Abstract
Our study aims to investigate the effects of bone marrow derived mesenchymal stem cells (BM-MSCs) in lipopolysaccharide (LPS)-induced acute lung injury (ALI) as well as the underlying mechanism. In our study, Wistar rats were randomly divided into four groups: control group; ALI group; ALI+MSCs group and ALI+MSCs claudin-4 siRNA group. MRC-5 and BEAS-2B cell lines were used for in vitro assay. Flow cytometry, western blot, hematoxylin and eosin (H&E) staining, CCK-8 assay, enzyme-linked immunosorbent assay (ELISA) were involved to measure the pathological changes in lung tissues. Results showed that in vivo MSCs administration significantly attenuated pulmonary edema (wet/dry ratio), inflammation cytokines levels (TGF-α), pathological alternations and cell apoptosis which were mediated by claudin-4 in LPS-induced acute lung injury in rats. In vitro experiment showed that hypoxia could induce the expression of claudin-4 in MSCs, and MSCs treatment showed significantly enhanced cell viability (by CCK-8 assay) and reduced cell apoptosis. In conclusion, the present study demonstrated that BM-MSCs can protect against LPS-induced ALI in vivo and in vitro, at least partly mediated by claudin-4.
Keywords: Acute lung injury, bone marrow derived mesenchymal stem cells, claudin-4
Introduction
The acute lung injury (ALI) is a relatively frequent clinical syndrome, which could result in acute respiratory failure in critically ill patients [1]. ALI and its severe form, acute respiratory distress syndrome (ARDS), were described by Ashbaugh and his colleagues in 1967 for the first time [2]. In experimental models, ALI can be trigged by either infectious or noninfectious stimuli, such as lipopolysaccharide (LPS) [3]. Despite the recent development of protective lung ventilation strategies are used in ALI patients, it is of great importance to find better treatment for ALI.
Mesenchymal stem cells (MSCs) are a multipotent endogenous population of progenitors with capabilities of high proliferation and self-renewal [4]. Researchers have isolated MSCs from many types of tissues and organs, including bone marrow, heart, lung, and umbilical cord blood [5-10]. MSCs isolated from bone marrow, adipose tissue, and umbilical cord blood have been widely reported in animal models or clinical trials for their regenerative ability [11]. Previous evidences have shown that bone marrow MSCs (BM-MSCs) could be used for treatment of lung diseases, ischemic cardiomyopathy, and osteonecrosis [12-14].
Claudins are a large family of integral membrane proteins and are essential components in the tight junctions’ formation [15,16]. Of note, claudin-4 was reported to be expressed by epithelia cells throughout the respiratory trees by immunohistochemistry of fetal and adult lungs detection [17,18]. In particular, claudin-4 shows protective role against lung injury [19]. Claudin-4 concentrations are associated with lung injury and loss of claudin-4 decreases alveolar fluid clearance in mice and patients [20]. In mice, the level of claudin-4 can be upregulated 12-16-fold in response to hyperoxia [21]. On the contrary, using blocking peptide can hamper the protective role of claudin-4, resulting in high alveolar permeability and impaired fluid clearance [22].
Therefore, the biological functions of MSCs in acute lung injury and the association between claundin-4, MSCs and ALI have not been well clarified. The present study was conducted to investigate the effects of MSCs on LPS-induced acute lung injury in rat model as well as the underlying mechanism.
Materials and methods
Cell culture
MRC-5 (human fetal lung fibroblast) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin, and maintained in a humidified atmosphere with 5% CO2 at 37°C. Hypoxia was induced by treating MRC-5 cells with 100 nM deferoxamine.
Osteogenic and adipogenic differentiation of MSCs
MSCs were isolated and cultured as described elsewhere [23]. When cells were approximately 80% confluent, MSCs were detached and plated in a 6-well culture plate at a concentration of 3×103 cells/well for osteogenic differentiation or 2×104 for adipogenic differentiation. For osteogenic differentiation, culture medium was discarded 24 h later and replaced with osteogenic induction medium (DMEM containing 10% FBS, 1% penicillin-streptomycin, glutamine, dexamethasone, β-Glycerophosphate disodium and vitamin C). The culture medium was refreshed every 3 days. After 2-3 weeks’ induction, cells were stained with Alizarin red S for the detection of calcium nodules. To induce adipogenic differentiation, culture medium was replaced with adipogenic induction medium A (DMEM containing 10% FBS, 1% penicillin-streptomycin, glutamine, insulin, 3-isobutyl-1-methylxanthine, dexamethasone and indometacin) when cells grew to 100% confluence. Three days later, adipogenic induction medium B (DMEM containing 10% FBS, 1% penicillin-streptomycin, glutamine and insulin) was added to replace medium A, and 24 h later, medium B was replaced with medium A. The above steps were repeated for three cycles. When lipid droplets were relative small, cells were maintained in medium B for 3-5 to allow the enlargement of lipid droplets. The lipid droplets were visualized by Oil Red O staining.
LPS-induced acute lung injury
All procedures involved in rats in this study were approved by the institutional animal care and research committee of Zhejiang Provincial People’s Hospital (Hangzhou, China). Specific pathogen-free Wistar rats, weighted about 200 g, were obtained from Shanghai SLAC Laboratory Animal Co.LTD (Shanghai, China). Rats were randomly divided into four groups. (1) Control group: negative control rats were treated with PBS; (2) ALI group: rats were treated with intratracheal injection of LPS (5 mg/kg) (Sigma, USA); (3) ALI+MSCs group: ALI rats were administered 1×106 MSCs by intraperitoneal injection (i.p.); (4) ALI+MSCs claudin-4 siRNA group: ALI rats were administered 1×106 MSCs i.p. which were treated with claudin-4 siRNA.
Histological analysis
Lung tissues of left middle lobe were fixed in 10% formalin and then embedded in paraffin. After that, the tissues were serial sectioned and stained with hematoxylin and eosin (H&E). The histological features were then examined under a light microscope (Leica Microsystems, Germany).
Measurement of wet/dry ratio and biochemical indexes
The freshly harvested left interior lobe was weighed and then dried in the oven at 60°C for 24 h. The lung tissues were weighed again after dehydration. The wet/dry ratio was then calculated to assess the severity of pulmonary edema.
Arterial oxygen pressure (PaO2), PH, base excess (BE) and other indicators were analyzed using automatic blood gas analyzer at 1 d, 3 d and 7 d after the surgery. Serum was obtained by centrifuging the blood. The supernatant was used for measurement of serum creatinine (Cr), urea nitrogen (BUN) and arterial oxygen pressure (PaO2) with automatic biochemical analyzer.
Measurement of myeloperoxidase (MPO) activity
100 mg frozen lung tissues were suspended in 4 ml of potassium phosphate buffer (20 mmol/L, pH 7.4) and homogenized in an ice bath at 4°C for 30 s. Precipitate was harvested by centrifugation at 30,000 g for 30 min. Precipitate was suspended with 4 ml of potassium phosphate buffer (50 mmol/L, pH 6.0) containing 0.5% HTAB. The suspension was then sonicated, incubated at 60°C for 2 h and then centrifuged at 40,000 g for 10 min. 0.1 ml of supernatant was mixed with 2.9 ml of reaction solution. Mixture was maintained at 25°C, and spectrophotometer was set at 460 nm for a 3 min time’s scanning. MPO activity was calculated based on the absorbance change during the 30 s to 90 s time course. The equation was that MPO activity (U/g) = ΔA460×13.5/lung wet weight (g). One unit of MPO activity was defined as the amount of enzyme required to catalyze 1 μmol H2O2 per minute at 25°C.
Enzyme-linked immunosorbent assay (ELISA)
The levels of TNF-α and TNF-β1 in the bronchoalveolar lavage fluid (BALF) were determined using ELISA kit according to the manufacturer’s instructions (Roche). The concentrations were calculated according to the absorbance of the samples and the standard curve.
TUNEL assay
Terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) assay was performed to evaluate the cell apoptosis in lung tissues. In brief, freshly harvest lung tissues were fixed in 4% paraformaldehyde (PFA). TUNEL assay was performed using TUNEL detection kit according to the manufacturer’s instructions (Roche). Sections were visualized under a light microscope (magnification, ×400; Leica Microsystems, Germany).
Cell viability assay
Cell viability was analyzed using Cell Counting Kit-8 (Sigma). Briefly, MRC-5 cells (5×103) suspended in the supernatant of MSCs after 24 h’s culture (100 μl). CCK-8 solution (10 μl) was added to each well and incubated for 3 h at 37°C. Absorbance at 450 nm was measured using the MRX II microplate reader (Dynex, USA).
Flow cytometry
MRC-5 Cells were collected and washed with ice cold PBS, and resuspended in ice cold PBS. 2~3×105 cells (100 μl) were collected and 5 μl of fluorescein isothiocyanate (FITC)-Annexin V (20 ug/ml) was added. The tubes were incubated for 30 min in dark at room temperature (RT). Then, 5 μl PI (50 μg/ml) was added and reacted for 5 min in dark at RT. The samples were then analyzed within 1 h using FACScan analyzer.
Statistical analysis
All statistical calculations were performed using SPSS10 statistical software. Data were represented as mean ± SD. The student’s t-test or one-way ANOVA were used to compare between groups. A P-value <0.05 was considered statistically significant.
Results
Identification of BM-MSCs
To confirm the mesenchymal nature of cells harvested, the surface antigens were evaluated by flow cytometry, including CD90, CD45, CD34, CD29, CD14 and CD31. Cells positively expressed CD90 and CD29, while they negatively expressed CD45, CD34, CD14 and CD31, which was in accordance with the expression pattern of MSCs (Figure 1A). Furthermore, in vitro adipogenic and osteogenic differentiation capabilities were analyzed as described in materials and methods. Cell morphology was changed after adipogenic induction and cells were positive to Oil Red O staining, suggesting that the BM-derived cells showed the ability to differentiate into adipogenic lineage (Figure 1B). In addition, BM-derived cells were able to form large calcium nodules and positive to Alizarin red S staining after the osteogenic induction medium, implying the osteogenic capability of BM-derived cells (Figure 1C). Taken together, these data confirmed the characteristics of MSCs in BM-derived cells used in this study.
Figure 1.

Characterization of BM-MSCs. A. Representative histograms showing the expression pattern of surface markers CD90, CD45, CD34, CD29, CD14 and CD31. B. Identification of adipogenic differentiation by Oil Red O staining. C. Identification of osteogenic differentiation by Alizarin red S staining.
Effect of MSCs on ICAM-1 expression in ALI
The expression levels of claudin-4 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (a house keeping protein) were detected by Western blot. As shown in Figure 2A, ALI significantly decreased protein levels of claudin-4 in compare to control group. Treatment with MSCs increased the levels of claudin-4 compared to ALI group, which implying a protective role of claudin-4 in MSCs treated group. Furthermore, claudin-4 siRNA was used and the protein levels of claudin-4 decreased with the time. Evidences showed that excess expression of intercellular adhesion molecule-1 (ICAM-1) could facilitate the recruitment of neutrophils and destroy the endothelial integrity [24]. PCR results showed that MSCs significantly increased the expression of ICAM compared to the control group, and with increase of time, the differences were more obvious (Figure 2B). In addition, the mRNA levels of ICAM-1 from ALI+MSCs were significantly lower than that from ALI group since 3 day after LPS-induced acute lung injury (Figure 2B). Of note, when claudin-4 siRNA was used, the MSCs couldn’t down-regulate the expression of ICAM-1 in lung tissues (Figure 2B). These results suggested that MSCs could reduce inflammatory infiltration in ALI and this might be mediated by up-regulation of claudin-4.
Figure 2.
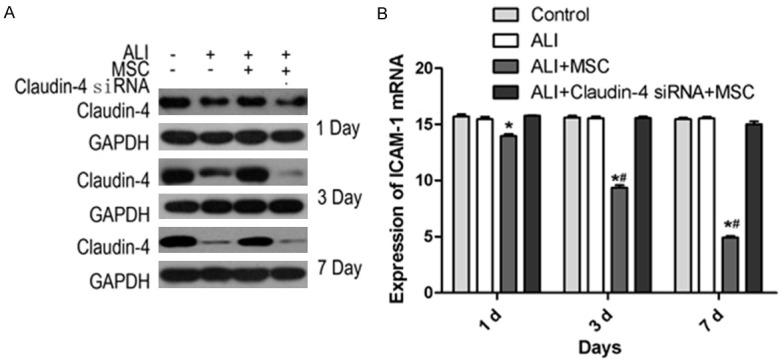
Effect of different treatments on ICAM-1 expression. A. Expression levels of claudin-4 and GAPDH were measured at day 1, 3 and 7 after LPS-induced ALI, using Western blot from control, ALI, ALI+MSCs and ALI+MSCs claudin-4 siRNA group. B. mRNA expression levels of ICAM-1 were detected in control, ALI, ALI+MSCs and ALI+MSCs claudin-4 siRNA group. *P<0.5 in comparison to control; #P<0.05 in comparison to ALI.
Effect of different treatments on PaO2, MPO activity, BUN, Cr and BE
The values of PaO2, MPO, BUN, Cr and BE in four groups were measured. The values of PaO2, MPO, BUN, Cr and BE of ALI group were significantly higher than those of the control group, whereas these values in ALI+MSCs group were significantly than those of the ALI group (Figure 3A-E). However, the values of PaO2, MPO, BUN, Cr and BE of ALI+MSCs claudin-4 siRNA group showed no significant differences in comparison to ALI group (Figure 3A-E).
Figure 3.
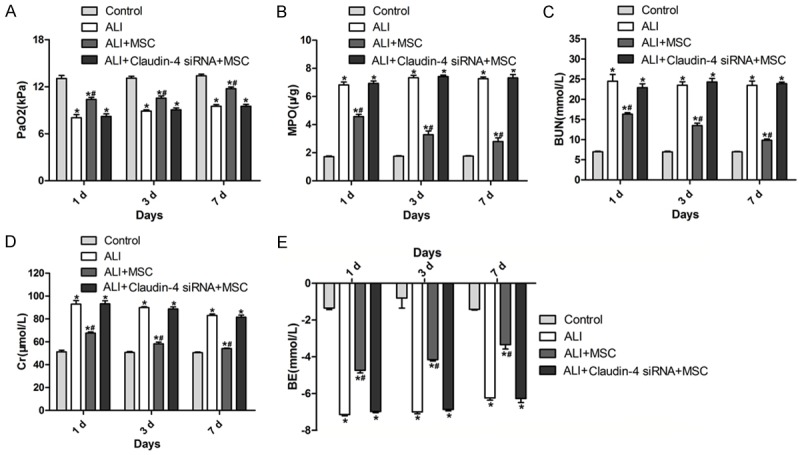
Measurement of PaO2, MPO, BUN, Cr and BE in different groups. A-E. PaO2, MPO, BUN, Cr and BE values in different groups. *P<0.5 in comparison to control; #P<0.05 in comparison to ALI.
Effect of MSCs on lung tissues in rats of LPS induced ALI in vivo
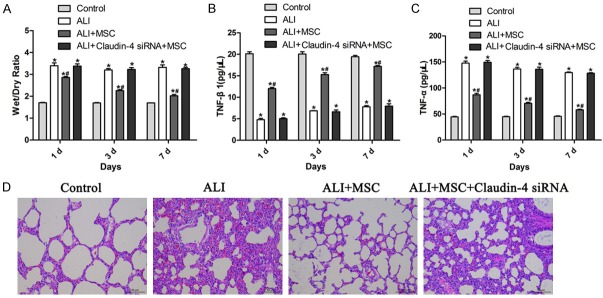
To detect the effect of MSCs on ALI, lung wet/dry values were measured to evaluate the pulmonary edema. Rats showed significant increase of wet/dry values after LPS induction (Figure 4A). While, when MSCs were administrated, wet/dry values were significantly lower than ALI or control group, indicating a protective role of MSCs (Figure 4A). However, this effect was disappeared when claudin-4 siRNA was employed to MSCs (Figure 4A). Moreover, ELISA was used to determine the levels of inflammatory cytokine TNF-α and anti-inflammatory cytokine TGF-β1 in BALF. TNF-β1 level of ALI group were significantly lower than that of control group (Figure 4B). ALI+MSCs group showed dramatically higher level of TNF-β1 than ALI group (Figure 4B). However, no obvious difference was observed between ALI+MSCs claudin-4 siRNA with ALI group (Figure 4B). On the contrary, the levels of inflammatory factor TGF-α were opposite to TNF-β1 (Figure 4C). In brief, LPS induction increased the level of TGF-α, and MSCs treatment reversed that, and claudin-4 siRNA treatment contacted the effect of MSCs (Figure 4C). We further evaluated whether MSCs treatment could attenuate lung injury by histological criteria. As expected, ALI group showed stronger lung hemorrhage, notable inflammatory cell influx; interstitial edema and interalveolar septal thickening (Figure 4D). However, MSCs treatment remarkably attenuated the pathological changes in lung tissues (Figure 4D). Furthermore, MSCs with claudin-4 siRNA treatment failed to relieve the pathological changes induced by LPS in rats (Figure 4D). Together, these results showed in vivo evidence of MSCs in protecting acute lung injury and this effect was mediated by claudin-4.
Figure 4.
MSCs attenuated lung injury through claudin-4. A. Wet/dry ratio determination of lung tissues in control, ALI, ALI+MSCs, and ALI+MSCs siRNA group. *P<0.5 vs control; #P<0.05 vs ALI. B. Levels of TNF-β1 in BALF detected by ELISA in control, ALI, ALI+MSCs, and ALI+MSCs siRNA group. *P<0.5 vs control; #P<0.05 vs ALI. C. Levels of TNF-α in BALF detected by ELISA in control, ALI, ALI+MSCs, and ALI+MSCs siRNA group. *P<0.5 vs control; #P<0.05 vs ALI. D. Representative hematoxylin and eosin (H&E) sections of lung tissues to show pathological changes in control, ALI, ALI+MSCs, and ALI+MSCs siRNA group. Legend = 50 μm.
Effect of MSCs on cell apoptosis of lung tissues
TUNEL staining was performed in lung tissues to detect the apoptosis of cells. After LPS treatment, the rats showed marked increase of apoptosis (Figure 5). However, rats that were treated with MSCs, showed significantly decrease of cell apoptosis, suggesting the alleviation of cell damage in lung tissues (Figure 5). Moreover, when claudin-4 was down-regulated, MSCs lost the function of reducing cell apoptosis (Figure 5). Together, these data suggested that MSCs perform their protective role partly through reducing cell apoptosis and this process was mediated by claudin-4.
Figure 5.
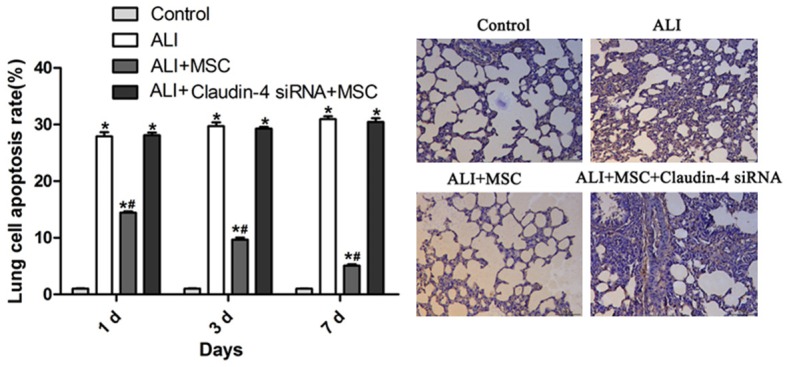
MSCs ameliorated cell apoptosis in lung tissues of ALI rats. Representative sections of TUNEL staining of lung tissues in control, ALI, ALI+MSCs, and ALI+MSCs siRNA group (right panel). Apoptosis rate was calculated based on the percent of TUNEL positive cells of total cells (left panel). *P<0.5 vs control; #P<0.05 vs ALI.
In vitro effect of MSCs on lung cell lines
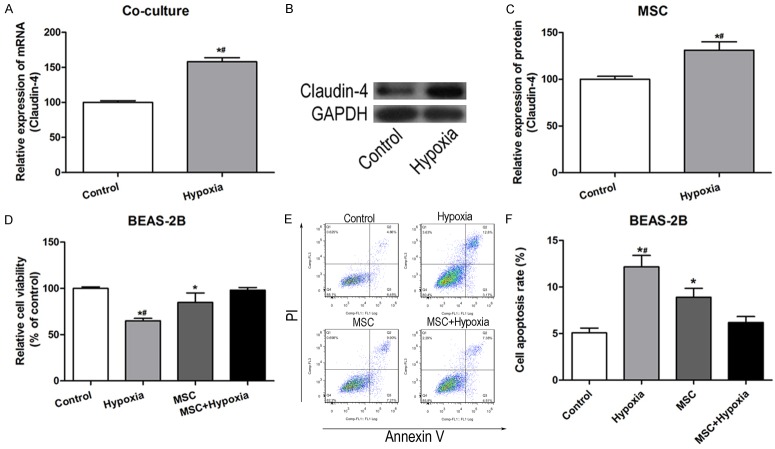
To confirm the mechanism of the protective role of MSCs, in vitro experiments using lung cell lines under hypoxia conditions were performed. Expression levels of claudin-4 in BM-MSCs were examined which were co-cultured with culture supernatant of normal MRC-5 or hypoxia induced MRC-5. The mRNA levels of claudin-4 was significantly up-regulated in BM-MSCs with culture supernatant of hypoxia induced MRC-5 (Figure 6A). In addition, the expression of claudin-4 of culture supernatant in normal cultured and hypoxia induced BM-MSCs were also detected. Of note, the mRNA and protein levels of claudin-4 were relative higher in hypoxia induced BM-MSCs than those in normal cultured BM-MSCs, indicating that hypoxia could induce the expression and release of claudin-4 (Figure 6B and 6C). Furthermore, cell viability was measured used CCK-8 assay and results showed that exposure to hypoxia decreased cell viability compared with control cells, but BM-MSCs culture supernatant treatment significantly reversed this alteration (Figure 6D). In addition, cell apoptosis was detected by Annexin V/propidium iodide (PI) staining. BM-MSCs supernatant co-culture significantly reduced the apoptosis percentage of cells cultured under hypoxia atmosphere, which was consistent with the in vivo study (Figure 6E and 6F). To be summary, these in vitro studies demonstrated that BM-MSCs could up-regulate claudin-4 expression under hypoxia condition, increase cell viability and reduce cell apoptosis.
Figure 6.
MSCs enhanced cell viability and reduced cell apoptosis in vitro. A. mRNA levels of claudin-4 in BM-MSCs co-cultured with culture supernatant of normal MRC-5 or hypoxia induced MRC-5. *P<0.5 vs control. B. Western blot analysis of claudin-4 in BM-MSCs and hypoxia induced BM-MSCs. C. mRNA levels of claudin-4 in BM-MSCs and hypoxia induced BM-MSCs. *P<0.5 vs control. D. Cellular viability determined by CCK-8 assay in control, hypoxia, MSCs and MSCs+hypoxia. *P<0.5 vs control; #P<0.05 vs MCSs+Hypoxia. E. Annexin V/PI staining of cells in control, hypoxia, MSCs and MSCs+hypoxia. Annexin V and PI double positive cells were at late-stage of apoptosis. F. Percentage of apoptosis cells (Annexin V and PI double positive cells) in control, hypoxia, MSCs and MSCs+hypoxia. *P<0.5 vs control; #P<0.05 vs MCSs+Hypoxia.
Discussion
ALI is featured for its high mortality rates that could be as high as 60%, which makes it urgent to study the pathological mechanisms and find therapeutic treatment for ALI [25,26]. LPS locates on the outer membrane of Gram-negative bacteria and could strongly stimulate the innate immunity [27]. In the current study, we used LPS-induced ALI in a rat model to study the therapeutic effect of MSCs and the related mechanism in this process.
MSCs are multipotent stem cells, and regenerative properties of them make them ideal for therapeutic use in vivo [28]. Studies regarding on the protective of MSCs in ALI are relatively limed. Therefore, we established an animal model of ALI and test the effect of MSCs on ALI. BM-MSCs were detected for the surface markers and in vitro differentiation of adipocytes and osteoblasts.
Claudin-4 was shown to maintain maximal epithelia barrier function and influence paracellular transport, together determining alveolar fluid clearance in mice and human lungs [19-31]. We found that the levels of claudin-4 decreased in ALI group with the increase of time. But, MSCs treatment could successfully reverse that change. Moreover, claudin-4 levels decreased when siRNA was used and the effect was stronger when time increased. The mRNA levels of ICAM-1 were measured to evaluate the extent of cell adhesion. Results showed that ALI treatment alleviated ICAM-1, but the effect was diminished by siRNA-mediated knockdown of claudin-4. These results suggest MSCs could reduce cell adhesion ability, resulting in decreased inflammation cell influx by regulating claudin-4.
Evidence showed that synergistic interaction between local pulmonary inflammatory factors and infiltrated neutrophils could damage pulmonary microvascular endothelium and exacerbate acute lung injury that resulting in damaged gas exchange [32]. The impaired gas exchange together with post-resuscitation hypotension may elicit a vicious cycle of systemic and pulmonary pro-inflammatory response [33]. Thus, we first examined biological indices in lung tissue or arterial blood gas, including PaO2, MPO, BUN, Cr and BE. Our experiment showed similar changes of these factors with previous studies after LPS-induced injury. However, the MSCs therapy successfully attenuated these changes and knockdown of claudin-4 abolished the effect of MSCs. Furthermore, wet/dry ratio was measured to evaluate the extent of pulmonary edema. Our data demonstrated MSCs ameliorated pulmonary edema in ALI and MSCs with siRNA of claudin-4 had no effect on that alteration. Moreover, MSCs could decrease the in-flammatory factor and increase the anti-inflammatory factor in BALF of ALI rats. Similarly, MSCs with down-regulated claudin-4 showed no significant changes on those molecular in ALI rats. MSCs therapy remarkably ameliorated the pathological changes in lung tissues. MSCs with siRNA of claudin-4 didn’t show that effect. These data served as strong in vivo evidence that MSCs could ameliorate acute lung injury mediated by claudin-4.
Recent studies have demonstrated that apoptosis contributes to pathogenesis of ALI [34]. Two major theories link apoptosis with the pathogenesis of ALI and ARDS in humans, which are epithelial cell apoptosis and the accumulation of neutrophils [34]. Apoptosis is defined as the process of programmed cell death, and the morphological changes often include cell shrinkage, nuclear fragmentation and chromatin condensation [34]. The increased alveolar epithelial cell apoptosis, together with neutrophil apoptosis, play a central role in the development and progression of ALI [34]. In our study, MSCs treatment significantly reduced the apoptosis rate compared with ALI group in a time-dependent manner; while knockdown of claudin-4 in MSCs abolished the effect, and showed similar apoptosis rate with the ALI group. These data indicated that MSCs can specifically ameliorate cell apoptosis in lung tissues in the progression of ALI in a time-dependent manner, and this process could be mediated by claudin-4.
Our in vivo experiments demonstrated the MSCs showed protective effect on acute lung injury. Our results showed that mRNA level of claudin-4 was significantly up-regulated in BM-MSCs with culture supernatant of hypoxia induced MRC-5. Furthermore, the mRNA and protein levels of claudin-4 were significantly higher in hypoxia induced BM-MSCs than those in normal cultured BM-MSCs. In addition, our data showed that MSCs significantly improved cell viabilities and decreased the apoptosis rate of hypoxia-induced BEAS-2B cells. These data demonstrated that MSCs therapy enhanced cell viability and inhibited cell apoptosis by claudin-4.
Taken together, the present study shows that BM-MSCs can protect against LPS-induced ALI in vivo and in vitro through mediation of claudin-4, suggesting that MSCs may be clinical therapeutic agent for ARDS in human.
Acknowledgements
This study was supported by the public welfare funds from the Science and Technology Department of Zhejiang province (grant no. 2013C33202), the general project funds from the Health Department of Zhejiang province (grant no. 2012KYB012) and the general project funds from the Administration of Traditional Chinese Medicine of Zhejiang province (grant no. 2012ZA018).
Disclosure of conflict of interest
None.
References
- 1.Koh Y. Update in acute respiratory distress syndrome. J Intensive Care. 2014;2:2–5. doi: 10.1186/2052-0492-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319–323. [Google Scholar]
- 3.Favarin DC, de Oliveira JR, de Oliveira CJ, Rogerio AP. Potential effects of medicinal plants and secondary metabolites on acute lung injury. Biomed Res Int. 2013;2013:576–589. doi: 10.1155/2013/576479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thanunchai M, Hongeng S, Thitithanyanont A. Mesenchymal Stromal Cells and Viral Infection. Stem Cells Int. 2015;15:860–867. doi: 10.1155/2015/860950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson S, Trial J, Soeller C, Entman ML. Cardiac mesenchymal stem cells contribute to scar formation after myocardial infarction. Cardiovasc Res. 2011;91:99–107. doi: 10.1093/cvr/cvr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foronjy RF, Majka SM. The potential for resident lung mesenchymal stem cells to promote functional tissue regeneration: understanding microenvironmental cues. Cells. 2012;1:874–885. doi: 10.3390/cells1040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV. Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013;14:11692–11712. doi: 10.3390/ijms140611692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong PP, Selvaratnam L, Abbas AA, Kamarul T. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res. 2012;30:634–642. doi: 10.1002/jor.21556. [DOI] [PubMed] [Google Scholar]
- 9.Banas A. Purification of adipose tissue mesenchymal stem cells and differentiation toward hepatic-like cells. Methods Mol Biol. 2012;826:61–72. doi: 10.1007/978-1-61779-468-1_6. [DOI] [PubMed] [Google Scholar]
- 10.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 11.Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013;34:747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Zhang J, Zhang F, Li J, Li Y, Tan Z, Hu J, Qi Y, Li Q. The Clinical Status of Stem Cell Therapy for Ischemic Cardiomyopathy. Stem Cells Int. 2015;20:135–164. doi: 10.1155/2015/135023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Wang Y, Meng HY, Yuan XL, Xu XL, Wang AY, Guo QY, Peng J, Lu SB. Application of bone marrow mesenchymal stem cells to the treatment of osteonecrosis of the femoral head. Int J Clin Exp Med. 2015;8:3127–3135. [PMC free article] [PubMed] [Google Scholar]
- 14.Houlihan DD, Hopkins LJ, Suresh SX, Armstrong MJ, Newsome PN. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:1891–1892. doi: 10.1002/hep.24722. [DOI] [PubMed] [Google Scholar]
- 15.Tokuhara Y, Morinishi T, Matsunaga T, Ohsaki H, Kushida Y, Haba R, Hirakawa E. Claudin-1, but not claudin-4, exhibits differential expression patterns between well- to moderately-differentiated and poorly-differentiated gastric adenocarcinoma. Oncol Lett. 2015;10:93–98. doi: 10.3892/ol.2015.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlingmann B, Molina SA, Koval M. Claudins: Gatekeepers of lung epithelial function. Semin Cell Dev Biol. 2015;42:47–57. doi: 10.1016/j.semcdb.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaarteenaho R, Merikallio H, Lehtonen S, Harju T, Soini Y. Divergent expression of claudin -1, -3, -4, -5 and -7 in developing human lung. Respir Res. 2010;11:59–64. doi: 10.1186/1465-9921-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaarteenaho-Wiik R, Soini Y. Claudin-1, -2, -3, -4, -5, and -7 in usual interstitial pneumonia and sarcoidosis. J Histochem Cytochem. 2009;57:187–195. doi: 10.1369/jhc.2008.951566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell LA, Overgaard CE, Ward C, Margulies SS, Koval M. Differential effects of claudin-3 and claudin-4 on alveolar epithelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2011;301:L40–49. doi: 10.1152/ajplung.00299.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rokkam D, Lafemina MJ, Lee JW, Matthay MA, Frank JA. Claudin-4 levels are associated with intact alveolar fluid clearance in human lungs. Am J Pathol. 2011;179:1081–1087. doi: 10.1016/j.ajpath.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kage H, Flodby P, Gao D, Kim YH, Marconett CN, DeMaio L, Kim KJ, Crandall ED, Borok Z. Claudin 4 knockout mice: normal physiological phenotype with increased susceptibility to lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;307:L524–536. doi: 10.1152/ajplung.00077.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wray C, Mao Y, Pan J, Chandrasena A, Piasta F, Frank JA. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;297:L219–227. doi: 10.1152/ajplung.00043.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baustian C, Hanley S, Ceredig R. Isolation, selection and culture methods to enhance clonogenicity of mouse bone marrow derived mesenchymal stromal cell precursors. Stem Cell Res Ther. 2015;6:151–156. doi: 10.1186/s13287-015-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neviere RR, Cepinskas G, Madorin WS, Hoque N, Karmazyn M, Sibbald WJ, Kvietys PR. LPS pretreatment ameliorates peritonitis-induced myocardial inflammation and dysfunction: role of myocytes. Am J Physiol. 1999;277:H885–892. doi: 10.1152/ajpheart.1999.277.3.H885. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Zhang H, Min D, Zhang R, Wu J, Qu H, Tang Y. Sox9 Activation is Essential for the Recovery of Lung Function after Acute Lung Injury. Cell Physiol Biochem. 2015;37:1113–1122. doi: 10.1159/000430236. [DOI] [PubMed] [Google Scholar]
- 26.Miyoshi K, Yanagi S, Kawahara K, Nishio M, Tsubouchi H, Imazu Y, Koshida R, Matsumoto N, Taguchi A. Epithelial Pten controls acute lung injury and fibrosis by regulating alveolar epithelial cell integrity. Am J Respir Crit Care Med. 2013;187:262–275. doi: 10.1164/rccm.201205-0851OC. [DOI] [PubMed] [Google Scholar]
- 27.Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zahringer U, Seydel U. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 28.Kobolak J, Dinnyes A, Memic A, Khademhosseini A, Mobasheri A. Mesenchymal stem cells: identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro engineering of their niche. Methods. 2015;5:1046–2023. doi: 10.1016/j.ymeth.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Allicin Protects against Lipopolysaccharide-Induced Acute Lung Injury by Up-Regulation of Claudin-4. Trop J of Pharma Res. 2014;4:321–327. [Google Scholar]
- 30.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107:1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rokkam D, Lafemina MJ, Lee JW, Matthay MA, Frank JA. Claudin-4 levels are associated with intact alveolar fluid clearance in human lungs. Am J Pathol. 2011;179:1081–1087. doi: 10.1016/j.ajpath.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shenkar R, Coulson WF, Abraham E. Hemorrhage and resuscitation induce alterations in cytokine expression and the development of acute lung injury. Am J Respir Cell Mol Biol. 1994;10:290–297. doi: 10.1165/ajrcmb.10.3.8117448. [DOI] [PubMed] [Google Scholar]
- 33.Kao MC, Yang CH, Sheu JR, Huang CJ. Cepharanthine mitigates pro-inflammatory cytokine response in lung injury induced by hemorrhagic shock/resuscitation in rats. Cytokine. 2015;76:442–448. doi: 10.1016/j.cyto.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Galani V, Tatsaki E, Bai M, Kitsoulis P, Lekka M, Nakos G, Kanavaros P. The role of apoptosis in the pathophysiology of Acute Respiratory Distress Syndrome (ARDS): an up-to-date cell-specific review. Pathol Res Pract. 2010;206:145–150. doi: 10.1016/j.prp.2009.12.002. [DOI] [PubMed] [Google Scholar]