Abstract
Ureter reconstruction is still a tough task for urologist. Cell-based tissue engineering serves a better technique for patients with long segments of ureter defect who need ureter reconstruction. In this study, we sought to evaluate the differentiation potential of adipose derived stem cells (ADSCs) into urothelial lineage and smooth muscle lineage and to assess the possibility of ureter reconstruction using differentiated cells seeded vessel extracellular matrix (VECM) in a rabbit model. ADSCs were isolated from adipose tissue and identified in vitro. Subsequently, they were cultured with induction medium for urothelium and smooth muscle phenotypes differentiation. After 14 days inducing, differentiation was evaluated by Quantitative PCR and western blot studies. After fluorescent protein labeling, the differentiated cells were seeded onto VECM and cultured under dynamic conditions in vitro. After 7 days culturing, the cell-seeded graft was tubularized and wrapped by two layers of the omentum in a rabbit. Three weeks later, the maturated graft was used for ureter reconstruction in vivo. The ADSCs were isolated and cultured in vitro. Flow cytometry demonstrated that the ADSCs expressed CD29 and CD90, but did not express CD34. After induction, urothelium phenotypes gene (cytokeratin 7) and smooth muscle expression gene (a-SMA and SM-MHC) was confirmed in mRNA and protein level. After cells seeding onto VECM, the induced urothelium cells formed a single epithelial layer, and the induced smooth muscle cells formed a few cell layers during dynamic culture. After 3 weeks of omental maturation, tubular graft was vascularized and comprised epithelial layer positively with cytokeratin 7, cytokeratin 20 on the luminal aspect. At 8 weeks post ureter reconstruction, histological evaluation showed a clearly layered structure of ureter with terminally differentiated multilayered urothelium positively with cytokeratin 20 and uroplakin III over connective smooth muscle tissue positively with a-SMA and SM-MHC. The labeled induced cells could be observed in the reconstructed ureter. We demonstrated that ADSCs could differentiate into urothelial and smooth muscle lineage. Tissue engineered graft by these differentiated cells seeded on VECM could be employed to long segments ureter reconstruction after omental maturation in vivo.
Keywords: Ureter, tissue engineering, adipose derived stem cells, differentiation, omental maturation
Introduction
Ureter reconstruction is still a tough task for urologist. Iatrogenic injuries are responsible for the largest number of ureter injures in surgery [1]. The result of the injury may be ureter fistula or obstruction of the urine passage that can lead to severe hydronephrosis [2]. Ureteroureterostomy was the choice for short segments of ureter defect. But ureter reconstruction is required for long segments of ureter defect to avoid urinary diversion. Gastrointestinal tract is commonly used for ureteral reconstruction. However, intestinal interposition is often associated with many complications, such as intestinal adhesions, chronic infections, urinary calculi, and secondary malignancies [3,4]. The use of tissue engineering techniques in ureter reconstruction presents a promising way to resolve these problems [5,6].
Cell-based tissue engineering may be a better technique for patients with long segments of ureter defect who need ureter reconstruction [5]. Baumert H et al. [7,8] used a sample of autologous cells from the host for cell resources of urothelial cells and smooth muscle cells. However, an organ tissue biopsy may not yield enough normal cells for expansion. Under such conditions, the stem cells may be the ideal resource for tissue engineering, due to their capability of self-renewal and tissue-specific differentiation. Among the stem cells, adipose derived stem cells (ADSCs) have the advantage of high proliferative potential, easy isolation and immunoprivilege [9].
Our previous study demonstrated the smooth muscle differentiation potential of ADSCs [10]. Recent studies show that ASDCs can differentiate toward epithelial lineage with expression of early epithelial-specific proteins under the co-cultured conditions [11-13]. As a continuing research, the aim of the current study is whether the ADSCs could serve as a potential substitute of urothelial cells and smooth muscle cells for ureter tissue engineering in vivo. Herein, ADSCs were inducted to urothelium and smooth muscle phenotype in vitro, and ureter reconstruction was performed using the differentiated cells seeded vessel extracellular matrix (VECM) in a rabbit model.
Materials and methods
Experimental strategy
The whole experimental procedure is comprised of five separate steps: adipose derived stem cell culture; urothelium and smooth muscle phenotype induction; sandwich co-culture of the vessel extracellular matrix (VECM) scaffold and the differentiated cells; omental maturation; ureter reconstruction.
Experimental animals and ethics statement
The animal studies were performed according to the guidelines established by the Medical Animal Care and Welfare Committee of the affiliated hospital, Jining Medical University. A total of 20 New Zealand white rabbits weighing about 3.5 kg were anesthetized with 3% isoflurane. All efforts were made to minimize suffering. The surgical procedures were done in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of China. A total of 30 female rabbits weighing about 3.5 kg were purchased from the Centre for Experimental Animal of Wuhan University (Wuhan, China), which has the production license for experimental animals (SCXK (E) 2008-0004). The rabbits were housed in cages in the animal facility and kept under controlled temperature of 20-25°C, humidity of 40-60%, 12 hour light/dark cycle conditions. The rats were fed with the standard rabbit food and provided tap water.
Cell culture and identification
Ten grams of adipose tissue was obtained from the dorsocervical subcutaneous region of each rabbit. The isolation and culture of ADSCs were performed as previously described [10]. The fresh adipose tissue was minced and then transferred into a centrifugation tube. Tissue digestion was performed using 0.1% collagenase I (Sigma Aldrich, St. Louis, MO, USA) under shaking for 60 mins in a 37°C incubator. After digestion, the suspension was centrifuged at 1,000 rpm for 10 min, and the cell pellet was resuspended in Dulbecco’s modified Eagle’s medium (Gibco, New York, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, New York, USA). Next, the cells were inoculated at a density of 4×104 cells/cm2 in a 25 cm2 culture flask in the incubator. After 2 d, the non-adherent cells were rinsed with phosphate-buffered solution (PBS). The cells were passaged at 80% confluence. The ADSCs of passage 3 were collected for flow cytometry analysis. Cells were analyzed on a fluorescence-activated cell sorter (Beckman Coulter, Brea, CA) to detect the surface markers, including CD29, CD90, and CD34. The antibodies were obtained from BD Biosciences (Franklin, NJ, USA). Data acquisition and analysis were performed by using Cell Quest software (BD, Franklin Lakes, NJ, USA).
Differentiation of ADSCs
For the smooth muscle phenotype differentiation, the induction medium (Dulbecco’s modified Eagle’s medium supplemented with 1% fetal bovine serum plus 100 U/mL heparin) was used. ADSCs were incubated in the medium for 14 d, exchanged every 2 d. The un-induced ADSCs were used as the control.
For the urothelium phenotype differentiation, the induction medium (Dulbecco’s modified Eagle’s medium supplemented with 2% fetal bovine serum plus 2.5 μM all-trans retinoic acid, 20 ng/ml epidermal growth factor, 10 ng/ml hepatocyte growth factor, 10 ng/ml keratinocyte growth factor, and 0.5 μg/ml hydrocortisone) was used. ADSCs were incubated in the medium for 14 d, exchanged every 2 d. The un-induced ADSCs were used as the control.
Gene expression evaluation
Quantitative PCR was carried out to evaluate gene expression. Total RNA was abstracted from cells using Trizol regent (Invitrogen, Carlsbad, CA, USA) and cDNA synthesis was performed using the reverse transcriptase-PCR Kit (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions. Quantitative PCR was performed using SYBR Green PCR Master Mix (DBI Bioscience, Shanghai, China). The primers used in this paper were listed in Table 1. The mRNA levels were evaluated with the comparative CT method and normalized to the mRNA levels of β-actin.
Table 1.
Primer sequences for Quantitative PCR
| Gene | Primer sequences | Product length (bp) | GenBank accession number |
|---|---|---|---|
| a-SMA | F: 5’-ACCGTATGCAGAAGGAAATCA-3’ | 211 | NM_001101682 |
| R: 5’-GCTAGAAACAGAGCAGGGAAGT-3’ | |||
| SM-MHC | F: 5’-GTTCATTCCCTAACCTCTGTGCC-3’ | 180 | NM_001082308 |
| R: 5’-GACCGTCCCTGTTCTCATCCA-3’ | |||
| Cytokeratin 7 | F: 5’-ATGCCGCCTACACGAAC-3’ | 207 | NM_001047870 |
| R: 5’-TCTCCTCATACTGGGCTTTG-3’ | |||
| Cytokeratin 20 | F: 5’-GCTCGTTATGCCAGCCAGTT-3’ | 171 | NM_173128 |
| R: 5’-TTCCAGAAGGCGGCGGTAG-3’ | |||
| Uroplakin III | F: 5’-CCCTGGCTCATGCCTTTATC-3’ | 182 | XM_008257977 |
| R: 5’-CTGTGGTTTGCTGGTTTGGA-3’ | |||
| β-actin | F: 5’-CTGCGTCTGGACCTGGATGG-3’ | 225 | NM_002712153 |
| R: 5’-CGATGGTGATGACCTGGCTGT-3’ |
Western blot was performed to evaluate gene expression at the protein level. Total protein was extracted from cells using M-PER reagent (Pierce, Rockford, IL, USA) and quantified using the BCA protein assay kit (Pierce, Rockford, IL, USA). 10% SDS-PAGE gel was used. Polyvinylidene difluoride membrane was incubated with primary antibody for 12 h at 4°C after being blocked with 5% fat-free milk for 1 h. Then the membranes were incubated with second antibody [horseradish peroxidase-conjugated goat-anti-mouse IgG (Boster, Wuhan, China)] for 1 h. Finally, the membranes were detected using the ECL reagent (Pierce, USA). β-actin was employed as an internal control. Primary antibodies SM-MHC and a-SMA (Abcam, Cambridge, UK) were used for the identification of induced smooth muscle cells. Primary antibodies cytokeratin 7 (CK7, Abcam, Cambridge, UK), cytokeratin 20 (CK20, Abcam, Cambridge, UK), and uroplakin III (UPIII, Santa Cruz, Dallas, USA) were used for the identification of induced urothelium cells.
Labeling of induced cells
The induced smooth muscle cells were transfected with pCDH-CMV-MCS-EF1-copGFP vector plasmid using recombinant lentivirus-mediated gene transfer system (Genechem, Shanghai, China), according to the manufacturer’s protocols. The induced urothelium cells were transfected with Ubi-MCS-3FLAG-RFP vector plasmid using the same gene transfer system. After 48 h transfection, cells were assessed for expression of green fluorescent protein (GFP) and red fluorescent protein (RFP) by fluorescence microscope.
VECM preparation and cell seeding
Abdominal aortas were aseptically obtained from 10 rabbits. The adventitia was dissected and the lumen of aorta was made a longitudinal incision before the decellularization process. VECM was prepared using a method described previously [10]. Next, the induced urothelium cells were seeded on the lumen aspect of the VECM. The culture medium was Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. When the cells had covered about 80% of the adventitia aspect of the graft, it was turned over and seeded with the induced smooth muscle cells on its adventitia aspect, as a “sandwich co-culture”. The grafts were placed onto a constant-temperature vibrator (Zhejiang shuxin Purification Equipment Co., Ltd, Zhejiang, China) at a constant inclination and speed (40 rpm) for 24 h. Then, the grafts were kept in an incubator for 6 days before omental maturation in vivo. The cellular morphology on the VECM was observed using a scanning electron microscope and fluorescence microscope.
Omental maturation in vivo
The seeded scaffold was tubularized around a home-made silicone drain, with the urothelial layer positioned toward the drain. Each end of the graft was fixed to the silicone drain with transfixion sutures to avoid its retraction during the in vivo maturation. The rabbits were anesthetized with 3% isoflurane. Through a ventral midline incision, the tabularized scaffold was transferred between the two layers of the omentum of the rabbit. Then the omentum was fixed around the graft by three interrupted 4/0 prolene sutures. Three weeks later, the grafts were harvested and evaluated by immunohistochemical (IHC) staining. Anti-CK7, anti-CK20, and anti-UPIII was used for visualization of urothelial cells; and anti a-SMA and anti SM-MHC for visualization of smooth muscle cells. Anti-VEGF (Santa Cruz, Dallas, USA) were used for vascular endothelial cells identification.
Ureter reconstruction
After three weeks, the rabbits were anesthetized with 3% isoflurane. Unilateral ureter was identified and mobilized from the abdomen via a ventral midline incision. Then an artificial ureter defects (approximately 50%-70% of the total ureter) was created by surgical excision. The silicone drain was removed from the maturated graft. Then the graft was trimmed and placed over the ureter defect. A home-made stent was placed in the ureter. The repair was performed with 8-0 vicryl sutures applied in incontinuous fashion under a surgical microscope (Shanghai Medical Instruments Co. Ltd, Shanghai, China). The home-made stent was removed at 4 weeks post-operation. At 8 weeks post-operation, the function of the reconstructed ureter was evaluated by intravenous urography. The grafts were gained for histological studies. IHC staining was performed to investigate the differentiation of the various layers of the graft. Anti-CK7, anti-CK20, and anti-UP III was used for visualization of urothelial cells; and anti a-SMA and anti SM-MHC for visualization of smooth muscle cells. The labeled cells were assessed by fluorescence microscope for detection of the ADSCs differentiated cells within the implants in vivo.
Statistical analysis
Data are presented as mean ± standard deviation and analyzed with SPSS version 17.0 (Chicago, IL, USA). Student’s t-test was used to determine differences. P-values < 0.05 were considered statistically significant.
Results
Characterization of ADSCs
After 2 days, the ADSCs presented as spindle-like morphology with large nucleus (Figure 1A). After 6 days, the cells proliferated rapidly scattered on the culture flask bottom (Figure 1B). Flow cytometry confirmed positive expression of CD90 (98.9%) (Figure 1C) and CD29 (97.8%) (Figure 1D), as well as negative expression of CD34 (0.8%) (Figure 1E).
Figure 1.
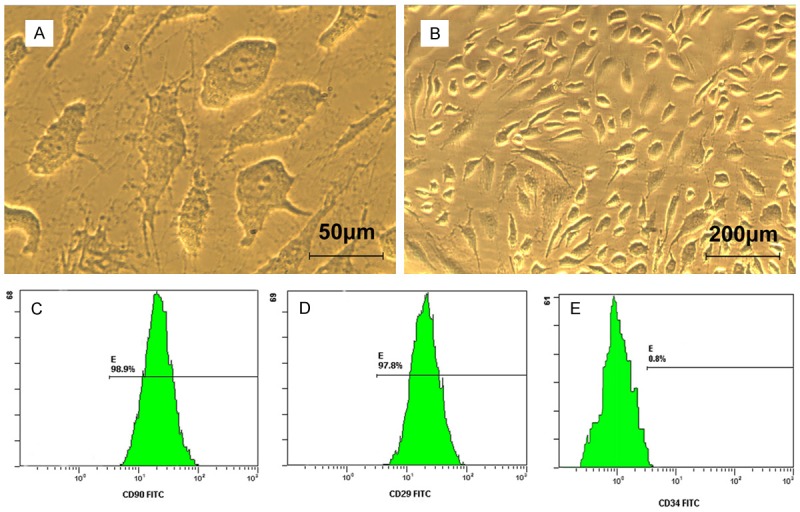
Morphological features and surface markers of ADSCs. A. ADSCs presented polygonal morphology with large nucleus. B. Cells proliferated rapidly after 6 days. C. ADSCs expressed high levels of marker CD90 by flow cytometry identification. D. ADSCs expressed high levels of marker CD29 by flow cytometry identification. E. ADSCs did not express CD34 by flow cytometry identification.
The morphological features and gene expression after induction
The morphological features of the induced urothelium cells and smooth muscle cells were showed in Figures 2 and 3. The induced smooth muscle cells exhibit with long spindle-shaped (Figure 2A) and the induced urothelium cells exhibit epithelium-shape after induction (Figure 3A). At the mRNA level, Quantitative PCR analysis shown that there is upregulation of a-SMA and SM-MHC expression (Figure 2B) in the induced smooth muscle cells compared with the control cells (P < 0.05). CK7 gene expression was higher in the induced urothelium cells than the control cells (P < 0.05). CK20 and UPIII gene expression has no significant difference compared with the control cells (P > 0.05) (Figure 3B). At translational level, western blot results revealed that the expression of a-SMA and SM-MHC increased in the induced smooth muscle cells when compared to the control (Figure 2C and 2D). For the urothelial specific proteins, significant amount of CK7 expression were identified in the induced urothelium cells (Figure 3C and 3D). However, the CK20 and UP III expression were not detected during the induction period (Figure 3C and 3D).
Figure 2.
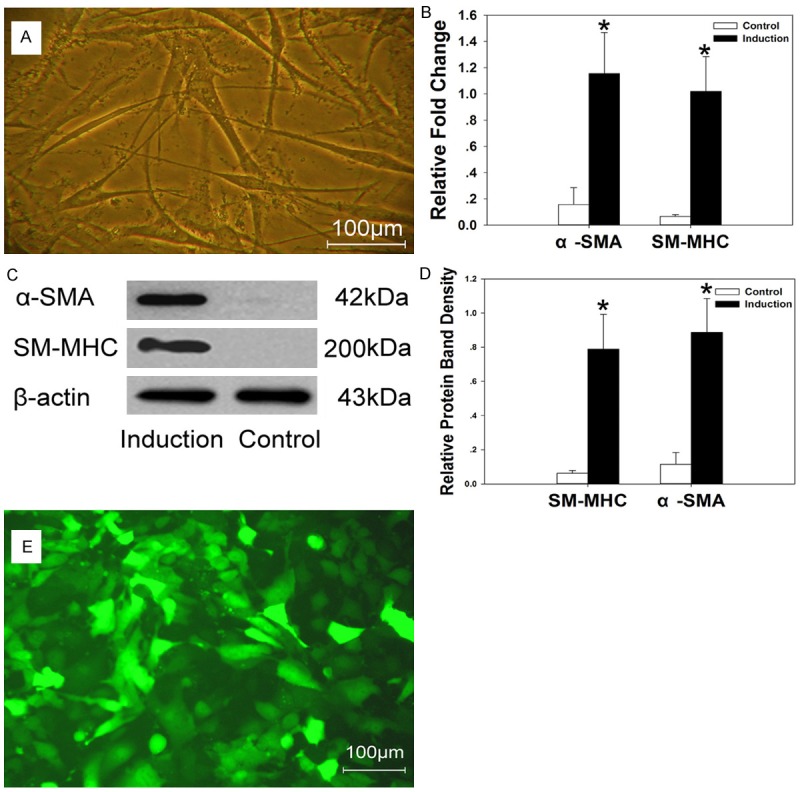
A. The induced smooth muscle cells exhibit with long spindle-shape. B-D. Higher expression of a-SMA and SM-MHC was identified in the induced smooth muscle cells by Quantitative PCR and western blot studies. E. The induced smooth muscle cells exhibited bright green fluorescence under fluorescence microscope. *P < 0.05.
Figure 3.
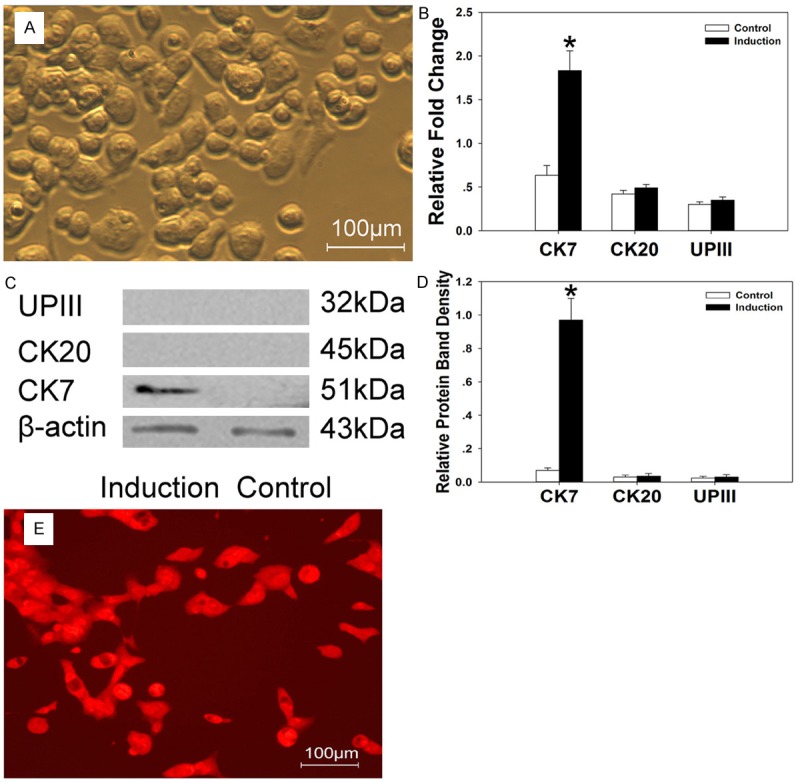
A. The induced urothelium cells exhibit epithelium-shape. B-D. Higher expression of CK-7 was identified in the induced urothelium cells by Quantitative PCR and western blot studies. CK-20 and UPIII expression were not detected. E. The induced urothelium cells exhibited bright red fluorescence under fluorescence microscope. *P < 0.05.
Labeling of induced cells
The induced cells could be transfected in vitro with lentivirus-mediated gene transfer system. Three days post-transfection, the percentage of GFP-positive cells was up to 80% in the induced smooth muscle cells (Figure 2E) and RFP-positive cells up to 86% in the induced urothelium cells (Figure 3E). After being passaged 3 times, a similar outcome was observed under the fluorescence microscope.
VECM and cells seeding
Under scanning electron microscope, VECM displayed a three dimensional structure of matrix fibers. The fibers appeared in a regular network, without cell fragments residual. They also provided a porous structure potential for cell infiltration (Figure 4A). After seeding, the induced cells were able to adhere and penetrate the matrix fibers under scanning electron microscope (Figure 4B). Under fluorescence microscope, the induced smooth muscle cells formed a few cell layers, presenting green fluorescent (Figure 4C), and the induced urothelium cells formed a single epithelial layer, presenting red fluorescent (Figure 4D). The primary three dimensional structures were obtained.
Figure 4.

A. VECM displayed a three dimensional structure of matrix fibers under scanning electron microscope. B. The induced cells adhered and penetrated the matrix fibers under scanning electron microscope. C. A single epithelial layer was formed by the induced urothelium cells, presenting red fluorescence under fluorescence microscope. D. The induced smooth muscle cells formed a few cell layers, presenting green fluorescent under fluorescence microscope.
Omental maturation
After 3 weeks of omental maturation, the tubular graft was formed with abundant blood supply. Immunohistochemically, the epithelial layer on the luminal aspect of the graft stained positively with CK7 (Figure 5A), CK20 (Figure 5B), but negative with UPIII (Figure 5C). The smooth muscle layers on the external aspect of the graft stained positively with a-SMA (Figure 5D) and SM-MHC (Figure 5E). The neovascularization were confirmed by positive expression of VEGF in vascular endothelial cells (Figure 5F).
Figure 5.
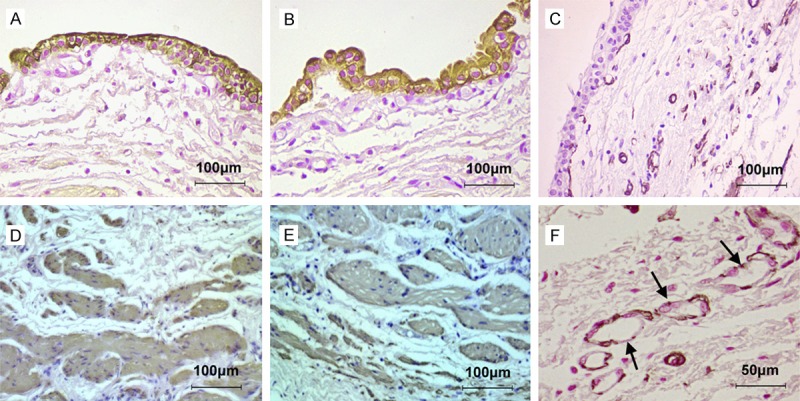
The histochemical features of the graft after omental maturation by immunohistochemical staining. A. The epithelial layer stained positively with CK7. B. The epithelial layer stained positively with CK20. C. The epithelial layer stained negatively with UPIII. D. The smooth muscle layers stained positively with a-SMA. E. The smooth muscle layers stained positively with SM-MHC. F. Vascular endothelial cells of blood vessels stained positively with VEGF.
Ureter reconstruction
After 8 weeks of ureter reconstruction, the hematoxylin-eosin staining showed a clearly layered structure of ureter, with a dense connective smooth muscle tissue, surrounding the multilayered urothelium (Figure 6A). No signs of inflammation were observed, which indicated no obvious evidence of fibrosis. The urothelium stained positively with CK20 and UPIII, which confirmed the terminal differentiation of the urothelial layer (Figure 6B and 6C). The muscular layer stained positively with a-SMA and SM-MHC (Figure 6D and 6E). Under fluorescence microscope, the labeled cells could be observed in the reconstructed ureter. The smooth muscle bundle presented green fluorescent (Figure 6F) and the multilayered urothelium presented red fluorescent (Figure 6G), which confirmed that the smooth muscle cells and urothelium cells differentiated from ADSCs participant in the ureter reconstruction. There was no ureteral abstruction or hydronephrosis in intravenous urography (Figure 6H).
Figure 6.
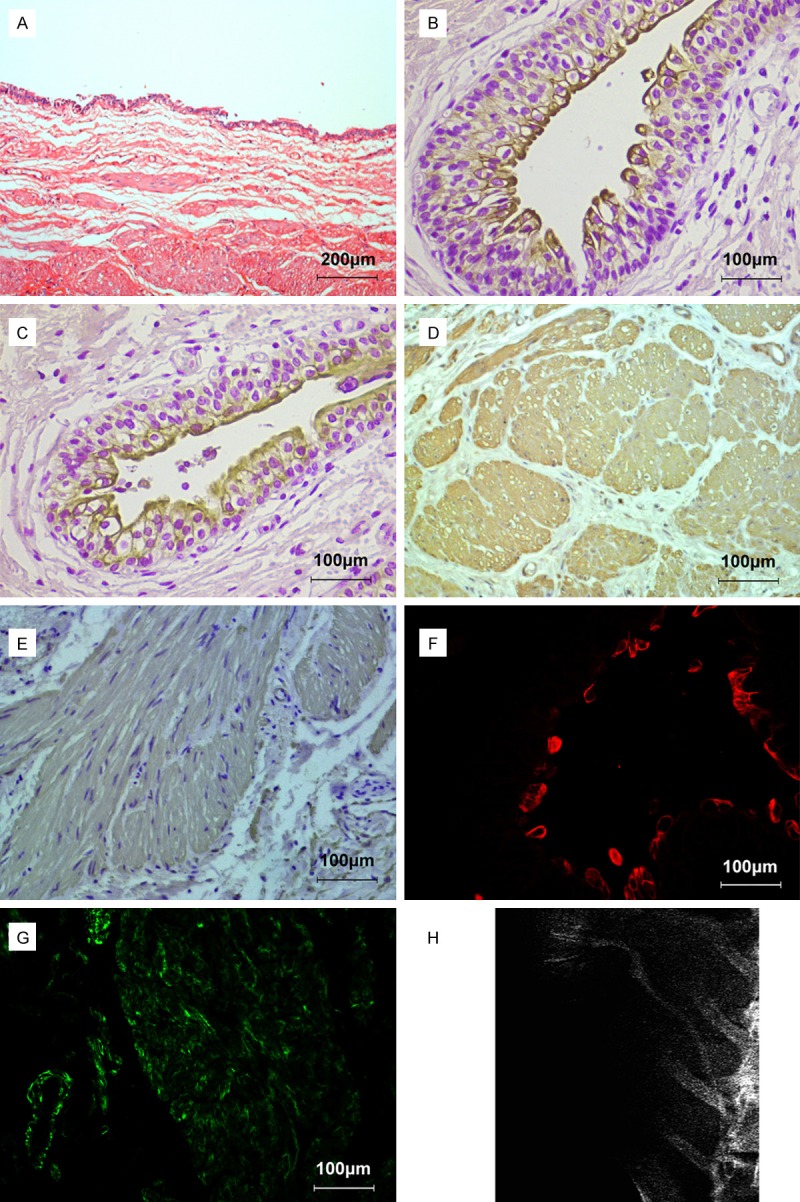
The histochemical features of the neoureter after ureter reconstruction. A. A dense connective smooth muscle tissue and multilayered urothelium formed the neoureter by hematoxylin eosin staining staining. B, C. The muscular layer stained positively with a-SMA and SM-MHC by immunohistochemical staining. D, E. The urothelium stained positively with CK20 and UPIII by immunohistochemical staining. F. The smooth muscle bundle presented green fluorescent under fluorescence microscope. G. The multilayered urothelium presented red fluorescent under fluorescence microscope. H. No ureteral obstruction or hydronephrosis was observed by intravenous urography.
Discussion
The field of tissue engineering is developing rapidly, and many encouraging achievements have been made in urinary tract reconstruction [14-16]. Tissue engineering may be a therapeutic alternative for patients with ureter defects. Generally, tissue engineering of urinary tract fall into two strategies, one is to use scaffolds, such as acellular matrices, the other is to use scaffolds seeding with cells. Cell-seeded scaffolds have been used successfully to reconstruct urethral tissues [15]. In our study, we demonstrated the ADSCs could be a potential source of seed cells for ureter reconstruction.
Among the stem cells used in tissue engineering, ADSCs may be a convenient source of stem cells, as fat tissue can be obtained conveniently and minimal-invasively. In the previous researches, ADSCs represented multi-lineage differentiation and were used to treat neurological dysfunctions, skin wounds, cardiovascular disease, and orthopedic injuries [17-20]. In our study, ADSCs were isolated successfully in the dorsocervical subcutaneous adipose tissue. Flow cytometry assays demonstrated that the isolated cells express mesenchymal stem cells’ markers CD90 and CD29, but but did not express hematopoietic stem cells’ marker CD34, indicating their mesenchymal origin.
The construction of the tissue engineered ureter requires two types of cells: smooth muscle and urothelium. Regeneration of the organized smooth muscle layer is necessary for preventing fistula and diverticula. In our previous study, we demonstrated that ADSCs could be differentiated into smooth muscle phenotype, so we don’t go into much detail here [10]. To prevent subepithelial damage and stone formation, the tissue engineering graft had better be covered with urothelium, particularly when long segments of urerter defect need be reconstructed [21]. As urothelial cells derived from the endoderm, whereas ADSCs are derived from the mesoderm, the cross-mesoderm differentiation of urothelial cells from ADSCs is more difficult than ADSCs differentiation towards SMCs [22]. Some researchers reported that ADSCs were differentiated into urothelium-like cells using a transwell co-culture system of ADSCs with urothelial cells. They suggested that cell-to-cell contact induced differentiation [23]. Zhang M et al. [13] reported that urothelial cell releases cytokines to neighboring stem cells, which alternate the microenvironment of the stem cells, thus alternate the fate of the stem cells in this system. In this microenvironment, epidermal growth factor, hepatocyte growth factor, and keratinocyte growth factor have key differentiation roles to assist the differentiation of ADSCs into urothelium-like cells. So we used these factors in the induction medium in our study. After 2 weeks of differentiation, expression of CK7, an early marker of urothelial cell, can be observed in the induced cells in the mRNA level and the protein level, which confirmed the urothelial phenotype differentiation. Previous studies reported that CK20 and UPIII can be observed in the intermediate and terminal course of urothelial cell differentiation respectively [24]. In our study, expression of CK20 and UPIII was not observed in the induced cells, which indicated that the urothelial differentiation of ADSCs can hardly reach the terminal stage in vitro. Baumert H et al. [7] reported omental maturation could support a specific microenvironment in favor of terminal differentiation of the urothelial layer. Therefore, we transferred the cell-seeded graft into the omentum in a rabbit model. Our results revealed that expression of CK20 was observed in the cell-seeded graft tissue by IHC staining after two weeks omental maturation, which demonstrated that the induced cells have the potential to be terminal differentiation in vivo. Nevertheless, the matured urothelium was observed after ureter reconstruction by the omental maturated graft as defined by positive expression of UP III. Uroplakins are used as specific markers of terminal urothelial differentiation. After ureter reconstruction, umbrella cells were formed due to contact with urine. Umbrella cells are important sign of matured urothelium. They are highly differentiated epithelial cells characterized by well-developed tight junctions, asymmetric unit membranes rich in uroplakins [25,26]. They play an important role in the physiology of the urothelial barrier that preventing epithelial and subepithelial inflammation [7].
Besides cell resource, another important issue for ureter tissue engineering is scaffold. The ideal scaffold should have excellent biocompatibility and biodegradability. VECM is a natural material, which possess the trait of containing native growth factors and a naturally three dimensional structure [12]. In our study, we demonstrated that VECM is compatible for cells survival. From the result of scanning electron microscopy, the seeded cells grown on the scaffold displayed a spreading appearance and a higher proliferation rate. In order to assist the seeded cells infiltrating into the scaffold, we adopt dynamic culture technique. Under the dynamic culture condition, cells infiltrated into the matrix and formed a multilayered tissue structure in vitro. Furthermore, dynamic culture conditions can provide constant nutrients for cells growth.
Sufficient blood supply is an essential factor for transplanted tissue survival after ureter reconstruction. The cell-seeded graft should be vascularized to minimize the risk of ureteral necrosis and recurrent strictures. The great omentum had the advantage of a rich blood supply, which can assist the cell-seeded graft vascularization as soon as possible [27]. We took advantage of the great omentum to construct a tubularized graft for the subsequent ureter reconstruction. This technique prevented tissue fibrosis and loss of contractility in the constructed ureter in our study. The histological results revealed a multilayered urothelium above a connective smooth muscle tissue in the tubularized graft.
Based on the observations from GFP and RFP labeling, the induced cells were detected both in vitro and in vivo. The induced urothelium cells labeled with RFP revealed positive expression of UPIII due to the contribution of urine stimulate microenvironment in vivo. Histological result demonstrated terminal differentiation was achieved after ureter reconstruction. The induced smooth muscle cells labeled with GFP revealed positive expression of SMA and SMHC and organized muscle bundles in the neoureter. These results demonstrated that our five steps strategy was feasible in ureter reconstruction by tissue engineering technique with ADSCs. As a major limitation, the ADSCs differentiation towards urothelium needs to be optimized to reach the terminal differentiation in vitro and the involved mechanism should be investigated. Also the possibility of long term functional evaluation of the reconstructed ureter should be conducted and followed up.
In conclusion, ADSCs served as a promising cell source used in uretel tissue engineering. We demonstrated that ADSCs differentiated into urothelial and smooth muscle lineage. Furthermore, we constructed a cell-seeded engineering graft after omental maturation and effectively applied it for ureter reconstruction in vivo. Hence, we proposed a five steps strategy on ureteral tissue engineering which might be applied in clinical research. Future researches may include the mechanism of ADSCs differentiation towards urothelium and long-term follow-up in a larger animal model.
Acknowledgements
This work was supported by grants from the National Natural Sciences Foundation of China (No. 81500517 and No. 81402119), Shandong Provincial Natural Science Foundation (NO. ZR2014HL071, and ZR2014HP055).
Disclosure of conflict of interest
None.
References
- 1.Pastore AL, Palleschi G, Silvestri L, Leto A, Autieri D, Ripoli A, Maggioni C, Al Salhi Y, Carbone A. Endoscopic rendezvous procedure for ureteral iatrogenic detachment: report of a case series with long-term outcomes. J Endourol. 2015;29:415–420. doi: 10.1089/end.2014.0474. [DOI] [PubMed] [Google Scholar]
- 2.Elliott SP, McAninch JW. Ureteral injuries: external and iatrogenic. Urol Clin North Am. 2006;33:55–66. doi: 10.1016/j.ucl.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Lazica DA, Ubrig B, Brandt AS, von Rundstedt FC, Roth S. Ureteral substitution with reconfigured colon: long-term followup. J Urol. 2012;187:542–548. doi: 10.1016/j.juro.2011.09.156. [DOI] [PubMed] [Google Scholar]
- 4.Ordorica R, Wiegand LR, Webster JC, Lockhart JL. Ureteral replacement and onlay repair with reconfigured intestinal segments. J Urol. 2014;191:1301–1306. doi: 10.1016/j.juro.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Kloskowski T, Kowalczyk T, Nowacki M, Drewa T. Tissue engineering and ureter regeneration: is it possible? Int J Artif Organs. 2013;36:392–405. doi: 10.5301/ijao.5000130. [DOI] [PubMed] [Google Scholar]
- 6.Engel O, Rink M, Fisch M. Management of iatrogenic ureteral injury and techniques for ureteral reconstruction. Curr Opin Urol. 2015;25:331–335. doi: 10.1097/MOU.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 7.Baumert H, Mansouri D, Fromont G, Hekmati M, Simon P, Massoud W, Molinié V, Malavaud B. Terminal urothelium differentiation of engineered neoureter after in vivo maturation in the “omental bioreactor”. Eur Urol. 2007;52:1492–1498. doi: 10.1016/j.eururo.2007.04.098. [DOI] [PubMed] [Google Scholar]
- 8.Baumert H, Hekmati M, Dunia I, Mansouri D, Massoud W, Molinié V, Benedetti EL, Malavaud B. Laparoscopy in ureteral engineering: a feasibility study. Eur Urol. 2008;54:1154–1163. doi: 10.1016/j.eururo.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Dai R, Wang Z, Samanipour R, Koo KI, Kim K. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem Cells Int. 2016;2016:6737345. doi: 10.1155/2016/6737345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Z, Yu H, Xiao F, Wang X, Yang S, Li S. Differentiation of adipose-derived stem cells promotes regeneration of smooth muscle for ureteral tissue engineering. J Surg Res. 2012;178:55–62. doi: 10.1016/j.jss.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Huang J, Lin T, Zhang C, Yin X. Cell-to-cell contact induces human adipose tissue-derived stromal cells to differentiate into urothelium-like cells in vitro. Biochem Biophys Res Commun. 2009;390:931–936. doi: 10.1016/j.bbrc.2009.10.080. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Peng Y, Zhou Z, Zhou J, Wang Z, Lu M. Differentiation of human adipose-derived stem cells co-cultured with urothelium cell line toward a urothelium-like phenotype in a nude murine model. Urology. 2013;81:465. doi: 10.1016/j.urology.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Xu MX, Zhou Z, Zhang K, Zhou J, Zhao Y, Wang Z, Lu MJ. The differentiation of human adipose-derived stem cells towards a urothelium-like phenotype in vitro and the dynamic temporal changes of related cytokines by both paracrine and autocrine signal regulation. PLoS One. 2014;9:e95583. doi: 10.1371/journal.pone.0095583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 15.Raya-Rivera A, Esquiliano DR, Yoo JJ, Lopez-Bayghen E, Soker S, Atala A. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet. 2011;377:1175–1182. doi: 10.1016/S0140-6736(10)62354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kloskowski T, Pokrywczyńska M, Drewa T. Artificial urinary conduit construction using tissue engineering methods. Cent European J Urol. 2015;68:109–114. doi: 10.5173/ceju.2015.01.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franco Lambert AP, Fraga Zandonai A, Bonatto D, Cantarelli Machado D, Pêgas Henriques JA. Differentiation of human adipose-derived adult stem cells into neuronal tissue: does it work? Differentiation. 2009;77:221–228. doi: 10.1016/j.diff.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Ebrahimian TG, Pouzoulet F, Squiban C, Buard V, André M, Cousin B, Gourmelon P, Benderitter M, Casteilla L, Tamarat R. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler Thromb Vasc Biol. 2009;29:503–510. doi: 10.1161/ATVBAHA.108.178962. [DOI] [PubMed] [Google Scholar]
- 19.Madonna R, Geng YJ, De Caterina R. Adipose tissue-derived stem cells: characterization and potential for cardiovascular repair. Arterioscler Thromb Vasc Biol. 2009;29:1723–1729. doi: 10.1161/ATVBAHA.109.187179. [DOI] [PubMed] [Google Scholar]
- 20.Lewallen EA, Jones DL, Dudakovic A, Thaler R, Paradise CR, Kremers HM, Abdel MP, Kakar S, Dietz AB, Cohen RC, Lewallen DG, van Wijnen AJ. Osteogenic potential of human adipose-tissue-derived mesenchymal stromal cells cultured on 3D-printed porous structured titanium. Gene. 2016;581:95–106. doi: 10.1016/j.gene.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodin A, Bharadwaj S, Wu S, Gatenholm P, Atala A, Zhang Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials. 2010;31:8889–901. doi: 10.1016/j.biomaterials.2010.07.108. [DOI] [PubMed] [Google Scholar]
- 22.Ning J, Li C, Li H, Chang J. Bone marrow mesenchymal stem cells differentiate into urothelial cells and the implications for reconstructing urinary bladder mucosa. Cytotechnology. 2011;63:531–539. doi: 10.1007/s10616-011-9376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi JG, Fu WJ, Wang XX, Xu YD, Li G, Hong BF, Wang Y, Du ZY, Zhang X. Tissue engineering of ureteral grafts by seeding urothelial differentiated hADSCs onto biodegradable ureteral scaffolds. J Biomed Mater Res A. 2012;100:2612–2622. doi: 10.1002/jbm.a.34182. [DOI] [PubMed] [Google Scholar]
- 24.Wu S, Cheng Z, Liu G, Zhao X, Zhong L, Zhu Y, Zhu J. Urothelial differentiation of human umbilical cord-derived mesenchymal stromal cells in vitro. Anal Cell Pathol (Amst) 2013;36:63–69. doi: 10.3233/ACP-130081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veranic P, Romih R, Jezernik K. What determines differentiation of urothelial umbrella cells? Eur J Cell Biol. 2004;83:27–34. doi: 10.1078/0171-9335-00351. [DOI] [PubMed] [Google Scholar]
- 26.Apodaca G. The uroepithelium: not just a passive barrier. Traffic. 2004;5:117–28. doi: 10.1046/j.1600-0854.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 27.Baumert H, Simon P, Hekmati M, Fromont G, Levy M, Balaton A, Molinié V, Malavaud B. Development of a seeded scaffold in the great omentum: feasibility of an in vivo bioreactor for bladder tissue engineering. Eur Urol. 2007;52:884–890. doi: 10.1016/j.eururo.2006.11.044. [DOI] [PubMed] [Google Scholar]


