Abstract
Tumors contain heterogeneous cell populations and achieve dominance by functioning as collective systems. The mechanisms underlying the aberrant growth and interactions between cells are not very well understood. The pre-B acute lymphoblastic leukemia cells we studied were obtained directly from a patient with Ph+ ALL. A new Ph+ ALL cell line (ALL3) was established from the leukemic cells growing as ascitic cells in his pleural fluid. The patient died of his disease shortly after the cells were obtained. ALL3 cells grow well at high cell densities (HD), but not at low cell densities. ALL3 cells are very sensitive to potent tyrosine kinase inhibitors (TKIs) such as Dasatinib and PD166325, but less sensitive to AMN 107, Imatinib, and BMS 214662 (a farnesyl transferase inhibitor). Here, we show that the growth of the LD ALL3 cells can be stimulated to grow in the presence of diffusible, soluble factors secreted by ALL3 cells themselves growing at high density. We also show that exosomes, part of the secretome components, are also able to stimulate the growth of the non-growing LD ALL3 cells and modulate their proliferative behavior. Characterization of the exosome particles also showed that the HD ALL3 cells are able to secret them in large quantities and that they are capable of inducing the growth of the LD ALL3 cells without which they will not survive. Direct stimulation of non-growing LD ALL3 cells using purified exosomes shows that the ALL3 cells can also communicate with each other by means of exchange of exosomes independently of direct cell-cell contacts or diffusible soluble stimulatory factors secreted by HD ALL3 cells.
Keywords: Acute lymphoblastic leukemia, quorum sensing, exosome, proliferation, collective behavior, tyrosine kinase inhibitor
Introduction
Collective behaviors are important features regulating many biological processes such as cell migration, stem-cell maintenance, maintenance of proper organ size, immune system regulations, hematopoiesis homeostasis and regeneration in higher organisms [1-10]. Individual cells use ‘autocrine’ and/or ‘paracrine’ factors to coordinate these beneficial collective behaviors. Secretomes include group of proteins or other molecules that are secreted into the extracellular space by cells, tissues, organs or organisms at any given time or in different conditions through known and unknown mechanisms [11,13]. These secreted proteins are an important class of molecules involved in various cellular interactions that can be defined on the basis of their functions such as cell motility factors, growth factors, extracellular proteases, cytokines, anti-survival proteins, angiogenic factors and other bioactive molecules. They are involved in various pathophysiological functions like cell differentiation, invasion, metastasis, autophagy, apoptosis, tissue organization, immune surveillance, angiogenesis, and cell- cell communications [12].
Cancer cells function at population levels to sustain excessive cell proliferation, initiate local invasion or more distant metastasis, neutralize invading immune cells or other immune defense mechanisms, and initiate mechanisms of therapeutic resistance, and metabolic reprogramming [1,14-17]. One of the most important questions in the field of cancer biology is how the cancer cells regulate their number by altering homeostatic quorum sensing regulatory mechanisms, but this is still not well understood [6]. It is also important to understand how cell-cell communication helps to regulate several key regulatory processes in cancer progression. There are several mechanisms of cell-cell communications. A direct contact between cells by integral membrane proteins is one of them. Another mechanism is indirect contact via the extracellular matrix. Cells also communicate with each other via soluble diffusible factors or via membrane vesicles. These interesting nanoscale sized membrane vesicles called “microvesicles” or “exosomes” are also involved in cell-cell communications.
Exosomes (nanometer-sized membrane vesicles) are secreted into extracellular space by numerous cell types including dendritic cells, B cells, T cells, mast cells, and tumor cells [18]. Circulating microvesicles were first described by Taylor and Doellgast in 1979 [19]. Trams and colleagues used the term “exosomes” for the first time in 1981 [20]. Exosomes are derived from an endosomal compartment and secreted as extracellular vesicles (EVs). EVs are formed by inward budding of late endosomes, and fusion with the plasma membrane leads to their release into the extracellular environment [21]. They are found in various body fluids such as urine, saliva, serum, peripheral blood, bronchoalveolar lavage fluid, and malignant pleural fluids [18,21-23]. Different studies confirm that exosomes derived from various cell types contain membrane and cytosolic components such as DNAs, proteins, lipids and RNAs [24]. Shuffling of these components between cells serve as a novel important and smart means of intra- and or inter- cell-to-cell communication. Several studies suggest that exosomes are involved in the induction of immune response [25,26], in the pathogenesis of neurodegenerative disease [27], in cell-cell communication between cancer cells, and in metastasis [28-30]. Exosomes also help the cells to determine their movement through tissues [31].
Leukemia is an excellent model system to study cancer cells’ collective behavior [32,35]. Several studies also suggest that exosomes have an important role in hematological malignancies. Exosomes derived from acute promyelocytic leukemia patients can be more effective in inducing cytotoxicity by dendritic cells [36]. Clarkson et al observed striking splenomegaly, granulopoietic stimulation and erythroblastosis in the chick embryo upon injection of several mouse leukemic cell lines, but was unable to identify the factors responsible for this effect [37,39]. It is possible that the P388, P815 and other mouse leukemic cell lines secrete exosomes that acts on host embryonic spleen stem cells to stimulate granulopoiesis and also to induce erythroblastosis [37]. In another study, acute myeloid leukemia patients’ sera derived exosomes have been shown to have a detrimental effect on natural killer cells’ (NK cells) ability to kill tumor cells [40,41]. Cross talk between endothelial cells and leukemic cells in the bone marrow via exosomes leads to an increase in neovascularization due to the presence of higher amount of angiogenic factors in CML exosomes [42]. In another study researchers found that K562 CML cell line derived exosomes induces angiogenesis in human umbilical endothelial cells (HUVEC) [43]. The CML cell line derived exosomes modulate the process of neovascularization by inducing expression of ICAM-1 and VCAM-1 cell adhesion molecules and down-regulating expression of VE-cadherin and β-catenin on the endothelial cell surface leading to an increase in endothelial cell motility [44]. K562 cell line derived exosomes are found to be more highly enriched for miRNAs than the whole K562 cells [45]. In acute myelogenous leukemia (AML) the exosomes secreted by leukemic cells also modulate bone marrow niche cells to support disease progression and therapy resistance at the expense of homeostasis. Huan et al found that primary AML cells and AML cell lines release exosomes carrying several coding and non-coding RNAs that alters and reprograms the proliferative, angiogenic, and migratory responses of stromal and hematopoietic progenitor cell lines [46].
In view of the above and the emerging importance of exosomes in leukemias, the objective of the present study was to determine if exosomes have any role in the collective stimulation of growth of Ph+ ALL3 cells at low cell density by factors secreted by the same or other cells growing at high cell density [6]. The ALL3 cell line provided a unique opportunity to investigate the mechanisms regulating the growth of these malignant cells that closely simulate the conditions in the pleural fluid ecosystem in which they were growing rapidly in ascetic form in the patient. In contrast to many other long-established human or murine BCR-ABL driven leukemic cell lines, ALL3 cells do not form colonies in methylcellulose, do not grow in liquid culture at low cell densities (~5000-10,000 cells/ml) (LD), and grow increasingly faster at progressively higher cell densities (HD) between 20,000 cells/ml and 3-4 × 105 cells/ml without stimulation by any growth factors (GFs) [6]. The ALL3 cells are unresponsive to any known hematopoietic cytokines, produce no clones in semi-solid media, not even tiny ones, and don’t grow as single cells in 60-well single cell cloning plates. The cell-free supernates from ALL3 cells grown at high starting cell densities (HDSN) were found to stimulate the growth of the ALL3 cells at LD at which they otherwise don’t grow. The ALL3 cells shortly enter apoptosis and die at LD, but the apoptosis of LD ALL3 cells can be repressed and some of the cells rescued and stimulated to resume proliferation in the presence of the HDSN. Labeling studies with Ki67, BrdU or EdU showed that the LD ALL3 cells are poised to begin proliferating but cannot do so without being triggered by HDSN from ALL3 cells or some other normal or leukemic cells growing at HD [6].
Our study reveals some clues about the underlying exosome mediated proliferative mechanisms responsible for the defective quorum sensing in ALL that may also be involved in other hematologic malignancies and solid cancers. It also provides strong evidence that the great majority of individual ALL3 cells by themselves have very limited self-renewal and proliferative potential, and are only able to proliferate continuously and achieve dominance in vitro and presumably in vivo because of collective communication and a continuously produced supply of growth promoting substances present in the exosomes and soluble growth factors secreted by rapidly proliferating HD ALL3 cells.
Materials and methods
Drug formulations
All the TKIs such as PD166326, Dasatinib, imatinib mesylate, AMN107, and BMS214662 were synthesized by Dr. Darren Veach in Clarkson Lab or by Chemical Synthesis core facility at MSKCC, NY.
ALL3 cells
The human p190BCR-ABL driven ALL cells line (ALL3) was derived from the rapidly growing Ph+ ALL leukemic cells growing in ascitic form in the pleural fluid of an adult patient with widely disseminated Ph+ ALL who died shortly thereafter [6]. Multiple aliquots of ALL3 cells were frozen to preserve the cells’ condition as closely as possible to their status in the pleural fluid. When experiments were planned, an aliquot was thawed about a month or so ahead of time as it took a few weeks or longer for the majority of cells surviving the freeze/thaw procedures to resume growing at about their original rate in the pleural fluid and shortly in vitro after collection of the pleural fluid by thoracentesis. After thawing an aliquot a portion of the thawed cells was refrozen for future use. During the course of a series of experiments the cells were passaged serially in liquid media for about 4-6 months during which they usually maintained their original growth characteristics, but with longer passage, they sometimes began to adapt to the liquid culture conditions and started to grow at lower cell densities at which they would not grow originally. During this optimal experimental period the cells were maintained in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Hyclone Laboratories, Logan, Utah, USA), 1% penicillin-streptomycin solution (PSN; 10,000 I.U. penicillin and 10,000 μg/ml streptomycin) (Corning # 30-001-CI, Fisher Scientific, Pittsburgh, PA, USA), 1% sodium pyruvate (Mediatech Inc, Manassas, VA, USA # 25-000-CI), 1% HEPES buffer (N-2-hydroxyethylpiperazine-N’-2-ethanesulphonic acid) (Mediatech # 25-060-CI), 1% non-essential amino acids (NEAA; Mediatech #25-025) and 0.1% β-mercaptoethanol (BME) (Life Technologies, Grand Island, NY, USA # 21985-023). Hereafter, we will use the term ALL3 media to describe the above culture medium used to culture the ALL3 cells.
For exosome collection, we used exosome-depleted FBS to prepare ALL3 media with 10% FBS. FBS was depleted of bovine exosomes by ultracentrifugation at 100,000 × g for 70 min. The ALL3 cells were grown for 72-96 hr for collecting supernatant and the cell-free supernates were subjected to exosome purification.
Collection and processing of supernatant
The ALL3 cells were washed with plain IMDM and suspended (0.5-1 × 106 cells/ml) in IMDM with 10% exosome-depleted FBS and cultured for 72-96 hr at 37°C in a 5% CO2 atmosphere. The cell culture supernates were harvested after 3-4 days of growth and the cells were centrifuged at 500 × g for 10 min. The cell-free supernatant was collected and filtered using Millipore 0.22 μm 50-ml filter tubes. The filtered-supernatant was used either for stimulation or exosome purification.
Exosome isolation
The ALL3 cells were cultured in media supplemented with 10% exosome-depleted FBS. We used a modified version of the technique developed by Théry et al [27] for exosome isolation. Supernatant fractions collected from 72-96 hr ALL3 cell cultures were pelleted by centrifugation at 500 × g for 10 min leaving the cells pellet behind. The supernatant was further centrifuged at 20,000 × g for 20 min to remove any cell debris. The exosomes were then harvested by centrifugation at 100,000 × g for 70 min. The exosome pellet was resuspended in 25 ml of PBS (Phosphate-buffered Saline, Invitrogen, Carlsbad, CA) and collected by ultracentrifugation at 100,000 × g for 70 min. The exosome pellet was suspended into 25 ml of PBS and loaded on a 4 ml 30% sucrose cushion in D2O (300 g/l sucrose, 24 g/l Tris base, pH 7.4) in a SW 28 ultracentrifuge tube. Samples were centrifuged at 165,000 × g for 1 hr at 37°C and, approximately 3.5 ml of the sucrose cushion, now containing exosomes, was collected from the lower phase and transferred to a fresh ultracentrifuge tube. The tube containing the sucrose cushion with exosomes was filled close to the rim with 25 ml of PBS and centrifuged again at 165,000 × g for 1 hr at 37°C. The supernatant was discarded and the pellets of exosomes were stored at -20°C until further use.
[3H]-Thymidine incorporation assay
The supernates or exosomes from cells growing at high starting cell densities were collected as described above. For growth assays, cells were counted using the trypan blue dye exclusion method and cells equivalent to 5,000-10,000 cells/ml (LD ALL3 cells) were placed into 15-ml falcon tubes. The adjusted ALL3 cells at the LD were washed with fresh ALL3 media once, and replaced with HDSN or exosome pellets collected from ALL3 cells grown at high starting cell density or with plain ALL3 media (as negative control). In each experiment the cell suspension volume was 6-7 ml. Then, the LD cells were resuspended thoroughly and dispensed into two 6-well flat bottom plates with 3 ml cell suspension per well in each plate and kept at 37°C, 5% CO2 in an incubator until harvesting them for assay. The day before harvesting the cells on a filter plate, 200 μl of cell suspensions were taken from the 6-well plate and seeded in triplicate in 96-well round bottom plates. Then, these cells were incubated with 20 μl (for each well) of 0.3 μCi of [3H]-thymidine (PerkinElmer Life Sciences, Shelton, CT, USA), and the cells were incubated for 18 hr at 37°C and 5% CO2. Next day, cells were harvested on Unifilter GF/C 96-well plates (Perkin Elmer Life Sciences # 1450-521) using Unifilter-96 cell harvester (Perkin Elmer Life Sciences # 961961) as described by manufacturer. The plates were allowed to air dry, and 20 μl Microscint-20 fluid (Perkin Elmer Life Sciences # 6013621) was added and plate was covered with transparent top-seal and opaque back-seal, and [3H]-thymidine radioactivity was measured in TopCount Microplate Scintillation Counter (Perkin Elmer Sciences). This assay system was used to determine stimulatory activity of different sources of supernates on the growth of the LD ALL3 cells.
[3H]-Thymidine proliferation assay for drug inhibition study
The effect of PD166326, Dasatinib, imatinib mesylate, AMN107, and BMS214662 on ALL3 proliferation was determined by [3H]thymidine assay as previously described [47]. Briefly, cells were plated in triplicate at a particular concentration in 96-well plates and treated with a range of TKI concentrations or control (DMSO). After 48 hours, [3H]thymidine was added to each well for 18 hours, and incorporation was quantitated by using a Packard scintillation counter (Packard Instrument, Downers Grove, IL). After correction for background, counts per minute (CPM) values were determined for each sample and normalized relative to control-treated samples. CPM as percentage of control was then plotted versus the concentration of drug.
Western blotting
ALL3 cells, K562, R10(-), Mo7 and CML CD34+ were lysed in RIPA buffer (50 mM Tris (tris(hydroxymethyl)aminomethane) pH 7.4, 150 mM NaCl, 1% sodium deoxycholate, 1 mM EDTA (ethylenediaminetetraacetic acid), 1% Triton-X, 0.1% SDS (sodium dodecyl sulfate)) containing 1 mM phenylmethylsulfonyl fluoride, 1% aprotinin (vol/vol), 25 mM sodium fluoride, 1 mM sodium orthovanadate, 1 μg/mL pepstatin, 5 μg/mL leupeptin, and 2 mM Pefabloc SC (Roche Diagnostics, Mannheim, Germany) as previously described [48]. RIPA lysates were normalized by OD595 (Bio-Rad Protein Assay; Bio-Rad Laboratories, Hercules, CA). For Bcr/Abl expression analysis, cells were lysed directly in 2X sample buffer, briefly sonicated, and centrifuged for 10 minutes at 12,000 × g at 4°C to remove insoluble debris. Cell lysates were resolved by SDS/polyacrylamide gel electrophoresis and electrophoretically transferred to a nitrocellulose membrane overnight and probed with antibody overnight. The antibody for BCR-ABL detection in ALL3 cell line was anti-Abl (Oncogene Research Products, Boston, MA.
Mouse transplantation
Animal studies and procedures were approved by the Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee. NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NOD/SCID) mice were either purchased from The Jackson Laboratory (Bar Harbor, ME, USA) or bred in-house, and were housed in specific pathogen-free facilities and fed a sulfatrim-containing diet. Mice, 6-8 weeks old, were irradiated (3 Gy) 3 h before transplantation and cells of interest were resuspended in 0.2 ml of QBSF and transplanted intravenously via the tail vein. Unless otherwise indicated, mice were sacrificed 5-6 weeks after transplant.
Bioluminescent imaging of mice
NOD-SCID mice (8-10 weeks of age; Jackson Laboratory) were sublethally irradiated with a single fraction of 300 rads, and 3-6 hr later, a total of 1 × 106 cells were administered by tail vein injection. Mice were imaged and total body luminescence quantified.
Atomic Force Microscopy (AFM)
AFM images were recorded at room temperature (RT) using an AFM in contact mode. The samples were prepared by applying a drop of the exosome solution (1:50 dilution of the original purified solution in PBS) onto mica, incubating for 10 min, and blowing dry with N2.
Measurement of particle size and concentration distribution with NTA
For particle size determination, nanoparticle tracking analysis (NTA) was performed with a NanoSight LM-10 instrument equipped with the NTA analytical software. Isolated exosomes from HD ALL3 cells were analyzed using the NanoSight LM-10 instrument (NanoSight Ltd, Amesbury, UK). The analysis settings were optimized and kept constant between samples, and each video was analyzed to give the mean, mode, median and estimated concentration for each particle size. All experiments were carried out at a 1:1000 dilution, yielding particle concentrations in the region of 1 × 108 particles per ml in accordance with the manufacturer’s recommendations. All samples were analyzed in quadruplicate. The concentrations of particles from HD ALL3 cells were expressed per cell numbers.
Measurement of particle size and concentration distribution with zetasizer
Dynamic light scattering was performed with a Zetasizer nanoseries instrument (Malvern Nano-Zetasizer, λ = 532 nm laser wavelength). The exosome size data refers to the scattering intensity distribution (z-average). Each experiment was carried out in quadruplicate. For dynamic light scattering, the exosomes that were initially dispersed in PBS after the isolation were diluted 1:1000 with PBS.
Statistical analysis
Unless otherwise indicated, analyses were performed on data generated from quadruplicate NTA and zetasizer results. Data were analysed using Prism6 (version 6).
Results
Karyotype and FISH analysis of ALL3 Cells
The human p190BCR-ABL driven ALL cell line (ALL3) was derived from the rapidly growing Ph+ ALL leukemic cells growing in ascitic form in the pleural fluid of an adult patient with widely disseminated Ph+ ALL who died shortly thereafter. 26.5% of ALL3 cells have 2 copies of bcr/abl and 73.5% have 3 copies (Figure 1A, 1B). ALL3 cells contain p190 bcr/abl protein as confirmed using western blotting (Figure 1C).
Figure 1.
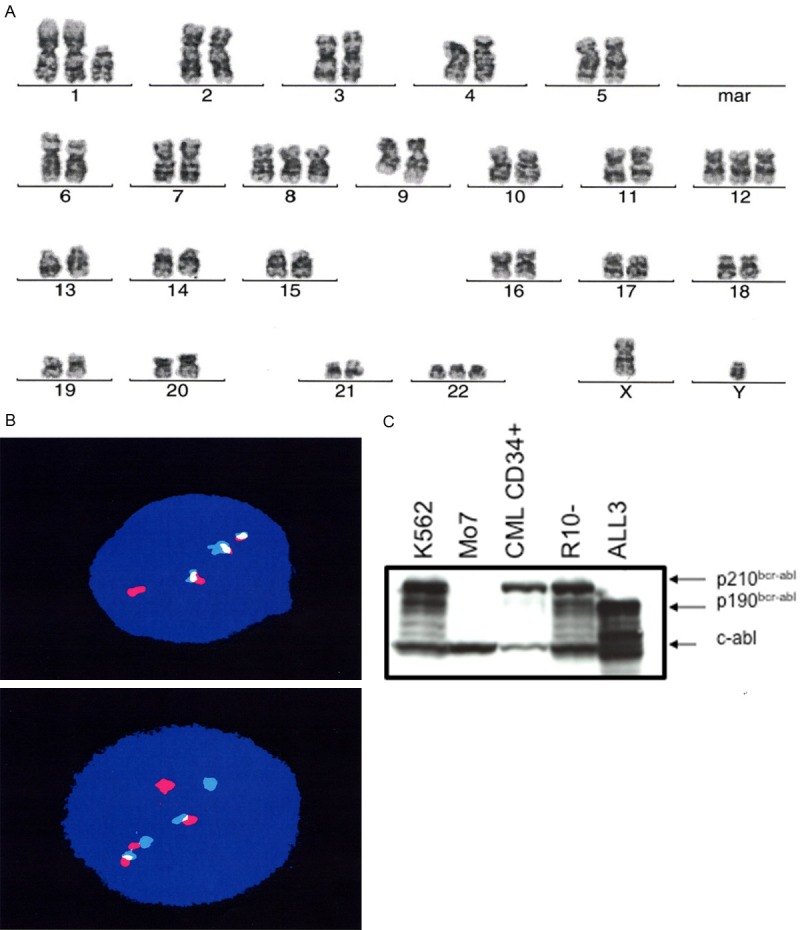
The karyotype and FISH analysis of ALL3 cells. A and B. 26.5% of ALL3 cells have 2 copies of bcr/abl and 73.5% have 3 copies of bcr/abl. C. Confirmation of size of BCR/ABL protein in ALL3 cells using western blot analysis. The ALL3 have a p190 kd size fusion protein as compared with other cell lines such as K562, R10(-) cell lines and CML CD34+ cells which contain a p210 kd BCR/ABL protein. Note: Analysis was performed by Cytogenetics core facility, MSKCC, NY.
Growth of ALL3 cells at different starting cell densities in-vivo in mice and in-vitro
We followed the growth of the ALL3 cells at different starting cell densities of 0.5 × 104, 1 × 104, 5 × 104, 1 × 105 and 5 × 105 cells/ml using the trypan-blue dye exclusion method. The hemocytometer cell counts are increasingly inaccurate at the low density. As shown in Figure 2A the ALL3 cells were growing faster at higher starting cell densities (HD) between 5 × 104-5 × 105 cells/ml with doubling times (DTs) of ~24 hr. At low starting cell densities of 0.5 × 104-1 × 104 cells/ml, ALL3 cells did not grow in liquid culture except very transiently during the first few days (Figure 2B). Mice injected S.C. with as few as 5 × 105 ALL3 cells developed tumors reaching 2 cm3 in size between 4 and 6 weeks after injection (Figure 3A), and mice injected i.v. with 5 × 105 or more ALL3 cells all developed disseminated leukemia within 5 weeks after injection (Figure 3B). However mice injected i.v. with 5 × 103, 2.5 × 104, and 5 × 104 ALL3 cells did not develop leukemia, unlike the established mouse BM185 cell line which caused disseminated leukemia in the majority of mice with 50-100 cells (data not shown). The cells grow increasingly faster at progressively higher cell densities between 20,000/ml and 1-3 × 106/ml and produce lethal tumors in NOD-SCID mice when injected either SC or IV in numbers as low as 105 cells (Figure 3C).
Figure 2.
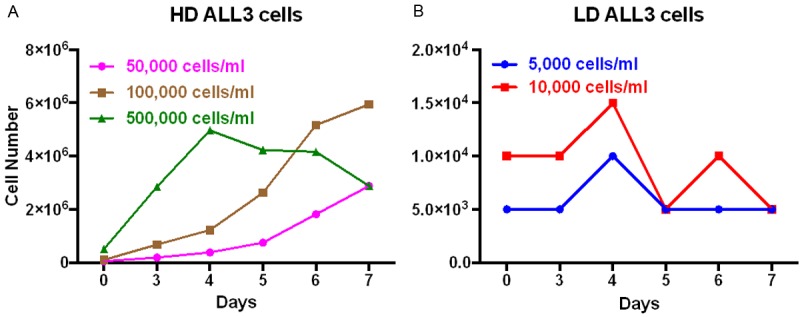
Growth of the ALL3 cells at different starting cell densities. The ALL3 cells were grown in the ALL3 medium as described in ‘Methods’. Comparative growth of the ALL3 cells at starting cell density of (A) HD of 5 × 104, 10 × 104 and 5 × 105 cells/ml and (B) 0.5 × 104 -1 × 104 cells/ml. Y-axis represents total number of viable cells on different days as determined using the trypan-blue exclusion method. Note: In, (A) and (B), the Y-axis has a different scale.
Figure 3.
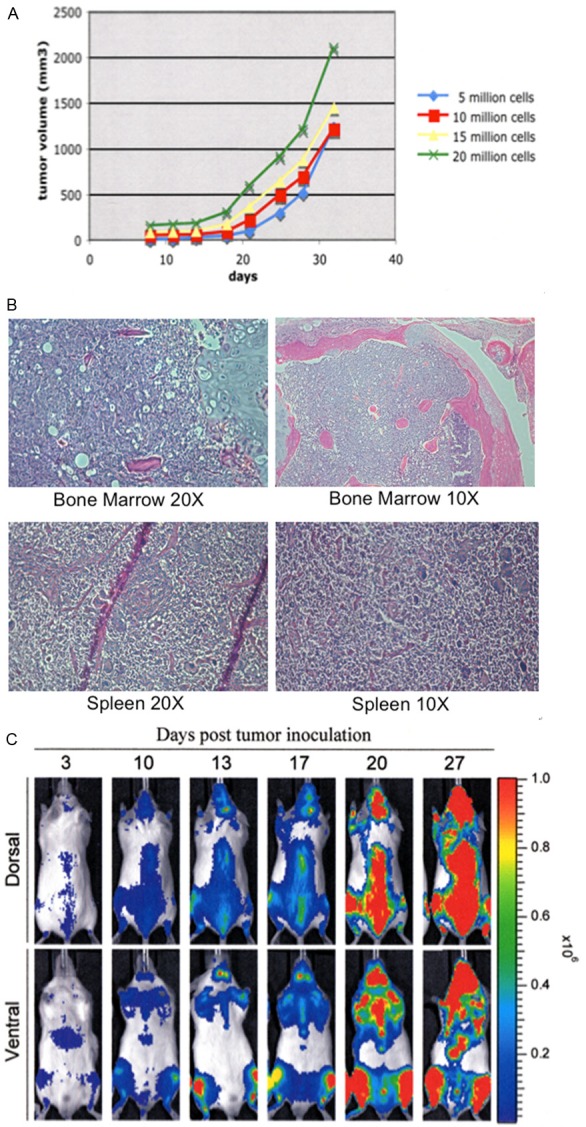
Growth of the ALL3 cells in mice. A. In-vivo growth rate of ALL3 cells implanted S.C. in NOD/SCID mice with 5, 10, 15, and 20 million ALL3 cells. B. In mice injected i.v. with 5 × 105 or more ALL3 cells widespread dissemination of the leukemic cells were observed in all mice (except the control), with infiltration of multiple organs such as spleen and bone marrow. C. Bioluminescence imaging of systemically injected ALL3 cells. Mice received 3Gy IR 3 hours before I.V. injection of ALL3 cells. Mice were sacrificed 35 days post injection and analyzed for signs of disease. Mice injected i.v. with ALL3 cells were sent to the Molecular Pathology Facility for complete necropsy either 5 or 6 weeks after injection. Widespread dissemination of neoplastic lymphocytes was observed in all mice (except the control), with infiltration of multiple organs as well as bone marrow, meninges, and epidural space. Despite bone marrow filtration, most of the animals had a normal WBC count and no sign of circulating atypical lymphocytes. Note: The mouse study was performed by Elisa DeStanchina, head of the MSKCC Anti-tumor Assessment Core Facility.
Comparison of inhibition of ALL3 cells in the presence TKIs
We then compared the sensitivities of the ALL3 cells to several different TKIs (Figure 4). The ALL3 cells were highly sensitive to inhibition of growth by Dasatinib and PD166326 as shown by 3H-thymidine uptake (Figure 4A), less sensitive to inhibition by Imatinib, BMS214662 and AMN107 (Figure 4B, 4C) and did not show any comparable beneficial synergistic effect to the combination of Dasatinib and BMS214662 (Figure 4D).
Figure 4.
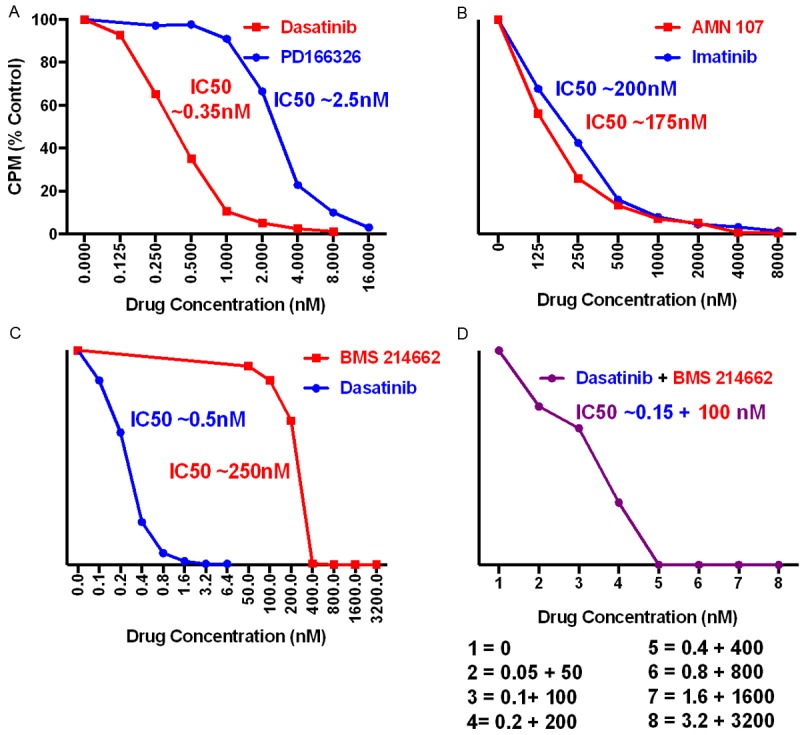
Inhibition of [3H]-Thymidine uptake by exponentially growing HD ALL3 cells by the TKIs. Comparison of the concentrations of (A) Dasatinib and PD166326 (B) AMN107 and Imatinib required to inhibit growth of HD ALL3 cells (40,000 cells/well) growing exponentially at a starting cell density of ~4 × 105 cells/ml. (C) Comparison of the concentrations of Dasatinib and BMS214662 required to inhibit growth of HD ALL3 cells (100,000 cells/well) growing exponentially at a starting cell density of ~5 × 105 cells/ml. (D) Lack of synergy combining Dasitinib + BMS214662. The approximate average IC50 values of the above TKIs for ALL3 cells respectively were: Imatinib = 200 nM, PD166326 = 2.5 nM, Dasatinib = 0.35 nM, AMN107 = 175 nM, and BMS214662 N = 250 nM.
Comparison of growth of ALL3 cells at different starting cell densities and per cm2 of surface area
As described earlier ALL3 cells do not grow at all in liquid media at starting cell densities at or below ~ 5000 cells/ml unless they are tightly packed together as in round-bottom 96 well plates. Proximity or close contact are clearly important for the growth of ALL3 cells, the ALL3 cells grown in 96-well round bottom plate at concentration of 0.5 × 104-5 × 104 cells/ml grow faster, whereas at very low starting cell densities (2000, 1000, and 500 cells/ml) they don’t grow well as shown using growth curve Figure 5A and [3H]-thymidine uptake assay (Figure 5B). We observed that 5000 cells failed to grow except transiently even at a density of 50,000 cells/ml and 14-15,000 cells per cm2 of surface area; 10,000 cells only grew slowly at cell densities of 105 cells/ml and ~30,000 cells per cm2; 20,000 cells grew poorly at 40,000 cells/ml and 27,000 cm2, and best at 2 × 105 cells/ml and ~60,000/cm2 (Figure 5C, 5D).
Figure 5.
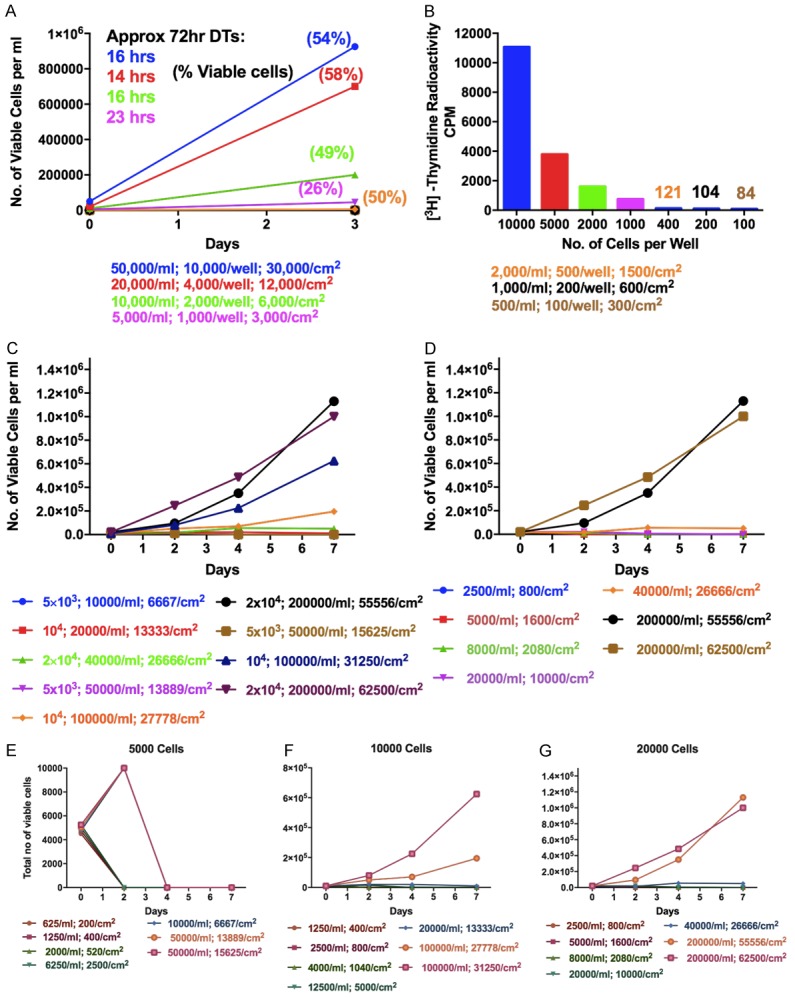
Growth of the ALL3 cells at different starting cell densities per ml and per cm2 of surface area. A. Comparison of the growth of ALL3 cells at different starting densities in 96-well round bottom plates. The y-axis represents total number of viable cells per ml. Note: Because the well volume is only 0.2 ml and the bottom surface area only 0.32 cm2, the total cell numbers per well are 1/5 of those shown per ml and 3 times the latter per cm2. The doubling times shown are very rough estimates because of low cell counts. B. The [3H]-Thymidine uptake assays showing comparison of ALL3 cells kept at different starting cell densities in 96-well round bottom plates. The y-axis represents the counts per minute (CPM). C. Comparison of growth of ALL3 cells at starting cell densities of 5000, 10000, and 20000 cells per ml and per cm2 of surface area, in different size flasks, petri dishes or wells. D. Growth of ALL3 cells at different cell densities per ml and per cm2 of bottom growing area in 24 well plates. E-G. Comparison of growth of ALL3 cells at starting cell densities of 5000, 10000, and 20000 cells/ml and per cm2 of surface area in different size flasks, petri dish or wells.
ALL3 cells usually don’t grow at all in liquid media at starting cell densities at or below ~5000 cells/ml unless they are tightly packed together as in round-bottom 96 well plates (Figure 5E). Total ALL3 cells at 10,000 or 20,000 only grew at the highest starting cell densities (104 or 2 × 104 cells/ml) and when closely packed together (~ 30,000/cm2 or 60,000/cm2), Figure 5F and 5G, and didn’t grow at 5000 cells/ml even at a starting cell density of 5 × 104 cells/ml and ~ 15,000 cells/cm2 (Figure 5E).
Figure 5 shows some of the important characteristics of the growth of ALL3 cells in liquid media, namely their failure to grow at low starting cell densities and faster growth at higher densities in 48 well plates, their still faster growth and to higher peak numbers at intermediate starting densities when closely packed together on bottom growing areas, and their slower growth at very high starting densities as they rapidly reach saturation densities. Thus both intimate cell contact as well as cell density are clearly important factors in enhancing their growth.
Comparison of growth of ALL3 cells in the presence of HDSN
Next, we used the filtered supernatant collected from the ALL3 cells growing at high starting density to see whether the HDSN can stimulate the growth of the LD ALL3 cells or not. We used [3H]-Thymidine incorporation assay as described in the “material and method section” to observe the stimulatory activity. The LD ALL3 at ~5,000 cells/ml grew very well in the presence of filtered HDSN providing maximum stimulatory activity around ~15-18 days (Figure 6). We also found that the HDSN is not able to stimulate growth of ALL3 cells at very low starting cell densities (100, 500, and 1000 cells/ml) (Figure 7). The ALL3 cells grow much better when the starting cell density is 5000 cells/ml compared to 2000 cells/ml and no growth at all was detectable below 2000 cells/ml (Figure 7). Thus, the diffusible, soluble, and secreted factors present in the HDSN are able to stimulate growth of the non-growing LD ALL3 cells but not if the latter’s cell number and density is below ~1-2 × 103 cells/ml. ALL3 cells at 5000 cells/ml or below without stimulation from HDSN failed to grow at all under the same condition.
Figure 6.
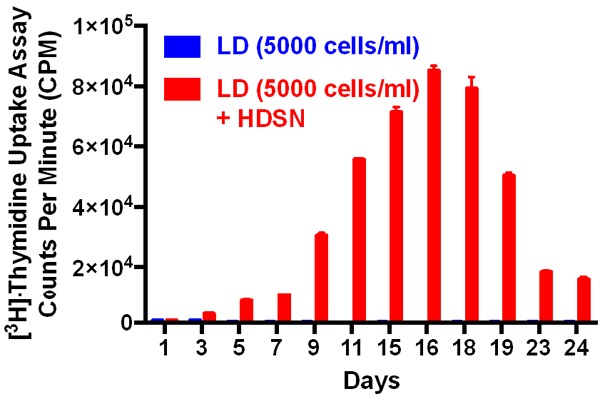
The [3H]-Thymidine uptake assays of the ALL3 cells in the presence of HDSN. The [3H]-Thymidine uptake assays showing the stimulatory effect of the HDSN collected from HD ALL3 cells on the growth of LD ALL3 cells (5000 cells/ml; red bar) to that of LD ALL3 cells without stimulation (blue bar). The y-axis represents the counts per minute (CPM).
Figure 7.
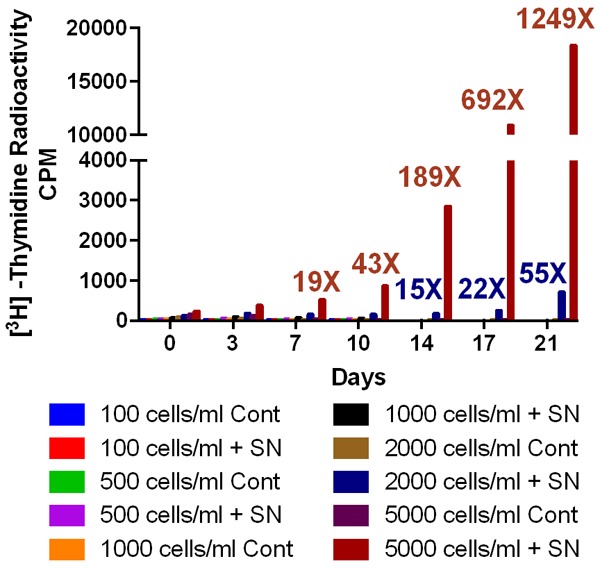
Stimulatory effect of the HDSN on the different numbers of low cell densities ALL3 cells. The HDSN was collected from HD ALL3 cells grown at 5 × 105 cells/ml after 3 days when cell count was 1.1 × 106 cells/ml and viability 85% (DT = ~52 hr). The [3H]-Thymidine uptake assays showing the stimulatory effect of the HDSN on different number of LD ALL3 cells (100-5000 cells/ml). The y-axis represents the counts per minute (CPM).
Comparison of growth of ALL3 cells in the presence of ultracentrifuged fractions
The stimulatory activity of the whole HDSN was due to several components of the HD ALL3 cells’ secretome. The cells’ secretomes include various soluble growth factors, proteins, small molecules, peptides and also exosomes. To determine which component of the secretome was responsible for the stimulatory activity, we designed the experiment as shown in Figure 8A. The supernates collected from exponentially growing HD ALL3 cells were filtered and centrifuged to remove cells, dead cells, and cell debris. Then the supernates were ultracentrifuged at high speed to collect top, middle, and pellet fractions as shown in Figure 8A. The pellet was further processed as described in the experimental section to enrich for purified exosomes as shown in Figure 8B. This experiment was designed to determine if the activity was due to the secretory proteins (upper part= lacking the exosomes), exosome (pellet containing the exosomes), and both. After obtaining all the fractions (upper, middle, purified enriched exosomes, and whole HDSN), we incubated them with the LD ALL3 cells to follow their effect in time-dependent manner using the [3H]-thymidine incorporation assay. As shown in Figure 9, we found that as expected the whole HDSN were able to stimulate the growth of the LD ALL3 cells (at 5000 cells/ml). We also found that the upper part (exosome depleted) also stimulated the LD ALL3 cells and the stimulatory activity profile on the LD ALL3 cells was similar to that of the HDSN. Surprisingly, the purified exosome (pellet) did not show any stimulatory activity until approximately two weeks, but after this the purified exosomes in the pellet first showed minimal stimulatory activity at day 16 and at day 21 the LD ALL3 cells were clearly stimulated and continued to grow until day 27 with maximal activity at day 24 (Figure 9). It is important to note that the LD ALL3 cells were only treated once with exosomes, whole HDSN, or upper HDSN lacking exosomes. Thus, in this study we were able to show that the stimulatory activity of the whole HDSN is present in both the soluble secretory proteins or other molecules as well as in exosomes, but the latter’s stimulation occurred later, probably due to the greater need for transport of exosomes across the cell membrane and intracellular processing.
Figure 8.
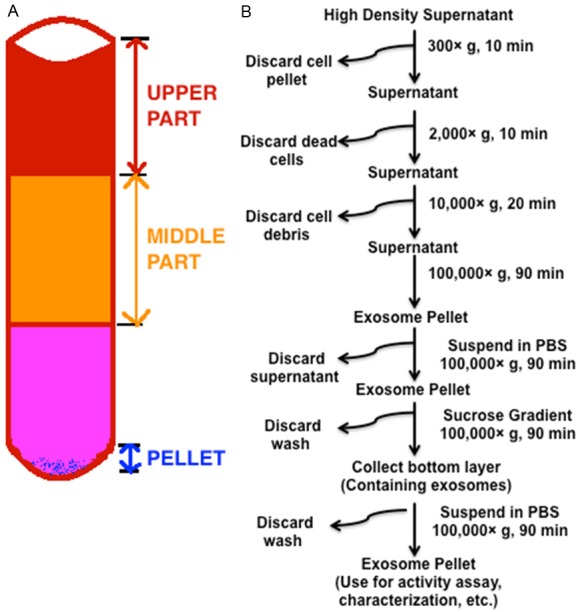
Design of experiment to determine activity of different part of HDSN. (A) Scheme used to collect HDSN and exosome. After removal of cells and cell debris the supernates were ultracentrifuged and the upper (representing supernate devoid of exosome), middle part (representing supernate devoid of exosome), and pellet (representing small extracellular vesicle = exosome) were collected. The pellet was further processed as shown in (B) to obtain more pure exosome. All the fractions were used to determine if they could stimulate the LD ALL3 cells or not. (B) Steps for the exosome purification procedure based on differential ultracentrifugation and sucrose gradients. The right side of the arrow indicates speed, time or washing solution used for each step. Pellets (cells, dead cells, cell debris) were discarded after each of the first three steps, and the supernatant was ultracentrifuged. Then the pellet was washed, and also purified using sucrose gradient and washed again with PBS. The washes were discarded and the pellet was incubated at -20°C until further use.
Figure 9.
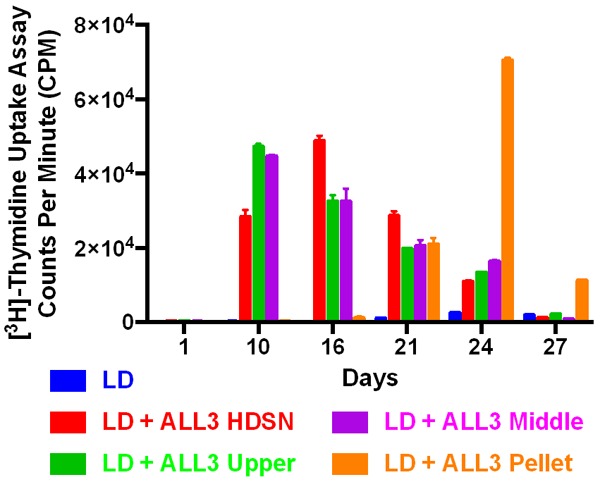
The [3H]-Thymidine uptake assays showing the stimulatory effect of different fractions collected from HD ALL3 cells on the growth of the LD ALL3 cells. Blue bar: the LD ALL3 cells (5000 cells/ml) without the HDSN; red bar: the LD ALL3 cells in the presence of the whole HDSN; green bar: the LD cells in the presence of upper part of the ultra-centrifuged HDSN without exosome; purple bar: middle fractions as control, and brown bar: the LD ALL3 cells stimulated with exosomes. The y-axis represents the counts per minute (CPM).
Characterization of exosomes
Next, we performed several analyses to confirm the presence of exosome vesicles in the pellet. We investigated the total number of exosomes and their size using nanoparticle tracking analysis (NTA) method. The exosomes were collected from five separately growing HD ALL3 cells after either three or four days of the growth. Appropriately diluted samples were kept on ice and applied to the sample chamber of the Nanosight LM-10 instrument. For each preparation the measurement were taken in triplicate. Using NTA we found that 0.0028 × 108 particles (average of all the five preparations) were secreted per million HD ALL3 cells. Exosome particles concentration for each preparation is shown in Figure 10A. Figure 10B shows the average mean size for exosome particles collected from five different preparations using the NTA method. The diameter of the exosome particles ranged from ~30 to 450 nm. The average particle diameter from all of the five preparation is ~148 nm. In NTA analysis, the light intensity of the larger particles causes overestimation of the smaller particle size exosome. Therefore, we also used Zetasizer to determi-ne the exosome particle size using the Dynamic Light Scattering (DLS) method. Figure 10C shows the Z-average size of the exosome particles collected from five different preparations; the average size from all the preparations was found to be ~87 nm. We also used AFM to confirm presence of the exosome particles (Figure 10D) and NTA video analysis to show representative images (Figure 10E).
Figure 10.
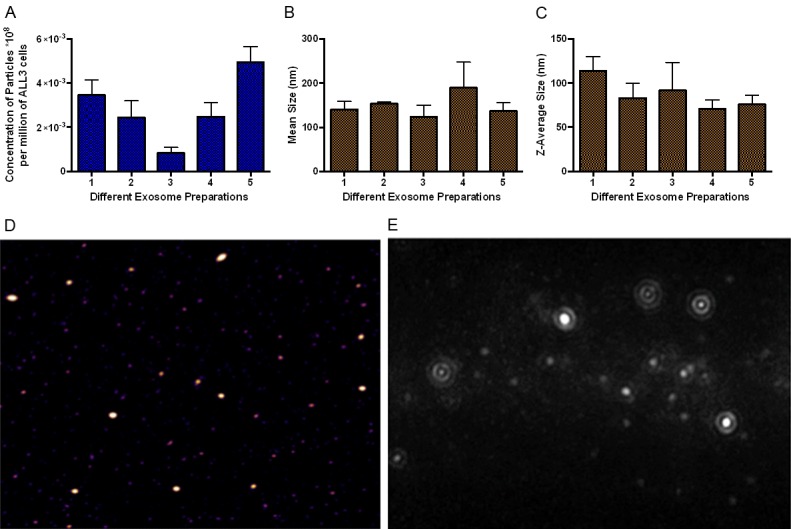
Typical characteristics of exosomes. (A) Nanoparticel tracking analysis (NTA) of the HD ALL3 cells. X-axis represents concentration (number) of exosome particles secreted per million of ALL3 cells from five individual preparations. The measurements were taken in quadriplicate (B) Average size of exosomes purified from ALL3 cells using NTA from five different preparations. (C) Z-mean average size of exosomes purified from HD ALL3 cells using Zetasizer from five different preparations. (D) Atomic force microscopic image of exosomes purified from ALL3 cells. (E) Representative images of exosome particles of different sizes purified from ALL3 cells from NTA video analysis.
The data indicate that the stimulation of the LD ALL3 cells are different for secretory factors and exosome fractions, suggesting that there may be different stimulatory factors or they are processed differently. The exosomal contents are enclosed in membranous structures and their uptake by the non-growing LD ALL3 cells require transport across the cell membrane and intracellular processing. Once they are processed the stimulatory factors act on the downstream growth promoting signaling pathways to further stimulate growth. In contrast the secreted proteins or other growth factors are readily available and once they are in sufficient quantity, they probably bind quickly to receptors and stimulate the growth of non-growing LD ALL3 cells.
Discussion
The Ph+ ALL3 cell line was used as a model system to try to understand how cells communicate with each other and with other cells and the abnormalities in quorum sensing that permit these S/P cells to far exceed normal homeostatic cell densities. The ALL3 cells do not grow at low staring cell densities but grow very well with good viability at high starting cell densities. This observation is nicely coordinate with older observations in bacteria in which light emission was determined to occur only at a high cell population density [49].
Although there are many potential interpretations of this finding, the most probable explanation is that both soluble factors and components of exosomes stimulate the growth of the non-growing LD ALL3 cells. With regard to exosomes, firstly RAB27A was ~2-4 fold up-regulated in the HDSN stimulated LD ALL3 cells [6] suggesting that this induces release and secretion of exosomes which contain factors that can stimulate the non-growing LD ALL3 cells to survive and resume proliferation. Rab27A is known to be involved in the exosome secretion pathways [50] and is highly expressed in melanocytes and hematopoietic and other secretory cells [51]. Its expression has been clinically related to hepatocellular carcinoma [52] and pancreatic cancer [53]. Raimondo et al have shown that CML-derived exosomes promote the proliferation and survival of tumor cells in an autocrine fashion by activating anti-apoptotic pathways [54].
Secondly, in our study we treated the LD ALL3 cells only once with exosomes from HD ALL3 cells. However, in vivo the exosomes are constantly secreted by neighboring leukemic cells or other cells present in microenvironments that can assist small number of semi-isolated leukemic S/P to survive, avoid apoptosis, and continue to grow even in adverse conditions such as during chemotherapy.
In summary, we have demonstrated that diffusible factors secreted or released by proliferating cells are capable of initiating and sustaining growth of Ph+ ALL3 cells at low cell densities at which they do not grow without these stimulatory factors but rather succumb to apoptosis, and death.
Acknowledgements
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30CA008748 at MSKCC. This work was supported in part by The Enid A Haupt Charitable Trust, the MeadRock Foundation, the E./S. Sindina Lymphoma Research Fund and the Albert C. Bostwick foundation. This research was funded in part through the NIH/NCI cancer center support grant P30 CA008748. We would like to thank Drs. Renier Brentjens and Mark Frattini for giving us the ALL3 cell line, Dr. Katia Manova (Molecular cytology core facility head) and Navid Paknejad for their help with AFM experiments and Carol Lambek, Dr. Chong-Yuan Liu, Dr. David Wisniewski and Dr. Su Dao for doing some of the quantitative cellular assays. We also want to thank Drs. David Lyden, Jacqueline Bromberg and Claudia Savini for help with exosome preparations, Dr. Darren Veach for synthesising TKI inhibitors, and Dr. Elisa De Stanchiana for conducting the animal study. We also want to thank Drs. Raju Chaganti, Stephen Larson for their support and Dr. Darren Veach for helpful scientific discussion. We thank Ms. Lucinda Lewis for her great assistance. Numerous colleagues at MSKCC have provided helpful support, advice and use of technical instruments and facilities in their laboratories without which many of the experiments could not have been performed. We are especially grateful to Drs. Minkui Luo, Ronald Hendrickson, Alex Kentsis, David Spriggs, Michael Kharas, Jan Hendricks, and Hediye Erdjument-Bromage for invaluable assistance. Finally we want to thank Drs. David Scheinberg, Geroge Bosl, Jose Baselga, Craig Thompson and Ms. Carol Slattery for support and encouragement without which it would not have been possible to complete this work.
Disclosure of conflict of interest
None.
References
- 1.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 2.Greaves M. Evolutionary determinants of cancer. Cancer Discov. 2015;5:806–820. doi: 10.1158/2159-8290.CD-15-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawano S, Otsu K, Kuruma A, Shoji S, Yanagida E, Muto Y, Yoshikawa F, Hirayama Y, Mikoshiba K, Furuichi T. ATP autocrine/paracrine signaling induces calcium oscillations and NFAT activation in human mesenchymal stem cells. Cell Calcium. 2006;39:313–324. doi: 10.1016/j.ceca.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Montaudouin C, Anson M, Hao Y, Duncker SV, Fernandez T, Gaudin E, Ehrenstein M, Kerr WG, Colle JH, Bruhns P, Daeron M, Freitas AA. Quorum sensing contributes to activated IgM-secreting B cell homeostasis. J Immunol. 2013;190:106–114. doi: 10.4049/jimmunol.1200907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel SJ, Dao S, Darie CC, Clarkson BD. Defective quorum sensing of acute lymphoblastic leukemic cells: evidence of collective behavior of leukemic populations as semi-autonomous aberrant ecosystems. Am J Cancer Res. 2016;6:1177–1230. [PMC free article] [PubMed] [Google Scholar]
- 7.Perie L, Aru J, Kourilsky P, Slotine JJ. Does a quorum sensing mechanism direct the behavior of immune cells? C R Biol. 2013;336:13–16. doi: 10.1016/j.crvi.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Shim J. Drosophila blood as a model system for stress sensing mechanisms. BMB Rep. 2015;48:223–228. doi: 10.5483/BMBRep.2015.48.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CC, Wang L, Plikus MV, Jiang TX, Murray PJ, Ramos R, Guerrero-Juarez CF, Hughes MW, Lee OK, Shi S, Widelitz RB, Lander AD, Chuong CM. Organ-level quorum sensing directs regeneration in hair stem cell populations. Cell. 2015;161:277–290. doi: 10.1016/j.cell.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szabo B, Szollosi GJ, Gonci B, Juranyi Z, Selmeczi D, Vicsek T. Phase transition in the collective migration of tissue cells: experiment and model. Phys Rev E Stat Nonlin Soft Matter Phys. 2006;74:061908. doi: 10.1103/PhysRevE.74.061908. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal GK, Jwa NS, Lebrun MH, Job D, Rakwal R. Plant secretome: unlocking secrets of the secreted proteins. Proteomics. 2010;10:799–827. doi: 10.1002/pmic.200900514. [DOI] [PubMed] [Google Scholar]
- 12.Patel S, Ngounou Wetie AG, Darie CC, Clarkson BD. Cancer secretomes and their place in supplementing other hallmarks of cancer. Adv Exp Med Biol. 2014;806:409–442. doi: 10.1007/978-3-319-06068-2_20. [DOI] [PubMed] [Google Scholar]
- 13.Ngounou Wetie AG, Sokolowska I, Woods AG, Wormwood KL, Dao S, Patel S, Clarkson BD, Darie CC. Automated mass spectrometry-based functional assay for the routine analysis of the secretome. J Lab Autom. 2013;18:19–29. doi: 10.1177/2211068212454738. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Friedl P, Locker J, Sahai E, Segall JE. Classifying collective cancer cell invasion. Nat Cell Biol. 2012;14:777–783. doi: 10.1038/ncb2548. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez Valencia AM, Wu PH, Yogurtcu ON, Rao P, DiGiacomo J, Godet I, He L, Lee MH, Gilkes D, Sun SX, Wirtz D. Collective cancer cell invasion induced by coordinated contractile stresses. Oncotarget. 2015;6:43438–43451. doi: 10.18632/oncotarget.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal. 2013;11:88. doi: 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor DD, Doellgast GJ. Quantitation of peroxidase-antibody binding to membrane fragments using column chromatography. Anal Biochem. 1979;98:53–59. doi: 10.1016/0003-2697(79)90704-8. [DOI] [PubMed] [Google Scholar]
- 20.Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 21.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willms E, Johansson HJ, Mager I, Lee Y, Blomberg KE, Sadik M, Alaarg A, Smith CI, Lehtio J, El Andaloussi S, Wood MJ, Vader P. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep. 2016;6:22519. doi: 10.1038/srep22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song J, Huang J, Chen X, Teng X, Song Z, Xing Y, Wang M, Chen K, Wang Z, Yang P, Hu S. Donor-derived exosomes induce specific regulatory T cells to suppress immune inflammation in the allograft heart. Sci Rep. 2016;7:20077. doi: 10.1038/srep20077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 27.Budnik V, Ruiz-Canada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016;17:160–172. doi: 10.1038/nrn.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, Garcia-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun. 2015;6:7164. doi: 10.1038/ncomms8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarkson B, Strife A. Linkage of proliferative and maturational abnormalities in chronic myelogenous leukemia and relevance to treatment. Leukemia. 1993;7:1683–1721. [PubMed] [Google Scholar]
- 33.Clarkson BD, Strife A, Wisniewski D, Lambek C, Carpino N. New understanding of the pathogenesis of CML: a prototype of early neoplasia. Leukemia. 1997;11:1404–1428. doi: 10.1038/sj.leu.2400751. [DOI] [PubMed] [Google Scholar]
- 34.Clarkson B, Strife A, Wisniewski D, Lambek CL, Liu C. Chronic myelogenous leukemia as a paradigm of early cancer and possible curative strategies. Leukemia. 2003;17:1211–1262. doi: 10.1038/sj.leu.2402912. [DOI] [PubMed] [Google Scholar]
- 35.Greaves M. Leukaemia ‘firsts’ in cancer research and treatment. Nat Rev Cancer. 2016;16:163–172. doi: 10.1038/nrc.2016.3. [DOI] [PubMed] [Google Scholar]
- 36.Shen C, Hao SG, Zhao CX, Zhu J, Wang C. Antileukaemia immunity: effect of exosomes against NB4 acute promyelocytic leukaemia cells. J Int Med Res. 2011;39:740–747. doi: 10.1177/147323001103900305. [DOI] [PubMed] [Google Scholar]
- 37.Clarkson B, Katz A, Dann MA, Karnofsky DA. Behavior of P388 Mouse Leukemia in the Chick Embryo and Hatched Chick. J Natl Cancer Inst. 1964;32:471–495. [PubMed] [Google Scholar]
- 38.Clarkson BD, Katz A. The behavior of P388 mouse leukemia in the chick embryo and hatched chick. Proc Amer Ass Cancer Res. 1960 [PubMed] [Google Scholar]
- 39.Clarkson BD, Ota K, O’connor A. Stimulation of leukopoiesis in the chick embryo by heterologous tumor cells. Proc Amer Ass Cancer Res. 1963:4. [Google Scholar]
- 40.Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica. 2011;96:1302–1309. doi: 10.3324/haematol.2010.039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whiteside TL. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes) Biochem Soc Trans. 2013;41:245–251. doi: 10.1042/BST20120265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corrado C, Flugy AM, Taverna S, Raimondo S, Guggino G, Karmali R, De Leo G, Alessandro R. Carboxyamidotriazole-orotate inhibits the growth of imatinib-resistant chronic myeloid leukaemia cells and modulates exosomes-stimulated angiogenesis. PLoS One. 2012;7:e42310. doi: 10.1371/journal.pone.0042310. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Mineo M, Garfield SH, Taverna S, Flugy A, De Leo G, Alessandro R, Kohn EC. Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a Src-dependent fashion. Angiogenesis. 2012;15:33–45. doi: 10.1007/s10456-011-9241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taverna S, Flugy A, Saieva L, Kohn EC, Santoro A, Meraviglia S, De Leo G, Alessandro R. Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis. Int J Cancer. 2012;130:2033–2043. doi: 10.1002/ijc.26217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng DQ, Huang B, Li J, Liu J, Chen XM, Xu YM, Chen X, Zhang HB, Hu LH, Wang XZ. Selective miRNA expression profile in chronic myeloid leukemia K562 cell-derived exosomes. Asian Pac J Cancer Prev. 2013;14:7501–7508. doi: 10.7314/apjcp.2013.14.12.7501. [DOI] [PubMed] [Google Scholar]
- 46.Huan J, Hornick NI, Shurtleff MJ, Skinner AM, Goloviznina NA, Roberts CT Jr, Kurre P. RNA trafficking by acute myelogenous leukemia exosomes. Cancer Res. 2013;73:918–929. doi: 10.1158/0008-5472.CAN-12-2184. [DOI] [PubMed] [Google Scholar]
- 47.Wisniewski D, Lambek CL, Liu C, Strife A, Veach DR, Nagar B, Young MA, Schindler T, Bornmann WG, Bertino JR, Kuriyan J, Clarkson B. Characterization of potent inhibitors of the Bcr-Abl and the c-kit receptor tyrosine kinases. Cancer Res. 2002;62:4244–4255. [PubMed] [Google Scholar]
- 48.Sokolowska I, Woods AG, Gawinowicz MA, Roy U, Darie CC. Identification of a potential tumor differentiation factor receptor candidate in prostate cancer cells. FEBS J. 2012;279:2579–2594. doi: 10.1111/j.1742-4658.2012.08641.x. [DOI] [PubMed] [Google Scholar]
- 49.Nealson KH, Hastings JW. Bacterial bioluminescence: its control and ecological significance. Microbiol Rev. 1979;43:496–518. doi: 10.1128/mr.43.4.496-518.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. sup pp 11-13. [DOI] [PubMed] [Google Scholar]
- 51.Tolmachova T, Anders R, Stinchcombe J, Bossi G, Griffiths GM, Huxley C, Seabra MC. A general role for Rab27a in secretory cells. Mol Biol Cell. 2004;15:332–344. doi: 10.1091/mbc.E03-07-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spiegelberg HL, Fishkin BG. Human myeloma IgA half-molecules. J Clin Invest. 1976;58:1259–1265. doi: 10.1172/JCI108580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Q, Ni Q, Wang X, Zhu H, Wang Z, Huang J. High expression of RAB27A and TP53 in pancreatic cancer predicts poor survival. Med Oncol. 2015;32:372. doi: 10.1007/s12032-014-0372-2. [DOI] [PubMed] [Google Scholar]
- 54.Raimondo S, Saieva L, Corrado C, Fontana S, Flugy A, Rizzo A, De Leo G, Alessandro R. Chronic myeloid leukemia-derived exosomes promote tumor growth through an autocrine mechanism. Cell Commun Signal. 2015;13:8. doi: 10.1186/s12964-015-0086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


