideal target genes to knock down in order to control pathogens that use sRNA effectors.
B. cinerea also infects many other plant species, in addition to Arabidopsis and tomato. Gray mold disease is a very serious problem in post-harvest management, as it destroys millions of fruits, vegetables and flowers during the packing, transportation and storage processes each year. Plant protection provided by cross-kingdom RNAi or HIGS is effective in some plant-pathogen interactions. However, this method is restricted to plants with established transformation protocols, while the majority of crop plants do not have available genetic manipulation tools. Moreover, genetically modified organisms (GMOs) are still a concern to many consumers. Therefore, we were motivated to explore whether RNA-based plant protection against eukaryotic pathogens can be achieved through a non-GMO approach. Uptake of RNAs from the environment, a phenomenon named environmental RNAi, was observed in C. elegans37-40 and certain insects41,42. Spraying dsRNAs targeting the actin gene of potato beetle Leptinotarsa decemlineata on potato leaves inhibited larval growth42. In vitro treatment of fungal pathogen F. graminearum with dsRNAs targeting CYP51 genes also inhibited fungal growth18, but there is no direct evidence demonstrating environmental dsRNAs move into fungal cells to silencing fungal CYP51 genes. Overall, it is unclear whether environmental RNAi works in fungi, plants, or mammals.
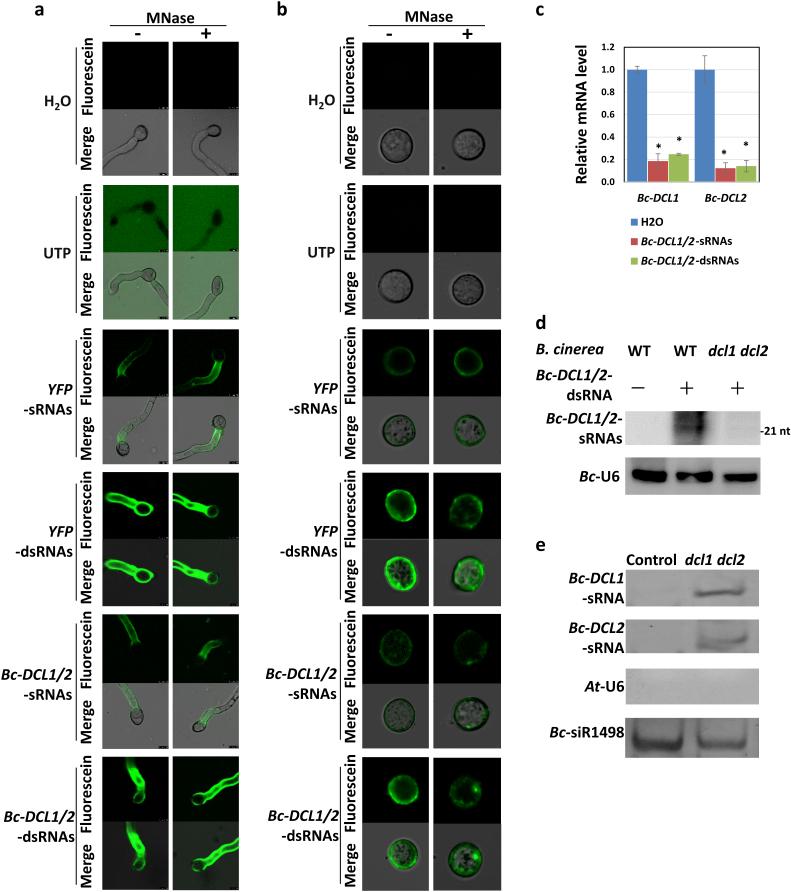
To investigate whether fungi can take up RNAs from the environment, we synthesized Fluorescein-labeled Bc-DCL1/2-dsRNAs by in vitro transcription using Fluorescein-12-UTP, and we generated Fluorescein-labeled Bc-DCL1/2-sRNAs by RNase III in vitro processing of the Fluorescein-labeled dsRNAs. These dsRNAs and sRNAs were applied on B. cinerea spores (Fig. 3a) and grown on agar medium to determine whether fungal cells could take up RNAs. Fluorescence signals accumulated within fungal cells 12 h after incubation (Fig. 3a). To exclude the possibility that fluorescent RNAs adhered to the exterior site of the fungal cells, we treated the fungus with Micrococcal nuclease (MNase). The fluorescence signals remained detectable (Fig. 3a), indicating that B. cinerea cells took up the dsRNAs and sRNAs. Fluorescein-12-UTP and water treatment were used as negative controls. To determine whether there is fungal preference for a type of RNAs or RNAs with specific sequences, we also tested Fluorescein-labeled YFP-sRNAs and YFP-dsRNAs. These RNAs were also efficiently taken into the fungal cells, suggesting that such RNA uptake is not likely to be selective. To confirm that the RNAs entered the cytoplasm of B. cinerea and were not buried in the cell wall matrix, fluorescent RNAs were added to liquid culture of B. cinerea geminating spores (Fig. 3b), which were subjected to protoplast preparation to remove all the cell walls after 20 h of incubation. The fluorescence signal was clearly detected within B. cinerea protoplasts even after MNase treatment (Fig. 3b). It is worth noting that the fluorescence intensities of labeled sRNAs were lower than that of dsRNAs (Supplementary Fig. 3). Furthermore, B. cinerea treated with Bc-DCL1/2-dsRNAs or -sRNAs clearly showed reduced Bc-DCL1 and Bc-DCL2 mRNA levels (Fig. 3c). Thus, fungal cells can take up dsRNAs and sRNAs from the environment, which then induces gene silencing in the fungal cells.
Figure 3. Environmental Bc-DCL1/2-sRNAs and -dsRNAs are taken into B. cinerea cells and where they silence fungal DCL genes; Bc-DCL1/2-sRNAs move from plants into fungal cells.
(a) Fluorescein-labeled Bc-DCL1/2-sRNAs and -dsRNAs, as well as YFP-sRNAs and -dsRNAs, were applied onto B. cinerea spores and fluorescent signals were detected in the B. cinerea cells at 12 h post culturing on agar (malt extract) ME medium. Fluorescence signals remained visible in the B. cinerea cells after MNase treatment. Fluorescein-UTP and water were used as controls. (b) Fluorescein-labeled Bc-DCL1/2-sRNAs and -dsRNAs, as well as YFP-sRNAs and -dsRNAs, were observed in B. cinerea protoplasts after MNase treatment. Fluorescent sRNAs or dsRNAs were applied onto germinated B. cinerea spores and protoplasts were isolated after culturing for 20 h. The fluorescent signals were detected within fungal protoplasts, and the MNase enzyme treatment did not reduce the fluorescent signal intensities. (c) Bc-DCL1 and Bc-DCL2 were down-regulated in Bc-DCL1/2-RNA-treated B. cinerea. (d) In vitro synthesized Bc-DCL1/2-dsRNAs were taken up and processed into sRNAs after co-culturing with the B cinerea WT strain but not with the dcl1 dcl2 mutant. (e) Bc-DCL1-sRNAs and Bc-DCL2-sRNAs were detected by stem-loop RT-PCR in B. cinerea dcl1 dcl2 protoplasts after infection on Arabidopsis Bc-DCL1/2-RNAi plants but not in mock-treated Arabidopsis Bc-DCL1/2-RNAi plants mixed with B. cinerea dcl1 dcl2 mycelium prior to protoplast formation. Similar results were obtained from two biological replicates.
Moreover, after entering WT B. cinerea cells, Bc-DCL1/2-dsRNAs were processed into Bc-DCL1/2-sRNAs by Bc-DCLs, but Bc-DCL1/2-sRNAs were not detected in the dcl1 dcl2 mutant (Fig. 3d). This provided an excellent system to determine whether sRNAs are mobile silencing signals transported from the plant into B. cinerea in the HIGS systems. Although various RNAi mobile signals, including sRNAs and dsRNA precursors, in HIGS have been proposed14,17,18,43,44, none have been clearly confirmed. In C. elegans, Drosophila and western corn rootworm (WCR, Diabrotica virgifera virgifera LeConte), it is the long dsRNAs instead of sRNAs that are taken up efficiently40,45,46. Transgenic Arabidopsis expressing hairpin Bc-DCL1/2-RNAs, which showed a high level of processed sRNAs (Fig. 2a), were inoculated with the dcl1 dcl2 mutant that was unable to process Bc-DCL1/2-dsRNAs into Bc-DCL1/2-sRNAs (Fig. 3d). At 48 hpi, we managed to isolate pure B. cinerea protoplasts from plant cells via sequential protoplast preparation by taking advantage of the different cell wall compositions between plants and fungi. The mock-treated Arabidopsis control mixed with B. cinerea dcl1 dcl2 mycelium prior to protoplast formation was used as a control to monitor the purity of the isolated B. cinerea protoplasts. Bc-DCL1/2-sRNAs were detected in the B. cinerea dcl1 dcl2-infected Arabidopsis sample but not in the control (Fig. 3e), supporting that Bc-DCL1/2-sRNAs indeed translocated into B. cinerea. The Bc-DCL-independent sRNA Bc-siR1498 that we previously identified5 was used as an internal control to show the amount of B. cinerea in each sample of the experiments. Thus, sRNAs are at least one of the major mobile signals for cross-kingdom RNAi in HIGS. We do not rule out that other forms of RNAs, such as long dsRNAs, could also be transported; however, they accumulated to a much lower level than sRNAs in planta, because most of the hairpin dsRNA precursors are efficiently processed into sRNAs.
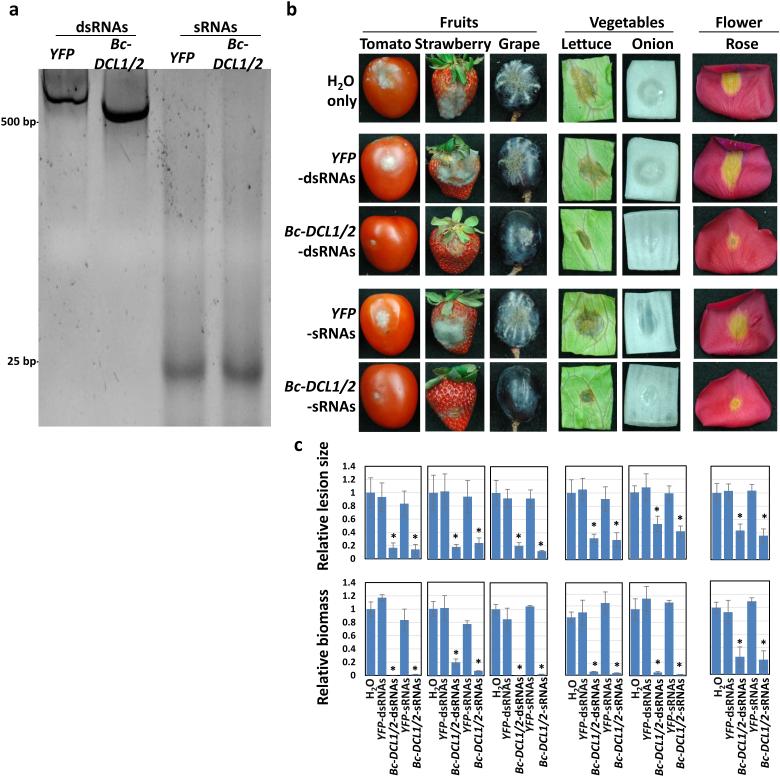
Since environmental RNAi can be achieved in fungi, we attempted to externally apply synthetic Bc-DCL1/2-sRNAs or Bc-DCL1/2-dsRNAs on the surface of various plants, to test whether B. cinerea is capable of taking up these dsRNAs and/or sRNAs from the environment and inducing silencing of its own genes. The same Bc-DCL1/2 RNAi fragment was transcribed in vitro from both ends and gave rise to Bc-DCL1/2-dsRNAs (Fig. 4a). The Bc-DCL1/2-dsRNAs were subjected to RNAse III treatment to generate Bc-DCL1/2-sRNAs in vitro (Fig. 4a). The Bc-DCL1/2-sRNAs and the precursor dsRNAs (20 ng/μl in pure water) were externally applied on fruits (tomato, strawberry, and grape), vegetables (lettuce and onion), flower petals (rose), and Arabidopsis leaves, and followed with B. cinerea infection. Water, YFP-dsRNAs and -sRNAs were used as controls. All the plants treated with Bc-DCL1/2-sRNAs and -dsRNAs developed much weaker disease symptoms (Fig. 4b,c and Supplementary Fig. 4a) and supported significantly less growth of B. cinerea (Fig. 4c and Supplementary Fig. 4a) compared to the controls treated with water, YFP-dsRNAs or YFP-sRNAs. Furthermore, the expression of Bc-DCL1 and Bc-DCL2 was suppressed by the treatment of Bc-DCL1/2-dsRNAs and -sRNAs in the B. cinerea isolated from the infection sites (Supplementary Fig. 4b), suggesting that externally applied Bc-DCL1/2-sRNAs and -dsRNAs can inhibit pathogen virulence, probably via the suppression of Bc-sRNA effector biogenesis and fungal growth. We also tested the efficiency of this plant protection using various concentrations of Bc-DCL1/2-sRNAs and -dsRNAs, and our data indicated that such RNAs remained functional at a concentration as low as 5 ng/μl, but not at 1 ng/μl (Supplementary Fig. 5), although the higher concentration of RNAs seems to provide stronger protection (Fig. 4b,c and Supplementary Fig. 5). We also examined the duration of plant protection against gray mold disease by inoculating the plant samples with B. cinerea 1, 3 and 5 days after RNA treatment, and pictures were taken 3 days after the fungal inoculation (dpi) for rose petals (6–8 days after RNA treatment), and 5 dpi for tomato (8–10 days after RNA treatment). We found that the RNAs remained effective for at least up to 8–10 days post RNA treatment, although plant protection was clearly reduced at later time point (Supplementary Fig. 6). It is worth noting that most fungicides contain various adjuvants, which help stabilize the active compounds, while we only used RNAs dissolved in water. Mixing the RNAs with reagents that stabilize RNAs could likely extend their effective period. We would pursue this in a follow-up study with a more applied focus.
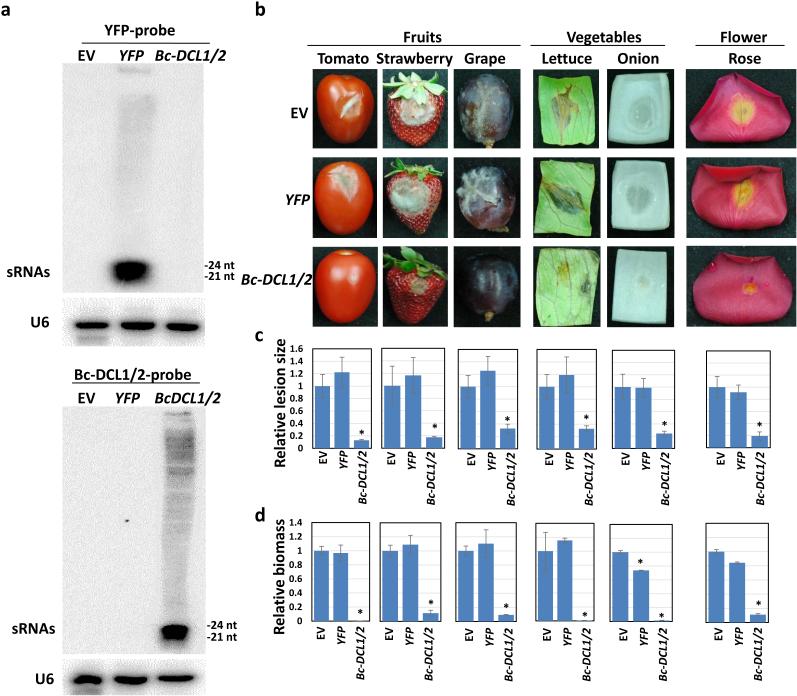
Figure 4. Externally applied Bc-DCL1/2-sRNAs and -dsRNAs inhibited pathogen virulence on fruits, vegetables, and flower petals.
(a) Bc-DCL1/2-dsRNAs and -sRNAs, as well as YFP-dsRNAs and -sRNAs, were synthesized and processed, and 100 ng of RNAs was analyzed on a native PAGE gel to check the quality. (b) External application of Bc-DCL1/2-dsRNAs and -sRNAs (20ul of 20 ng/μl synthetic RNAs) inhibits the virulence of B. cinerea on fruits (tomato, strawberry, and grape), vegetables (lettuce and onion), and flower petals (rose), compared to the treatments using water, YFP-dsRNAs and -sRNAs. (c) The relative lesion sizes and fungal biomass were measured 3 dpi for lettuce, onion, rose, and strawberry and 5 dpi for tomato and grape fruits using ImageJ software and quantitative PCR, respectively. Error bars indicate the SD of 10 samples and three technical repeats for the relative lesion sizes and relative biomass, respectively. Asterisks indicate statistically significant differences (P < 0.01). Similar results were obtained from three biological replicates.
Although external treatment of Bc-DCL1/2-sRNAs and the precursor Bc-DCL1/2-dsRNAs can inhibit fungal disease, in vitro RNA synthesis is too costly. In order to obtain a large amount of Bc-DCL1/2-sRNAs and -dsRNAs at a much lower cost, we expressed the Bc-DCL1/2-RNAi construct in N. benthamiana, which yielded a large amount of Bc-DCL1/2-sRNAs, but with a low level of Bc-DCL1/2-dsRNAs and intermediate processing products (Fig. 5a and Supplementary Fig. 7). The plants expressing the Hellsgate empty vector (EV) or the YFP-RNAi construct, which also yielded a large quantity of YFP-sRNAs and a very low level of YFP-RNAs with longer lengths, were used as controls. The purified total RNAs were sprayed onto the surface of fruits, vegetables, and rose petals and followed with B. cinerea infection. All the plants treated with the RNA extracts from Bc-DCL1/2-RNA plants developed less severe disease symptoms and showed decreased fungal growth compared to the RNA extracts from plants expressing EV or YFP-RNAs (Fig. 5b-d). This result demonstrates that the Bc-DCL1/2-RNAs, but not N. benthamiana total RNAs, can protect plants from B. cinerea infection.
Figure 5. Treatment with N. benthamiana RNA extracts containing Bc-DCL1/2-sRNAs and -dsRNAs reduces gray mold disease symptoms caused by B. cinerea.
(a) Total RNA extracted from the N. benthamiana plants expressing Hellsgate empty vector (EV), YFP or Bc-DCL1/2 RNAi constructs was examined by Northern blot analysis to measure the expression levels of YFP- and Bc-DCL1/2 sRNAs. (b) N. Benthamiana total RNAs containing Bc-DCL1/2-RNAs (20 ng/μl) were sprayed onto fruits (tomato, strawberry, and grape), vegetables (lettuce and onion), and flower petals (rose) reduced grey mold disease symptoms, when compared with the application of N. Benthamiana total RNA extracts from plants expressing YFP-sRNAs and -dsRNAs or no sRNAs (EV). (c) The relative lesion sizes were measured 3 dpi for lettuce, onion, rose and strawberry, and 5 dpi for tomato and grape fruits using imageJ, and error bars represent the SD of 10 plant samples. The relative biomass of B. cinerea was also calculated with quantitative PCR, and error bars represent the SD of three technical replicates. Asterisks indicate statistically significant differences (P < 0.01). Similar results were observed from three biological replicates.
Such an RNAi-based disease control method could be powerful if it is effective against multiple pathogens. For example, we could design DCL-targeting dsRNAs or sRNAs to silence DCL genes of multiple pathogens that utilize sRNA effectors. To prove the concept, we chose to examine the soil borne fungal pathogen V. dahilae that displayed reduced virulence on Arabidopsis ago1-27 mutant47, just like B. cinerea. V. dahilae is another economically important fungal pathogen that causes wilt disease on many plant species, including herbaceous annuals, perennials, and woody species24,25. So far, there is no effective control method other than toxic fungicide application.
To investigate whether V. dahilae also utilizes cross-kingdom RNAi to suppress host immunity genes by hijacking host AGO1 for its virulence, and whether V. dahilae sRNAs are indeed loaded into host AGO1 protein during infection, we performed RNA immunoprecipitation (RIP) assay on V. dahilae-infected Arabidopsis roots using Arabidopsis AGO1- and AGO2-antibodies as described previously5,48. Arabidopsis ago1-27 mutant is less susceptible to V. dahilae than WT plants in both soil and root culture conditions (Fig. 6a,b). The ago2-1 mutant shows no difference compared to WT plants, suggesting that At-AGO2 is not involved in the gene regulation during plant - V. dahilae interaction (Fig. 6b). Therefore, we used AGO2-RIP as a control. Using a stringent target prediction program and 100 reads per million (RPM) total Vd-sRNAs as a cutoff5, we found that 99 At-AGO1-associated Vd-sRNAs have Arabidopsis target genes, whereas only 13 At-AGO2-associated Vd-sRNAs have predicted Arabidopsis targets (Supplementary Table 2,3). These results suggest that V. dahilae also utilizes sRNA effectors and cross-kingdom RNAi for successful infection; thus, targeting Vd-DCL genes could also be a potential strategy for controlling verticillium wilt disease.
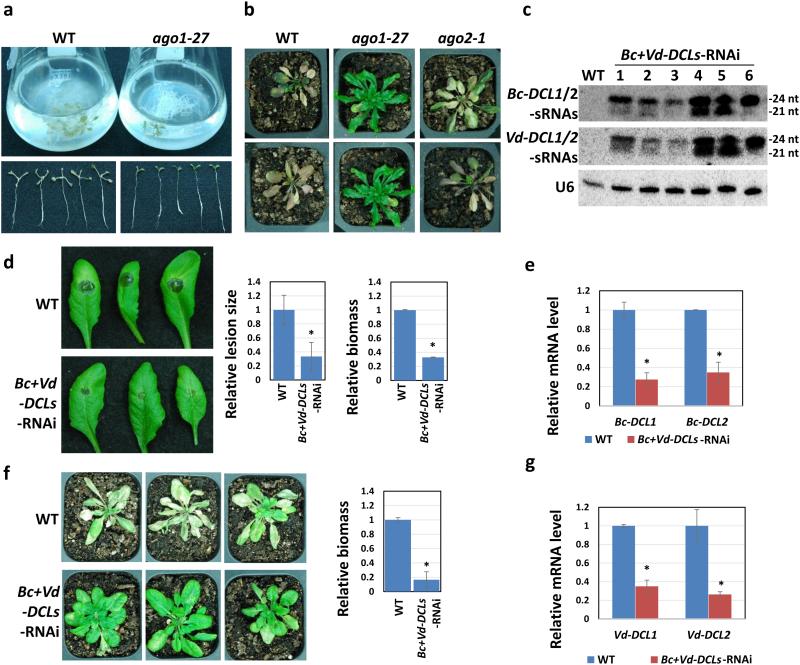
Figure 6. Arabidopsis plants expressing hairpin RNAs that simultaneously target DCL genes of B. cinerea and V. dahilae show enhanced disease resistance to both pathogens.
(a) The Arabidopsis ago1-27 mutant was more resistant to V. dahilae compared with WT plants in root culture conditions. (b) Arabidopsis ago1-27, but not ago2-1 mutant, was less susceptible to V. dahilae when compared with WT plants grown in soil. (c) The expression levels of Bc-DCL1/2-sRNAs and Vd-DCL1/2-sRNAs in the Arabidopsis Bc+Vd-DCLs-RNAi transgenic plants were examined by Northern blot analysis. (d) Arabidopsis Bc+Vd-DCLs-RNAi plants display reduced disease after infection of B. cinerea. Relative lesion sizes were measured 3 dpi using ImageJ, and error bars indicate the SD of 10 leaves. B. cinerea biomass was measured 3 dpi by quantitative PCR, and error bars indicate the SD of three technical replicates. (e) Quantitative RT-PCR showed that Bc-DCL1 and Bc-DCL2 were silenced in B. cinerea-infected Arabidopsis Bc+Vd-DCLs-RNAi plants compared with WT plants. (f) Arabidopsis Bc+Vd-DCLs-RNAi plants were less susceptible to V. dahilae compared to WT plants. Relative biomass of V. dahilae was measured at 3 weeks post inoculation, and error bars indicate the SD of three technical replicates. (g) The expression level of Vd-DCL1 and Vd-DCL2 were suppressed in V. dahilae-infected Arabidopsis Bc+Vd-DCLs-RNAi plants. Asterisks represent statistically significant differences (P < 0.01). Similar results were obtained from three biological replicates (a-g).
We generated Arabidopsis transgenic plants that express hairpin RNAs targeting DCL genes of both V. dahilae and B. cinerea to test whether the RNAi-based method can simultaneously control both fungal diseases. V. dahilae also has two DCL genes. Again, we were not able to find a DNA region outside the conserved domains that has sufficient homology between Vd-DCL1 and Vd-DCL2 (Vd-DCL1/2), or between the DCLs of V. dahilae and B. cinerea to silence two or more DCLs. Thus, we had to select two DNA segments, each from Vd-DCL1 and Vd-DCL2, which had 2.2% to 3.1% identity to the four At-DCLs, (Supplementary Fig. 8a), and fused them to the fragments of Bc-DCL1 and Bc-DCL2 for RNAi vector construction. These transgenic plants expressed high levels of both Bc-DCL1/2-sRNAs and Vd-DCL1/2-sRNAs (Fig. 6c). As expected, the expression of Arabidopsis DCL genes was not affected in these transgenic lines (Supplementary Fig. 8b). Pathogen infection assays revealed that these transgenic plants are indeed more resistant to both B. cinerea and V. dahilae (Fig. 6d-g). To determine whether the silencing effect is indeed gene-specific, we infected Bc-DCL1/2-RNAi plants with V. dahilae. These plants only express Bc-DCL1/2-sRNAs but not Vd-DCL1/2-sRNAs. They displayed severe wilt disease symptoms just like the WT plants (Supplementary Fig. 9a,b), indicating that Bc-DCL1/2 RNAs could not silencing V. dahilae DCL genes. Thus, these Bc-DCL DNA regions chosen from outside the conserved regions for RNAi are very specific to B. cinerea DCL genes and do not cause off-target effects. Thus, specific target region selection could circumvent potential off-target problem. This RNAi-based disease management strategy targeting specific regions of pathogen DCL genes is efficient and safe in controlling multiple fungal pathogens that use sRNA effectors.
Discussion
We previously discovered that B. cinerea delivered sRNA effectors into plant cells to induce cross-kingdom RNAi of host plant immunity genes5. Here, we show that another plant fungal pathogen V. dahilae also uses sRNAs as effectors and these sRNAs are loaded into Arabidopsis AGO1 for silencing of host genes. A similar virulence mechanism was recently observed in animal systems as well. For example, animal parasites, such as H. polygyrus, deliver miRNAs to mammalian cells and silence host genes involved in innate immunity8. Thus, cross-kingdom RNAi has evolved in pathogens and pests of both plant and animal systems as a conserved virulence mechanism. Furthermore, we demonstrate that sRNAs generated from the host plant cells are also transferred into fungal B. cinerea cells, which provided the first example of bidirectional cross-kingdom RNAi and sRNA trafficking between a plant host and a fungal pathogen. Plant hosts expressing Bc-DCL-targeting sRNAs can effectively control gray mold disease.
In this study, we provide solid evidences to demonstrate that environmental RNAi also exists in fungi. We show that fungal pathogen B. cinerea is capable of taking up external sRNAs and long dsRNAs. In C. elegans, RNA uptake from the environment requires dsRNAs that are longer than 50 bp, shorter dsRNAs or mature sRNAs cannot be effectively taken up by C. elegans37-40,49. Some herbivorous insects are also capable of taking up dsRNAs that are longer than 50–60 bp, but not sRNAs41,46. Here, we show that B. cinerea can take up both sRNAs and dsRNAs directly, and both can induce silencing of B. cinerea genes, suggesting that the RNA uptake channels or pathways may differ among organisms. Consistent with that, the Systemic RNAi-deficient (SID) genes that are important for RNA uptake and transport37-40,49,50 are mostly C. elegans-specific and not present in plants, fungi, oomycete, or even other animals50. Future studies are needed to identify the sRNA and dsRNA trafficking pathways between pathogens and hosts and to elucidate the mechanisms of RNA uptake in fungi.
Eukaryotic pathogens, including fungi and oomycetes, cause billions of dollars in crop loss annually. Currently, fungicide and chemical spray is still the most common disease control strategy, yet they pose serious threats to human health and environments. Over the last few years, the stable plant transformation-based HIGS system was proven to efficiently control certain pests, nematodes, filamentous pathogens, and parasitic plants in various plant models and crop species13-15,17,18,21. However, these successful HIGS studies relied on a plant transformation system that is not available for many crops. Our discovery of RNA uptake by B. cinerea makes it possible to directly use such external dsRNAs and sRNAs for disease management. We show that applying Bc-DCL1/2-sRNAs and -dsRNAs on the surface of various fruits, vegetables, and flowers can efficiently control gray mold diseases. This RNAi-based new generation of “RNA fungicides” could circumvent the technical limitation of transformation and the public’s concerns about GMOs and provide an easy-to-use and environmentally friendly disease management tool for crop production and storage. Furthermore, such RNA-based strategies could be easily designed to target multiple pathogens.
Materials and Methods
Plasmid construction
The plasmids pHellsgate-Bc-DCL1/2 and pHellsgate-Bc+Vd-DCLs were constructed following the methods outlined for the Hellsgate8.0 system51. The Bc-DCL1/2 RNAi fragment was obtained by integrating Bc-DCL1 (252 bp) and Bc-DCL2 (238 bp) via overlapping PCR. The Bc+Vd-DCLs RNAi fragment was obtained by integrating the 315 bp Bc-DCL1/2 RNAi fragment (164 bp of Bc-DCL1 and 151 bp of Bc-DCL2) and RNAi fragments of Vd-DCL1 (156 bp) and Vd-DCL2 (156 bp) via overlapping PCR. The RNAi fragments were cloned separately into pDONR207 using Gateway® BP clonase (Life Technologies, Carlsbad, CA), then into the destination vector pHellsgate8.0 using Gateway® LR clonase (Life Technologies, Carlsbad, CA). For the pTRV2-Bc-DCL1/2 plasmid, the pDONR207-Bc-DCL1/2 vector was used to do LR reaction with pTRV2-EV to obtain pTRV2-Bc-DCL1/2 using LR clonase (Life Technologies, Carlsbad, CA)5.
In vitro synthesis of dsRNAs and sRNAs
Following the MEGAscript® RNAi Kit instructions (Life Technologies, Carlsbad, CA), T7 promoter sequence was introduced into both 5’ and 3’ ends of the RNAi fragments by PCR, respectively. After purification, the DNA fragments containing T7 promoters at both ends were used for in vitro transcription. To obtain sRNAs the synthesized dsRNAs were digested with ShortCut® RNase III (NEB, Ipswich, MA), and sRNAs were subsequently cleaned using the mirVana™ miRNA Isolation Kit (Life Technologies, Carlsbad, CA).
In vitro RNA fluorescence labeling for confocal microscopy
Bc-DCL1/2-dsRNAs were labeled using the Fluorescein RNA Labeling Mix Kit following the manufacture’s instructions (Sigma, St. Louis, MO). The fluorescent Bc-DCL1/2-sRNAs were obtained from the digestion of fluorescent Bc-DCL1/2-dsRNAs with the ShortCut® RNase III enzyme (NEB Ipswich, MA). For confocal microscopy examination of fluorescent RNA trafficking into fungal mycelium, 4 μl of 100ng/μl fluorescent RNAs were applied onto 4 μl of 105 spores/ml geminating spores and grew on ME agar medium that prepared on microscopic slides. After 12 hours, the mycelium were treated by KCl buffer or 75 U Micrococcal Nuclease enzyme (Thermo Scientific, Waltham, MA) at 37°C for 30 minutes. The fluorescent signal was analyzed using a Leica SP5 confocal microscope.
To detect fluorescent RNA trafficking into B. cinerea protoplasts, 15 μl of 100 ng/μl fluorescent RNAs were added into 107 B. cinerea spores cultured in 5 ml liquid yeast extract peptone dextrose (YEPD). After 20 h, the protoplasts were isolated using lysing enzyme from Trichoderma harzianum (Sigma, St. Louis, MO), followed by treatment with KCl buffer or 75 U Micrococcal Nuclease enzyme (Thermo scientific, Waltham, MA) at 37°C for 30 min. Fluorescence was examined using a Leica SP5 confocal microscope.
To autocorrect artificial fluorescence and/or auto-fluorescence of fungal mycelium and protoplasts, the confocal microscope settings were adjusted using the water-treated control samples (mycelium or protoplast), and the same settings (except for the focus) were applied to all the samples.
Isolation of B. cinerea protoplasts from infected Arabidopsis plants
Arabidopsis plants were spray-infected with B. cinerea dcl1 dcl2 mutant strain as well as inoculation medium for 48 hours, and then the leaf tissues were subjected to sequential protoplast preparation based on the different compositions of cell walls between fungus and plant. First, plant protoplast extraction from the leaf tissues was performed as described previously52. The plant protoplasts were sheared using a 1% Triton X-100 (Sigma, St. Louis, MO) treatment, and were washed 5 times to remove plant contents. Then the fungal protoplasts were isolated using lysing enzyme from Trichoderma harzianum (Sigma, St. Louis, MO) to obtain pure fungal protoplasts.
Preparation of N. Benthamiana total RNA carrying Bc-DCL1/2-sRNAs and -dsRNAs, as well as YFP-sRNAs and -dsRNAs
Four-week old N. benthamiana plants were infiltrated with A. tumefaciens strain carrying pHellsgate-EV, pHellsgate-Bc-DCL1/2 or pHellsgate-YFP. The leaf tissue was harvested 2 dpi and used for total RNA extraction.
External application of RNAs on the surface of plant materials
All RNAs were adjusted to a concentration of 20 ng/μl with RNase-free water before use. For the concentration series, RNAs were diluted to 10, 5, and 1 ng/μl. For in vitro synthetic YFP-sRNA and -dsRNAs, and Bc-DCL1/2-sRNAs and -dsRNA, 20 μl RNA (20 ng/μl) was dropped onto the surface of each plant specimen. Then the B. cinerea inoculum was applied on the same spot. For N. benthamiana total RNA extracts, which is easy and cheap to obtain large quantity, the surface of each plant specimen was evenly sprayed with approximately 400μl of 20 ng/μl RNAs.
Supplementary Material
Acknowledgements
We thank Herve Vaucheret for the ago1-27 seeds, Michael Coffey for the V. dahilae JR2 strain, Isgouhi Kaloshian for providing the growth room space for VIGS experiments, and Yifan Lii for editing the paper. This work was supported by grants from National Institute of Health (R01 GM093008), National Science Foundation (IOS-1257576, IOS-1557812) and an AES-CE Award (PPA-7517H) awarded to HJ.
Footnotes
Author contributions
HJ conceived the idea. MW and HJ designed the experiments. MW performed most of the experiments and analyzed data. AW profiled the sRNAs from dcl1 dcl2 and WT strains and analyzed the data. FML and HDH conducted bioinformatics analysis on sRNA libraries. BT provided Vd genome sequence for JR2 strain. MW, AW and HJ wrote the manuscript.
Competing interests
The authors declare no competing financial interests.
References
- 1.Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat. Rev. Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baulcombe D. RNA silencing in plants. Nature. 2004;431:356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- 3.Vaucheret H. Plant ARGONAUTES. Trends Plant Sci. 2008;13:350–358. doi: 10.1016/j.tplants.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Hutvagner G, Simard MJ. Argonaute proteins: key players in RNA silencing. Nat. Rev. Mol. Cell Biol. 2008;9:22–32. doi: 10.1038/nrm2321. [DOI] [PubMed] [Google Scholar]
- 5.Weiberg A, et al. Fungal Small RNAs Suppress Plant Immunity by Hijacking Host RNA Interference Pathways. Science. 2013;342:118–123. doi: 10.1126/science.1239705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiberg A, Jin HL. Small RNAs - the secret agents in the plant-pathogen interactions. Curr. Opin. Plant Biol. 2015;26:87–94. doi: 10.1016/j.pbi.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiberg A, Wang M, Bellinger M, Jin H. Small RNAs: a new paradigm in plant-microbe interactions. Annu. Rev. Phytopathol. 2014;52:495–516. doi: 10.1146/annurev-phyto-102313-045933. [DOI] [PubMed] [Google Scholar]
- 8.Buck AH, et al. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat. Commun. 2014;5:5488. doi: 10.1038/ncomms6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng GF, Luo R, Hu C, Cao J, Jin YX. Deep sequencing-based identification of pathogen-specific microRNAs in the plasma of rabbits infected with Schistosoma japonicum. Parasitology. 2013;140:1751–1761. doi: 10.1017/S0031182013000917. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Silva MR, et al. Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitol. Res. 2014;113:285–304. doi: 10.1007/s00436-013-3655-1. [DOI] [PubMed] [Google Scholar]
- 11.Zamanian M, et al. Release of Small RNA-containing Exosome-like Vesicles from the Human Filarial Parasite Brugia malayi. PLoS Negl. Trop. Dis. 2015;9:e0004069. doi: 10.1371/journal.pntd.0004069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quintana JF, et al. Extracellular Onchocerca-derived small RNAs in host nodules and blood. Parasit Vectors. 2015;8:58. doi: 10.1186/s13071-015-0656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baum JA, et al. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007;25:1322–1326. doi: 10.1038/nbt1359. [DOI] [PubMed] [Google Scholar]
- 14.Mao YB, et al. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007;25:1307–1313. doi: 10.1038/nbt1352. [DOI] [PubMed] [Google Scholar]
- 15.Huang GZ, Allen R, Davis EL, Baum TJ, Hussey RS. Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc. Natl. Acad. Sci. USA. 2006;103:14302–14306. doi: 10.1073/pnas.0604698103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Todd TC, Oakley TR, Lee J, Trick HN. Host-derived suppression of nematode reproductive and fitness genes decreases fecundity of Heterodera glycines Ichinohe. Planta. 2010;232:775–785. doi: 10.1007/s00425-010-1209-7. [DOI] [PubMed] [Google Scholar]
- 17.Nowara D, et al. HIGS: Host-Induced Gene Silencing in the Obligate Biotrophic Fungal Pathogen Blumeria graminis. Plant Cell. 2010;22:3130–3141. doi: 10.1105/tpc.110.077040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch A, et al. Host-induced gene silencing of cytochrome P450 lanosterol C14 alpha-demethylase-encoding genes confers strong resistance to Fusarium species. Proc. Natl. Acad. Sci. USA. 2013;110:19324–19329. doi: 10.1073/pnas.1306373110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jahan SN, et al. Plant-mediated gene silencing restricts growth of the potato late blight pathogen Phytophthora infestans. J. Exp. Bot. 2015;66:2785–2794. doi: 10.1093/jxb/erv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vega-Arreguin JC, Jalloh A, Bos JI, Moffett P. Recognition of an Avr3a Homologue Plays a Major Role in Mediating Nonhost Resistance to Phytophthora capsici in Nicotiana Species. Mol. Plant Microbe Interact. 2014;27:770–780. doi: 10.1094/MPMI-01-14-0014-R. [DOI] [PubMed] [Google Scholar]
- 21.Nunes CC, Dean RA. Host-induced gene silencing: a tool for understanding fungal host interaction and for developing novel disease control strategies. Mol. Plant Pathol. 2012;13:519–529. doi: 10.1111/j.1364-3703.2011.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williamson B, Tudzynsk B, Tudzynski P, van Kan JAL. Botrytis cinerea: the cause of grey mould disease. Mol. Plant Pathol. 2007;8:561–580. doi: 10.1111/j.1364-3703.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 23.van Kan JAL. Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 2006;11:247–253. doi: 10.1016/j.tplants.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Fradin EF, Thomma BP. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006;7:71–86. doi: 10.1111/j.1364-3703.2006.00323.x. [DOI] [PubMed] [Google Scholar]
- 25.Klosterman SJ, Atallah ZK, Vallad GE, Subbarao KV. Diversity, pathogenicity, and management of verticillium species. Annu. Rev. Phytopathol. 2009;47:39–62. doi: 10.1146/annurev-phyto-080508-081748. [DOI] [PubMed] [Google Scholar]
- 26.Pliego C, et al. Host-Induced Gene Silencing in Barley Powdery Mildew Reveals a Class of Ribonuclease-Like Effectors. Mol. Plant Microbe Interact. 2013;26:633–642. doi: 10.1094/MPMI-01-13-0005-R. [DOI] [PubMed] [Google Scholar]
- 27.Whigham E, et al. Broadly Conserved Fungal Effector BEC1019 Suppresses Host Cell Death and Enhances Pathogen Virulence in Powdery Mildew of Barley (Hordeum vulgare L. Mol. Plant Microbe Interact. 2015;28:968–983. doi: 10.1094/MPMI-02-15-0027-FI. [DOI] [PubMed] [Google Scholar]
- 28.Panwar V, McCallum B, Bakkeren G. Host-induced gene silencing of wheat leaf rust fungus Puccinia triticina pathogenicity genes mediated by the Barley stripe mosaic virus. Plant Mol. Biol. 2013;81:595–608. doi: 10.1007/s11103-013-0022-7. [DOI] [PubMed] [Google Scholar]
- 29.Ghag SB, Shekhawat UK, Ganapathi TR. Host-induced post-transcriptional hairpin RNA-mediated gene silencing of vital fungal genes confers efficient resistance against Fusarium wilt in banana. Plant Biotechnol. J. 2014;12:541–553. doi: 10.1111/pbi.12158. [DOI] [PubMed] [Google Scholar]
- 30.Cheng W, et al. Host-induced gene silencing of an essential chitin synthase gene confers durable resistance to Fusarium head blight and seedling blight in wheat. Plant Biotechnol. J. 2015 doi: 10.1111/pbi.12352. [DOI] [PubMed] [Google Scholar]
- 31.Sanju S, et al. Host-mediated gene silencing of a single effector gene from the potato pathogen Phytophthora infestans imparts partial resistance to late blight disease. Funct. Integr. Genomics. 2015;15:697–706. doi: 10.1007/s10142-015-0446-z. [DOI] [PubMed] [Google Scholar]
- 32.Lee HC, et al. Diverse pathways generate microRNA-like RNAs and Dicer-independent small interfering RNAs in fungi. Mol. Cell. 2010;38:803–814. doi: 10.1016/j.molcel.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin HL, Zhu JK. How Many Ways Are There to Generate Small RNAs? Mol. Cell. 2010;38:775–777. doi: 10.1016/j.molcel.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helliwell CA, Waterhouse PM. Constructs and methods for hairpin RNA-mediated gene silencing in plants. Methods Enzymol. 2005;392:24–35. doi: 10.1016/S0076-6879(04)92002-2. [DOI] [PubMed] [Google Scholar]
- 35.Liu YL, Schiff M, Dinesh-Kumar SP. Virus-induced gene silencing in tomato. Plant J. 2002;31:777–786. doi: 10.1046/j.1365-313x.2002.01394.x. [DOI] [PubMed] [Google Scholar]
- 36.Song JQ, et al. Gene RB cloned from Solanum bulbocastanum confers broad spectrum resistance to potato late blight. Proc. Natl. Acad. Sci. USA. 2003;100:9128–9133. doi: 10.1073/pnas.1533501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winston WM, Sutherlin M, Wright AJ, Feinberg EH, Hunter CP. Caenorhabditis elegans SID-2 is required for environmental RNA interference. Proc. Natl. Acad. Sci. USA. 2007;104:10565–10570. doi: 10.1073/pnas.0611282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whangbo JS, Hunter CP. Environmental RNA interference. Trends Genet. 2008;24:297–305. doi: 10.1016/j.tig.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 39.McEwan DL, Weisman AS, Huntert CP. Uptake of Extracellular Double-Stranded RNA by SID-2. Mol. Cell. 2012;47:746–754. doi: 10.1016/j.molcel.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feinberg EH, Hunter CP. Transport of dsRNA into cells by the transmembrane protein SID-1. Science. 2003;301:1545–1547. doi: 10.1126/science.1087117. [DOI] [PubMed] [Google Scholar]
- 41.Ivashuta S, et al. Environmental RNAi in herbivorous insects. RNA. 2015;21:840–850. doi: 10.1261/rna.048116.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.San Miguel K, Scott JG. The next generation of insecticides: dsRNA is stable as a foliar-applied insecticide. Pest Manage. Sci. 2015 doi: 10.1002/ps.4056. [DOI] [PubMed] [Google Scholar]
- 43.Baulcombe DC. VIGS, HIGS and FIGS: small RNA silencing in the interactions of viruses or filamentous organisms with their plant hosts. Curr. Opin. Plant Biol. 2015;26:141–146. doi: 10.1016/j.pbi.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Govindarajulu M, Epstein L, Wroblewski T, Michelmore RW. Host-induced gene silencing inhibits the biotrophic pathogen causing downy mildew of lettuce. Plant Biotechnol. J. 2015;13:875–883. doi: 10.1111/pbi.12307. [DOI] [PubMed] [Google Scholar]
- 45.Saleh MC, et al. The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nat. Cell Biol. 2006;8:793–802. doi: 10.1038/ncbl439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolognesi R, et al. Characterizing the mechanism of action of double-stranded RNA activity against western corn rootworm (Diabrotica virgifera virgifera LeConte) PLoS One. 2012;7:e47534. doi: 10.1371/journal.pone.0047534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellendorff U, Fradin EF, de Jonge R, Thomma BPHJ. RNA silencing is required for Arabidopsis defence against Verticillium wilt disease. J. Exp. Bot. 2009;60:591–602. doi: 10.1093/jxb/ern306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, et al. Arabidopsis Argonaute 2 regulates innate immunity via miRNA393( *)-mediated silencing of a Golgi-localized SNARE gene, MEMB12. Mol. Cell. 2011;42:356–366. doi: 10.1016/j.molcel.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hinas A, Wright AJ, Hunter CP. SID-5 Is an Endosome-Associated Protein Required for Efficient Systemic RNAi in C. elegans. Curr. Biol. 2012;22:1938–1943. doi: 10.1016/j.cub.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang M, Weiberg A, Jin H. Pathogen small RNAs: a new class of effectors for pathogen attacks. Mol. Plant Pathol. 2015;16:219–223. doi: 10.1111/mpp.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Helliwell C, Waterhouse P. Constructs and methods for high-throughput gene silencing in plants. Methods. 2003;30:289–295. doi: 10.1016/s1046-2023(03)00036-7. [DOI] [PubMed] [Google Scholar]
- 52.Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2007;2:1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.